Abstract
Hair shaft melanin components (eu- or/and pheomelanin) are a long-lived record of precise interactions in the hair follicle pigmentary unit, e.g., between follicular melanocytes, keratinocytes, and dermal papilla fibroblasts. Follicular melanogenesis (FM) involves sequentially the melanogenic activity of follicular melanocytes, the transfer of melanin granules into cortical and medulla keratinocytes, and the formation of pigmented hair shafts. This activity is in turn regulated by an array of enzymes, structural and regulatory proteins, transporters, and receptors and their ligands, acting on the developmental stages, cellular, and hair follicle levels. FM is stringently coupled to the anagen stage of the hair cycle, being switched-off in catagen to remain absent through telogen. At the organ level FM is precisely coupled to the life cycle of melanocytes with changes in their compartmental distribution and accelerated melanoblast/melanocyte differentiation with enhanced secretory activity. The melanocyte compartments in the upper hair follicle also provides a reservoir for the repigmentation of epidermis and, for the cyclic formation of new anagen hair bulbs. Melanin synthesis and pigment transfer to bulb keratinocytes are dependent on the availability of melanin precursors, and regulation by signal transduction pathways intrinsic to skin and hair follicle, which are both receptor dependent and independent, act through auto-, para- or intracrine mechanisms and can be modified by hormonal signals. The important regulators are MC1 receptor its and adrenocorticotropic hormone, melanocyte stimulating hormone, agouti protein ligands (in rodents), c-Kit, and the endothelin receptors with their ligands. Melanin itself has a wide range of bioactivities that extend far beyond its determination of hair color.
Keywords: follicular melanocytes, hair pigmentation, melanin, melanogenesis
Abbreviations: ASP, agouti protein; BMP, bone morphogenic proteins; COMT, catechol-O-methyltransferase; CRH, corticotropin releasing hormone; CTSL, cysteine protease cathepsin L; DCT, dopa-chrome tautomerase; DHI, dihydroxyindole; DHICA, dihydroxy-indole carboxylic acid; DHICA-CF, DHICA conversion factor; DP, dermal papilla; EPR, electron paramagnetic resonance; ER, endoplasmic reticulum; ET, endothelin; FM, follicular melanogenesis; l-dopa; l-3,4-dihydroxyphenylalanine; MC1, melanocortin receptor 1; MIF, macrophage migration inhibitory factor; MITF, microphtalmia-associated transcription factor; MRP, melanogenesis-related proteins; MSH, melanocyte stimulating hormone; OCA, oculocutaneous albinism; PAH, phenylalanine hydroxylase; PAR2, protease-activated receptor 2; ROS, reactive oxygen species; SCF, stem cell factor; TGN, trans-Golgi network; TRP, tyrosinase-related protein
Overview
Follicular pigmentation is under complex genetic control, as determined from studies in mostly murine models. In the mouse, coat color is regulated by more than 150 alleles at over 90 loci (Hearing, 1999; Nakamura et al, 2002) (S1) (Mouse Genome Database). Protein products of these loci have a wide array of cellular targets, and functions, acting as enzymes, structural proteins, transcriptional regulators, transporters, or receptors and their ligands (Hearing, 1999; Slominski et al, 2004) (S5). This organization allows control of melanin synthesis at all levels: cellular (follicular melanocyte), organ (hair follicle), and developmental steps (neural crest, melanoblast migration, targeting to skin, differentiation to melanocytes, and melanocyte proliferation and survival).
In the adult hair follicle, pigmentation results from precise sequential interactions between follicular melanocytes, matrix keratinocytes, and dermal papilla (DP) fibroblasts (Slominski and Paus, 1993). It involves the melanogenic activity of follicular melanocytes, the transfer of their product, melanin granules, into cortical and medullary keratinocytes, and the formation of pigmented hair shafts. Hair is actively pigmented only during the anagen stage of the hair cycle, to which the melanogenic activity of follicular melanocytes is stringently coupled; melanin formation is switched-off in catagen remaining absent through telogen (Slominski and Paus, 1993S). Pigmentation lags behind the expression of melanogenesis-related proteins (MRP) that in turn exhibits a time-frame restricted, differential pattern of transcription, translation and functional activity during the development of anagen follicles (Slominski et al, 1991, 1994, 1996; Slominski and Paus, 1993). Thus, follicular melanogenesis (FM) is characteristically cyclic in nature, as opposed to the continuous melanogenesis of epidermal pigmentation.
Melanin synthesis and pigment transfer to bulb keratinocytes are to a large extent controlled by signals intrinsic to skin and represented by products of keratinocytes, immunocytes, fibroblasts, and endothelial cells (Slominski and Paus, 1993; Slominski et al, 1993; Tobin et al, 1999). Melanocytes can reciprocally affect the surrounding cells, e.g., by direct melanosome transfer (to keratinocytes), or by production and secretion of functional regulators (Slominski et al, 1993). Thus, anagen-coupled melanogenesis and its regulatory network control hair growth and pigmentation, leaving the pigmented hair shaft (a uniquely mammalian trait) as a visible, long-lived record of complex epithelial–mesenchymal–neuroectodermal interactions. The melanocyte component of this tissue interactive cell system in hair follicles is more sensitive to aging influences than melanocytes in the epidermis, resulting in hair graying/canities; likely, this reflects differences in the epidermal and follicular microenvironments (Tobin and Paus, 2001).
Development of the Follicular Melanin Unit
Cutaneous melanocytes originate from the neural crest where lineage commitment is determined by microphthalmia-associated transcription factor (MITF), fibroblast growth factor-2, endothelin 3 (ET3), and others (Dupin and Le Douarin, 2003). Melanoblasts migrate from the neural crest (S2) and populate the basal layer of the epidermis and then non-randomly enter the developing hair follicles. Melanogenic melanocytes can be detected at all stages of hair morphogenesis, from hair germ stage onwards (Peters et al, 2002) (S2a). Melanoblasts expressing c-Kit migrate into the stem cell factor (SCF)-supplying hair follicle epithelium and differentiated c-Kit-positive melanocytes target the bulb when “SCF-positive” (S2a) (Peters et al, 2002). The importance of this signaling pair is evident from the ability of a Kit blocking antibody to induce apoptosis in murine follicular melanocytes (Ito et al, 1999). C-Kit-negative melanoblasts invade the outer root sheath and bulge region in the fully developed hair follicle (Peters et al, 2002). Post-natally, c-Kit is required during the hair growth cycle for activation of melanocyte, although in stem cell compartment appears to exhibit SCF/c-Kit independence (Botchkareva et al, 2001).
In the fully developed human scalp, anagen follicle melanocytes can be assigned to distinct anatomic compartments of region-specific differentiation status (Fig 1). In the mature hair follicle, melanotic dopa (dihydroxyphenylalanine)-positive melanocytes are readily detectable in the basal layer of the infundibulum and around the upper DP; moderately differentiated melanocytes may also be detected in the basal layer of the sebaceous gland (Fig 1). Dopa-negative amelanotic melanocytes appear in the mid-to-lower outer root sheath. Some amelanotic dopa-negative melanocytes may also be distributed in the periphery of the bulb and the most proximal matrix. The presence of immature melanocytes (melanoblasts) has been clearly documented in the adult hair follicle (Horikawa et al, 1996; Tobin and Bystryn, 1996). All the dopa-positive cells, and also some dopa-negative melanocytes of the mid outer root sheath are (pre)melanosome gp100 positive (Horikawa et al, 1996). Amelanotic hair follicle melanocytes are devoid of dopa-oxidase activity, although low levels of the tyrosinase protein itself may be detected in some cells (S3). Similarly, c-Kit and Bcl-2 reactive amelanotic melanocytes are present in this hair follicle compartment (Grichnik et al, 1995), but do not express the melanogenic enzymes TRP (tyrosinase-related protein)1 and TRP2 (Horikawa et al, 1996).
