Abstract
Orbitofrontal cortex (OFC) has been implicated in the use of outcome expectancies to guide behavior. The present study used a devaluation task to examine this function. Rats first received light-food pairings, followed by food-toxin pairings designed to devalue the food. After either excitotoxic or sham lesions of OFC, responding to the light was reassessed. Sham lesioned rats showed reduced responding to the light, relative to behavioral controls that had received food and toxin unpaired. In contrast, OFC-lesioned rats showed no such reductions. Combined with previous data from our laboratories, these results indicate that OFC is critical for either the maintenance of information about the current incentive value of reinforcers, or the use of that information to guide behavior.
Considerable evidence indicates that the orbitofrontal cortex (OFC) and amygdala are important for forming and using expectancies of future events in a flexible manner to guide goal-directed behavior (Holland & Gallagher, 2004). Patients with damage to these brain regions show performance deficits in tasks that require reassessment of the expected values of reinforcers (Bechara et al., 1994; 1999), and in a recent neuroimaging study (Gottfried, O’Doherty, & Dolan, 2003) these regions showed activation changes when normal participants were tested in a reinforcer devaluation task in which the value of cues predicting specific outcomes were reduced by satiation.
OFC and amygdala are critical for proper performance in devaluation tasks in a number of animal models. In one example of this task (Hatfield et al., 1996), rats were first given Pavlovian light-food pairings in a standard conditioning chamber. Then, the food reinforcer was devalued by establishing an aversion to it, via food-toxin pairings in another context (home cage). When later tested in the experimental chamber, normal rats show reduced responding to the light, presented in the absence of food, relative to behavioral control rats that did not receive food-toxin pairings. However, rats with bilateral excitotoxic lesions of either basolateral amygdala (BLA; Hatfield et al, 1996) or OFC (Gallagher, McMahan, & Schoenbaum, 1999) failed to reduce responding to the light conditioned stimulus (CS) after taste-aversion learning. Importantly, comparable deficits have also been obtained under instrumental training conditions. Bilateral BLA lesions impair rats (Balleine, Killcross & Dickinson, 2003) and monkeys (Malkova, Gaffan, & Murray, 1997) in instrumental devaluation tasks. Similar deficits have also been found in monkeys using a disconnection preparation that includes unilateral lesions of amygdala and OFC in contralateral hemispheres (Baxter et al., 2000).
A recent study indicates that OFC and BLA play different roles in the devaluation task. Pickens et al. (2003) found that if OFC lesions were made after light-food training, but before taste aversion training, rats failed to reduce responding to the light in testing. By contrast, rats with BLA lesions made after light-food training showed no such deficits in devaluation performance. These results suggest that the role of BLA is limited to learning the light-food information necessary for this task, whereas OFC is responsible for either maintaining light-food information, integrating the light-food and food-sickness information, expressing this knowledge in behavior, or any combination of these processes.
The present experiment further investigated the role of OFC in the devaluation task by making OFC lesions after taste aversion training, but before test. If OFC is involved solely in the integration of light-food and food-sickness information, then post-taste-aversion lesions should have no effect on normal devaluation performance. If OFC, however, has a role in either maintenance or expression of information, then the lesions should impair performance.
Methods
Subjects: The subjects were 48 male Long-Evans rats (Charles River Laboratories, Raleigh, North Carolina), which weighed 300–325 g when they arrived in the laboratory vivarium. The rats were maintained for 1 week with free access to food and water in individual cages before they were food deprived to 85% of their ad lib weights. The vivarium was illuminated from 6 a.m. to 8 p.m.
Apparatus: The behavioral training apparatus consisted of the eight individual chambers (22.9 × 20.3 × 20.3 cm) described in Pickens et al. (2003). A 6-w lamp, which served as the source of the visual CS, was mounted on the inside wall of the shell, 10 cm above the experimental chamber and even with the end wall opposite the food cup. A dimly illuminated food cup was recessed in the center of one end wall. An infrared photocell placed just inside the food cup was polled (1 kHz) by computer circuitry.
