Abstract
Sitosterolaemia is a rare autosomal recessive disease characterized by increased intestinal absorption of plant sterols, decreased hepatic excretion into bile and elevated concentrations in plasma phytosterols. Homozygous or compound heterozygous loss of function mutations in either of the ATP-binding cassette (ABC) proteins ABCG5 and ABCG8 explain the increased absorption of plant sterols. Here we report a Swiss index patient with sitosterolaemia, who presented with the classical symptoms of xanthomas, but also had mitral and aortic valvular heart disease. Her management over the last 20 years included a novel therapeutic approach of high-dose cholesterol feeding that was semi-effective. Mutational and extended haplotype analyses showed that our patient shared this haplotype with that of the Amish-Mennonite sitosterolaemia patients, indicating they are related ancestrally.
Keywords: Sitosterolaemia, plant sterols, Amish-Mennonites, ABC95, ABC98, valvular xanthomas
Sitosterolaemia is a rare autosomal recessive disease, first described in 1974 (1). The characteristic feature of this disease is the presence of large quantities of plant sterols in the plasma and tissues measured by using either capillary gas liquid or high-performance liquid chromatography, because routine enzymatic cholesterol determination does not distinguish between phytosterols or cholesterol (2–4). The normal western diet contains daily similar amounts of cholesterol and plant sterols. Normal individuals absorb approximately 40–50% of intestinal (from diet and bile) cholesterol, but less than 5% of plant sterols are absorbed and principally excreted by the liver into bile (5–8). Patients with sitosterolaemia absorb more than 50% of plant sterols and show an inability to excrete these sterols in their bile (5, 9–12).
Clinical features of sitosterolaemia include the presence of tendon and tuberous xanthomas, especially on Achilles tendon and on extensor tendons of the hands, premature atherosclerotic disease, arthritis and arthralgia and haemolytic anaemia (1, 2, 9, 13, 14).
Pedigree assembly and genetic analyses facilitated the localization of the disease locus (STSL) to chromosome 2p21 (15, 16). Not one but two genes comprise the STSL locus, ABCG5 and ABCG8, and homozygous or compound heterozygous mutations in either gene cause the disease (15, 17, 18). The proposed function of these proteins is to pump plant sterols and cholesterol back out into the biliary system or the intestinal lumen (18, 19). Clinical management of sitosterolaemia has included a low-phytosterol and low-cholesterol diet, resin therapy and ileal bypass (2, 5, 9, 20–27).
Ezetimibe is the most promising new agent for management in sitosterolaemia, as it specifically inhibits all dietary sterol absorption from the intestinal lumen (28–30).
Here we report the management of a young sitosterolaemic patient over a period of 20 years. Because the absorption of both plant sterols and cholesterol is dependent upon solubilization into micelles and that these sterols compete with each other for solubilization (31, 32), we hypothesized that high-dose cholesterol should be able to prevent plant sterols from absorption. This therapy was semi-effective. Mutational analysis showed that our patient was homozygous for a mutation that has been described in the Amish-Mennonite community in the USA (16, 33). Extended haplotype analyses showed a remarkable degree of conservation, suggesting that our patient’s ancestors and the Amish-Mennonite ancestors are connected. Lineage tracing of our proband’s family suggests that there may be more sitosterolaemia cases in a limited geographic area around the Swiss border.
Materials and methods
All clinical analyses other than plant sterols, cholesterol and genetic analysis were performed using standard methods at the Laboratory of the University Hospital of Bern, Switzerland. Cholesterol and plant sterols analyses were performed by gas chromatography – mass spectroscopy as previously described (30). For genotyping, genomic DNA was isolated from whole blood. Genomic DNA was amplified with microsatellite marker-specific radiolabelled primers as previously described (15, 17). Single nucleotide polymorphisms (SNPs) were determined by restriction enzyme digestion pattern or direct sequencing as previously described (16, 17).
Case report
Our patient was born in 1976. The diagnosis of sitosterolaemia was recorded at the age of 10 years because of increasing tendon xanthomas by effective low-cholesterol therapy. Unfortunately, no data are available from the time of this diagnosis from her medical records. Consequently, therapy with cholestyramine (4 g daily) was initiated, and the patient was advised to adhere to low-phytosterol and low-cholesterol diets.