Figure 1. Melanocyte distribution in the human anagen scalp hair follicle.
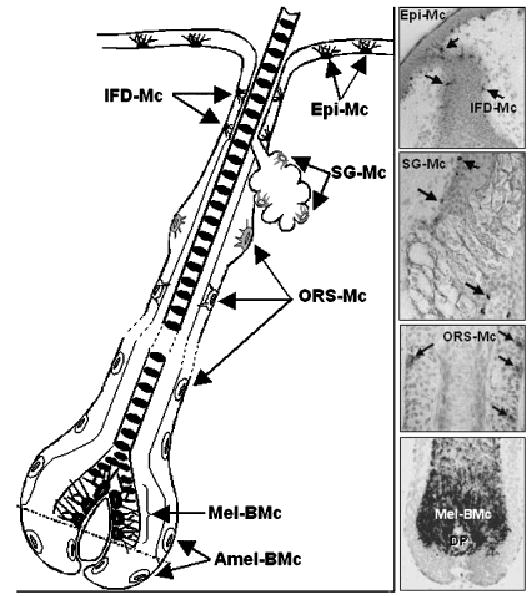
Melanocytes in frozen scalp sections were detected using the antibody against gp100. Epi-MC, epidermal melanocytes; IFD-Mc, infundibular melanocytes; SG-Mc, sebaceous gland melanocytes; ORS-Mc, outer root sheath melanocytes; Mel-BMc, melanogenic bulbar melanocytes; Amel-BMc, amelanotic bulbar melanocytes; DP, dermal papilla.
The hair bulb is the only site of pigment production for the hair shaft; it contains highly melanogenic melanocytes and a minor sub-population of poorly differentiated pigment cells (Tobin and Bystryn, 1996). It has been proposed that amelanotic hair bulb melanocytes may represent “transient” melanocytes that migrate from precursor melanocytes stores in the upper outer root sheath (Tobin and Bystryn, 1996; Nishimura et al, 2002) (S4). Melanogenically active melanocytes are restricted to the upper hair matrix of the anagen hair follicle, below the pre-cortical keratinocyte population, correlating with the anagen transfer of melanin predominantly to the hair shaft cortex, less to the medulla, and, only rarely to the hair cuticle. Melanogenically active hair bulb melanocytes form functional units with neighboring immature pre-cortical keratinocytes that receive melanized secretory granules and ultimately form the pigmented hair shaft. The intimate nature of the relationship between bulbar melanocytes and the DP is evidenced by their separation via only a very thin and permeable basal lamina at the interface between the matrix and the mesenchymal DP.
Molecular and Cellular Biology of Melanogenesis
A common biochemical apparatus determines both follicular and epidermal melanogenesis (Fig S1), initiated by either hydroxylation of l-phenylalanine to l-tyrosine (Schallreuter et al, 1998a) or directly from l-tyrosine (Lerner and Fitzpatrick, 1950). Melanocyte melanogenesis therefore requires direct transport of l-tyrosine from the extracellular space (S5), or intracellular hydroxylation of l-phenylalanine by phenylalanine hydroxylase (PAH) (EC 1.14.16.1) (Schallreuter et al, 1998a). The latter depends on the essential cofactor (6R)-l-erythro 5,6,7,8 tetrahydrobiopterin (6BH4), which melanocytes have full capacity for synthesis de novo, and for regulation of its recycling (Schallreuter et al, 1997). 6BH4 may act as an allosteric inhibitor of tyrosinase and its abiogenic isomer, 7BH4, may inhibit PAH (S6).
Tyrosinase (EC 1.14.18.1), the product of the c locus, catalyzes the hydroxylation of l-tyrosine to l-3,4-dihydroxyphenylalanine (l-dopa), which is followed by its oxidation to dopaquinone (common step in the eu- and pheomelanogenic pathways) (Hearing, 1999) (S7). Alternative mechanisms for l-dopa formation are represented by the reduction of l-dopaquinone back to l-dopa (Cooksey et al, 1997), or even by a direct hydroxylation of tyrosine by tyrosine hydroxylase isoform I (TH I, EC 1.14.16.2) (Marles et al, 2003; Gillbro et al, 2004) (T1). Once l-dopa is formed, melanogenesis can proceed further through oxido-reduction reactions and intramolecular transformations occurring spontaneously, at rates determined by local concentrations of hydrogen ion, metal cations, thiols and other reducing agents, hydrogen peroxide, and oxygen (S7, S8). In the eumelanogenic pathway, the velocity and specificity of the reactions are regulated by the TRP, of which the most important is tyrosinase (also catalyzes the oxidation of dihydroxyindole (DHI)) (Korner and Pawelek, 1982; Hearing, 1999) (S7).
Tyrosinase protein structure is highly conserved among different species and shows high homology with other TRP including TRP1, a product of TYRP1 (human) or b (mouse) locus and TRP2 product of TYRP2/DCT (dopachrome tautomerase) (human), and slaty locus (mice). The gene sequences for these proteins have been established, and post-transcriptional processing of the corresponding mRNA generates several alternatively spliced products (reviewed in (Slominski et al, 2004)). In mice but not humans, TRP1 acts as a 5,6 dihydroxyindole carboxylic acid (DHICA) oxidase to generate indole-5,6-quinonecarboxylic acid (S5, S9). In humans, it can exhibit tyrosine hydroxylase activity at low concentrations of substrate (Sarangarajan and Boissy, 2001). TRP1 appears to be important for eumelanogenesis, as suggested by its lack or defective expression in pheomelanogenic cells (Hearing, 1999). It also appears to ensure the appropriate processing of tyrosinase, stabilization of its enzymatic activity, and maintenance of melanosome structural integrity (Hearing, 1999; Sarangarajan and Boissy, 2001) (S9). TRP2//DCT can act as DCT (EC 5.3.2.3) catalyzing transformation of dopachrome to DHICA (S5). TYR-2 can also stabilize tyrosinase activity and has recently been shown to support melanocyte survival (S9).
Other enzymatic regulators of melanogenesis include PMEL17 protein (HMB-45/gp100/silver locus), catechol-O-methyltransferase (COMT) (EC 2.1.1.6), peroxidase (EC 1.11.1.7), and macrophage migration inhibitory factor (MIF) (Hearing, 1999) (S5, S7). It has been proposed that PMEL17 catalyzes the polymerization of DHICA to melanin, and that PMEL17/GP100 may act in melanosomes as scaffold for the deposition of melanin, and for stabilizing melanin intermediates (S5, S9). COMT is responsible for O-methylation of dopa and its DHI intermediates, whereas peroxidase catalyzes the oxidation of DHI and DHICA (S5, S7). MIF expresses d-dopachrome tautomerase activity and transforms d-dopachrome, dopaminechrome or its derivatives to their indole compounds (S9). Enzymes indirectly affecting melanogenesis are glutathione reductase and glutathione peroxidase that regulate glutathione reduced/oxidized levels (S5, S7), and catalase which regulates hydrogen peroxide removal (Schallreuter et al, 1998a).