Surgical procedures: After the end of behavioral acquisition training, aseptic surgery was performed under isoflurane inhalation anesthesia (Isovet; Mallinckrodt, Mundelein, IL). Bilateral OFC lesions were made in 24 rats with 20 mg/ml N-methyl-D-aspartate (NMDA) (Sigma, St. Louis, MO) to remove neurons in a target area that included ventrolateral, and lateral orbital regions and both dorsal and ventral agranular insular cortex. One set of injections was made at stereotaxic coordinates 4.0 mm anterior to bregma, 4.2 mm ventral from the skull surface at bregma, with infusions of 0.08 μl at sites 2.2 mm and 3.7 mm from the midline. Another set of injections were made 3.0 mm anterior to bregma, 5.2 mm ventral from the skull surface at bregma with infusions of 0.08 μl at sites 4.2 mm from midline and 0.05 μl at sites 3.2 mm from midline. In the sham surgeries (n=23), the injector was lowered to the corresponding depths and phosphate buffered saline (PBS) vehicle was injected in 6 of the rats in a manner comparable to lesion injections whereas 17 of the rats simply had the injector raised up again without any vehicle infusion. The NMDA or vehicle was infused at a rate of 0.1 μl/minute using a glass micropipette attached by a length of plastic tubing to a picospritzer (General Valve Corporation, Fairfield, NJ).
Histological procedures: After completion of behavioral testing, rats were deeply anaesthetized with Nembutal (150 mg/kg), and perfused with 0.1-M PBS, followed by 10% (v/v) formalin. The brains were removed and stored in 0.1-M PBS with 20% (w/v) sucrose and 1% (w/v) dimethyl sulfoxide (DMSO) at 4° C for 24–48 hr. Sections (40-μm) were taken from each brain, and every third section was mounted on slides and Nissl-stained to verify lesions.
Behavioral training procedures: The rats were first trained to eat from the recessed food cup in a single 64-min session that included 16 deliveries of the reinforcer, two 45-mg Noyes food pellets (P.J. Noyes, Manchester, N.H.). Next, in each of eight daily 64-min sessions, the rats received sixteen 10-s presentations of the house light, each followed immediately by the delivery of two 45-mg food pellets.
The rats were then assigned to Paired (n=24) and Unpaired (n=24) groups for flavor aversion training, which took place in the animals’ home cages. All rats received two exposures to food pellets and two injections of lithium chloride (LiCl), but the Paired groups received the injections immediately following the food consumption and the Unpaired group received the LiCl and food 24 hr apart. This equated both groups for the number of exposures to the food pellets and the lithium chloride while giving only the Paired group a taste aversion. On each of the two days before aversion training began, all animals received 1-hour exposure to the white ceramic bowls used in the aversion training to minimize neophobia to the bowls. On the first and third days of aversion training, the rats in the Paired group received, in their home cages, 10-min access to a ceramic bowl containing 100 food pellets (identical to those delivered in the acquisition chambers). Immediately following food consumption by the Paired animals, all rats received an injection of 0.3 M LiCl solution (5 ml/kg i.p.). On the second and fourth days of aversion training, the rats in the Unpaired group received 10-min access to a ceramic bowl containing 100 food pellets (identical to those delivered in the acquisition chambers), but these were not followed by injections. On the second day of consumption for each group, rats that did not eat at least 20 pellets in either consumption session (4 Paired, 4 Unpaired) were given up to three additional 10-minute sessions to reach a total of 20 pellets consumed on that day. The first extra session was given in the rat’s home cage and the two additional sessions, if necessary, were given in a beddingless cage. All of the consumption sessions were given before LiCl injection if the rat was in the Paired group. If a rat did not eat a total of at least 20 pellets on either consumption day (n=1), it was excluded and did not undergo surgery. The rats were then assigned to surgical conditions and received surgery 3–12 days later, followed by 11–16 days of recovery. Neurotoxic lesions were made in 11 Paired and 13 Unpaired rats and sham lesions were made in 12 Paired and 11 Unpaired rats.
The rats then received a single 64-min test session in the experimental chamber. This session included 16 presentations of the 10-second house light, but no food pellets were delivered. Consistent with previous studies, we reported only the data from the first 8 of these trials. Approximately 6 hours later, the rats received 10-minute access to 50 45-mg food pellets (those used as the reinforcer) placed in the food cup of the experimental chamber, in order to assess the level of generalization of the taste aversion from the home cage to the experimental chamber. On the final day of testing, the rats received, in their home-cages, 10-min access to a ceramic bowl containing 100 food pellets, as in the flavor aversion training.