Despite the continued therapy and a normal TS1 level (166 mg/dl), at the age of 19 (1995), xanthomas reached a maximal thickness of 5 cm on both elbows and of 3 cm on both Achilles tendons. The patient admitted a poor compliance for the proposed low-phytosterol diet. Furthermore, she discontinued cholestyramine treatment in 1998 because of increased bloating and constipation. The cholesterol-poor diet alone maintained TS levels at levels lesser than 200 mg/dl. Because both cholesterol and phytosterols compete with each other for entry into micelles, it was reasoned that a high-cholesterol intake may reduce phytosterol absorption, and thus xanthomas progression. At the age of 23 years, empiric therapy with cholesterol tablets, 500 mg/day for 6 weeks, increasing to 1000 mg/day was initiated. Initially TS levels peaked to 276 mg/dl, but after 2 months, they returned to pre-treatment levels (<200 mg/dl). Unfortunately, we do not have cholesterol and plant sterol values before initiation of therapy, but blood sample analysed by GC 3 weeks after initiation showed a cholesterol value of 239 mg/dl and a sitosterol value of 26.4 mg/dl, confirming sitosterolaemia. Interestingly, with continued therapy, there was progressive fall in cholesterol (25%) and sitosterol (33%) (Fig. 1). High-density lipoprotein sterol (HDL-S) followed a similar pattern as TS (Fig. 1). The TS/HDL-S ratio gradually increased from 3.7 to 5.2. During this therapy, her xanthomas remained unchanged and large.
Fig. 1.
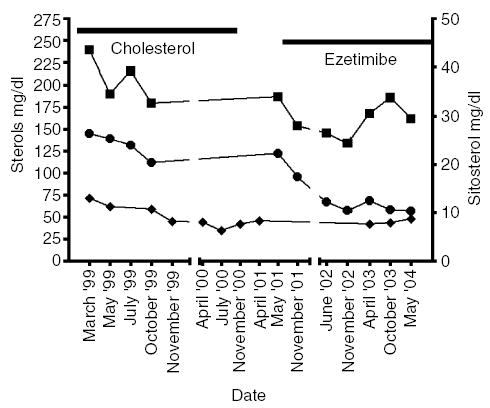
Cholesterol, plant sterol and high-density lipoprotein sterol (HDL-S) profiles on oral cholesterol and ezetimibe therapies. The cholesterol (mg/dl) (▪) and sitosterol (mg/dl) (∘) profiles, as determined by gas chromatography (GC), on samples during oral cholesterol therapy (indicated by the horizontal line) and on ezetimibe monotherapy. Because the enzymatic test was used to determine the HDL-cholesterol values (mg/dl) (♦), the term HDL-S is used. After an initial decrease, the plasma cholesterol values remained around 190 mg/dl on cholesterol tablets and 170 mg/dl on ezetimibe. In contrast, note that the plant sterol values fell on oral cholesterol therapy. The greatest and most persistent falls (more than 50%) were during therapy with ezetimibe, with lowering of sitosterol to the lowest values our patient has ever achieved (10 mg/dl).
From October 2000 onwards, cholesterol therapy was discontinued; despite biochemical improvement, there was no clinical improvement. Until April 2001, she received no medication, and her xanthomas remained stable, although TS (245 mg/dl) and HDL-S rose by 25%.
In May 2001, she began therapy with ezetimibe 10 mg/day as part of a clinical trial (30). As a result of this therapy, her TS (176 mg/dl), cholesterol, sitosterol and TS/HDL-S decreased by 28%, 13%, 50% and 32%, respectively (Fig. 1). During ezetimibe therapy, her xanthomas were overall reduced to less than 1 cm.
At the age of 20 (1997), a new 2/6 grade diastolic murmur was noticed. Colour-flow Doppler echocardiography showed a thickening and sclerosis of the mitral valve ring and of the aortic valve leaflets associated with grade I valvular regurgitation across both valves (Fig. 2). There was no history of rheumatic fever. From 1997 until 2001, yearly echocardiograms showed a slight increase in the aortic regurgitation to grade II. Since the beginning of ezetimibe in 2001, her cardiac findings showed neither progression nor regression.
Fig. 2.
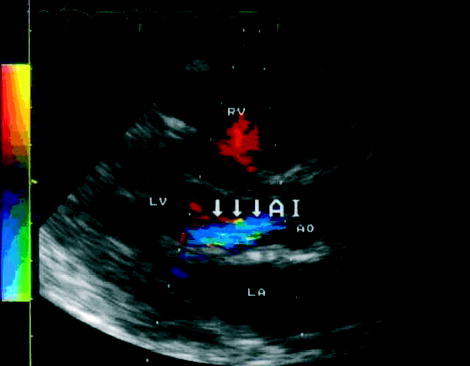
Doppler colour echocardiogram demonstrating the valvular heart disease in our patient. The left ventricle (LV), left atrium (LA), right ventricle (RV) and the aorta (Ao) are as indicated. The colour flow patterns show the aortic valve regurgitation (blue and indicated by the arrows).
