Abstract
Cerebellar parallel fibers are among the thinnest known vertebrate axons and represent an extreme anatomical adaptation. Until now a systematic examination of their properties across species has not been carried out. We used transmission electron microscopy and light microscopy to compare parallel fibers in mammals of different brain sizes. From mouse to macaque, the average unmyelinated parallel fiber diameter was 0.2–0.3 μm, consistent with the idea that they are evolutionarily selected for compactness. Average unmyelinated parallel fiber diameter scaled up slightly with brain size, and across species the estimated total conduction time is 5–10 ms. However, these conduction times can vary by milliseconds, and unmyelinated PFs consume large amounts of energy per action potential. These functional disadvantages are overcome in myelinated parallel fibers, which we found in the deep regions nearest the Purkinje cell layer in marmoset, cat and macaque. These axons were 0.4–1.1 μm wide, have expected conduction times of 0.5–1.0 ms, and may convey fast feedfoward inhibition via basket cells to Purkinje cells.
Keywords: brain evolution, cerebellum, optimization, perceptron
Abbreviations: MPFs, myelinated parallel fibers; PFs, parallel fibers; TEM, transmission electron microscopy
Introduction
The layout and microstructure of cerebellar parallel fibers (PFs) may provide clues about their functional role. PFs are granule cell axons, which ascend through the Purkinje cell layer to the molecular layer, bifurcate, and pass through the dendritic trees of the Purkinje neurons (Fig. 1A). In the rat (Harvey & Napper, 1991) each granule cell forms hundreds of synapses on Purkinje neurons and interneurons, and each Purkinje cell receives approximately 200 000 PF synapses and inhibitory inputs. Axons from the Purkinje cells form the only efferent pathways from the cerebellum. This massive divergence and convergence motivated Marr (1969), Albus (1971) to propose that the cerebellar cortex acts as a pattern detector in which the number of Purkinje cell inputs is very high as part of a sparse coding scheme.
Fig. 1.

Unmyelinated and myelinated parallel fibers of the cerebellum. (A) Cerebellar anatomy (Ramon y Cajal, 1911). (B–D) Electron micrographs from marmoset cerebellum. All images are sagittal sections from lobule VI (×3150 direct magnification). Pc, Purkinje cell; Pcd, Purkinje cell dendrite; bc axon, basket cell axon; mpf, myelinated PF. (B) Lower molecular layer, adjacent to a Purkinje cell. (C) Middle molecular layer, approximately 90 μm from Purkinje cell. (D) Upper molecular layer, approximately 40 μm from the pial surface. (E) Unmyelinated PF diameters (average and SD) for five different species as a function of brain diameter. Brain diameters were obtained as described in Shultz et al. (2003), and were (in cm): mouse 0.9, rat 1.4, marmoset 2.4, cat 3.8, macaque 5.5. (F) Unmyelinated PF diameter as a function of position in molecular layer in cat. (G) Unmyelinated PF diameter as a function of distance from the Purkinje cell layer in mouse, marmoset, and macaque. In (F) and (G), each point represents data from one TEM image.
In this ‘perceptron’ model, maximizing the number of PFs would maximize combinatorial possibilities. Therefore PFs may be narrow in order to fit large numbers of axons into a limited space. An axon’s size determines its functional properties, so the size of PFs may be influenced not only by combinatorial needs, but also by selection pressure for speed and volume. Unmyelinated PFs conduct action potentials slowly, raising the possibility that PFs can also be used as time delay devices (Braitenberg, 1967), albeit somewhat variable ones (Gardner-Medwin, 1972; Llinás, 1982). However, unmyelinated axons have high capacitance and are energetically costly to operate per action potential.
These trade-offs might be better understood by comparing brains of different sizes, as variation in size may impose different functional constraints. Until now no systematic comparison of PFs has been carried out. We examined five species of mammals with brain sizes from 0.4 cm3 (mouse) to 90 cm3 (macaque). In addition to unmyelinated PFs, we characterized a subpopulation of myelinated PFs, which should have faster conduction velocity and less capacitance, thus offseting disadvantages found in unmyelinated PFs.
