Abstract
There has been substantial research activity in the past decade directed at phenotyping macrophage lineages and defining macrophage functional subsets or patterns of activity. The emphasis over the past 2–3 years has been to divide macrophage functional patterns into type 1 (Th1-driven) or type 2 (Th2-driven) functions. However, a huge array of environmental factors (including cytokines, chemokines, pattern recognition receptors, hormones) differentially regulates macrophage response patterns, resulting in the display of numerous distinct, functional phenotypes. Upon stimulation, a macrophage does not display just a single set of functions but rather displays a progression of functional changes in response to the progressive changes in its microenvironment. The remarkable ability of monocytes and tissue macrophages to adapt to changes in their microenvironment challenges the thesis that macrophages displaying unique tissue-specific or response-specific, functional patterns represent distinct lineages. With the exception of mature osteoclasts and mature dendritic cells, evidence supporting stable differentiation as the basis for macrophage functional heterogeneity is equivocal. The concept of whether macrophages develop into functional subsets as opposed to continuously adapting their functional pattern in response to the changing environment of a progressive inflammatory response is important to resolve from the perspectives of therapeutic targeting and understanding the role of macrophages in disease pathogenesis.
Keywords: inflammation, cytokines, regulation, differentiation
INTRODUCTION
Macrophages are present in and provide innate immune surveillance for every tissue in the body. Macrophages are derived from myeloid precursors in bone marrow (BM), spleen, and fetal liver. Newly formed “inexperienced” macrophages, termed monocytes, leave the unique environment of the BM and enter the blood, where they are exposed to a plethora of agents [including cytokines, chemokines, adrenergic and cholinergic agonists, fatty acids, hormones, immunoglobulins (Igs)], which are capable of impacting their functional and phenotypic characteristics. These monocytes selectively home to different tissues, presumably under the influence of chemokines or other tissue-specific homing factors [1]. Upon entry into a tissue, the monocyte/macrophage migrates into the tissue parenchyma, the environment of which significantly influences the function of macrophages (Fig. 1) such that macrophages resident in different tissues display different patterns of function [2, 3]. Upon inflammatory insult to the tissue, these resident tissue macrophages can contribute to the innate immune response by expression of a variety of inflammatory and effector activities, the pattern of which is differentially regulated by the microenvironment of the different tissues.
Fig. 1.
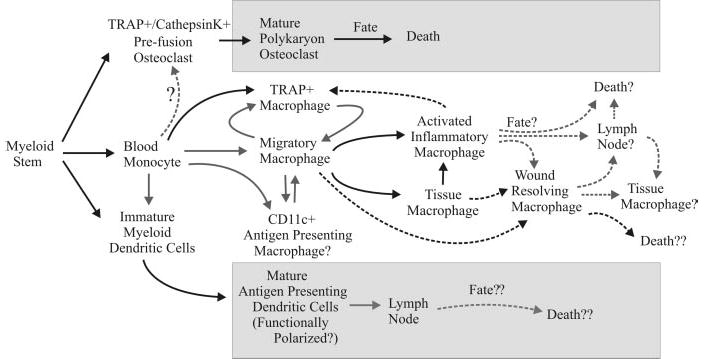
Development, migration, activation, and fate of macrophages. Shaded boxes contain pathways dependent on stable differentiation. Solid arrows indicate established pathways. Dashed arrows represent questionable pathways. TRAP, Tartrate-resistant acid phosphatase.
Macrophages are renown for their apparent phenotypic heterogeneity and for the diverse activities in which they engage [4, 5]. Many of these activities appear to be opposing in nature: proinflammatory versus anti-inflammatory activities, immunogenic versus tolerogenic activities, and tissue-destructive versus tissue-restorative activities [4, 6, 7]. Historically, until the 1990s, research on mechanisms of macrophage activation had focused on induction of inflammatory and effector functions. The discovery that T cells elaborated cytokines that contributed to macrophage activation and the identification of interferon-γ (IFN-γ) as the cytokine that enhanced macrophage cytotoxicity [8] established the concept that the quality and intensity of macrophage activation depended on the nature of the signals generated by the activating agent [e.g., Toll-like receptor (TLR) or CD40 ligation] and the modulating cytokine [9]. The development of the T helper cell type 1/2 (Th1/Th2) paradigm and the emphasis on the inflammatory and cytotoxic functions of macrophages maintained the perception that only Th1 cytokines such as IFN-γ and tumor necrosis factor α (TNF-α) enhanced macrophage activation, whereas Th2 cytokines such as interleukin (IL)-4 and IL-10 inhibited macrophage activation [4, 6].
