Abstract
Following up on our previous findings that the skin possesses steroidogenic activity from progesterone, we now show widespread cutaneous expression of the full cytochrome P450 side-chain cleavage (P450scc) system required for the intracellular catalytic production of pregnenolone, i.e. the genes and proteins for P450scc enzyme, adrenodoxin, adrenodoxin reductase and MLN64. Functionality of the system was confirmed in mitochondria from skin cells. Moreover, purified mammalian P450scc enzyme and, most importantly, mitochondria isolated from placenta and adrenals produced robust transformation of 7-dehydrocholesterol (7-DHC; precursor to cholesterol and vitamin D3) to 7-dehydropregnenolone (7-DHP). Product identity was confirmed by comparison with the chemically synthesized standard and chromatographic, MS and NMR analyses. Reaction kinetics for the conversion of 7-DHC into 7-DHP were similar to those for cholesterol conversion into pregnenolone. Thus, 7-DHC can form 7-DHP through P450scc side-chain cleavage, which may serve as a substrate for further conversions into hydroxy derivatives through existing steroidogenic enzymes. In the skin, 5,7-steroidal dienes (7-DHP and its hydroxy derivatives), whether synthesized locally or delivered by the circulation, may undergo UVB-induced intramolecular rearrangements to vitamin D3-like derivatives. This novel pathway has the potential to generate a variety of molecules depending on local steroidogenic activity and access to UVB.
Keywords: 7-dehydrocholesterol, 7-dehydropregnenolone, cytochrome P450scc, skin, ultraviolet radiation
Abbreviations: 7-DHC, 7-dehydrocholesterol; 7-DHP, 7-dehydropregnenolone; P450scc, cytochrome P450 side-chain cleavage; FDX1, adrenodoxin; FDXR, adrenodoxin reductase; MO-TMS, methyloxime-trimethylsilyl
The skin, the largest body organ, maintains internal homeostasis by not only separating the external environment from the internal milieu, but also through its immune and neuroendocrine activities [1–3]. Cutaneous elements can in addition have powerful systemic actions as is the case for vitamin D3 [1], which regulates calcium metabolism, and modulates immune and neuroendocrine activities and proliferation and differentiation in cells of different lineages [4–6]. Vitamin D3 is formed from the precursor steroid 7-dehydrocholesterol (7-DHC) localized mostly on the plasma membrane of basal epidermal keratinocytes (80% of skin 7-DHC content). Upon stimulation with photons of UVB (wavelength 290–320 nm), 7-DHC undergoes photolysis to generate previtamin D3, which, at normal skin temperature, undergoes internal rearrangement to vitamin D3 [4,7].
Cytochrome P450 side-chain cleavage (P450scc) is a product of the CYP11A1 locus thought until recently to use solely cholesterol as substrate, which is then hydroxylated and cleaved on the side chain. The reaction takes place at a single active site on the cytochrome to produce pregnenolone [8]. Electrons for the hydroxylations are provided by NADPH through the electron transfer proteins adrenodoxin reductase and adrenodoxin [8,9]. This biochemical pathway may be operative in the skin, as it expresses the related CYP11A1, CYP17, CYP21A2 and MC2-R genes [10]. Furthermore, skin and skin cells can rapidly and selectively metabolize progesterone and deoxycorticosterone to a number of intermediates that include deoxycorticosterone, 18-hydroxy deoxycorticosterone and corticosterone, consistent with active local steroidogenesis [11–14].
Interest in the P450scc system has been renewed by recent findings in patients with the rare Smith–Lemli–Opitz syndrome whose cholesterol synthesis from 7-DHC is impaired because of a deficiency of the 7-DHC Δ7 reductase [15,16]. Patients with Smith–Lemli–Opitz syndrome accumulate 7-DHC and also have noticeable amounts of 7-dehydropregnenolone (7-DHP) (and its metabolites) suggesting enzymatic production from 7-DHC [17,18]. Furthermore, in most recent studies with an in vitro system of reconstituted P450scc, 7-DHC and vitamin D3 were found to serve as alternative substrates for cytochrome P450scc [19].
Within the context above, the skin presents the unique situation of having readily available all the potential substrates for P450scc, e.g. cholesterol, 7-DHC and vitamin D3, thus providing the background for a systematic investigation on the cutaneous expression of each of the components of the P450scc enzymatic system. In addition, we tested reconstituted and mitochondrial P450scc systems for their ability to convert 7-DHC into 7-DHP.
Materials and methods
Biological materials
Tissue.
Human skin and placenta were obtained from discarded biopsy material or surgical specimens, or after delivery. The corresponding protocols were reviewed and approved by the University of Tennessee Institutional Review Board as an exempt protocol [under 45CFR46.102(F)] entitled ‘Skin as a neuroendocrine organ’ with original IBR date of approval of 19 July 2000.
RNA from C57BL/6 mice was isolated at the Albany Medical College and stored at −80 °C. The skin and internal organs were harvested from female C57BL/6 mice aged 8 weeks at telogen and anagen stages of the hair cycle as described previously [20]. Adrenals were obtained from Wistar rats killed under anesthesia. Male rats aged 3 months were obtained from the vivarium of the Department of Biotestings of Bioorganic Chemistry Institute (Minsk, Belarus) (detailed protocols were described previously) [21]. The Institutional Animal Care and Use Committee at AMC approved the original protocol, and a similar protocol for mice was approved at UTHSC; for LC/MS assays the experiments were approved by the Belarus University Animal Care and Use Committee.
Cell lines.
Cultures of normal and immortalized keratinocytes, dermal fibroblasts, melanocytes and melanoma cells were carried out according to standard protocols described previously [12,22,23]. Normal human epidermal keratinocytes and melanocytes, and dermal fibroblasts were obtained from Cascade Biologics, Inc. (Portland, OR, USA) and cultured as described previously [24].
Mitochondrial fractions and enzymes.
Mitochondrial fractions of the test tissue (skin, adrenals or placenta) were prepared by homogenizing the tissue in 5 vols ice-cold 0.25 m sucrose containing protease inhibitor cocktail (Sigma) [25]. The homogenate was centrifuged at 600 g for 10 min at 4 °C, and the resulting supernatant was centrifuged at 6000 g (placenta) or 9000 g (skin and adrenals) for 20 min at 4 °C to sediment the mitochondrial fraction. The pellet was resuspended in 0.25 m sucrose, and the mitochondrial fraction was again sedimented under the same conditions. The washed mitochondrial fraction was resuspended in 0.25 m sucrose and used for enzymatic reaction. For cultured skin cells, the above procedure was carried out after the cells had been swelled for 30 min in 20 mm HEPES, pH 7.4, before homogenization.
Bovine cytochrome P450scc, adrenodoxin and adrenododoxin reductase were isolated from adrenals [26,27]. Human cytochrome P450scc and adrenodoxin were expressed in Escherichia coli and purified as described before [28].
Synthesis of 7-DHP
The 7-DHP standard was synthesized from pregnenolone acetate following protocols described in [29]. The chemical structure of the standard had been confirmed by NMR analysis. The standard was further purified by RP-HPLC and stored at −70 °C.
Enzymatic assays
Side-chain cleavage of 7-DHC.