Materials and methods
Electron microscopy
Cerebella were obtained from one mouse (Mus musculus, strain B6CBAF1/J, adult male, age 11 months), one rat (Rattus norvegicus, Sprague–Dawley, Taconic, young adult male, age 1.5 months), one marmoset (Callithrix jacchus, adult male, age 3.5 years), one cat (Felis domesticus, adult male, age 17 months), and one macaque monkey (Macaca mulatta, adult male, age unknown). Under deep anaesthesia, the animals were transcardially perfused manually (except the cat, which was gravity-perfused) with Dulbecco’s modified Eagle medium at 37 °C followed by Karnovsky’s fixative (1.5% glutaraldehyde, 2.0% paraformaldehyde, 2.0 mm CaCl2 in 0.1 m sodium cacodylate buffer, pH 7.2) at 37 °C. Brains were removed immediately and postfixed in full-strength Karnovsky’s at room temperature for 2 h, then overnight at 4 °C, and stored at 4 °C in 0.5% glutaraldehyde in 0.1 m sodium cacodylate with 5.0% sucrose. For each specimen, the cerebellum was sectioned at the midline and 1 × 2 × 3 mm blocks were dissected from the vermal region of lobule VI, left hemisphere.
Tissue blocks were osmicated on ice for 30 min with 2.0% OsO4 in 0.1 m sodium cacodylate, reduced with 10 mg/mL potassium ferrocyanide, and rinsed with 0.1 m sodium cacodylate. Blocks were then serially dehydrated in ethanol followed by infiltration with propylene oxide and overnight incubation in 1:1 propylene oxide: Embed-812 resin, and finally embedded in Epon epoxy resin in sagittal orientation. Thick sections (0.5 μm) were stained with toluidine blue or Azure II stain and examined with a light microscope to select an area for ultrathin sectioning. Ultrathin sections (80 nm) were placed on 150 cc carbon grids and stained with 2% uranyl acetate and a modified Sato’s lead solution. Grids were examined using a Zeiss 912 AB transmission electron microscope. The Purkinje cell layer was located, then serial transects of overlapping images, each approximately 9 μm × 9 μm (×3150 direct magnification) or 5.5 μm × 5.5 μm (×5000 direct magnification), were made with an AMT XR-100 2600-line CCD camera starting from the Purkinje cell layer and continuing upward through the molecular layer. Some transects started at the pia in order to explore the superficial molecular layer.
Electron micrograph analysis
Axon morphometry was performed using ImageJ (http://rsb.info.nih.gov/ij/). Axons containing mitochondria or more than three synaptic vesicles were excluded from analysis (Napper & Harvey, 1988; Sultan, 2000). PF profiles were traced using a tablet PC and fitted with ellipses. The diameter was taken as the average of the major and minor axes of the ellipse if the major axis to minor axis ratio was less than 1.5, and as the minor axis if the ratio was larger. Diameters were not corrected for shrinkage. For calculation of conduction velocities and times, diameters were corrected assuming a tissue shrinkage estimate of 15% due to perfusion and tissue preparation.
Myelin staining and light microscopy
Using a Leica VT1000S microtome, 50-μm coronal sections were taken from rat (all lobules), cat (lobules IV and V), and macaque (lobules III and V). Sections were stained for myelin using a modification of a previously described procedure (McNally & Peters, 1998). Sections were incubated in 0.5% Triton-X in Tris phosphate-buffered saline (TPBS; 100 mm Tris and 50 mm sodium phosphate, pH 7.6) for 36–48 h, rinsed twice in TPBS, reacted in 2.5% DAB for 15 min, rinsed in TPBS, incubated in 8.0% thioglycolic acid for 4 h, rinsed four times in 2.0% sodium acetate, developed for 10–15 min in a solution containing 2.5% sodium acetate, 1.0% ammonium nitrate, 1.0% silver nitrate, 5.0% tungstolic acid, and 0.07% formaldehyde, incubated in 1.0% acetic acid for 5 min, reacted in 0.05% gold chloride for 15 min, rinsed in 2.0% sodium acetate, incubated in 3.0% sodium thiosulphate, and rinsed in 0.1 m sodium phosphate buffer. The sections were mounted onto coated slides, air-dried overnight, dehydrated, cleared, and coverslipped. Slides were examined using a Nikon Eclipse E800 microscope using a CCD camera and IPLab software.