Response measures: As in previous studies, the primary measure of appetitive conditioning to the house light CS was the percentage of time the rat spent with its head in the food cup during the last 5 s of the 10-s CS, and during the 5-s empty interval immediately before each CS, as indicated by disruption of the photocell beam. Previous data (Holland, 1977) show that food cup behaviors during 10 second visual CSs are concentrated in the last 5 seconds of the cue. For the test session, we also report the percentage of time the rat spent with its head in the food cup in the 5 seconds after the light ended, when food would normally be presented. Consumption of food pellets in the home cage was determined by counting the number of pellets in the bowl after 10 minutes. Consumption in the experimental chamber test was determined by counting the number of pellets in the food cup and tray beneath the floor after 10 minutes.
The level of statistical significance adopted was p = 0.05.
Results and discussion
Two sham rats died during or soon after surgery and one had excessive surgical damage, as revealed by histology. All remaining sham control rats (10 Paired and 10 Unpaired) were included in the analysis. One lesion rat was excluded due to post-surgical illness. Of the remaining lesioned rats, 9 of the Paired animals and 9 of the Unpaired animals had acceptable lesions. Lesions were excluded (n=5) if there was less than 30% damage to the target area in either hemisphere. Acceptable lesions averaged ~65% damage at the center of the lesion and ~45% of the entire rostrocaudal axis of the target region (Figure 1). There was no significant difference in the amount of damage to OFC in the Paired (***%) and Unpaired (***%) lesion groups (t(16) = 0.50)). Many lesions had damage to cortical areas dorsal to OFC along the area where the injector was positioned with some rats exhibiting damage extending to the lateral borders of the brain. This was typically unilateral and limited to primary motor cortex. However, in some rats, damage was bilateral or included parts of primary somatosensory cortex. Overall, there was no significant correlation between lesion size and the behavioral measures reported, rs ≤ 0.18. Finally, although there was substantial variation in the placement of these lesions as well, lesion placements were comparable in the rats in the Paired and Unpaired conditions.
Figure 1.
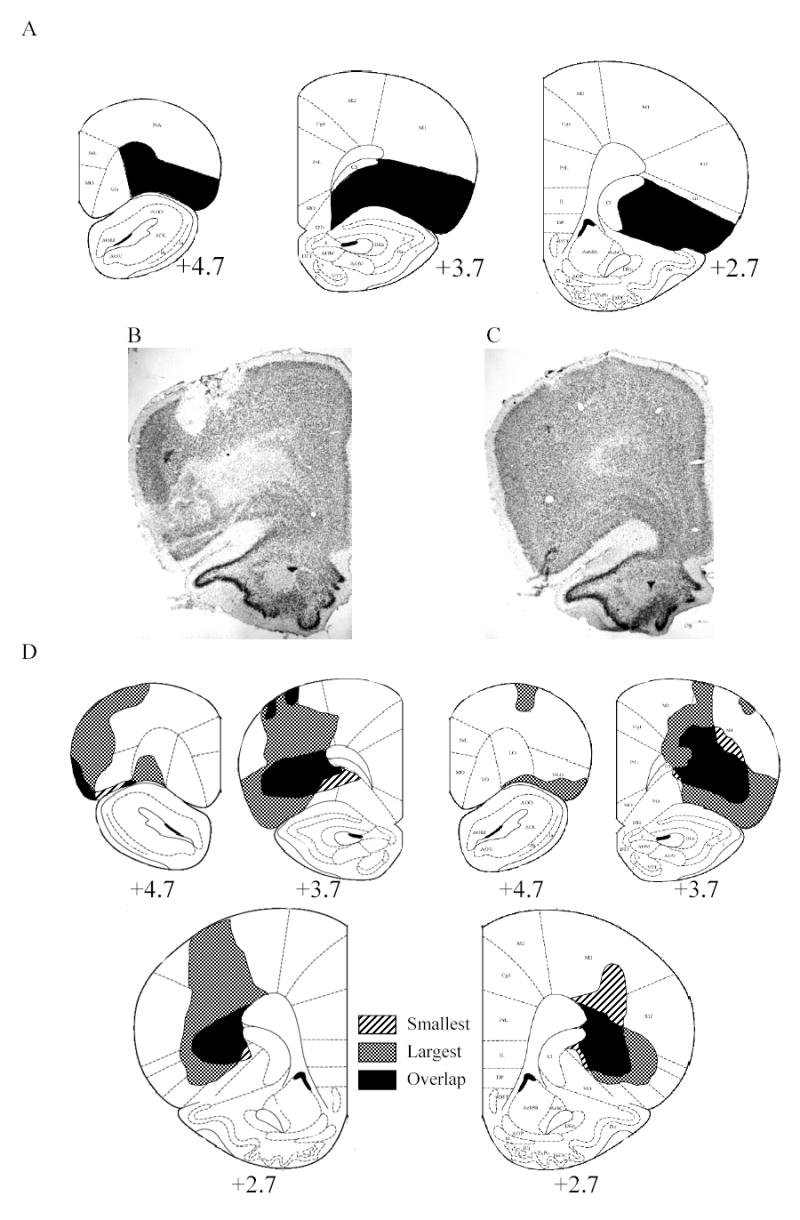
A: Diagram with ideal lesion shaded in black. B: Neurotoxic lesion of orbitofrontal cortex (OFC). C: Sham lesion of OFC. D: Extent of minimum and maximum lesions of OFC at various distances anterior to bregma. The stripes show the position of the smallest lesion, the hatching shows to the position of the largest lesion and black shows the area where the smallest and largest lesions overlap. The target region of OFC includes: lateral orbital (LO), dorsolateral orbital (DLO), lateral parts of ventral orbital (VO), dorsal agranular insular cortex and ventral agranular insular cortex.