Synthesis of pheomelanin starts with the conjugation of dopaquinone with cysteine or glutathione yielding cysteinyldopa and glutathionyldopa (S7). After oxidation, cysteinyldopa undergoes ring closure to yield 1,4-benzothiazinylalanines that may couple through a peroxidase/H2O2-promoted reaction or tyrosinase-catalyzed oxidation; the multistep process ends with the formation of pheomelanin (S7). An alternative route for cysteinyldopa formation is represented by the conjugation of dopaquinone with glutathione followed by glutamyltranspeptidase catalyzed hydrolysis of the resultant glutathionyldopa. The main regulatory mechanism switch from eu- to pheomelanogenesis employs dopaquinone as a key molecule controlling the activity of glutathione reductase and blocking pheomelanogenesis at high tyrosinase activity, and high eumelanogenesis rate (Oyehaug et al, 2002). The velocity of post-cysteinyldopa steps of melanogenesis is increased by peroxidase and tyrosinase that are involved in the transformation of benzothiazinylalanines (S7).
In melanocytes, melanin synthesis is restricted to melanosomes that are structurally assembled via a process resembling lysosome biogenesis (Jimbow et al, 2000; Marks and Seabra, 2001) (S10, S11). Although the exact sequence of events leading to melanosome formation is under intense research, there is a consensus that melanosome structure correlates with the type of melanin produced—eumelanosomes are elliptical and contain fibrillar matrix, whereas pheomelanosome shape is variable but predominantly spherical and contain a vesiculoglobular matrix. Morphologically their development and maturation proceeds via 4 stages: stage I characterized by early matrix organization; stage II when this matrix is complete, and melanin formation has not yet commenced in eumelanosomes (but is already detectable in pheomelanosomes); stage III typified by melanin deposition; and stage IV where the organelle is saturated with melanin. Recent evidence suggests that cleavage of the melanocyte-specific integral membrane glycoprotein PMEl17 by prohormone convertases contributes to melanosome biogenesis by regulating the nucleation of intralumenal fibrous striations onto which melanin is later deposited (Berson et al, 2003).
Melanosome biogenesis appears to be essentially similar in follicular and epidermal melanocytes. Black-hair follicles melanocytes contain the largest number of melanosomes (eumelanosomes) that are electron-dense. Brown-hair bulb melanosomes are somewhat smaller, in blonde hair melanosomes are poorly melanized, and, often only the melanosomal matrix is visible. Red-hair pheomelanosomes contain a vesicular matrix, but melanin is deposited irregularly as flocculent material. Both eumelanogenic and pheomelanogenic melanosomes can co-exist in the same human cell (Inazu and Mishima, 1993), but not within the same melanogenic pathway, e.g., there is a switch committing melanosomes to either eu- or pheomelanin synthesis (Oyehaug et al, 2002).
The initial step in melanin synthesis, hydroxylation of l-tyrosine to l-dopa as well as l-dopa stability require proton pump-associated acidification of premelanosomes (Puri et al, 2000; Marles et al, 2003) (S11). It was proposed that the product of pink-eyed dilution gene (p) and/or its human P homologue facilitate melanin synthesis by acting as an ion exchange protein in the melanosomal membrane (Puri et al, 2000). The fundamental role of this protein in melanin pigmentation is unequivocal since its mutations lead to decreased pigmentation in mice, and to type II oculocutaneous albinism in humans (OCA2) (S5). The mouse p (pink-eyed dilution) and human P genes encode a protein involved not only in regulating melanosome biogenesis but also in the post-translational processing of tyrosinase and its intracellular transport. Mutations in these genes result in melanosomal defects that include OCA2 (Chen et al, 2002). Once l-dopa is accumulated under acidic conditions, it requires an increased pH (preferably neutral or basic) for the efficient formation of melanin pigment (Ancans et al, 2001), since acidification inhibits melanin synthesis (S11).
Defects in the tyrosinase gene lead to the tyrosinase-negative OCA type 1 (OCA1), resulting from mutations in hot spots (copper binding domains), and missense, nonsense, frameshift, and splicing abnormalities (S12) (http://albinismdb.med.umn.edu). If translated, mutant tyrosinase proteins are routed for degradation by proteasomes (due to the retention of misfolded proteins in the endoplasmic reticulum (ER)) (Kushimoto et al, 2003). In OCA3, the mutated TYRP1 is retained and appropriate processing of normal tyrosinase is aborted leading to early proteasomal degradation and a significant reduction in pigmentation (Kushimoto et al, 2003). In OCA2 (mutation of P) or OCA4 (mutation of MATP) tyrosinase sorting from trans-Golgi network (TGN) to melanosomes is also disrupted (Costin et al, 2003; Kushimoto et al, 2003) (S13). Thus, the defects underlying OCA1 through OCA4 strongly imply that in vivo melanogenic activity depends mainly on post-translational pathways, the most important of which is the effective processing of tyrosinase. Other sites of potential deregulation of the activity are: defects in melanosome biogenesis with resulting accumulation of this enzyme in TGN, or blockades in the translocation of tyrosinase from the TGN to the melanosomes (Slominski et al, 1989) (S14).
Transfer of melanin granules to cortical and medullary keratinocytes in the growing hair shaft is presumed to involve the same mechanism as occurs in the epidermis, although differing in the degradation processes (Bell, 1967). Other differences include melanosome quality; bulbar follicular melanocytes commonly contain a high proportion of fully mature stage IV melanosomes, whereas their proportion is low in epidermal melanocytes, as these are, upon maturation, swiftly transferred to keratinocytes. Melanosome transfer has been explained by the “cytophagic” theory (where keratinocytes phagocytose the tips of dendrites containing stage IV melanosomes, Garcia, 1979), the “discharge” theory (where mature melanosomes are released into the intercellular space to be internalized by adjacent keratinocytes), and the “fusion” theory (where mature melanosomes pass from melanocyte to keratinocyte via fusion of their respective plasma membranes, Okazaki et al, 1976). More recently, filopodia from melanocyte dendrites have been observed to act as conduits for melanosome transfer to keratinocytes (Scott et al, 2002), suggesting melanocyte dendricity as an important phenotypic regulator of melanin transfer to keratinocytes. Notably, the molecular motor myosin Va encoded by the dilute gene, when mutated, can be associated with dilution of hair color in the Griscelli Syndrome (Menasche et al, 2003). It has also been proposed as the driving force for melanosome movement in mammalian melanocytes (Hara et al, 2000). Phagocytosis of melanosomes by keratinocytes is mediated via the activation of the protease-activated receptor 2 (PAR2) on keratinocytes and PAR2 inhibitors retard melanosome transfer (Seiberg et al, 2000).