Large-scale reactions (50 mL) to cleave the side chain of 7-DHC were performed with purified bovine P450scc and its electron- transfer system in a manner similar to that described for cholesterol [28]. The incubation mixture comprised 510 μm phospholipid vesicles (dioleoyl phosphatidylcholine plus 15 mol% cardiolipin) with a substrate to phospholipid molar ratio of 0.2, 50 μm NADPH, 2 mm glucose 6-phosphate, 2 U·mL−1 glucose 6-phosphate dehydrogenase, 0.3 μm adrenodoxin reductase, 6.5 μm adrenodoxin, 1.0 μm cytochrome P450scc and buffer, pH 7.4 [28]. After incubation at 37 °C for 3 h, the mixture was extracted three times with 50 mL methylene chloride and dried under nitrogen at 35 °C. Products were purified by preparative TLC on silica gel G with three developments in hexane/ethyl acetate (3 : 1, v/v) and products eluted from the silica gel with chloroform/methanol (1 : 1, v/v). Samples were dried under nitrogen and shipped for analysis on dry ice. Small-scale reactions (0.25 mL) to determine the kinetics of 7-DHC and cholesterol metabolism were performed with either bovine or human cytochrome P450scc as described for cholesterol [28]. The amount of 7-DHP produced from 7-DHC was measured by RIA [25] using purified 7-DHP as standard.
Side-chain cleavage by mitochondria isolated from skin cells.
[4-14C]Cholesterol (58 mCi·mmol−1; Amersham Bioscience) was purified before its use as a substrate by mitochondria, by TLC on silica gel G plates in hexane/acetone (7 : 3, v/v). Isolated mitochondria (0.5 mg protein) were then preincubated (15min at 37 °C) with purified [4-14C] cholesterol (1 μCi, 34 μm) in 0.5mL medium comprising 0.25 m sucrose, 50 mm HEPES, pH 7.4, 20 mm KCl, 5 mm MgSO4, 0.2mm EDTA 0.4 μm adrenodoxin reductase, 6 μm adrenodoxin, 5 μm N-62 StAR protein (gift from W. Miller, University of California, San Francisco, CA, USA) and 8 μm cyanoketone. The reaction was started by adding NADPH (0.5 mm) and isocitrate (5 mm), and samples were incubated at 37 °C for 150 min. The reaction was stopped by the addition of 1 mL ice-cold methylene chloride, and the incubation mixture extracted twice more with 1 mL methylene chloride. The fractions were combined, dried under nitrogen, and subjected to TLC on silica gel G plates and developed with hexane/acetone (7 : 3, v/v). Radiolabelled products were visualized using a phosphoimager, the steroids eluted from the plate with chloroform/methanol (1 : 1, v/v), and the associated radioactivity measured by scintillation counting.
Side-chain cleavage of 7-DHC by placental and adrenal mitochondria.
Incubations were carried out as described for skin mitochondria except that radiolabelled cholesterol was replaced with 200 μm 7-DHC, exogenous adrenodoxin and adrenodoxin reductase were not added, and the incubation volume was 1.0 mL (placenta) or 0.5 mL (adrenal). Extracted products from placenta incubation were analyzed by TLC on silica gel G plates developed three times with hexane/ethyl acetate (3 : 1, v/v) and visualized by charring; products from adrenal incubations were dissolved in methanol and subjected to LC/MS analysis.
RT-PCR amplifications
Tissues and cells were homogenized in Trizol (Invitrogen), and the isolation of RNA followed the manufacture’s protocol. The synthesis of first-strand cDNA was performed using the Superscript preamplification system (Invitrogen). Either 5 μg of total or 0.05 μg of poly(A) mRNA per reaction was reverse-transcribed according to the manufacturer’s protocol using oligo(dT) as the primer.
All samples were standardized for the analysis by amplification of the housekeeping gene GAPDH as described previously [30]. Human and mouse CYP11A1, FDX1 and FDXR cDNAs were routinely amplified by a single PCR (30 cycles), and in selected experiments human CYP11A1 was also amplified by nested PCR. The sequence and exonal localization of the primers in the corresponding genes are presented in Table 1. The reaction mixture (25 μL) contained 2.5 mm MgCl2, 0.25mm each dNTP, 0.4 μm each primer, 75 mm Tris/HCl (pH 8.8), 20 mm (NH4)2SO4, 0.01% (v/v) Tween 20 and 1.25 U Taq polymerase (Promega). The mixture was heated to 94 °C for 2.5 min, and then amplified for 30 cycles as specified: 94 °C for 30 s (denaturation), 55 °C for 20 s (annealing), and 72 °C for 40 s (extension). For nested PCR an aliquot was transferred to a new tube for amplification with the nested pair of primers.
Table 1. Primer sequences.
| Gene | Primers | Primer sequences | Primer location | Amplified band (bp) |
|---|---|---|---|---|
| Human genes | ||||
| FDX1 | P553 | GTGATTCTCTGCTAGATGTTG | Exon 2 | 257 |
| P554 | GGCACTCGAACAGTCATATTG | Exon 4 | ||
| FDXR | P557 | ATTAAGGAGCTTCGGGAGATG | Exon 7 | 380 |
| P558 | CTCTTATACCCAATGCTGCTG | Exon 10 | ||
| CYP11A | First pair | |||
| P561 | GCCTTTGAGTCCATCACTAAC | Exon 4 | 628 | |
| P562 | CCAGTGTCTTGGCAGGAATC | Exon 8 | ||
| Nested pair | ||||
| P563 | ATGTGGCTGCATGGGACGTG | Exon 4 | 390 | |
| P564 | TCTGCAGGGTCACGGAGATG | Exon 7 | ||
| Mouse genes | ||||
| FDX1 | P581 | AAATTGGCGACTCTCTGCTAG | Exon 2 | 295 |
| P582 | CTTGCTCATGTCAACAGACTG | Exon 4 | ||
| FDXR | P583 | CTTGGAGTCATCCCCAACAC | Exon 10 | 281 |
| P584 | TGGCCTCGAGAGACTTCCTC | Exon 12 | ||
| CYP11A | P585 | AGACTTCTTTCGACTCCTCAG | Exon 4 | 693 |
| P586 | CTGAAGTTCTCCAGCAGATTG | Exon 8 | ||
Amplification products were separated by agarose electrophoresis and visualized by ethidium bromide staining [30]. The identified PCR products were excised from the agarose gel and purified using a GFX PCR DNA and gel band purification kit (Amersham-Pharmacia-Biotech). PCR fragments were cloned in pGEM-T easy vector system (Promega) and purified with a plasmid purification kit (Qiagen). Sequencing was performed at the Molecular Resource Center at the University of Tennessee HSC (Memphis, TN, USA) using Applied Biosystems 3100 Genetic Analyzer and BigDyeTM Terminator Kit.