In 1992, Stein et al. [10] published a clear demonstration that IL-4 could enhance the expression of macrophage mannose receptor. Subsequent studies established that IL-4 could up-regulate a distinctive set of genes, establishing a functional pattern quite different from that induced by IFN-γ [7]. The distinctive effect of IL-4 was underscored by coining the term “alternative activation,” emphasizing the contrast with “classical activation” by IFN-γ. The concept of alternatives to classical activation inspired a virtual avalanche of reports describing modifications of the functional pattern displayed by macrophages by treatment with IL-10, transforming growth factor-β(TGF-β), glucocorticoids, or ligation of Fc receptor for IgG (FcγR) [7, 11–13]. These reports provided insight into the physiological basis of macrophage functional heterogeneity and the major role of microenvironmental influences on macrophage function. However, the emphasis of many of the reports was to categorize the responses into two generalized groups that corresponded to the Th1/Th2 dichotomy of type 1 proinflammatory/cytotoxic response (classical) and type 2 anti-inflammatory/humoral response (alternative) [7, 11–13]. The ability of IL-4 to enhance the production of inflammatory cytokines [14] clearly erodes the concept of inflammatory versus suppressive macrophages being driven by Th1 versus Th2 cytokines, respectively. In the sections below, we present our perspective about the extent and origin of the phenotypic and functional heterogeneity within macrophage populations, the role of the tissue microenvironment in diffentially regulating macrophage function, and therapeutic targeting of macrophages in disease pathogenesis [4, 5, 15].
MACROPHAGES EXHIBIT AN ENORMOUS VARIETY OF FUNCTIONAL PATTERNS
Our research group has been examining modulation of macrophage function with just a limited number of parameters, and nonetheless, we have found over a dozen different functional patterns displayed by the macrophages (unpublished data). Gene array by Lang et al. [16] revealed that lipopolysaccharide (LPS) induced 259 of the screened genes and that IL-10 repressed 62 of these and enhanced 15 others [16]. Williams et al. [17] reported that gene array analysis indicated that treatment of human monocytes with IL-10 induced a pattern of gene expression that was different than the pattern of gene expression induced by IL-4 or TGF-β. A gene array study by Wells et al. [18] demonstrated that the pattern of LPS-inducible genes differed significantly among macrophages from C57Bl/6J, DBA/2J, and BALB/cJ mice. All of these studies have indicated that differential regulation of a large number of genes contributes to murine and human macrophage phenotypic heterogeneity and the pattern of functions expressed by macrophages.
The functional patterns described above represented static profiles of macrophages. In fact, the response of macrophages to a stimulus is not static. The study by Wells et al. [18] described abundant differences between the genes expressed early (within the first 6 h) or late (12–24 h or later) after LPS stimulation. Therefore, the functional pattern expressed by macrophages changes with time as the response progresses. It should be noted that the macrophages can be stimulated by early cytokines that they produce; so, with time, their functional pattern may shift in accordance with the cytokine milieu of their microenvironment [4]. Macrophages treated overnight with IL-4 prior to stimulation with LPS, display enhanced production of TNF-α and IL-12, in stark contrast to the reduced production of these cytokines observed upon stimulation with LPS in the presence of IL-4 [14]. We have corroborated this finding and expanded it to other cytokines, demonstrating that cytokines can synergize or antagonize in their impact on macrophages, depending on the sequence of treatment and time of activating stimulus.