Results
We used transmission electron microscopy (TEM) to examine sagittal sections of cerebellar samples taken from lobule VI of mouse, rat, marmoset, cat, and macaque (Fig. 1A). Sections were rich in unmyelinated PFs (Fig. 1B–D), which were identifiable by their microtubules, electron-light cytoplasm, and polygonal appearance in cross-section (Palay & Chan-Palay, 1974). Unmyelinated PF diameters were small (range 0.08–0.62 μm) and increased weakly as a function of brain size (Fig. 1E; mouse 0.19 ± 0.06 μm, n = 179; rat 0.17 ± 0.04 μm, n = 555; marmoset 0.27 ± 0.09 μm, n = 381; cat 0.23 ± 0.09 μm, n = 337; and macaque 0.28 ± 0.10 μm, n = 132; all measurements are mean ± SD; correlation coefficient of rank order with respect to rank order of brain size ρ = 0.8, P = 0.1).
Unmyelinated PFs have previously been reported to be wider in deep regions nearer to the Purkinje cell layer (Palkovits et al., 1971; Palay & Chan-Palay, 1974; Sultan, 2000). We confirmed this in cat (Fig. 1F, rank order of average diameter by location correlated with rank order of distance from the Purkinje cell layer all the way to the pia, ρ = 0.51, 312 unmyelinated PFs in 14 locations, P < 0.1), mouse (Fig. 1G; up to 200 μm away from Purkinje cell layer, ρ = 0.96, 179 unmyelinated PFs in seven locations, P < 0.001, two-tailed test), and rat (Fig. 1G; up to 70 μm away, ρ = 0.86, 555 unmyelinated PFs in eight locations, P < 0.01), but not marmoset (Fig. 1G, up to 100 μm away, ρ = 0.30, 316 unmyelinated PFs in eight locations, P = 0.5). Overall, the mean change in unmyelinated PF diameter relative to depth was weak (mouse, 0.05 ± 0.01 μm increase in diameter per 100 μm depth; rat, 0.03 ± 0.01 μm per 100 μm; cat, 0.03 ± 0.01 μm per 100 μm).
We also found a population of longitudinally oriented myelinated axons with near-circular profiles in marmoset, cat, and macaque (Fig. 2). These axons were found in the lower molecular layer and were 0.4–1.1 μm wide (Fig. 2B; marmoset 0.75 ± 0.16 μm, 63 axons; cat 0.64 ± 0.15 μm, 25 axons; macaque 0.64 ± 0.11 μm, five axons). They were less common in images taken from regions higher in the molecular layer (Fig. 2A) and were entirely absent in the upper molecular layer (Fig. 1D). In total we found 93 longitudinally oriented myelinated axons in individual micrographs (marmoset, 63 axons in eight 78-μm2 micrographs, cat, 25 axons in nine 32-μm2 micrographs, and macaque, five axons in four 32-μm2 micrographs) and more such axons in montages (macaque, Fig. 2G). Only one such axon was found in 13 mouse micrographs (32 μm2 per image) and none in eight rat electron micrographs (32 μm2 per image).
Fig. 2.