Western blotting
The methodology followed standard protocols described in our laboratories [21,31]. Briefly, mitochondrial fractions prepared as described above for detection of P450scc or adrenodoxin reductase or proteins extracted with 1% (v/v) Triton X-100 (to test StAR expression) from placenta, skin or cultured cells were dissolved in Laemmli buffer and separated on an SDS/12% polyacrylamide gel, transferred to an Immobilon P [poly(vinylidene difluoride)] membrane (Millipore Corp, Bedford, MA, USA); nonspecific binding sites were blocked by incubation in 5% (w/v) nonfat powdered milk in buffer containing 50 mm Tris/HCl, pH 7.5, 150 mm NaCl, and 0.01% (v/v) Tween-20, for 3 h at room temperature. Membranes were incubated overnight at 4 °C with 4182 A. Slominski et al. (Eur. J. Biochem. 271) polyclonal antisera raised in rabbits as follows: anti- (bovine P450scc) diluted 1 : 1000, anti-(porcine adrenodoxin reductase) diluted 1 : 1000, or anti-StAR protein diluted 1 : 2000 [32]. In parallel incubations, nonimmune serum was used as the control. Next day, membranes were washed and incubated for 1 h with goat anti-rabbit IgG coupled to horseradish peroxidase, diluted 1 : 10000 (Santa Cruz Biotechnology). Membranes were washed, and bands were visualized with ECL reagent (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. For the blots with anti-StAR serum the secondary antibody was coupled to alkaline phosphatase (1 : 2000 dilution) and color developed as before [32].
NMR
Samples were dissolved in CDCl3 (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) and referenced to the residual solvent signal (δ7.24 p.p.m). Proton and proton-detected 2D spectra (gradient-enhanced correlation spectroscopy, gradient heteronuclear multiple quantum coherence and gradient heteronuclear multiple bond correlation) were recorded on a Bruker DRX 500-MHz NMR spectrometer equipped with a Nalorac 3 mm inverse Z-axis gradient probe (MIDG-500). Carbon and distortionless enhancement by polarization transfer spectra were recorded on a Varian Unity Inova 600-MHz spectrometer equipped with a Nalorac 3 mm direct detect probe (MDBC600F). The NMR data were processed using XWINNMR 3.5 running on Red Hat Linux 7.3.
GC/MS analysis
Derivatization of the products of 7-DHC metabolism was carried out using a modified version of previously published methods [17,18,33]. The methyloxime-trimethylsilyl (MO-TMS) derivatives were dissolved in 200 μL cyclohexane and transferred to the autosampler vial. GC/MS was carried out on a 5890 gas chromatograph coupled with a 5971 MSD (Hewlett-Packard, Palo Alto, CA, USA) equipped with a DB-1 cross-linked methyl silicone column (15 m ×0.25 mm internal diameter; film thickness 0.25 μm; J&W Scientific, Folsom, CA, USA). Other conditions were as described elsewhere [17,18].
LC/MS analysis
RP-HPLC and MS analysis was performed on a high-performance liquid chromatography mass spectrometer LCMS-QP8000α (Shimadzu, Tokyo, Japan) equipped with a Restec Allure C18 column (150 ×4.6 mm; 5 μm particle size; 60 Å pore size), UV/VIS photodiode array detector (SPD-M10Avp) and quadrupole mass spectrometer. The LC/MS workstation class-8000 software was used for system control and data acquisition (Shimadzu). Elution was carried out isocratically at a flow rate of 0.5 mL·min−1 and temperature of 40 °C. The mobile phase from 0 to 30 min consisted of 85% (v/v) methanol and 0.1% (v/v) acetic acid, and from 30 to 75 min of 98%(v/v) methanol and 0.1%(v/v) acetic acid. The mass spectrometer was operated in atmospheric pressure chemical ionization; positive ion mode was used with nitrogen as the nebulizing gas. The MS parameters were as follows: nebulizer gas flow rate 2.5 L·min−1; probe high voltage 3.5 kV; probe temperature 300 °C; curved desolvation line heater temperature 230 °C. Analyses were carried out in the scan mode from m/z 310–415.
Results and Discussion
For cholesterol side-chain cleavage to proceed in vivo, P450scc must receive electrons from NADPH, via the proteins adrenodoxin reductase and adrenodoxin [8], and cholesterol, via transport by StAR protein or MLN64 [34,35]. In agreement with our previous detection of CYP11A1 gene expression in human skin biopsy samples [10], we have now documented expression of the gene coding for P450scc (CYP11A1) by direct PCR (30 cycles) in a wider assortment of human skin samples (transcript of 628 bp; Table 1). These include skin biopsy specimens, subcutaneous adipose tissue, epidermal and dermal cell lines [normal epidermal keratinocytes, immortalized keratinocytes (HaCaT), dermal fibroblasts, squamous cell carcinoma, five human melanomas at different levels of progression; not shown]. Nested RT-PCR revealed general CYP11A1 gene expression; it was below the level of detectability only in human epidermal melanocytes and in a single melanoma line (SKMEL-188; representative panel Fig. 1A). The lower band detected in keratinocytes, squamous cell carcinoma and melanoma cells (Fig. 1A, lanes 2, 3, 8, 10 and 11) represents an additional alternatively spliced CYP11A1 isoform of 229 bp (GeneBank No. AY603498). Again, direct RT-PCR (30 cycles) showed expression of CYP11A1 in anagen and telogen murine skin and the Cloudman S91 mouse melanoma line (Fig. 1D). The genes for adrenodoxin (FDX1) and adrenodoxin reductase (FDXR) were consistently expressed in all samples tested by direct PCR (30 cycles) (Fig 1B,C,E,F). In Fig. 1C, in addition to the correct transcript of 380 bp (confirmed by sequencing), there are additional bands that may represent either alternatively spliced variants or nonspecific DNA fragments (these bands were not sequenced). Figure 2 shows that the corresponding mRNAs have been further translated into proteins producing immunoreactive species recognized by specific antibodies. These immunoreactive products had molecular masses compatible with those expected for processed P450scc (50–55 kDa; Fig. 2A,B) and adrenodoxin reductase (48 kDa; Fig. 2C). These panels are representative of several experiments performed with extracts from tissues, and cultured skin cells of normal, immortalized or malignant origin. As regards the protein components of P450scc, these were detected in control placenta, whole human skin, normal epidermal and immortalized keratinocytes, dermal fibroblasts, squamous cell carcinoma and five human melanomas. Thus, these data clarify in detail the cutaneous expression of the P450scc system; they also amplify and extend recent information on an active P450scc system present in immortalized sebocytes, and on detection of P450scc by immunocytochemistry in human epidermis and hair follicle [36].
Fig. 1. Nested RT-PCR showing that human and mouse skin express P450scc (CYP11A1) (A, D), adrenodoxin (FDX1) (B,E) and adrenodoxin reductase (FDXR) genes (C,F).