Given the number of genes contributing to diversity of macrophage function, the synergistic and antagonistic effects of different cytokines and ligands on differential expression of those genes, and the number of cytokines, chemokines, hormones (including adrenergic and cholinergic agonists), TLR ligands, and other endogenous ligands (e.g., histamine, integrin ligands, peroxisome proliferator-activated receptor ligands, apoptotic cells) that modulate macrophage gene expression, it is our opinion that macrophages are capable of displaying a large number of distinct, functional patterns that have not yet been truly appreciated. An important concept to note is that the above studies clearly indicate that a macrophage will display a progression of functional changes (early and late gene expression) upon stimulation. Another important concept to consider is that identical macrophages placed in different modulating environments do not simply display different functional patterns but display different progressions of functional patterns in response to a common stimulus. From our perspective, this places restraints on our ability to establish that macrophages displaying different phenotypic or functional patterns are actually distinct lineages, as opposed to differentially regulated macrophages of common lineage. In the context of microenvironmental stimulation, the liver, burdened with clearing endotoxin and particulate material from the portal circulation [2], provides radically different environmental stimuli for macrophages compared with anti-inflammatory, privileged sites, such as the eye or the brain [3, 19].
FUNCTIONAL HETEROGENEITY OF MACROPHAGES IS A RESULT OF DIFFERENTIAL REGULATION BY THE MICROENVIRONMENT
Treatment of macrophages with cytokines such as IFN-γ or IL-4 initiates a signal cascade that results in differential modulation (enhancement or inhibition) of different genes at the transcriptional or post-transcriptional level (e.g., stabilization or destabilization of mRNA). It has been our experience that unless the signal cascade has initiated an apoptotic cascade, macrophages will eventually revert to their original, functional status after the cytokine signaling ceases. For example, in vivo or in vitro treatment of macrophages with cytokine alters their functional response pattern to LPS. However, if the macrophages are washed after cytokine treatment and held in the absence of cytokine for 1–2 days before LPS stimulation, the functional response pattern is essentially identical to that of macrophages that had not been treated with cytokine. A similar reversion to basal macrophage phenotype is observed when human monocyte-derived, immature dendritic cells (DC) are removed from IL-4 + granulocyte macrophage-colony stimulating factor (GM-CSF) and placed in a neutral environment [20]. We have been able to corroborate this observation with immature DC (CD11c+) derived by IL-4 + GM-CSF treatment of murine BM-derived and peritoneal macrophages (unpublished data). The point is that the majority of type 1 and type 2 cytokines does not seem to induce differentiation of macrophages into stable subsets but rather induces regulatory cascades that transiently alter the functional pattern of response of macrophages.
Macrophages from the lung (interstitial and alveolar), peritoneum, liver (Kupffer cells), and brain (microglia) are usually considered to be separate lineages of macrophages with distinct and unique functions [2, 3]. Originally, these populations (peritoneal macrophages being the exception) were thought to be maintained during adult life by precursors that seeded the organs during development. Current evidence indicates that the slow turnover of these long-lived populations is maintained, at least in part, by immigration of blood monocytes [2, 3]. Establishment of these populations as distinct, differentiation lineages is based on the distinct pattern of functional responsiveness and pattern of surface molecules expressed [2, 3]. There does not appear to be a single membrane molecule that by itself could phenotypically distinguish these tissue macrophage populations. A comparative summary of the functional and phenotypic characteristics of these populations, adapted from Guillemin and Brew [3], Hanisch [21], and Laskin et al. [2], is presented in Table 1. The majority of differences is quantitative differences in level of expression of a molecule. Thus, each population displays a unique functional and phenotypic pattern. However, the macrophages within each of these populations do not uniformly display the same functional and phenotypic pattern. Significant heterogeneity exists within the macrophage population of each tissue. For example, the functional phenotype of Kupffer cells depends on their proximity to the portal vein [2]. Density gradient fractionation of lung interstitial and alveolar macrophage populations yields subpopulations differing in phenotype (e.g., degree of expression of FcγR and class II MHC) and phagocytic function [2]. Each population is fully capable of changing its functional pattern, as evidenced by the response to infectious or inflammatory insult [2, 3, 22]. The most dramatic are the microglia that display a ramified morphology and support neuronal survival by producing cytokines such as brain-derived neurotrophic factor and TGF-β [21, 23]. In vitro or during inflammatory responses in the brain, microglia lose their characteristic morphology, become migratory, and produce abundant oxidative radicals and inflammatory cytokines [3, 22]. How many of the distinctions between the various tissue macrophage populations are a result of reversible adaptation to the microenvironment of the tissue? If these macrophage populations are placed in identical microenvironments for several days, how many of the functional and phenotypic differences will be retained? As stated in the previous section, each of these tissues provides different environmental stimuli for macrophages. As stable acquisition of a trait is the hallmark of differentiation, we currently are pursuing this line of experimentation in an attempt to determine which of the functional traits of these tissue macrophages are a result of reversible adaptation to the host tissue’s microenvironment and which are a result of differentiation.