Conditioning to the light
The rats in all groups increased their food cup responses during the light over the course of training. Performance on the final conditioning session did not differ as a function of later lesion status or assignment to Paired or Unpaired taste aversion conditions (responding to the light: sham-Paired: 80.0 ± 2.9%, sham-Unpaired: 79.4 ± 3.8%, lesion-Paired: 79.0 ± 5.3%, lesion-Unpaired: 74.8 ± 4.9%). Separate Lesion X Taste Aversion ANOVAs of responding during both the CS and pre-CS periods found no significant main effects or interactions.
Taste aversion learning
Taste aversion training reduced food consumption in the Paired groups but not in the Unpaired groups. The effects of this training were not affected by subsequent lesion status. A Lesion X Taste Aversion X Trial ANOVA for the two training trials (left side of Figure 2a) found a significant interaction of Taste Aversion X Trial (F(1,34) = 21.99). No other main effects or interactions were significant.
Figure 2.
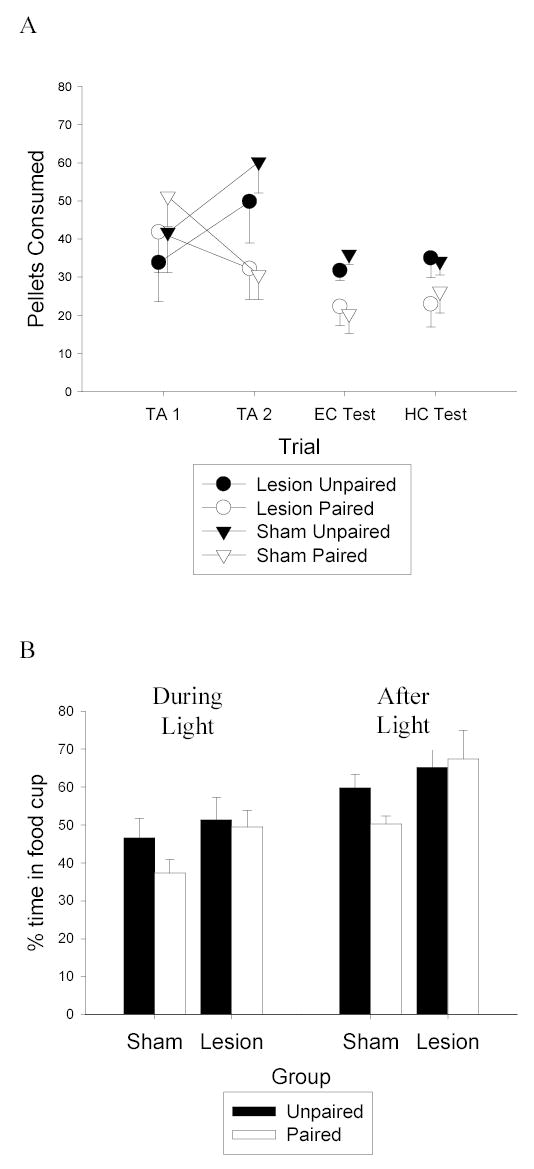
A: Consumption of food pellets during the two taste aversion (TA) trials in the homecage is shown on the left portion of the figure. The amount of food eaten in the experimental chamber (EC) during the consumption test is shown in the point in the middle of the figure. The amount of food eaten in the homecage (HC) consumption test is shown in the points on the right portion of the figure. Black symbols refer to Unpaired groups, white symbols refer to Paired groups, circles represent lesion groups and triangles represent sham groups. B: Responding during the first 8 trials of the devaluation test. Responding during the last 5 seconds of the light cue is shown on the left and responding in the five seconds after the light is shown on the right. Black bars represent Unpaired groups and white bars represent Paired groups.
The taste aversion transferred readily from the home cage, where it was established, to the experimental chamber. Furthermore, that transfer was unaffected by the OFC lesions, which were made prior to this test. A Lesion X Taste Aversion ANOVA of the experimental chamber consumption test (middle points in Figure 2a) showed a significant effect of Taste Aversion (F(1,34) = 9.82), and no other main effects or interactions were significant. A Lesion X Taste Aversion ANOVA of the final home cage consumption test (rightmost point in Figure 2a) showed a marginal effect of Taste Aversion (F(1,34) = 3.79, p = 0.06). Again, there was no effect of the OFC lesions on performance in this final test; neither the main effect of Lesion nor the Lesion X Taste Aversion interaction was significant. It is notable that the two consumption tests were conducted 18–25 days after taste aversion training, after surgery and recovery, which may account for the smaller taste aversion effect in this experiment compared to previous studies that used similar procedures but with no delay between taste aversion training and testing (e.g, Gallagher et al, 1999; Hatfield et al, 1996; Pickens et al, 2003).