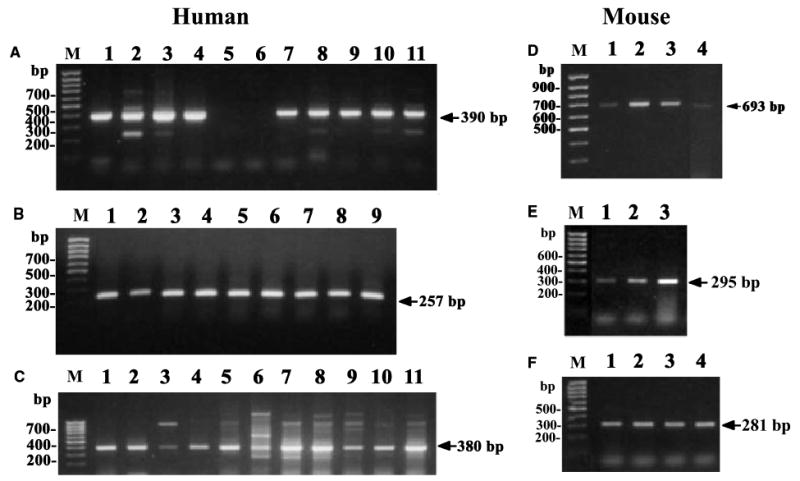
(A–C)Human samples; (D–F) mouse samples. Arrows indicate size of amplified message. DNA ladder is marked M. (A) HaCaT keratinocytes (lane 1); normal epidermal keratinocytes (lane 2); C1–4 squamous cell carcinoma (lane 3); dermal fibroblasts (lane 4); epidermal melanocytes (lane 5); melanoma lines SKMEL-188 (lane 6); SBCE2 (lane 7); WM35 (lane 8); WM98 (lane 9); WM164 (lane 10) and WM1341D (lane 11). (B) HaCaT keratinocytes (lane 1); normal epidermal keratinocytes (lane 2); dermal fibroblasts (lane 3); epidermal melanocytes (lane 4); C1–4 squamous cell carcinoma (lane 5); melanoma lines SKMEL-188 (lane 6); SBCE2 (lane 7); WM35 (lane 8); WM98 (lane 9). (C) HaCaT keratinocytes (lane 1); normal epidermal keratinocytes (lane 2); C1–4 squamous cell carcinoma (lane 3); dermal fibroblasts (lane 4); epidermal melanocytes (lane 5); melanoma lines SKMEL-188 (lane 6); SBCE2 (lane 7); WM35 (lane 8); WM98 (lane 9); WM164 (lane 10) and WM1341D (lane 11). (D,F) Pituitary (lane 1); anagen skin (lane 2); telogen skin (lane 3); S91 melanoma (lane 4). (E) Anagen skin (lane 1); telogen skin (lane 2); S91 melanoma (lane 3).
Fig. 2. Expression of P450scc protein (A and B) and adrenodoxin reductase (C) in human skin.
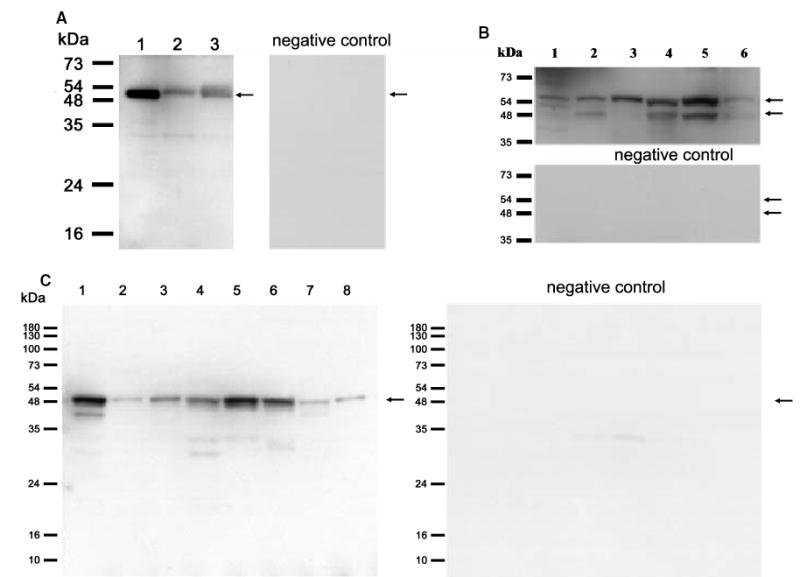
Blots incubated with specific antibodies are on the left (A and C) or upper (B) panels, while controls (primary antibody omitted) are on the right (A and C) or bottom (B). Size of molecular mass markers is on the left, and arrows indicate immunoreactive proteins. (A) Placenta (lane 1); skin from white (lane 2) or black (lane 3) patients. (B) HaCaT keratinocytes (lane 1); C1–4 squamous cell carcinoma (lane 2); dermal fibroblasts (lane 3); normal epidermal keratinocytes (lane 4); melanoma lines WM1341D (lane 5) and SBCE2 (lane 6). (C) Placenta (lane 1); skin from white (lane 2) or black (lane 3) patients; melanoma WM 35 (lane 4); normal epidermal keratinocytes (lane 5); HaCaT keratinocytes (lane 6); C1–4 squamous cell carcinoma (lane 7); dermal fibroblasts (lane 8).
The cholesterol substrate for P450scc is transported into mitochondria by specific cholesterol-transporting proteins, StAR in testis, adrenal and ovary, and probably MLN64 in the placenta [34,35,37]. Cholesterol transport by MLN64 in mitochondria may require proteolytic processing to release the 27-kDa cholesterol-binding domain from the full-length form associated with late endosomes [35,37]. There is also evidence that the full-length (55 kDa) form of MLN64 is associated with placental mitochondria [38]. Using specific antibodies that recognize a common epitope for both MLN64 and StAR [35], we detected the expected protein (arrow) in the 48–55-kDa range corresponding to MLN64 [38] in placenta, human skin, and human, mouse and hamster melanoma cells (Fig. 3). Two major bands in the size range 48–55 kDa are present in the placenta, as reported previously [37]. The multiple bands are believed to result from proteolytic processing. These bands and other smaller ones are also seen when the MLN64 gene is transfected into COS-1 cells [37]. The relative proportions of the two bands in the 48–55-kDa range in human skin are similar to that in the human placenta (Fig. 3, lanes 2 and 3), but the proportion varies in the different cell types tested, indicating different levels of processing. The additional immunoreactive proteins of lower molecular mass (37 kDa and 18 kDa) present in some melanoma lines represent either further products of MLN54 processing [35,37] and/or the full-length StAR protein [34].
Fig. 3. Expression of MLN64 protein (arrow) in human skin (left) and human and rodent skin cells (right).
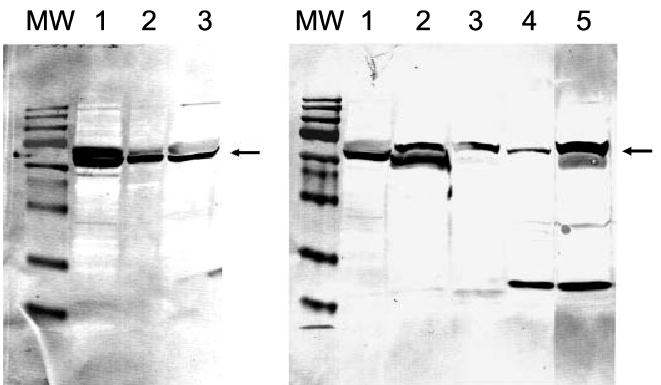
Molecular mass (MW) markers are 180, 130, 73, 54, 48, 35, 24, 16 and 10 kDa. Left: placenta (lanes 1 and 2); skin (lane 3). Right: human SBCE2 (lane 1), WM35 (lane 2), hamster AbC-1 (lane 3) and mouse S-91 (lane 4) melanomas; placenta (lane 5). The amount of protein loaded on gels was 5 and 1 μg for placenta (lanes 1 and 2, respectively) and 20 μg for the skin samples.