Morphologic and Biophysical Aspects of Pigmented Hair Shaft Formation
Although follicular melanocytes are derived from epidermal melanocytes during hair follicle morphogenesis, these pigment cell sub-populations diverge as they reach their respective distinct anatomic compartments (Peters et al, 2002). The “follicular-melanin unit” resides in the immune-privileged proximal hair bulb and consists of hair matrix melanocytes and keratinocytes, partly regulated by DP fibroblasts (reviewed in Slominski and Paus, 1993; Stenn and Paus, 2001). There is one melanocyte for every five keratinocytes in the hair bulb as a whole with the ratio of almost 1:1 in the basal epithelial layer next to the DP (Tobin and Paus, 2001). Hair bulb melanogenic melanocytes differ from epidermal ones by being larger, more dendritic, with more extensive Golgi and rough ER, and by producing larger melanosomes (Bell, 1967) (see Table 1 in Tobin and Paus, 2001). Melanin granules transferred into hair cortical keratinocytes remain minimally digested, contrasting with the epidermis, where melanin is almost completely degraded in the differentiating keratinocytes (Bell, 1967). As a consequence of differential processing, eumelanic white individuals may have black hair but very fair skin and blue eyes. Although hair follicle melanocytes remain much less completely characterized than epidermal melanocytes, considerable progress has been made recently (Table S1).
Hair bulb melanocytes are activated cyclically, with melanogenesis being tightly coupled to the hair growth cycle (Slominski and Paus, 1993). Hair grows throughout a finite period of hair shaft formation (anagen; ~3–5 y on average in human scalp), followed by a brief regression phase that results in the apoptosis-driven resorption of up to 70% of the hair follicle (catagen ~1–2 wk in human scalp), and by a relatively quiescent period (telogen ~3 mo) (reviewed in Stenn and Paus, 2001)). Early signs of imminent hair follicle regression become apparent in the hair follicle pigmentary unit toward the end of anagen VI. These include retraction of melanocyte dendrites and attenuation of melanogenesis. Since keratinocyte proliferation continues for some time, the most proximal telogen hair shaft (the so-called club hair) remains unpigmented. Melanogenically active melanocytes, so conspicuous during high anagen (anagen VI), are no longer morphologically detectable during catagen. The hair bulb melanocyte system has, however, long been perceived as self-perpetuating whereby melanocytes involved in the pigmentation of one generation are also involved in the pigmentation of the next wave (Sugiyama, 1979). For this to be so, melanocytes would have needed to survive/avoid the extensive apoptosis-driven regression of the hair bulb (Lindner et al, 1997). However, fully differentiated bulbar melanocytes undergo apoptosis during catagen and are removed from the regressing hair follicle (Tobin et al, 1998). Still, some less-differentiated, hair bulb melanocytes appear to survive catagen (Commo and Bernard, 2000). The multi-functionality of follicular melanocytes is further attested by their complex responses to chemotherapy (Slominski et al, 1996; Tobin et al, 1999).
The “re-differentiating” melanocytes of early anagen are likely newly recruited immature melanocytes derived from a melanocyte reservoir (Nishimura et al, 2002) (S15) located in the upper, “permanent,” outer root sheath. This view is supported by the observation that melanocyte stem cells (TRP2 positive) located at the base of the permanent part of hair follicle are not only immature, but also slow cycling, self-maintaining and are fully competent to regenerating progeny at early anagen (Nishimura et al, 2002). Moreover, melanocyte stem cells in this location appear to have the capacity to enter vacant niches, including, via migration to, the epidermis.
Telogen hair follicles do not show melanin synthesis (Slominski et al, 1991) in their undifferentiated melanocytes/melanoblasts, which, when stimulated at the start of anagen, respond by increasing the cell volume. This anagen signal predates the melanogenic stimulus to be delivered during anagen III, which is followed by subsequent transfer of mature melanosomes into pre-cortical keratinocytes. Melanocytes reach the S-phase of the cell cycle as early as in anagen II, with significant proliferation apparent by anagen III (Sugiyama et al, 1995; Commo and Bernard, 2000). Ultrastructural changes in bulbar melanocytes that accompany progression to full anagen VI include: (1) increased dendricity, (2) development of Golgi and rough ER, (3) increased size/number of melanosomes, and (4) transfer of mature melanosomes to pre-cortical keratinocytes.
Melanins belong to rare biological substances with stable paramagnetic properties (Commoner et al, 1954). Furthermore, hair shafts are among the few biological products with low content of water, a major interference in the assay of biological samples by electron paramagnetic resonance spectrometry (EPR). Thus, EPR enables us to access the qualitative diversity of paramagnetic properties of various types of melanins (Sealy et al, 1982), together with quantitative assays (Slominski et al, 1994) (S16). As EPR can also provide information on the inner microenvironment of the pigment, in particular—for the presence of paramagnetic metal ions (iron, copper, manganese) (Felix et al, 1978), it becomes a principal option for the method developed and adopted for hair research by Ito (Ito and Fujita, 1985) (S17). This method is based on chemical degradation of pigments followed by chromatographic analysis of the products, and is complementary to EPR as being particularly suitable for the detection of low levels of pheomelanin, e.g., in white wool (S18).
The partition between eu- and pheomelanin can be expressed quantitatively by EPR through the a/b factor (Fig 2; Fig S2), together with the integral signal intensity allowing the classification of rodent fur into five types: (1) homogenously black furs containing high amounts of black eumelanins; (2) other homogenously colored furs containing low amounts of eumelanin (brown, platine, pink-eye-dilution, and dilution phenotypes); (3) albino furs that do not contain melanin, but reveal a very weak and broad free radical EPR signal; (4) “agouti” fur containing heterogenous collections of pigments with eumelanin, and pheomelanin stripes; and (5) yellow fur with predominance of pheomelanin, and clear, well-resolved hyperfine structure of the EPR signal. It can be appreciated that there is always some contribution of eumelanin even in “pheomelanin-only” phenotypes (e.g., Ay/a). In fact, there may be no absolute switch-off product in the pathway toward eumelanin synthesis, as even the yellowest fur always reveals eumelanin contamination in the EPR spectrum, as judged by their a/b factor (Fig 2). The integral intensities for yellow fur are always below black fur. This indicates that in hair follicles pheomelanins are only generated under low melanogenic activity of the pigmentary apparatus, but never with total inhibition of tyrosinase activity or eumelanogenesis. By contrast, during eumelanogenesis synthesis of pheomelanin can be switched off completely.
Figure 2 (Fig S2). Biophysical characteristics of rodent hairs.