Devaluation test
Figure 2b shows the primary data from this experiment, food cup responding evoked by the light CS alone in the final test session. The left bars show responding during the last 5 s of the light CS and the right bars show responding during the 5-s interval after the CS, the normal time of US delivery. Unlike sham rats, rats with OFC lesions failed to show a devaluation effect. Responding of lesion-Paired rats did not differ significantly from that of lesion-Unpaired during either the CS (left bars) or post-CS (right bars) periods (Fs < 1). Although lesion and sham rats in the Paired condition showed equivalent aversions to the food, that aversion had less effect on conditioned food cup responding evoked by the light in the lesioned rats. Responding was significantly greater in the lesion-Paired rats than in the sham-Paired rats in both CS and post-CS periods, Fs (1, 17) = 4.66 and 5.26, respectively. At the same time, responding of lesioned-Unpaired and sham-Unpaired rats did not differ (Fs <1). Finally, responding in the sham-Paired rats was lower than in the sham-Unpaired rats, although that difference was statistically significant only in the post-CS periods, Fs (1, 17) = 2.09 and 5.63 for the CS and post-CS periods, respectively.
Compared to the effects we have observed in previous studies (e.g.. Pickens, et al, 2003) the devaluation effect observed in sham rats was small. Using the same response measure as we reported in earlier studies (responding during the last 5 sec of the light CS), the reduction of responding observed here after food-toxin pairings was smaller both numerically and statistically that we observed in previous studies. In the present study, post-CS responding was more sensitive to the effects of devaluation. Conditioned responding is often timed to peak near the time of reinforcer delivery (Holland, 2002); indeed conditioned eyeblink research often introduces nonreinforced “catch” trials during training to assess responding during the time interval in which the reinforcer would normally be delivered (e.g.: Rogers, Britton, & Steinmetz, 2001; Woodruff-Pak, 1993). We suspect that the relatively small size of the devaluation effect observed here was related to the smaller magnitude taste aversion, which in turn may have been related to the long delay between taste aversion training and devaluation testing in this study, as previously discussed. Regardless of the origins of the smaller-than-usual devaluation effect in sham rats, it is notable that the critical difference that defines the lesion effect, greater responding in lesion-Paired rats than in sham-Paired rats, was statistically significant with both response measures.