Lastly, when mitochondria from skin cells (immortalized and malignant keratinocytes) were incubated with [4-14C] cholesterol, it resulted in the production of steroids that migrated at the same rate as the pregnenolone and progesterone standards (not shown). The calculated rates of conversion of [4-14C] cholesterol into pregnenolone and progesterone in cutaneous mitochondria were 0.14% and 0.04%, respectively, ≈1% of the conversion reported for placental mitochondria [39]. Pregnenolone was also detected by RIA in the culture medium of skin cells incubated for 18h with 25 μm 22R-hydroxycholesterol (not shown). These results are in agreement with recent findings of Thiboutot et al. [36] of 22R-hydroxycholesterol conversion into 17-hydroxypregnenolone in cultured sebocytes. Thus, not only do the whole skin and a wide spectrum of skin cells express the genes and proteins necessary for the activity of the P450scc system in vivo, but this cutaneous P450scc system is functional as it does exhibit cholesterol sidechain shortening activity leading to actual production of pregnenolone.
As 7-DHC is normally present in the skin, we tested this sterol as an alternative substrate for cytochrome P450scc. This required the chemical synthesis of a 7-DHP standard the identity of which was confirmed by NMR analysis (not shown). Purified P450scc enzyme supplemented with adrenodoxin and adrenodoxin reductase did indeed transform 7-DHC to a product identical with the 7-DHP standard, as determined by identical migration rate on TLC, retention time on RP-HPLC, and UV absorption spectrum (not shown). A UV spectrum of this biotransformation product showed the characteristic pattern of bands at 272, 282, and 294 nm with a shoulder at 263 nm, in full agreement with the published data for 7-DHP [29,40,41]. GC/MS analysis of this product also showed the mass spectra pattern expected for 7-DHP (Fig. 4), identical with that reported most recently by Guryev et al. [19]. Thus, our GC/MS analysis showed two major peaks with the mass spectrum and retention time of authentic 7-DHP, characterized as the TMS (peak B) and MO-TMS (peak C) derivatives (Fig. 4). Figure 4B illustrates mass spectra of isolated 7-DHP-TMS, and the synthesized standard. The molecular ion is at m/z 386 with prominent fragments at m/z 296 (M+ – 90), 281 (M+ – 90–15) and 255 (M+ – 131). The loss of mass 131 results from the scission of the C1–C2 and C4–C5 bonds. Figure 4C illustrates mass spectra of isolated 7-DHP-MO-TMS, and the synthesized standard. The molecular ion is at m/z 415, and distinctive ions are formed by loss of the silylated hydroxy, methyl and oxime groups at m/z 310 (M+ – 90–15) and 294 (M+ – 90–31). The distinctive ion at m/z 100, formed by cleavage of the C13– C17 and C15–C16 bonds, is also important. The ion at m/z 126, characteristic of 7-DHP, is also present. Thus, both Guryev et al. [19] and our analysis provide MS evidence that the main product of 7-DHC in the reaction catalyzed by cytochrome P450scc is 7-DHP. Definitive proof of chemical structure was obtained with NMR which showed all resonance signals characteristic of 7-DHP (Fig. 5). The 1H-NMR spectrum of the biotransformation product is in agreement with that of the chemically synthesized standard (Fig. 5) and with data from the literature [41]. Thus, the two angular methyl groups (18-CH3 and 19-CH3) showed the resonance signals at 0.56 and 0.90 p.p.m., respectively. The methyl group in the side chain (21-CH3) gave the singlet at 2.12 p.p.m. because of the presence of an adjacent keto group atC-20. The signal of the methine proton (3αH) at the secondary alcohol was shown as a multiplet at 3.61 p.p.m. Finally, two very characteristic signals for the steroidal 5,7-diene system (6-H and 7-H) appeared as an AB quartet at 5.40 and 5.52 p.p.m. with the coupling constants J1 = 6Hz and J2 = 0.5 Hz. Lastly, 13C-NMR and 2D NMR data (COSY, HMQC, and HMBC) fully and unequivocally confirmed the structure of the product generated by the reaction of 7-DHC with P450scc as 7-DHP.
Fig. 4. GC/MS analysis of product of P450scc-mediated side-chain cleavage of 7-DHC.
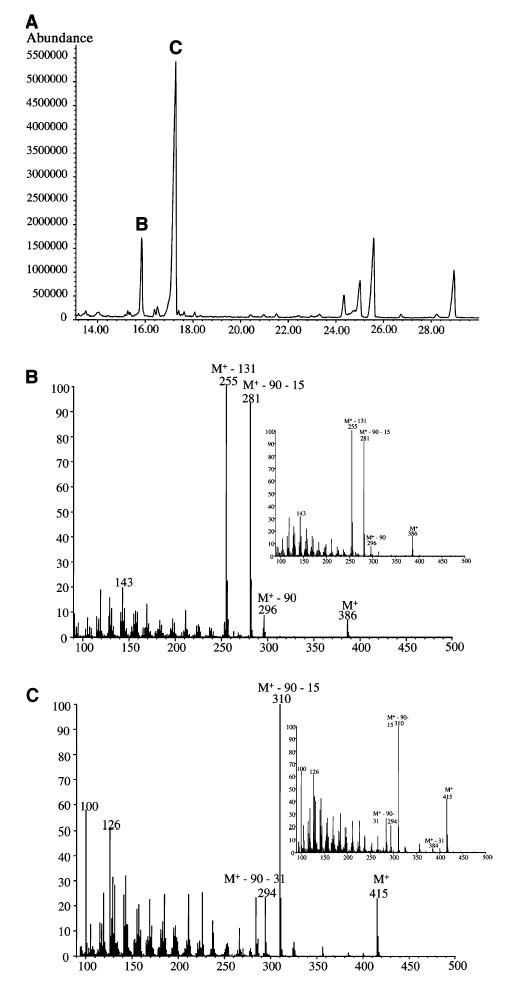
(A) Total ion current chromatogram. (B) Mass spectra of 7-DHP (TMS derivative) corresponding to peak B with an inset showing the synthetic reference material. (C) Mass spectra of 7-DHP (MO-TMS derivative) corresponding to peak C in comparison with that obtained from synthetic reference material (inset).
Fig. 5. 1H-NMR (500 MHz) spectrum of the product of P450scc-mediated side-chain cleavage of 7-DHC.
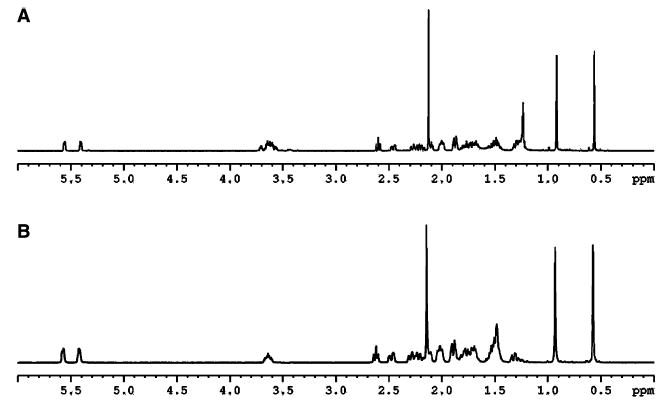
(A) Spectrum of the enzymatic side-chain cleavage of 7-DHC; (B) spectrum of 7-DHP synthetic standard.