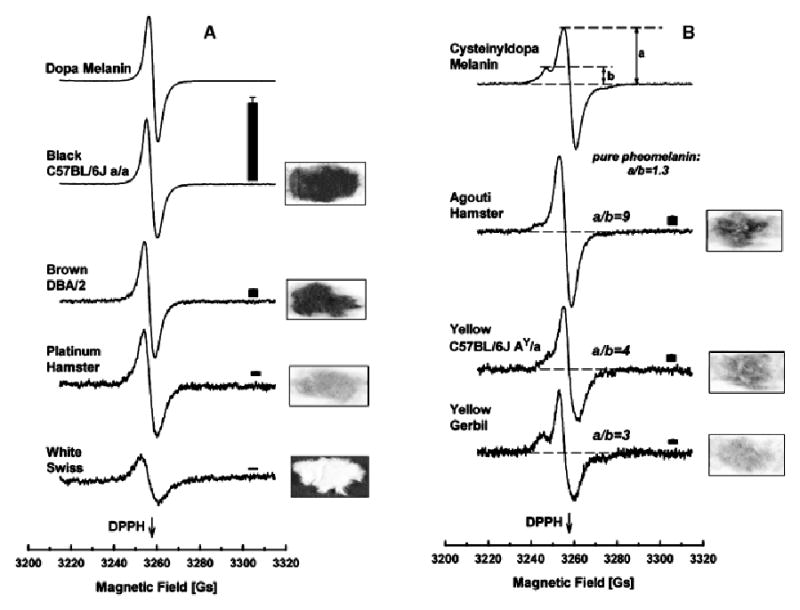
Electron paramagnetic resonance spectrometry signals show eumelanin character (A) and pheomelanin (B) contribution of the hair shaft pigments. Bars on the right above each spectrum show relative free-radical (melanin) concentration per 20 mg of dry fur. Pheomelanin contribution is further quantified by the a/b ratio (see hyperfine splitting of the EPR signal in B). Position of the free-radical standard 1,1-diphenyl-2-picrylhydrazyl (DPPH; g = 2.0037) is indicated by the arrow. All the spectra were registered at room temperature at X-band (9.2 GHz), 1 mW microwave power, 2 Gs modulation amplitude, and variable gain (dopa melanin, C57BL/6J a/a—12,500; cysteinyldopa melanin 62,000; agouti hamster—320,000; brown DBA/2, and C57BL/6J Ay/a – 400,000; other samples—800,000); for bar plots (means of n = 5 ± SEM) modulation amplitude was 5 Gs, and all the integral amplitudes were recalculated for the constant gain 12,500.
Melanogenesis Is Coupled to Anagen: Lessons from the C57BL/6 Mouse
Mice are particularly suited for studies of FM: not only are melanogenic truncal skin melanocytes confined to the hair follicles, but melanogenic activity is strictly coupled to the anagen stage of the hair cycle (Slominski and Paus, 1993; Slominski et al, 1994, 1996; Tobin et al, 1999; Botchkareva et al, 2001). The C57BL/6J mouse melanogenic apparatus is restrictedly coded by the genotype aaBBCCDDEEPP (S1). Their hair follicles produce exclusively eumelanin during spontaneous or depilation induced hair growth, and the melanin production rate is closely correlated with activities of tyrosinase and dopachrome tautomerase (Slominski et al, 1994) (S16), but not of DHICA conversion factor activity. Neither tyrosinase mRNA nor protein, TRP1 (b-locus protein), nor is new melanin synthesized in telogen skin (Slominski et al, 1991).
In the telogen follicle, some melanocytes/melanoblasts from the secondary germ are immunohistochemically positive for TRP2; and of these, a subpopulation also expresses c-Kit (Botchkareva et al, 2001). All melanocytes are mitotically quiescent as assessed by Ki67 immunostaining; during anagen I (day 1 and 2 after anagen induction), tyrosinase mRNA and tyrosinase protein become detectable, but barely, and melanin, TRP1, and tyrosine hydroxylase- and dopa oxidase activities of tyrosinase remain still undetectable (at the biochemical level). Some TRP2 positive melanocytes begin to express TRP1 at this stage (detected by immunocytochemistry), especially melanocytes located proximally, i.e., closer to the forming hair bulb. At the same time, melanocytes residing in the infundibulum (site of the presumptive germ cell reservoir) remain TRP1 negative, whereas some cells expressing TRP1 or TRP2 together with c-Kit may show proliferative activity.
During anagen II, tyrosine hydroxylase and dopa oxidase activities of tyrosinase, tyrosinase mRNA, protein, TRP1, and melanin are readily detectable, to increase rapidly during anagen IIIc (day 5 after anagen induction) reaching their highest levels during anagen V and early anagen VI (days 8–12) (Slominski et al, 1991). By anagen IV, when the hair pigmentary unit becomes fully functional with respect to melanin synthesis, melanocytes distribute into discrete locations throughout the hair follicle. Melanocytes localized to the HF bulge (site of presumptive reservoir) express only TRP2, lacking TRP1, c-kit, and Ki67 immunoreactivities. Melanocytes located in the elongating outer root sheath are TRP2 + and c-Kit + and in some cases are also positive for the proliferation marker Ki67, but express little TRP1 and no tyrosinase (Botchkareva et al, 2001). Only melanocytes distributing to the hair follicle melanogenic zone, i.e., the matrix above the DP, express TRP1, TRP2, tyrosinase, c-Kit, and also Ki67 in the majority of cells. There are also sequential changes in expression of dopa oxidase (tyrosinase) proteins along with anagen development (Slominski et al, 1994). Both activity and concentration of tyrosinase remain constant during mid to late anagen VI (days 12–17), and decrease rapidly during the anagen VI/catagen transition phase to become undetectable or very low in catagen. The expression of other MRP follows a similar pattern, but the decrease during late anagen VI is more gradual with TRP2 reaching its lowest level during catagen. DHICA conversion factor (DHICA-CF) activity, after decrease during the anagen–catagen transformation, rebounds to basal levels during catagen.
Melanocytes from the fully developed anagen VI hair follicles express TRP2 only when located in the hair bulge, TRP2 and c-Kit only when located in the outer root sheath, and all three proteins, together with c-Kit, when located in the hair bulb matrix. Melanocyte proliferation, however, ceases by this sub-stage of anagen. The physiologic decrease in FM may reflect two possible mechanisms for termination of melanogenesis, e.g., exhaustion of an active signaling system that stimulates melanogenesis, and/or production of inhibitors of melanocyte activity. Active depletion of differentiated hair bulb melanocytes via apoptosis also contributes to termination of melanogenesis during late anagen/catagen transition phase (Tobin et al, 1999).
Control of the supply of l-tyrosine via 6BH4 and PAH and setting of the redox conditions also show hair cycle coupling (Schallreuter et al, 1998b). Moreover, human DP cells actively transport and accumulate high concentrations of l-phenylalanine without its turnover to l-tyrosine (Schallreuter et al, 1998b). In anagen I, there are very high levels of 6BH4 (inhibitory of tyrosinase), GTP-cyclohydrolase I (GTP-CH-I) (the rate-limiting enzyme for the de novo synthesis of 6BH4), and PAH activity, thus generating ideal conditions for the production of high concentrations of l-tyrosine from l-phenylalanine (a prerequisite for melanogenesis). All above values drop significantly by anagen III (Schallreuter et al, 1998b). Thus, 6BH4 levels are low preventing allosteric inhibition of tyrosinase by this cofactor. Similarly, low thioredoxin reductase (TR, EC 1.6.4.5) activities during anagen II and III promote more oxidizing conditions necessary for the initiation of melanogenesis. The gradual increase of TR during anagen V up to catagen could neutralize reactive oxygen species (ROS) produced by melanogenesis (Schallreuter et al, 1998b). In catagen pterin synthesis and PAH activities are low, whereas TR levels remain elevated, providing a reducing environment. This would decrease the supply of l-tyrosine that, together with the reducing environment, would generate conditions unfavorable for active melanogenesis.