TABLE 1.
Phenotypic and Functional Comparison of Macrophages from Different Tissues
| Characteristic | Elicited peritoneal macrophages | Alveolar macrophages | Kupffer cells | Blood-derived macrophages | Activated microglial cells |
|---|---|---|---|---|---|
| CD11b | high | low | high | minimal | minimal |
| CD14 | low | low | minimal | high | minimal |
| CD68 | high | high | high | high | high |
| FcγR | high | low | low | low | low |
| MHC-II | high | low | minimal | low | low |
| Ag presentation | high | low | low | yes | |
| Esterase | low | high | low | low | minimal |
| Phagocytosis | high | low | high | low | low |
| IFN-γ | high | low | low | yes | |
| TNF-α | high | high | low | yes | |
| IL-1 | high | low | low | yes | |
| IL-6 | high | low | low | yes | |
| MIP-1 | low | high | low | yes | |
| MCP-1 | high | low | low | yes | |
| IL-10 | high | ? | minimal | yes |
The functional activities of elicited macrophages, Kupffer cells, and alveolar cells are adapted from the review by Laskin et al. [2] and blood-derived macrophages and microglial cells, from the reviews by Guillemin and Brew [3] and Hanisch [21]. MHC, Major histocompatibility complex; Ag, antigen; MIP-1, macrophage-inflammatory protein-1; MCP-1, monocyte chemoattractant protein-1.
Osteoclasts are the clearest example of a distinct, differentiated lineage of macrophage. Trance (receptor activator of nuclear factor-κB ligand) is a potent inducer of osteoclasto-genesis [24] and apparently, also the interesting synergistic pairing of TNF-α and TGF-β [25]. The key genes that are expressed uniquely in osteoclasts are for the TRAP, calcitonin receptor, and cathepsin K [24]. The end-stage cell is clearly differentiated in that it is the polykaryon product of cell fusion that has developed clear cell-body polarity. However, macrophages can be readily driven to express the osteoclast genes and display bone-resorbing activity (Fig. 1) [25]. Can prefusion, single-cell osteoclasts removed from bone revert to a macrophage functional phenotype given the appropriate environment? The role of prefusion osteoclasts versus macrophages with bone-resorption activity in atherogenic plaque decalcification is addressed below.