The reaction kinetics for the conversion of 7-DHC into 7-DHP by bovine P450scc, as determined with the substrate dissolved in the membrane of phospholipid vesicles, were similar to those for the conversion of cholesterol into pregnenolone (Table 2), with the catalytic rate constant (kcat) for 7-DHC being 62% of that for cholesterol. Human P450scc had a kcat value for 7-DHC 70% of that for cholesterol and a lower Km. This gives human P450scc a slightly higher kcat/Km value with 7-DHC as substrate compared with that for cholesterol (Table 2). In comparison, Guryev et al. [19] recently reported that bovine P450scc had the same Vmax for 7-DHC and cholesterol in an assay of P450scc where cholesterol was held in solution with 2-hydroxypropyl-β-cyclodextrin. It must also be noted that in a reconstituted in vitro system, both MLN64 and StAR can interact with 7-DHC and transport it from donor to acceptor vesicles with efficiency similar to that for cholesterol (R. C. Tuckey, unpublished data).
Table 2. Kinetic parameters for side-chain cleavage of 7-DHC and cholesterol by bovine and human cytochromes P450scc.
Kinetic parameters were determined with substrates and P450scc incorporated into phospholipid (PL) vesicles prepared from dioleoyl phosphatidylcholine containing 15 mol% cardiolipin. Values for kcat and Km are ± SE and are expressed as min−1 and mol sterol·mol PL−1, respectively. They were obtained from fitting hyperbolic curves to the kinetic data using KALEIDAGRAPH.
| Human P450scc
|
Bovine P450scc
|
|||||
|---|---|---|---|---|---|---|
| Substrate | Km | kcat | kcat/Km | Km | kcat | kcat/Km |
| Cholesterol | 0.164 ± 0.009 | 19.0 ± 0.4 | 116 | 0.078 ± 0.011 | 39.3 ± 1.7 | 504 |
| 7-DHC | 0.103 ± 0.006 | 13.3 ± 0.4 | 129 | 0.069 ± 0.010 | 24.4 ± 1.1 | 353 |
The P450/7-DHC pathway must be operative in living cells as mitochondria purified from human placenta and rat adrenal do transform 7-DHC to 7-DHP, as identified by TLC, LC/MS, and LC with UV absorption spectra analysis (Figs 6 and 7). Thus the use of 7-DHC as substrate for P450scc provides the likely explanation for the humoral accumulation of 7-DHP and its metabolites in Smith– Lemli–Opitz syndrome [17,18], thereby indicating pathway activation in vivo, at least under pathological conditions. Epidermal availability of 7-DHC in conjunction with the presence of an active P450scc system makes it probable that 7-DHP is produced in the skin. The level of 7-DHP production and its hypothetical conversion into other metabolites (including 17-, 20-, 21- and 11-hydroxy-7- DHP) are the subject of investigations in our laboratories (Fig. 8). In this context, the unsaturated B ring of 7-DHP susceptibility to cleavage by UVB of its 9,10 carbon bond, and to further temperature-dependent conversion, supports the 7-DHP transformation into the vitamin D3-like compound 5Z,7E-3β-hydroxy-9,10-secopregna-5,7,10(19)trien- 20-one as reported by others [29]. Therefore, we propose that UVB-induced molecular rearrangements, similar to that occurring in 7-DHC, could affect 7-DHP hydroxy derivatives generating vitamin D3-like compounds (Fig. 8). Such putative conversion would explain the lack of increase in vitamin D3 concentrations in spite of 7-DHC tissue accumulation in patients with Smith–Lemli–Opitz syndrome [42]. Indeed, the skin (exposed to solar radiation) would be the site of choice for production of vitamin D3-like compound from 7-DHP or its hydroxy derivatives [43].
Fig. 6. Conversion of 7-DHC into 7-DHP by mitochondria from the human placenta.

Mitochondria (1.4 mg·mL−1) were incubated with 200 μM 7-DHC, 5.0 μM N-62 StAR protein and 10 μM cyanoketone for 2 h at 37 °C. Reaction products were analyzed by TLC. Control (incubation without NADPH and isocitrate) (lane 1); experimental incubation with NADPH and isocitrate (lane 2); 7-DHC and 7-DHP standards (lane 3); marked on the left by arrows are cholesterol, 7-DHC, pregnenolone and 7-DHP.
Fig. 7. Conversion of 7-DHC into 7-DHP by rat adrenal mitochondria.
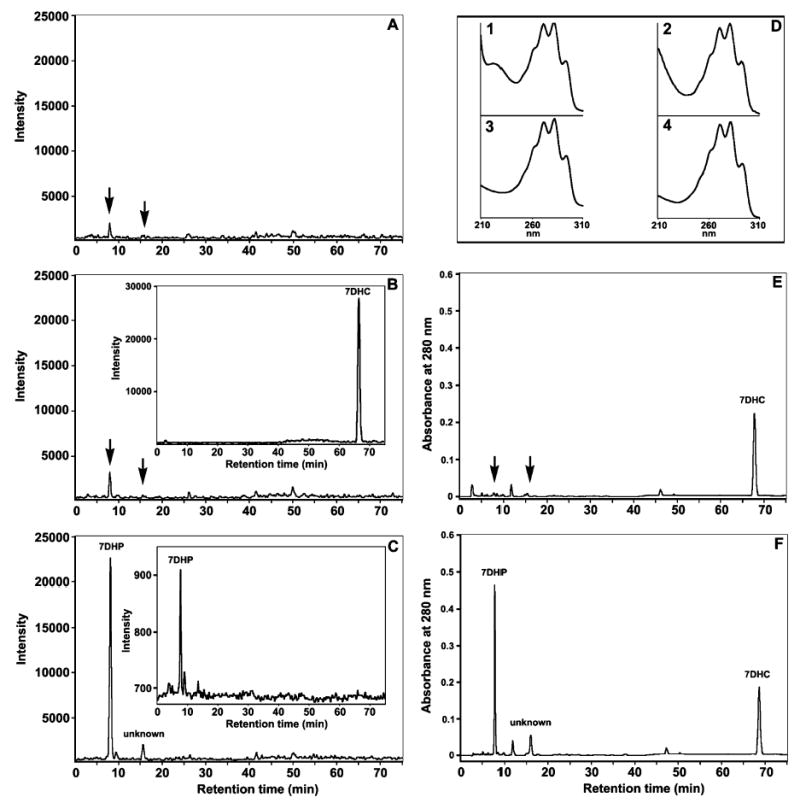
Samples were analyzed by LC/MS (A–C) or LC with UV spectrophotometry (D–F). Incubation of mitochondria with NADPH and isocitrate (C and F) yielded two peaks of ion [M + H] with m/z 315.3 at retention time 8.1 and 15.6 min. The first peak had m/z, retention time and UV spectra (inset 3 in D) corresponding to the 7-DHP standard (inset 2 in D and inset in C). The product was at the limits of detectability in the control sample with the reaction stopped at time 0 (A) and in mitochondria incubated in the absence of NADPH and isocitrate (B and E). The second peak (unknown) had a retention time 15.6 min and UV spectra (inset 4 in D) similar to those of the first product, and probably represents an additional product of 7-DHC transformation (C). Differing from these reaction products were the parameters for the 7-DHC; the retention time for its ion with m/z 385.3 and UV spectra are shown in the inset in (B) and in inset 1 in (D).