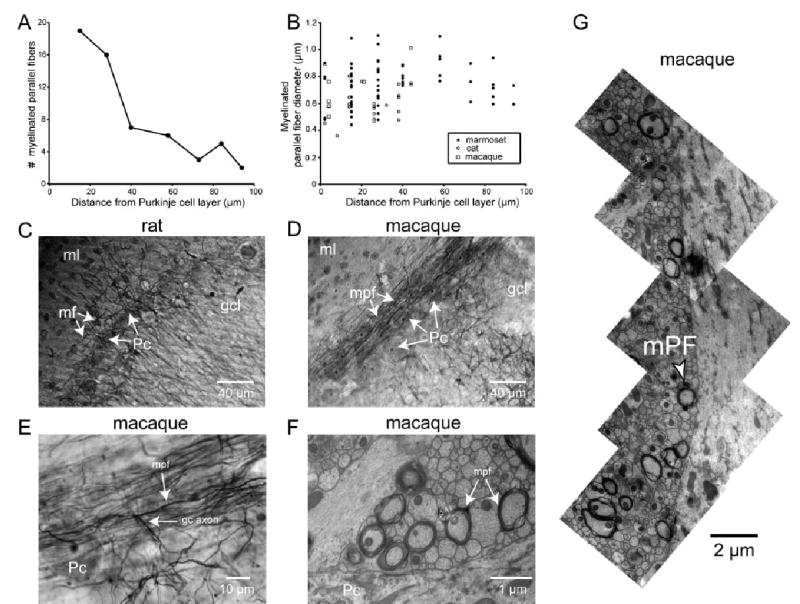
Myelinated parallel fibers. (A) Number of myelinated PFs as a function of position in the molecular layer. Marmoset cerebellum, lobule VI. Individual counts were taken from 78 μm2 TEM images (×3150 direct magnification, ×20 000 print magnification). (B) Myelinated PF diameter as a function of position in the molecular layer for marmoset, cat, macaque (lobule VI for all). Diameters were measured from ×20 000 and ×32 000 TEM prints (×3150 and ×5000 direct magnification). (C) Brightfield image of rat cerebellum, coronal section from crus II (×200 direct magnification), stained for myelin. Pc, Purkinje cell; gcl, granule cell layer; ml, molecular layer; mf, myelinated fiber. Note the sparse plexus of myelinated fibers in the lower molecular layer, putatively corresponding to recurrent collaterals of Purkinje cell axons and/or Lugaro cell axons. (D and E) Brightfield images of coronal sections of macaque cerebellum lobule III stained for myelin taken at (D) ×200 direct magnification and (E) ×1000 direct magnification. Pc, Purkinje cell; gcl, granule cell layer; gc axon, granule cell axon; ml, molecular layer; mpf, myelinated parallel fiber. Note the many myelinated PFs coursing through the lower molecular layer above the Purkinje cell layer and the T-junction in E. (F) TEM micrograph of macaque cerebellum, lobule VI, ×5000 direct magnification. Pc, Purkinje cell; mpf, myelinated PF. (G) Montage of multiple micrographs (×5000 direct magnification) of macaque lobule VI tissue in the lower molecular layer to show multiple myelinated PFs. The bottom of the montage begins approximately 5 μm from the top of the Purkinje cell layer.
To further examine these myelinated fibers, we stained 50-μm-thick sections of rat (all lobules), cat (lobules IV and V) and macaque (lobules III and V) cerebellar tissue for myelin and examined the sections with brightfield light microscopy. In cat and macaque tissue we found a dense band of myelinated fibers just above the Purkinje cell layer (Fig. 2D). In contrast, in rat tissue we observed only sparse staining (Fig. 2C), with no visible plexus of longitudinally oriented myelinated fibers in any lobule.
Myelinated axons (Fig. 2D and E) had key characteristics of parallel fibers. They ran parallel to the Purkinje cell layer along the longitudinal axis of the folium. Individual fibers ran for up to several hundred microns within the plane of each section and could often be traced back to a T-junction. Many ascending axons could be traced well into the granule cell layer (Fig. 2E). Fibers varied little in diameter and were myelinated all along their length. Unlike Lugaro cell axons, which are branched and can give rise to longitudinally running collaterals (Lainé & Axelrad, 1996, 2002), the axons we observed did not branch in the molecular layer. Taken together, these observations indicate that most of the myelinated fibers in the lower molecular layer are myelinated parallel fibers (mPFs).
We also found a second population of myelinated axons distinct from the mPFs. Like mPFs, these axons were found mainly in the lower molecular layer. However, they were less abundant, were several times thicker (1–2 μm wide), and ran for only 15–50 μm before turning and leaving the plane of section (Fig. 2C). These axons were visible in all three species examined by light microscopy and may be Lugaro cell axons.