DIFFERENTIAL REGULATION OF MACROPHAGES DURING INFLAMMATORY DISEASE
The concept of whether macrophages develop into functional subsets as opposed to continuously adapting their functional pattern in response to the changing environment of a progressive inflammatory response is important to resolve from the perspectives of therapeutic targeting and understanding the role of macrophages in disease pathogenesis. In the example of a simple inflammatory response, the early stages are dominated by macrophages displaying inflammatory and tissue-destructive activities (including metalloproteinase and oxidative radicals), and the late stages are dominated by macrophages displaying tissue-restructuring activities. Do these represent two distinct, sequential cellular infiltrates or a single infiltrate that progressively changes its function (Fig. 1)? The answer could increase our understanding of the anti-inflammatory efficacy of corticosteroids and could impact how we approach therapy of chronic inflammation. A second example is the tumor-associated “suppressor” macrophage [26]. Many murine and human tumors promote the development of macrophages whose functional activities are anti-inflammatory, tolerogenic, and proangiogenic [26]. If these are a developmental subset of macrophages, one approach to tumor therapy would be to eliminate these suppressor macrophages. However, if the macrophages are displaying an anti-inflammatory and proangiogenic, functional pattern as an adaptive response to IL-10, TGF-β, or other tumor products in the environment, one therapeutic approach would be to convert these macrophages to a proinflammatory, proimmunogenic, functional pattern by targeting the regulatory factors in the tissue environment. A third example is found in atherogenesis. Calcification of the atheroma appears to be controlled by “osteoblast-like” and “osteoclast-like” cells [27]. Does the environment of the atheroma secrete chemokines that attract osteoblasts and prefusion osteoclasts or does it promote macrophage expression of a functional pattern that includes expression of TRAP and cathepsin K? A fourth example is the deterioration of macrophage function resulting from immunosenescence. We have found that macrophages from aged mice are capable of responding to stimuli such as LPS or CD40 ligation. The difference we observe between peritoneal and splenic macrophages from young and aged mice is that the macrophages from aged mice display a different functional pattern upon activation compared with macrophages from young mice. Removing the macrophages from the aged environment results in a change in functional pattern to one that is more similar to the functional pattern displayed by macrophages from young mice–i.e., the macrophages “reset” or recover from the regulatory influence of the aged environment. This suggests that targeting the regulatory factors of the aged environment might restore, at least transiently, inflammatory and proimmunogenic function of macrophages in the elderly, a concept that would not have been considered from the perspective that macrophages differentiate into distinct, functional subsets during inflammatory responses. A final example addresses whether microglia are differentiated or differentially regulated by the central nervous system (CNS) environment. This impacts on the question of why chronic relapsing-remitting multiple sclerosis converts into a secondary, progressive, fatal disease. Did the immune system dysregulate or did the CNS lose its ability to regulate the pattern of functions expressed by its glial elements [19, 23, 28]?
CONCLUDING REMARKS
We postulate that a serious effort to categorize macrophage functional patterns will reveal a plethora of distinct phenotypes based on the many ligands that can alter macrophage responsiveness and phenotype, the antagonistic or synergistic interactions between these ligands, and the changing effects of these ligands depending on the temporal relationship of modulating versus activating ligands. All of these microenvironmental influences on macrophages are likely to be major contributing causes to macrophage heterogeneity in tissues and in inflammation. Considering the progressive/regressive course of inflammatory lesions from initiation to resolution, it is likely that an inflamed tissue would contain macrophages displaying a wide variety of functional patterns, representing different stages of their evolving response to a changing microenvironment. Therefore, although definitions of the many functional phenotypes of macrophages may have scientific and diagnostic value, the perspective should be maintained that macrophages are not static and that the functional and phenotypic changes occurring during inflammatory responses may not be differentiation into stable, defined, functional phenotypes but an evolving shift of functional activities in response to a changing environment. Even if tissue macrophages all represent distinct myeloid lineages, the heterogeneity observed within each population underscores that they too undergo progressive shifts in functional activities in response to regulatory oscillations in their environment. In this context, defining macrophage subsets by membrane protein and functional phenotype during an inflammatory episode might be akin to defining chameleons by the color pattern they display as they move across an artist’s paint palette.
Acknowledgments
This research was supported by grants from the National Institute for Aging (R. D. S.), the Kentucky Lung Cancer Research Fund (R. D. S.), the National Institute of Allergy and Infectious Disease (J. S.), the National Multiple Sclerosis Society (J. S.), the Arthritis Foundation (J. S.), and the Commonwealth of Kentucky Research Challenge Trust Fund (R. D. S. and J. S).