Fig. 8. Transformation of 7-DHC (1) to 7-DHP (2), followed by a proposed sequence for the enzymatic transformation of 7-DHP to its hydroxy derivatives (3–10), and/or to secosteroids (11–19) generated by the action of UVB radiation.
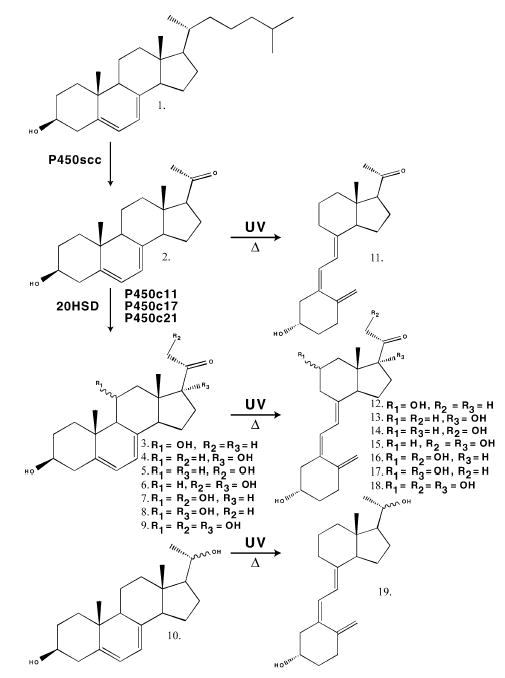
3, 3β,11α- or β-Dihydroxypregna- 5,7-dien-20-one; 4, 3β,17β-dihydroxypregna-5,7-dien-20-one; 5, 3β,21-dihydroxypregna-5,7-dien-20-one; 6, 3β,17β,21-trihydroxypregna- 5,7-dien-20-one; 7, 3β,11α- or 11β,21-trihydroxypregna-5,7-diene- 20-one; 8, 3β,11α- or 11β,17-trihydroxypregna-5,7-diene-20-one; 9, 3β,11α-or β,17β,21-tetrahydroxypregna-5,7-dien-20-one; 10, 3β,20αor β-dihydroxypregna-5,7-diene; 11, 5Z,7E-3β-hydroxy-9,10-secopregna- 5,7,10(19)trien-20-one; 12, 5Z,7E-3β, 11α- or β-dihydroxy- 9,10-secopregna-5,7,10(19)trien-20-one; 13, 5Z,7E-3,17β-dihydroxy- 9,10-secopregna-5,7,10(19)trien-20-one; 14, 5Z,7E-3,β,21-dihydroxy- 9,10-secopregna-5,7,10(19)trien-20-one; 15, 5Z,7E-3β,17β,21-trihydroxy- 9,10-secopregna-5,7,10(19)trien-20-one; 16, 5Z,7E-3β,11α- or 11β,21-trihydroxy-9,10-secopregna-5,7,10(19)trien-20-one; 17, 5Z,7E- 3β,11α- or 11β,17-trihydroxy-9,10-secopregna-5,7,10(19)trien-20-one; 18, 5Z,7E-3β,11α- or β,17β,21-tetrahydroxy-9,10-secopregna- 5,7,10(19)-trien-20-one; 19, 5Z,7E-3β,11α- or β-dihydroxy-9,10-secopregna- 5,7,10(19)triene.
Conclusions
We document that the genes and proteins required for the P450scc system are expressed concomitantly in the skin and skin cells. Moreover, using an array of methods including chemical synthesis with TLC and HPLC separation, NMR, LC/MS and GC/MS, we demonstrate that mammalian P450scc transforms 7-DHC to 7-DHP with high efficiency. As 7-DHC is readily available in human skin, it represents a natural substrate for P450scc, yielding 7-DHP. Moreover, regardless of whether they originate from local synthesis or from delivery to the skin by the circulation, 5,7-steroidal dienes (7-DHP and its hydroxy derivatives) may also undergo UVB-induced intramolecular rearrangements to vitamin D3-like compounds (Fig. 8).
Supplementary Material
Acknowledgments
The project was supported by grants from the Center of Excellence in Connective Tissue (to A.S. and J.Z.) and Center of Excellence in Genomics and Bioinformatics (to A.S.), UTHSC, and NIH grants 1R01-AR047079-01A2 (to A.S.) and RR017854. We thank Professor Cedric Shackelton, Children’s Hospital Oakland Research Institute, Oakland, CA, USA for performing GC/MS analyses, and Professor Walter Miller, University of California, San Francisco for the gift of N-62 StAR protein and StAR protein antiserum. The excellent secretarial skills of Ms. Christine Crawford are also acknowledged.
Footnotes
Supplementary material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/EJB/EJB4356/EJB4356sm.htm
Fig. S1. 13C-NMR (A), homonuclear shift correlation- COSY (correlation spectroscopy) (B), heteronuclear multiple- quantum correlation (HMQC) (C) and heteronuclear multiple-bond correlation (HMBC) (D) spectra of the product of 7-DHC side-chain cleavage.