These results extend our previous findings (Pickens et al., 2003) by showing that lesions of OFC made after taste aversion training impair performance of rats in the devaluation task. Thus, OFC is critical to expressing appropriate performance in this task, even if all of the information necessary for that performance is acquired while the animal is intact. Together with our previous findings, these results support a model in which BLA and OFC work together in establishing and using expectancies of reinforcers, but with specialized functions for each region. BLA is responsible for establishing an associative structure wherein a cue maintains access to the current incentive value of the reinforcer, and OFC is responsible for maintaining that information, or translating it into appropriate action (or both).
This model is also supported by electrophysiological recording data from our laboratories using a go/no-go task in rats, and by recent functional magnetic resonance imaging (fMRI) studies with humans. In our recording studies, neurons in BLA showed differential responding to cues that predict different outcomes through the course of learning before accurate behavioral performance in reached, whereas neurons in OFC exhibited differential firing only after the criterion behavioral performance was achieved (Schoenbaum, Chiba, & Gallagher, 1999). Furthermore, OFC neurons failed to acquire stimulus-outcome selectivity in rats with lesions of BLA (Schoenbaum et al, 2003), as if BLA performed a training function on those neurons. Likewise, in a recent fMRI study, Gottfried, O’Doherty, and Dolan (2002) found activation in a subregion of amygdala that could correspond to part of the basolateral complex that was related to the presentation of visual signals for pleasant odors early in visual-odor pairing training, but not with extended training. In contrast, OFC activation by the visual cues was maintained throughout. Finally, in another fMRI study, Arana et al. (2003) found activation of subregions of OFC by verbal labels for preferred food items only when participants had to choose among those items, but activation of amygdala and other OFC subregions regardless of whether a choice response was necessary. These findings all support a model in which BLA is involved in coding and learning relationships between emotional events and OFC is involved in using these relationships to guide behavior.
The results of our study suggest that OFC is needed for proper devaluation performance at the time of action. This does not, however, preclude a role for OFC at earlier stages of the devaluation task. Gottfried and colleagues (2002) found activation of human OFC in learning an appetitive relationship between cues and outcomes. This suggests that OFC is involved in learning information about appetitive relationships, but the results do not allow us to determine if OFC is necessary in learning the information needed for proper devaluation performance. Future research will focus on the role of OFC in learning and maintenance of information needed for later devaluation performance by utilizing transient inactivation methods to disrupt the integrity of OFC during earlier stages of training with intact OFC function during the devaluation test.
Acknowledgments
C.L.P and M.P.S. contributed equally to this work. This research was supported by National Institute of Mental Health Grants MH60179 (MG), MH53667 (PCH) and National Science Foundation Graduate Research Fellowship DGE-0202743 (CLP). We would like to thank Erin Kerfoot, Frank Groshek and Vanessa McKenna for excellent technical support and Geoffrey Schoenbaum for advice and assistance to support this work.
References
- Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. Journal of Neuroscience. 2003;29:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital frontal cortex. Journal of Neuroscience. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. Journal of Neuroscience. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. Journal of Neuroscience. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;15:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesion of the basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer-devaluation effects. Journal of Neuroscience. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. The conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Trial and intertrial durations in appetitive conditioning in rats. Animal Learning and Behavior. 2000;28:121–135. [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Malkova L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. Journal of Neuroscience. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RF, Britton GB, Steinmetz JE. Learning-related interpositus activity is conserved across species as studied during eyeblink conditioning in the rat. Brain Research. 2001;905:171–177. doi: 10.1016/s0006-8993(01)02532-x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. Journal of Neuroscience. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends on output from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Eyeblink classical conditioning in H.M.: delay and trace paradigms. Behavioral Neuroscience. 1993;107:911–925. doi: 10.1037//0735-7044.107.6.911. [DOI] [PubMed] [Google Scholar]