Discussion
Parallel fibers (PFs) are often considered to be exclusively unmyelinated, and myelinated PFs (mPFs) are a neglected subject. A dense ‘supraganglionic plexus’ (Jakob, 1928) of myelinated fibers near the Purkinje cell layer has been observed in cats (Eccles et al., 1967), macaques (Fox et al., 1964) and humans (Jakob, 1928). At the time, these myelinated fibers were attributed to recurrent collaterals of Purkinje cell axons (Ramon y Cajal, 1911; Fox et al., 1964; Eccles et al., 1967). In contrast, Mugnaini (1972) reported that in cats a large fraction of the myelinated fibers in the lower molecular layer are PFs based on ultrastructure and the presence of ascending and bifurcating myelinated granule cell axons.
We find that the composition of the supraganglionic plexus varies by species. In rat it contains only a few longitudinally running myelinated fibers, in agreement with Palay and Chan-Palay (1974). These fibers are likely to be either recurrent Purkinje cell axon collaterals (Chan-Palay, 1971) or Lugaro cell axons (Lainé & Axelrad, 1996; Dieudonné & Dumoulin, 2000; Lainé & Axelrad, 2002). In the larger-brained animals these thick fibers are outnumbered by the thinner mPFs.
Myelinated PFs may arise from mechanisms for glial ensheathment triggered when axons pass a diameter threshold. Lange (1978) found that in kittens the supraganglionic plexus contains mainly recurrent Purkinje cell collaterals; he did find abundant mPFs but only in adults (Lange, 1976, 1978). In the central nervous system unmyelinated and myelinated axons are approximately separated by a diameter threshold (Hirano & Llena, 1995). For PFs our results indicate that this threshold is approximately 0.4 μm. This raises the possibility that myelinated PFs may simply represent the endpoint of a gradient of PF diameters (see Fig. 1F and Sultan, 2000) without necessarily having a special function.
When mPFs fire is unknown. If these axons have the same length as the unmyelinated PFs, their conduction time would be approximately 0.5 ms (Fig. 3, dashed line). This is much shorter than measured cerebellar response latencies to auditory-evoked potentials; 8–18 ms in cat (Huang & Liu, 1985; Fig. 3, arrowhead) and 22 ms in macaque (Mortimer, 1975). These physiological latency measurements reflect summed delays from brainstem synapses, conduction along mossy fibers to granule cells, and transmission through parallel fibers. The shortest latencies, 8 ms, can be accounted for if the total measured latency is dominated by conduction delays in unmyelinated PFs (Fig. 3, arrowhead).
Fig. 3.
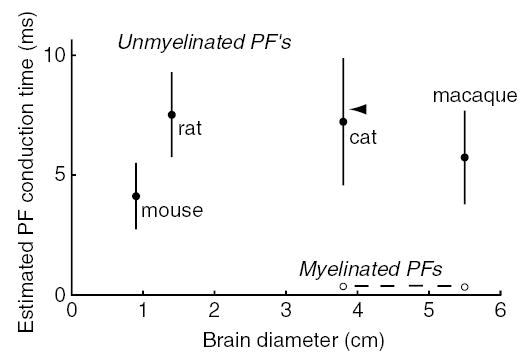
Estimated parallel fiber conduction times. Estimated conduction times based on measured PF diameters corrected for shrinkage and literature values for PF lengths (mouse, Soha et al., 1997; rat, Pichitpornchai et al., 1994; cat, Brand et al., 1976; macaque, Mugnaini, 1983), halved to give an estimate of the distance from the T-junction to the end of the PF. Conduction velocities were calculated as v = 0.75 × diameter for unmyelinated axons (Hoffmeister et al., 1991), and as v = 5.7 × diameter for myelinated axons (Hursh, 1939) (diameters in μm, velocities in m/s). Arrowhead, range of auditory evoked potential latencies in Purkinje cell simple spikes in cat (Huang & Liu, 1985).