References
- 1.Slominski A, Wortsman, J Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 2.Slominski A, Wortsman J, Luger T, Paus R, Solomon, S Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 3.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei, E.T Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 5.Kamradt J, Ra. L, Mitschele T, Meineke V, Gartner BC, Wolfgang T, Holick MF, Reichrath, J Analysis of the vitamin D system in cutaneous malignancies. Recent Results Cancer Res. 2003;164:259–269. doi: 10.1007/978-3-642-55580-0_19. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 7.fTian X.Q.& Holick, M.F A liposomal model that mimics the cutaneous production of vitamin D3. Studies of the mechanism of the membrane-enhanced thermal isomerization of pre-vitamin D3 to vitamin D3. J Biol Chem. 1999;274:4174–4179. doi: 10.1074/jbc.274.7.4174. [DOI] [PubMed] [Google Scholar]
- 8.Lambeth JD, Seybert DW, Lancaster JR, Jr, Salerno JC, Kamin, H Steroidogenic electron transport in adrenal cortex mitochondria. Mol Cell Biochem. 1982;45:13–31. doi: 10.1007/BF01283159. [DOI] [PubMed] [Google Scholar]
- 9.Tuckey RC, Sadleir, J The concentration of adrenodoxin reductase limits cytochrome p450scc activity in the human placenta. Eur J Biochem. 1999;263:319–325. doi: 10.1046/j.1432-1327.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 10.Slominski A, Ermak G, Mihm, M ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 11.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455:364–366. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 12.Slominski A, Wortsman J, Foecking MF, Shackleton C, Gomez-Sanchez C, Szczesniewski, A Gas chromatography/mass spectrometry characterization of corticosteroid metabolism in human immortalized keratinocytes. J Invest Dermatol. 2002;118:310–315. doi: 10.1046/j.0022-202x.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- 13.Rogo. D, Gomez-Sanchez CE, Foecking MF, Wortsman J, Slominski, A Steroidogenesis in the human skin: 21- hydroxylation in cultured keratinocytes. J Steroid Biochem Mol Biol. 2001;78:77–81. doi: 10.1016/s0960-0760(01)00076-0. [DOI] [PubMed] [Google Scholar]
- 14.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochim Biophys Acta. 2000;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 15.Nowaczyk MJ, Waye, J.S The Smith–Lemli–Opitz syndrome: a novel metabolic way of understanding developmental biology, embryogenesis, and dysmorphology. Clin Genet. 2001;59:375–386. doi: 10.1034/j.1399-0004.2001.590601.x. [DOI] [PubMed] [Google Scholar]
- 16.Tint GS, rons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen, G Defective cholesterol biosynthesis associated with the Smith–Lemli–Opitz syndrome. N Engl J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 17.Shackleton CH, Roitman E, Kelley, R Neonatal urinary steroids in Smith–Lemli–Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids. 1999;64:481–490. doi: 10.1016/s0039-128x(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 18.Shackleton C, Roitman E, Guo LW, Wilson WK, Porter, F.D Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosteroldelta 7-reductase deficiency (Smith–Lemli–Opitz syndrome) J Steroid Biochem Mol Biol. 2002;82:225–232. doi: 10.1016/s0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 19.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook, R.W A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci USA. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slominski A, Paus R, Costantino, R Difierential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J Invest Dermatol. 1991;96:172–179. doi: 10.1111/1523-1747.ep12460956. [DOI] [PubMed] [Google Scholar]
- 21.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman, J Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur J Biochem. 2003;270:3335–3344. doi: 10.1046/j.1432-1033.2003.03708.x. [DOI] [PubMed] [Google Scholar]
- 22.Slominski A, Moellmann G, Kuklinska E, Bomirski A, Pawelek, J Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, 1-tyrosine and 1-dopa. J Cell Sci. 1988;89:287–296. doi: 10.1242/jcs.89.3.287. [DOI] [PubMed] [Google Scholar]
- 23.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, Jing C, Johansson, O Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16:896–898. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- 24.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman, J Difierential expression of a cutaneous CRH system. Endocrinology. 2003;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuckey RC, Cameron, K.J Side-chain specificities of human and bovine cytochromes P-450scc. Eur J Biochem. 1993;217:209–215. doi: 10.1111/j.1432-1033.1993.tb18235.x. [DOI] [PubMed] [Google Scholar]
- 26.Tuckey RC, Stevenson, P.M Properties of ferredoxin reductase and ferredoxin from the bovine corpus luteum. Int J Biochem. 1984;16:489–495. doi: 10.1016/0020-711x(84)90165-4. [DOI] [PubMed] [Google Scholar]
- 27.Tuckey RC, Stevenson PM. Properties of bovine luteal cytochrome P-450scc incorporated into artificial phospholipid vesicles. Int J Biochem. 1984;16:497–503. doi: 10.1016/0020-711x(84)90166-6. [DOI] [PubMed] [Google Scholar]
- 28.Woods ST, Sadleir J, Downs T, Triantopoulos T, Headlam MJ, Tuckey, R.C Expression of catalytically active human cytochrome p450scc in Escherichia coli and mutagenesis of isoleucine-462. Arch Biochem Biophys. 1998;353:109–115. doi: 10.1006/abbi.1998.0621. [DOI] [PubMed] [Google Scholar]
- 29.Noboru, K., Katsuhito, M., Kiyoshige, O., Isao, M. & Eigoro, M. (1990) Vitamin D derivatives, process for producing the same and their use in the difierentiation of tumor cells. US Patent 4891364.
- 30.Pisarchik A, Slominski, A.T Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their difierential expression. FASEB J. 2001;15:2754– 2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 31.Slominski A, Pisarchik A, Johansson O, Jing C, Semak I, Slugocki G, Wortsman, J Tryptophan hydroxylase expression in human skin cells. Biochim Biophys Acta. 2003;1639:80–86. doi: 10.1016/s0925-4439(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 32.Tuckey RC, Kostadinovic Z, Cameron, K.J Cytochrome P-450scc activity and substrate supply in human placental trophoblasts. Mol Cell Endocrinol. 1994;105:103–109. doi: 10.1016/0303-7207(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 33.Guo LW, Wilson WK, Pang J, Shackleton, C.H Chemical synthesis of 7- and 8-dehydro derivatives of pregnane- 3,17alpha,20-triols, potential steroid metabolites in Smith–Lemli– Opitz syndrome. Steroids. 2003;68:31–42. doi: 10.1016/s0039-128x(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 34.Stocco DM. Intramitochondrial cholesterol transfer. Biochim Biophys Acta. 2000;1486:184–197. doi: 10.1016/s1388-1981(00)00056-1. [DOI] [PubMed] [Google Scholar]
- 35.Bose HS, Whittal RM, Huang MC, Baldwin M.A.& Miller, W.L N-218 MLN64, a protein with StAR-like steroidogenic activity, is folded and cleaved similarly to StAR. Biochemistry. 2000;39:11722–11731. doi: 10.1021/bi000911l. [DOI] [PubMed] [Google Scholar]
- 36.Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, Clawson, G Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1) J Invest Dermatol. 2003;120:905–914. doi: 10.1046/j.1523-1747.2003.12244.x. [DOI] [PubMed] [Google Scholar]
- 37.Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL, Rio MC, Baker ME, Strauss JF., III MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci USA. 1997;94:8462–8467. doi: 10.1073/pnas.94.16.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uribe A, Strauss JFIII, Martinez F. Contact sites from human placental mitochondria: characterization and role in progesterone synthesis. Arch Biochem Biophys. 2003;413:172–181. doi: 10.1016/s0003-9861(03)00097-3. [DOI] [PubMed] [Google Scholar]
- 39.Boguslawski W. Cytochrome P-450 and steroidogenic activities of the human placental mitochondria. J Steroid Biochem. 1983;18:771–775. doi: 10.1016/0022-4731(83)90258-3. [DOI] [PubMed] [Google Scholar]
- 40.Tait AD, Santikarn S, Allen, W.R Identification of 3beta-hydroxy-5,7-pregnadien-20-one and 3beta-hydroxy-5,7- androstadien-17-one as endogenous steroids in the fetal horse gonad. J Endocrinol. 1983;99:87–92. doi: 10.1677/joe.0.0990087. [DOI] [PubMed] [Google Scholar]
- 41.Murari MP, Londowski JM, Bollman S, Kumar, R Synthesis and biological activity of 3beta-hydroxy-9, 10-secopregna- 5,7,10[19]-triene-20-one: a side chain analogue of vitamin D3. J Steroid Biochem. 1982;17:615–619. doi: 10.1016/0022-4731(82)90562-3. [DOI] [PubMed] [Google Scholar]
- 42.Irons M, Elias ER, Abuelo D, Bull. MJ, Greene CL, Johnson VP, Keppen L, Schanen C, Tint GS, Salen, G Treatment of Smith–Lemli–Opitz syndrome: results of a multicenter trial. Am J Med Genet. 1997;68:311–314. [PubMed] [Google Scholar]
- 43.Slominski, A., Stewart, J., Tuckey, R., Wortsman, J. & Zjawiony, J. (2004) Method of producing 7-dehydropregnenolone, vitamin D3-like compounds and derivatives thereof Provisional US patent D6555; filed 7 January 2004.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