References
- 1.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 2.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70:163–170. [PubMed] [Google Scholar]
- 3.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 4.Stout, R. D., Suttles, J. (1995) T cell signaling of macrophage activation. Cell Contact-Dependent and Cytokine Signals, Austin, TX, R. G. Landes, Springer-Verlag.
- 5.Gordon S, Fraser I, Nath D, Hughes D, Clarke S. Macrophages in tissues and in vitro. Curr Opin Immunol. 1992;4:25–32. doi: 10.1016/0952-7915(92)90119-y. [DOI] [PubMed] [Google Scholar]
- 6.Stout RD, Suttles J. T cell signaling of macrophage function in inflammatory disease. Front Biosci. 1997;2:d197–d206. doi: 10.2741/a183. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber RD. Identification of γ-interferon as a murine macrophage-activating factor for tumor cytotoxicity. Contemp Top Immunobiol. 1984;13:171–198. doi: 10.1007/978-1-4757-1445-6_9. [DOI] [PubMed] [Google Scholar]
- 9.Adams, D. O., Hamilton, T. A. (1992) Molecular basis of macrophage activation: diversity and its origins. In The Macrophage (C. E. Lewis, J. O. McGee, eds.), Oxford, UK, Oxford University Press, 75–114.
- 10.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209 –212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 12.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 13.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. [Google Scholar]
- 14.D’Andrea A, Ma X, Aste Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor α production. J Exp Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKnight AJ, Gordon S. Membrane molecules as differentiation antigens of murine macrophages. Adv Immunol. 1998;68:271–314. doi: 10.1016/s0065-2776(08)60562-3. [DOI] [PubMed] [Google Scholar]
- 16.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 17.Williams L, Jarai G, Smith A, Finan P. IL-10 expression profiling in human monocytes. J Leukoc Biol. 2002;72:800–809. [PubMed] [Google Scholar]
- 18.Wells CA, Ravasi T, Faulkner GJ, Carninci P, Okazaki Y, Hayashizaki Y, Sweet M, Wainwright BJ, Hume DA. Genetic control of the innate immune response. BMC Immunol. 2003;4:5–23. doi: 10.1186/1471-2172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka KF, Kashima H, Suzuki H, Ono K, Sawada M. Existence of functional β1- and β2-adrenergic receptors on microglia. J Neurosci Res. 2002;70:232–237. doi: 10.1002/jnr.10399. [DOI] [PubMed] [Google Scholar]
- 20.Hausser G, Ludewig B, Gelderblom HR, Tsunetsugu-Yokota Y, Akagawa K, Meyerhans A. Monocyte-derived dendritic cells represent a transient stage of differentiation in the myeloid lineage. Immunobiology. 1997;197:534–542. doi: 10.1016/S0171-2985(97)80085-X. [DOI] [PubMed] [Google Scholar]
- 21.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 23.Kempermann G, Neumann H. Neuroscience. Microglia: the enemy within? Science. 2003;302:1689–1690. doi: 10.1126/science.1092864. [DOI] [PubMed] [Google Scholar]
- 24.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 25.Fox SW, Fuller K, Bayley KE, Lean JM, Chambers TJ. TGF-β 1 and IFN-γ direct macrophage activation by TNF-α to osteoclastic or cytocidal phenotype. J Immunol. 2000;165:4957–4963. doi: 10.4049/jimmunol.165.9.4957. [DOI] [PubMed] [Google Scholar]
- 26.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 27.Doherty TM, Uzui H, Fitzpatrick LA, Tripathi PV, Dunstan CR, Asotra K, Rajavashisth TB. Rationale for the role of osteoclast-like cells in arterial calcification. FASEB J. 2002;16:577–582. doi: 10.1096/fj.01-0898hyp. [DOI] [PubMed] [Google Scholar]
- 28.De Keyser J, Zeinstra E, Mostert J, Wilczak N. β 2-Adrenoceptor involvement in inflammatory demyelination and axonal degeneration in multiple sclerosis. Trends Pharmacol Sci. 2004;25:67–71. doi: 10.1016/j.tips.2003.12.002. [DOI] [PubMed] [Google Scholar]