Mutational analysis showed that our patient was homozygous for the Gly574Arg mutation in ABCG8. This mutation has been found previously in the Amish-Mennonite patients where a founder effect has been reported (34). Haplotype analyses using microsatellite markers spanning 4.9 cM on chromosome 2 showed that our patient and the two Amish-Mennonite probands were identical for markers D2S2174, D2S1761, D2S4009, D2S4014, D2S4015 and D2S4016 encompassing the STSL locus (33) (data reviewed but not shown). We have previously reported a number of SNPs of the STSL locus (16, 17). We compared poly SNPs at the STSL locus of our patient to that of two Amish-Mennonite probands (two families). The Swiss proband and one Amish proband shared identical SNPs, with a minor difference between the two Amish-Mennonite probands (Table 1).
Table 1.
Single nucleotide polymorphisms (SNP) of ABCG5 and ABCG8 of the Swiss/German proband and the two Amish-Mennonite probands
| Gene | SNP | Position | refSNP | Swiss/German | Amish 1 | Amish 2 |
|---|---|---|---|---|---|---|
| ABCG5 | EXI | P9P | CC | CC | CC | |
| EX2 | R50C | rs6756629 | CC | CC | CC | |
| EX11 | V523I | AA | AA | AA | ||
| EX13 | C600Y | GG | GG | GG | ||
| EX13 | Q604E | rs6720173 | GG | GG | GG | |
| EX13 | V622M | AA | AA | AA | ||
| ABCG8 | 5′UTR | 41 | CC | CC | CC | |
| 5′UTR | 19 | rs11887534 | GG | GG | TTa | |
| EX1 | P17P | CC | CC | CC | ||
| EX1 | D19H | GG | GG | GG | ||
| INT1 | 21 | CA | CA | CA | ||
| INT1 | 7 | CT | CT | TTa | ||
| EX2 | C54Y | rs4148211 | GG | GG | GG | |
| EX6 | E238L | GG | GG | GG | ||
| EX6 | A259V | CC | CC | CC | ||
| EX7 | Q340E | GG | GG | GG | ||
| EX8 | T400K | rs9282575 | CC | CC | CC | |
| INT9 | 19 | CC | CC | CC | ||
| INT10 | 50 | CC | CC | CC | ||
| EX11 | A565A | rs4148217 | CC | CC | CTa | |
| EX11 | G575R | GG | GG | GG | ||
| EX13 | A632V | rs6544718 | CC | CC | CC |
The minor differences in one of the Amish-Mennonite proband.
Note the high degree of SNP conservation as well as homozygosity of the SNPs at the STSL locus.
A family history from the patient indicated that most of her relatives lived during the last 300 years in a small region between the northeast of German-speaking Switzerland and southern Germany (between the lake of Constance and the Obersaxen in the Canton of Grison). These data support the contention that the Gly574Arg mutation in the Amish-Mennonite originated in Europe and is likely more than 250 years old.
Discussion
Initially, the patient adhered to a cholesterol-poor diet alone or in combination with cholestyramine, but this was insufficient to relieve her symptoms and led to increased side-effects. Every attempt to introduce a phytosterol-poor diet was unsuccessful. We reasoned if plants sterols compete with cholesterol for micellar solubilization when given in high concentrations (and this reduce absorption), the reverse would also be true (31, 32). This is the first report of using high doses of cholesterol to reduce plant sterol levels in sitosterolaemia. Despite the decrease of phytosterols in the blood, some caution about this therapy should be expressed. In contrast to the following treatment with ezetimibe, our patient did not report any significant improvement in her xanthomas (despite the relatively short term), and the TS/HDL-S ratio worsened.
In our patient also, we report valvular heart disease limited to the aortic and mitral valves. After exclusion for other causes, we presume that this is a clinical manifestation of her sitosterolaemia. Deposition of phytosterols-enriched macrophages into the valvular tissue might have caused the valvular heart disease in this patient. Xanthomatosis involving the mitral valve and the entire aorta with occlusion of coronary ostia has been reported at autopsy in a sitosterolaemic girl (35). Interestingly, the valvular regurgitations have remained stable over the 3 years since therapy with ezetimibe was initiated.