Regulation of FM
There is a complex regulatory control of the biosynthetic machinery involved in melanogenesis. It involves hormones, neurotransmitters, cytokines, growth factors, eicosanoids, cyclic nucleotides, nutrients, and the physicochemical milieu; the subject has been recently reviewed (Slominski et al, 2004). Briefly, soluble melanogenesis regulators may reach the skin through local production, nerve endings release, or circulatory transport (Slominski and Wortsman, 2000). Positive regulators (melanogenesis stimulators) include α-MSH (melanocyte stimulating hormone), adrenocorticotrophic hormone (ACTH), β-endorphin, prostaglandins, leukotrienes, ET1 and ET3, histamine, SCF, estrogens, androgens, vitamin D3, and bone morphogenic proteins (BMP) (reviewed in (Hearing, 1999; Slominski et al, 2000, 2004) (S5). These act sequentially or in parallel, through pathways involving activation of G-protein receptors, receptors coupled to kinase activities or nuclear receptors. In addition, the nutritional factors l-tyrosine and l-dopa may not only function as substrates for melanin, but also as positive regulators of the melanogenic apparatus in a proper genetic and environmental background (Slominski and Paus, 1994; Slominski et al, 2004). The predominant modifier and inhibitor of melanin synthesis in animal, but not in human hair follicles is agouti protein (ASP) (Hearing, 1999; Barsh et al, 2000).
Studies have documented that signal transduction pathways involving SCF/c-Kit and ET1, ET3/ETA, and ETB play a crucial role in normal FM (Ito et al, 1999; Slominski et al, 2000) (S1, S5, S19). Its pro-pigmentary activity is initiated by binding of locally produced proopiomelanocortin (POMC)-derived ACTH, α-MSH, and β-MSH peptides (reviewed in Slominski et al, 2000). We have proposed that the POMC complementary system, namely, the β-endorphin/μ-opiate receptor system also participates in the regulation of both human epidermal and follicular melanocyte biology, by inducing changes in dendricity, proliferation, and melanogenesis (Kauser et al, 2004) (S20). Nevertheless, the action of POMC peptides on hair follicle melanin is dependent on the environmental context and genetic background. For example, POMC knockout mice expressing ASP show predominance of pheomelanogenesis (reviewed in Slominski et al, 2000), whereas similar POMC deficiency affecting a/a C57BL/6 mice is associated with maintenance of the eumelanogenic phenotype (Smart and Low, 2003; Slominski et al,1 submitted). Humans with POMC gene mutations leading to defective production of the POMC protein have red-hair phenotype (Krude et al, 1998). Corticotropin releasing hormone (CRH) is also produced locally in the human skin (Slominski et al, 2000) (S21), and can modify the phenotype of human hair follicle melanocytes in vitro by upregulating cell dendricity and pigmentation levels (Kauser et al, in preparation), though the mechanism for this activity (direct or indirect) has not been established.
Transient synthesis of the melanogenesis inhibitor ASP, switches eumelanogenesis to pheomelanogenesis, a change that can be associated with decreased tyrosinase activity (Hearing, 1999; Barsh et al, 2000). ASP acts also as a direct antagonist to melanocortins and as an inverse agonist at the MC1-R (Hearing, 1999; Barsh et al, 2000), being both qualitative modulator and/or inhibitor of melanogenesis (Hearing, 1999). Among the factors modifying ASP action are mahogany (mg) or Attractin (Atrn) (required for the action of ASP) and mahoganoid (md), also known as Mahogunin (Mgrn1), which prevents hair follicle melanocytes from responding to the ASP (He et al, 2003).
Additional factors providing negative regulation of melanogenesis include melatonin, interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β, interferon gamma (INF-γ), glucocorticoids, triiodothyronine, and dopaminergic and cholinergic agonists (reviewed in Slominski et al, 2004). Again, these factors act through cell receptors (membrane-bound or nuclear), or through metabolic modification.
Given the similar biogenic derivation of melanosomes and lysosomes, it is notable that the absence of lysosomal enzymes can affect the hair follicle pigmentary unit. For example, in the absence of the papain-like lysosomal cysteine protease cathepsin L (CTSL), hair bulb melanocytes in the regenerating anagen hair follicle exhibit marked vacuolation during melanosome organellogenesis, suggesting that these organelles may become unstable in the absence of this lysosomal enzyme (S22). Thus, CTSL knockout mice provide us with an intriguing model system to assess the role of such proteases in melanosome biogenesis and/or the initiation of melanogenesis. The absence of CTSL in such an oxygen radical-rich organelle may reduce their stability, as under normal circumstances the level of such hydrolases is elevated during melanogenesis (Diment et al, 1995).
Aging of the Hair Follicle Pigmentary Unit
Although a relatively small number of melanocytes can, in a single hair growth cycle, potentially produce sufficient melanin to intensely pigment up to 1.5 m of hair shaft, this remarkable synthetic capacity is realized only during youth (Tobin and Paus, 2001). Individual scalp hair follicles go through approximately 7–15 melanocyte seeding/replacements cycles in the average “gray-free” life span of 45 y (reviewed in Tobin and Paus, 2001). Age of onset of graying also appears to be hereditary, developing usually in late fourth decade. Thus, the average age for Whites is mid-30s, for Orientals late-30s, and for Africans mid-40s, such that by 50 years of age, 50% of people have 50% gray hair. Canities can affect individual hair follicles with either gradual loss of pigment over several cycles, gradual loss of pigment along the same hair shaft (i.e., within the anagen phase of a single hair cycle), or the hair fiber may grow fully de-pigmented. Pigment loss in graying hair follicles is due to a marked reduction in melanogenically active melanocytes in the hair bulb of gray anagen hair follicles (reviewed in Tobin and Paus, 2001). True gray hairs show a much reduced, but detectable, dopa oxidation reaction (indicator of tyrosinase activity), whereas white hair bulbs are broadly negative.
There appears also to be a specific defect of melanosome transfer in graying hair follicles, as keratinocytes may lack melanin granules despite their close proximity to melanocytes with melanosomes. The remaining hair bulb melanocytes in canities-affected anagen hair follicles often appear enlarged, although this may reflect a reduction in dendricity rather than an overall increase in cell volume. Ultrastructural analysis of the human gray hair matrix reveals melanocytes with heterogeneous degrees of melanogenesis (reviewed in (Tobin and Paus, 2001)). Melanocytes of gray/white hair bulbs contain fewer and smaller melanosomes and less supporting organelles, e.g., Golgi apparatus. Interestingly, the remaining melanosomes may be packaged within auto-phagolysosomes suggesting that these melanosomes are defective, perhaps even leaking reactive melanin metabolites. Melanocytes in graying and white hair bulbs may be vacuolated, a common cellular response to increased oxidative stress, and may disappear very rapidly (Commo et al, 2004). A parallel increase in dendritic cells (including Langerhans cells) and their shift from upper to lower hair follicles may represent response to degenerative changes within melanocytes. Gray hair is often unable to hold a permanent or temporary set and may be more resistant to incorporating artificial color, suggesting reprogramming of matrix keratinocytes in aging hair follicles to alter cortical keratinocyte differentiation.
Acknowledgments
The authors thank Dr Eva Peters for help with the design of Fig 1. The support of following grants is acknowledged: NIH 1R01-AR047079 to A. S. and D. J. T.; Center of Excellence in Molecular Biotechnology (5FP, European Union, project “BIER”, contract no. ICA1-CT-2000-70012), SPUB-M 3018 from the Polish Ministry of Science and Informatization (Workpackage 6) to P. M. P.; and by STIEFEL International to K. U. S.