Finally, our proband and two probands from the Amish-Mennonite community in the US share the same homozygous mutation of ABCG8, and haplotype analysis links these patients (34). We have reported previously that there may be a founder effect for the mutation in the US Amish-Mennonite community (34). Interestingly, while similar founder effects have been proposed for other diseases (36, 37), this is the first direct evidence linking two existing communities. If this is true, there may be more undiagnosed individuals in this region, and attempt to identify at-risk individuals should be made.
Acknowledgments
We thank Klaus Von Bergmann for helping us with the sterol analysis and Eric Klett and Radu Tutuian for the critical discussion of the manuscript. This work was supported by grants from Novartis Foundation, formerly the Ciba-Geigy Jubilee Foundation Basel/CH; Balli Foundation Bellinzona/CH, the Ruth De Bernardi Foundation Bern/CH and by the National Institutes of Health grant HL 060613 (SBP).
Footnotes
We use the term TS to indicate all sterols measured enzymatically (the standard ‘cholesterol’ test) to distinguish between gas chromatography (GC) determined cholesterol or plant sterols.
References
- 1.Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53(4):1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res. 1992;33(7):945–955. [PubMed] [Google Scholar]
- 3.Tvrzicka E, Mares P, Pisarikova A, Novakovic J, Hrabak P. Simplified gas chromatographic method for the simultaneous determination of phytosterols and cholesterol. J Chromatgr. 1991;563(1):188–192. doi: 10.1016/0378-4347(91)80294-m. [DOI] [PubMed] [Google Scholar]
- 4.Kuksis A, Myher JJ, Marai L, Little JA, McArthur RG, Roncari DA. Usefulness of gas chromatographic profiles of plasma total lipids in diagnosis of phytosterolemia. J Chromatgr. 1986;381(1):1–12. doi: 10.1016/s0378-4347(00)83559-8. [DOI] [PubMed] [Google Scholar]
- 5.Salen G, Shore V, Tint GS, et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res. 1989;30(9):1319–1330. [PubMed] [Google Scholar]
- 6.Salen G, Ahrens E, Jr, Grundy SM. Metabolism of beta-sitosterol in man. J Clin Invest. 1970;49(5):952–967. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould RG, Jones RJ, LeRoy GV, Wissler RW, Taylor CB. Absorbability of beta-sitosterol in humans. Metabolism. 1969;18(8):652–662. doi: 10.1016/0026-0495(69)90078-x. [DOI] [PubMed] [Google Scholar]
- 8.Morton GM, Lee SM, Buss DH, Lawrence P. Intakes and major dietary sources of cholesterol and phytosterols in the British diet. J Hum Nutr Dietetics. 1995;8:429–440. [Google Scholar]
- 9.Miettinen TA. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur J Clin Invest. 1980;10(1):27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 10.Lutjohann D, Bjorkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res. 1995;36(8):1763–1773. [PubMed] [Google Scholar]
- 11.Shulman RS, Bhattacharyya AK, Connor WE, Fredrickson DS. Beta-sitosterolemia and xanthomatosis. New Eng J Med. 1976;294(9):482–483. doi: 10.1056/NEJM197602262940907. [DOI] [PubMed] [Google Scholar]
- 12.Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler Thromb. 1992;12(5):563–568. doi: 10.1161/01.atv.12.5.563. [DOI] [PubMed] [Google Scholar]
- 13.Salen G, Horak I, Rothkopf M, et al. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J Lipid Res. 1985;26(9):1126–1133. [PubMed] [Google Scholar]
- 14.Bjorkhem I, Boberg KM. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic basis of inherited disease, New York: McGraw-Hill Inc, 1995: 2073–2102.