Footnotes
Supplementary Material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/JID/JID23528/JID23528sm.htm
Figure S1 Scheme illustrating common pathway for follicular and epidermal melanogenesis. GSH, glutathione; Cys, cysteine; 1, phenylalanine hydroxylation; 2, tyrosine hydroxylation; 3, dopa oxidation; 4, dopa-chrome tautomerization; 5a, dihydroxyindole carboxylic acid (DHICA) oxidation; 5b, dihydroxyindole (DHI) oxidation; A, hydrolysis of glutathionyldopa; B, oxidation of cysteinyldopa; C, intramolecular cyclization of cysteinyldopaquinone.
Figure S2 Biophysical characteristics of rodent hairs (color version). For explanation, see Fig 2 legend in the text.
Table S1. Characteristics of melanocytes in human and murine anagen hair follicles. Recently low expression of tyrosine hydroxylase mRNA or its absence was reported in one study that used RT-PCR methodology (S23). However, these authors did not measure the actual enzyme activity and they cannot entirely exclude alternative splicing mechanism as the reason for the negative results. Thus, further experimentation is necessary to reconcile these differences.
References S1–S23
Slominski A, Plonka PM, Pisarchik A, Smart JL, Tolle V, Wortsman J, Low MJ: Preservation of eumelanin hair pigmentation in POMC-gene knockout mice on a non-agouti (a/a) genetic background. Submitted for publication, 2004.
References
- Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res. 2001;268:26–35. doi: 10.1006/excr.2001.5251. [DOI] [PubMed] [Google Scholar]
- Barsh G, Gunn T, He L, Schlossman S, Duke-Cohan J. Biochemical and genetic studies of pigment-type switching. Pigment Cell Res. 2000;13(Suppl 8):48–53. doi: 10.1034/j.1600-0749.13.s8.10.x. [DOI] [PubMed] [Google Scholar]
- Bell, M. Ultrastructure of differentiating hair follicles. In: Montagna W, Dobson RL (eds). Advances in Biology of Skin. Oxford: Pergamon Press, 1967
- Berson JF, Theos AC, Harper DC, Tenza D, Raposo G, Marks MS. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J Cell Biol. 2003;161:521–533. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkareva NV, Khlgatian M, Longley BJ, Botchkarev VA, Gilchrest BA. Scf/c-Kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J. 2001;15:645–658. doi: 10.1096/fj.00-0368com. [DOI] [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell. 2002;13:1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commoner B, Townsend J, Pake GW. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- Commo S, Bernard BA. Melanocyte subpopulation turnover during the human hair cycle: An immunohistochemical study. Pigment Cell Res. 2000;13:253–259. doi: 10.1034/j.1600-0749.2000.130407.x. [DOI] [PubMed] [Google Scholar]
- Commo S, Gaillard O, Bernard BA. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br J Dermatol. 2004;150:435–443. doi: 10.1046/j.1365-2133.2004.05787.x. [DOI] [PubMed] [Google Scholar]
- Cooksey CJ, Garratt PJ, Land EJ, Pavel S, Ramsden CA, Riley PA, Smit NP. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J Biol Chem. 1997;272:26226–26235. doi: 10.1074/jbc.272.42.26226. [DOI] [PubMed] [Google Scholar]
- Costin GE, Valencia JC, Vieira WD, Lamoreux ML, Hearing VJ. Tyrosinase processing and intracellular trafficking is disrupted in mouse primary melanocytes carrying the underwhite (UW) mutation. A model for oculocutaneous albinism (OCA) type 4. J Cell Sci. 2003;116:3203–3212. doi: 10.1242/jcs.00598. [DOI] [PubMed] [Google Scholar]
- Diment S, Eidelman M, Rodriguez GM, Orlow SJ. Lysosomal hydrolases are present in melanosomes and are elevated in melanizing cells. J Biol Chem. 1995;270:4213–4215. doi: 10.1074/jbc.270.9.4213. [DOI] [PubMed] [Google Scholar]
- Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22:3016–3023. doi: 10.1038/sj.onc.1206460. [DOI] [PubMed] [Google Scholar]
- Felix C, Hyde J, Sarna T, Sealy R. Interaction of melanins with metal ions. Electron spin resonance evidence for chelate complexes of metal ions with free radicals. J Am Chem Soc. 1978;100:3922–3926. [Google Scholar]
- Garcia RI. Ultrastructure of melanocyte-keratinocyte interactions. Pigment Cell Res. 1979;4:299–310. [Google Scholar]
- Gillbro JM, Marles LK, Hibberts NA, Schallreuter KU. Autocrine catecholamine synthesis and the beta 2 adrenoceptor signal promote pigmentation in human epidermal melanocytes. J Invest Dermatol. 2004;123:346–353. doi: 10.1111/j.0022-202X.2004.23210.x. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Crawford J, Jimenez F, et al. Human recombinant stem-cell factor induces melanocytic hyperplasia in susceptible patients. J Am Acad Dermatol. 1995;33:577–583. doi: 10.1016/0190-9622(95)91274-6. [DOI] [PubMed] [Google Scholar]
- Hara M, Yaar M, Byers HR, Goukassian D, Fine RE, Gonsalves J, Gilchrest BA. Kinesin participates in melanosomal movement along melanocyte dendrites. J Invest Dermatol. 2000;114:438–443. doi: 10.1046/j.1523-1747.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24–28. doi: 10.1038/sj.jidsp.5640176. [DOI] [PubMed] [Google Scholar]
- He L, Eldridge AG, Jackson PK, Gunn TM, Barsh GS. Accessory proteins for melanocortin signaling: Attractin and mahogunin. Ann NY Acad Sci. 2003;994:288–298. doi: 10.1111/j.1749-6632.2003.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Horikawa T, Norris DA, Johnson TW, et al. Dopa-negative melanocytes in the outer root sheath of human hair follicles express premelanosomal antigens but not a melanosomal antigen or the melanosome-associated glycoproteins tyrosinase, TRP-1, and TRP-2. J Invest Dermatol. 1996;106:28–35. doi: 10.1111/1523-1747.ep12326989. [DOI] [PubMed] [Google Scholar]
- Inazu M, Mishima Y. Detection of eumelanogenic and pheomelanogenic melanosomes in the same normal human melanocyte. J Invest Dermatol. 1993;100:172S–175S. [PubMed] [Google Scholar]
- Ito M, Kawa Y, Ono H, et al. Removal of stem cell factor or addition of monoclonal anti-c-Kit antibody induces apoptosis in murine melanocyte precursors. J Invest Dermatol. 1999;112:796–801. doi: 10.1046/j.1523-1747.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Fujita K. Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal Biochem. 1985;144:527–536. doi: 10.1016/0003-2697(85)90150-2. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Park J, Kato F, Hirosaki K, Toyofuku K, Hua C, Yamashita T. Assembly, target-signaling and the intracellular transport of tyrosinase gene family proteins in the initial stages of melanosome biogenesis. Pigment Cell Res. 2000;13:222–229. doi: 10.1034/j.1600-0749.2000.130403.x. [DOI] [PubMed] [Google Scholar]
- Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. Beta-endorphin as a regulator of human hair follicle melanocyte biology. J Invest Dermatol. 2004;123:184–195. doi: 10.1111/j.0022-202X.2004.22724.x. [DOI] [PubMed] [Google Scholar]