- 15.Patel SB, Salen G, Hidaka H, et al. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J Clin Invest. 1998;102(5):1041–1044. doi: 10.1172/JCI3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu K, Lee M-H, Hazard S, et al. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8 respectively. Am J Hum Genet. 2001;69:278–290. doi: 10.1086/321294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M-H, Lu K, Hazard S, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat General. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berge KE, Tian H, Graf GA, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290(5497):1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 19.Graf GA, Li WP, Gerard RD, et al. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110(5):659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen L, Salen G, Shefer S, Shore V, Tint GS, Ness G. Unexpected failure of bile acid malabsorption to stimulate cholesterol synthesis in sitosterolemia with xanthomatosis. Comparison with lovastatin. Arteriosclerosis. 1990;10(2):289–297. doi: 10.1161/01.atv.10.2.289. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharyya AK, Connor WE, Lin DS, McMurry MM, Shulman RS. Sluggish sitosterol turnover and hepatic failure to excrete sitosterol into bile cause expansion of body pool of sitosterol in patients with sitosterolemia and xanthomatosis. Arterioscler Thromb. 1991;11(5):1287–1294. doi: 10.1161/01.atv.11.5.1287. [DOI] [PubMed] [Google Scholar]
- 22.Skrede B, Bjorkhem I, Bergesen O, Kayden HJ, Skrede S. The presence of 5 alpha-sitostanol in the serum of a patient with phytosterolemia, and its biosynthesis from plant steroids in rats with bile fistula. Biochim Biophys Acta. 1985;836(3):368–875. doi: 10.1016/0005-2760(85)90141-9. [DOI] [PubMed] [Google Scholar]
- 23.Salen G, Kwiterovich P, Jr, Shefer S, et al. Increased plasma cholestanol and 5 alpha-saturated plant sterol derivatives in subjects with sitosterolemia and xanthomatosis. J Lipid Res. 1985;26(2):203–209. [PubMed] [Google Scholar]
- 24.Lutjohann D, Bjorkhem I, Ose L. Phytosterolaemia in a Norwegian family: diagnosis and characterization of the first Scandinavian case. Scand J Clin Laboratory Invest. 1996;56(3):229–240. doi: 10.3109/00365519609088612. [DOI] [PubMed] [Google Scholar]
- 25.Belamarich PF, Deckelbaum RJ, Starc TJ, Dobrin BE, Tint GS, Salen G. Response to diet and cholestyramine in a patient with sitosterolemia. Pediatrics. 1990;86(6):977–981. [PubMed] [Google Scholar]
- 26.Nguyen LB, Cobb M, Shefer S, Salen G, Ness GC, Tint GS. Regulation of cholesterol biosynthesis in sitosterolemia: effects of lovastatin, cholestyramine, and dietary sterol restriction. J Lipid Res. 1991;32(12):1941–1948. [PubMed] [Google Scholar]
- 27.Cobb MM, Salen G, Tint GS, Greenspan J, Nguyen LB. Sitosterolemia: opposing effects of cholestyramine and lovastatin on plasma sterol levels in a homozygous girl and her heterozygous father. Metab: Clin Exp. 1996;45(6):673–679. doi: 10.1016/s0026-0495(96)90130-4. [DOI] [PubMed] [Google Scholar]
- 28.Altmann SW, Davis HR, Jr, Zhu LJ, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303(5661):1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 29.Van Heek M, France CF, Compton DS, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283(1):157–163. [PubMed] [Google Scholar]
- 30.Salen G, von Bergmann K, Lutjohann D, et al. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109(8):966–971. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda I, Sugano M. Some aspects of mechanism of inhibition of cholesterol absorption by beta-sitosterol. Biochim Biophys Acta. 1983;732(3):651–658. doi: 10.1016/0005-2736(83)90243-2. [DOI] [PubMed] [Google Scholar]
- 32.Child P, Kuksis A. Investigation of the role of micellar phospholipid in the preferential uptake of cholesterol over sitosterol by dispersed rat jejunal villus cells. Biochem Cell Biol. 1986;64(8):847–853. doi: 10.1139/o86-113. [DOI] [PubMed] [Google Scholar]
- 33.Lu K, Lee M-H, Carpten JD, Sekhon M, Patel SB. High-resolution physical and transcript map of human chromosome 2p21 containing the sitosterolemia locus. Eur J Hum Genet. 2001;9:364–374. doi: 10.1038/sj.ejhg.5200627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipid. 2001;12(2):141–149. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mymin D, Wang J, Frohlich J, Hegele RA. Image in cardiovascular medicine. Aortic xanthomatosis with coronary ostial occlusion in a child homozygous for a nonsense mutation in ABCG8. Circulation. 2003;107(5):791. doi: 10.1161/01.cir.0000050545.21826.ad. [DOI] [PubMed] [Google Scholar]
- 36.Puffenberger EG. Genetic heritage of the old order Mennonites of southern Pennsylvania. Am J Med Genet Part C. 2003;121C:18–31. doi: 10.1002/ajmg.c.20003. [DOI] [PubMed] [Google Scholar]
- 37.McKusick VA. Ellisvan Creveld syndrome and the Amish. Nat General. 2000;24:203–204. doi: 10.1038/73389. [DOI] [PubMed] [Google Scholar]