- Korner A, Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982;217:1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Kushimoto T, Valencia JC, Costin GE, et al. The melanosome: An ideal model to study cellular differentiation. Pigment Cell Res. 2003;16:237–244. doi: 10.1034/j.1600-0749.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Fitzpatrick TB. Biochemistry of melanin formation. Physiol Rev. 1950;30:1–126. doi: 10.1152/physrev.1950.30.1.91. [DOI] [PubMed] [Google Scholar]
- Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression (catagen) Am J Pathol. 1997;151:1601–1617. [PMC free article] [PubMed] [Google Scholar]
- Marks M, Seabra M. The melanosome: Membrane dynamics in black and white. Nat Rev Mol Cell Biol. 2001;2:1–11. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- Marles LK, Peters EM, Tobin DJ, Hibberts NA, Schallreuter KU. Tyrosine hydroxylase isoenzyme I is present in human melanosomes: A possible novel function in pigmentation. Exp Dermatol. 2003;12:61–70. doi: 10.1034/j.1600-0625.2003.120108.x. [DOI] [PubMed] [Google Scholar]
- Menasche G, Ho CH, Sanal O, et al. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (gs3) or a myo5a fexon deletion (gs1) J Clin Invest. 2003;112:450–456. doi: 10.1172/JCI18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Tobin DJ, Richards-Smith B, Sundberg JP, Paus R. Mutant laboratory mice with abnormalities in pigmentation: Annotated tables. J Dermatol Sci. 2002;28:1–33. doi: 10.1016/s0923-1811(01)00158-x. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Uzuka M, Morikawa F, Toda K, Seiji M. Transfer mechanism of melanosomes in epidermal cell culture. J Invest Dermatol. 1976;67:541–547. doi: 10.1111/1523-1747.ep12664554. [DOI] [PubMed] [Google Scholar]
- Oyehaug L, Plante E, Vage DI, Omholt SW. The regulatory basis of melanogenic switching. J Theor Biol. 2002;215:449–468. doi: 10.1006/jtbi.2001.2521. [DOI] [PubMed] [Google Scholar]
- Peters EM, Tobin DJ, Botchkareva N, Maurer M, Paus R. Migration of melanoblasts into the developing murine hair follicle is accompanied by transient c-Kit expression. J Histochem Cytochem. 2002;50:751–766. doi: 10.1177/002215540205000602. [DOI] [PubMed] [Google Scholar]
- Puri N, Gardner JM, Brilliant MH. Aberrant ph of melanosomes in pink-eyed dilution (p) mutant melanocytes. J Invest Dermatol. 2000;115:607–613. doi: 10.1046/j.1523-1747.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- Sarangarajan R, Boissy RE. Tyrp1 and oculocutaneous albinism type 3. Pigment Cell Res. 2001;14:437–444. doi: 10.1034/j.1600-0749.2001.140603.x. [DOI] [PubMed] [Google Scholar]
- Schallreuter K, Slominski A, Pawelek JM, Jimbow K, Gilchrest BA. What controls melanogenesis? Exp Dermatol. 1998a;7:143–50. doi: 10.1111/j.1600-0625.1998.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Schallreuter K, Beazley WD, Hibberts NA, Tobin DJ, Paus R, Wood JM. Pterins in human hair follicle cells and in the synchronized murine hair cycle. J Invest Dermatol. 1998b;111:545–550. doi: 10.1046/j.1523-1747.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Schulz-Douglas V, Bunz A, Beazley WD, Korner C. Pteridines in the control of pigmentation. J Invest Dermatol. 1997;109:31–35. doi: 10.1111/1523-1747.ep12276418. [DOI] [PubMed] [Google Scholar]
- Scott G, Leopardi S, Printup S, Madden BC. Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci. 2002;115:1441–1451. doi: 10.1242/jcs.115.7.1441. [DOI] [PubMed] [Google Scholar]
- Sealy R, Hyde J, Felix C, Menon I, Prota G. Eumelanins and phaeomelanins: Characterization by electron spin resonance spectroscopy. Science. 1982;217:545–547. doi: 10.1126/science.6283638. [DOI] [PubMed] [Google Scholar]
- Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, Shapiro SS. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162–167. doi: 10.1046/j.1523-1747.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Moellmann G, Kuklinska E. l-tyrosine, l-dopa and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in Bomirski Ab amelanotic melanoma. Pigment Cell Res. 1989;2:109–116. doi: 10.1111/j.1600-0749.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R. Melanogenesis is coupled to murine anagen: Toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R. Towards defining receptors for l-tyrosine and l-dopa. Mol Cell Endocrinol. 1994;99:C7–C11. doi: 10.1016/0303-7207(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Costantino R. Differential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J Invest Dermatol. 1991;96:172–179. doi: 10.1111/1523-1747.ep12460956. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Plonka P, Handjiski B, Maurer M, Chakraborty A, Mihm M. Pharmacological disruption of hair follicle pigmentation as a model for studying the melanocyte response to and recovery from cytotoxic damage in situ. J Invest Dermatol. 1996;106:1203–1211. doi: 10.1111/1523-1747.ep12348479. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Plonka P, Maurer M, Chakraborty A, Pruski D, Lukiewicz S. Melanogenesis during the anagen-catagentelogen transformation of the murine hair cycle. J Invest Dermatol. 1994;102:862–869. doi: 10.1111/1523-1747.ep12382606. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Schadendorf D. Melanocytes as sensory and regulatory cells in the epidermis. J Theor Biol. 1993;164:103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1122. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocrine Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Salomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Smart JL, Low MJ. Lack of proopiomelanocortin peptides results in obesity and defective adrenal function but normal melanocyte pigmentation in the murine C57BL/6 genetic background. Ann NY Acad Sci. 2003;994:202–210. doi: 10.1111/j.1749-6632.2003.tb03181.x. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Sugiyama S. Mode of redifferentiation and melanogenesis of melanocytes in mouse hair follicles. An ultrastructural and cytochemical study. J Ultrastruct Res. 1979;67:40–54. doi: 10.1016/s0022-5320(79)80016-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Saga K, Morimoto Y, Takahashi M. Proliferating activity of the hair follicular melanocytes at the early and anagen III stages in the hair growth cycle: Detection by immunocytochemistry for bromodeoxyuridine combined with dopa reaction cytochemistry. J Dermatol. 1995;22:396–402. doi: 10.1111/j.1346-8138.1995.tb03413.x. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Bystryn JC. Different populations of melanocytes are present in hair follicles and epidermis. Pigment Cell Res. 1996;9:304–310. doi: 10.1111/j.1600-0749.1996.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Hagen E, Botchkarev VA, Paus R. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)? J Invest Dermatol. 1998;111:941–947. doi: 10.1046/j.1523-1747.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Paus R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Slominski A, Botchkarev V, Paus R. The fate of hair follicle melanocytes during the hair growth in mice. J Investig Dermatol Symp Proc. 1999;4:323–332. doi: 10.1038/sj.jidsp.5640239. [DOI] [PubMed] [Google Scholar]