What information might myelinated PFs convey? Myelinated PFs synapse predominantly onto basket cells (Mugnaini, 1972), thus providing a disynaptic pathway for feedforward inhibition to arrive at the soma and axon of Purkinje neurons faster than monosynaptic excitation via unmyelinated PFs. Such a pathway might not only allow myelinated PFs to counterbalance excitation (Bower, 2002), but arrive quickly enough to veto excitatory input from unmyelinated PFs. To our knowledge, such short-latency sensory inhibitory events have not been reported in large-brained mammals (though see measurements in rat in Korn & Axelrad, 1980; and in Mittmann et al., 2004).
In the case of unmyelinated PFs, expected conduction velocities can be calculated from the diameter distributions using the relationship between unmyelinated PF diameter and conduction velocity (Hoffmeister et al., 1991) and compared with physiological measurements. Calculation from the anatomical diameter distribution gives a range of 0.18–0.27 m/s for PF diameters of 0.2–0.3 μm, while the physiologically reported speed is somewhat higher, 0.3 m/s (Eccles et al., 1967; their Figure 33). This small discrepancy could be due the use of hindlimb nerve axons by Hoffmeister et al., (1991), which might have different membrane properties such as sodium channel density (Ritchie, 1995). Thus, calculations of conduction speed and time may be more useful for comparative purposes than as absolute values.
Selection pressure for conduction speed in large-brained mammals would not have been predicted from cerebellar anatomy alone. Unmyelinated PF lengths are not proportional to brain diameter (2.8 mm in mouse, Soha et al., 1997; 4.5 mm in rat, Pichitpornchai et al., 1994; 6.0 mm in cat, Brand et al., 1976; 5.7 mm in macaque, Mugnaini, 1983). These values, halved to account for the fact that conduction begins at the T-junction, can be combined with our measurements and the relationship between unmyelinated PF diameter and conduction velocity (Hoffmeister et al., 1991) to obtain estimates of total conduction time (Fig. 3, filled symbols). The resulting conduction times are largely in the 5–10-ms range in all species examined, and do not increase systematically with brain size. Selection pressure for speed may be imposed by other structures such as the neocortex, where long-distance conduction can reach hundreds of milliseconds (Swadlow & Waxman, 1976).
The maximal packing density of unmyelinated PFs creates a new problem – metabolic cost. Unmyelinated PFs are potentially metabolically expensive per unit volume because of their capacitance. However, the cerebellum does not use more energy than other brain regions (Sokoloff, 1983), suggesting that unmyelinated PFs fire sparsely and at low average rates, as suggested for neocortex (Lennie, 2003) and consistent with the low spontaneous firing rate of cerebellar granule cells in vivo under anaesthesia (Shambes et al., 1978; Chadderton et al., 2004). This would be consistent with the suggestion that sensory input triggers activity in a sparse population of PFs (Marr, 1969; Albus, 1971).
In summary, unmyelinated parallel fibers constitute a compact pathway that is well-suited to convey a sparse code. This may come at the expense of precise spike timing, indicating that millisecond or submillisecond processing operations would have to occur at earlier stages in the mossy fiber pathway. In contrast, myelinated fibers can conduct quickly and fire precisely. The appearance of these fibers in large-brained animals allows the possibility of an additional parallel fiber pathway in cerebellar cortex, one that rapidly inhibits Purkinje cells.
Acknowledgments
We thank Kimberly Harrison and Nick Hastings for technical advice and assistance; Peggy Bisher, Joe Goodhouse and Jane Woodruff for TEM training and guidance; Eric Wieschaus for use of the Nikon Eclipse E800 microscope; David Tank for use of the Leica VT1000S microtome; Enrico Mugnaini for discussions; and Megan Sullivan for reading the manuscript. This work was supported by an Alfred P. Sloan Foundation Research Fellowship and National Institutes of Health grant NS045193 to S.S.-H.W.
References
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- Bower JM. The organization of cerebellar cortical circuitry revisited: implications for function. Ann NY Acad Sci. 2002;978:135–155. doi: 10.1111/j.1749-6632.2002.tb07562.x. [DOI] [PubMed] [Google Scholar]
- Braitenberg V. Is the cerebellar cortex a biological clock in the millisecond range? Prog Brain Res. 1967;25:334–346. doi: 10.1016/S0079-6123(08)60971-1. [DOI] [PubMed] [Google Scholar]
- Brand S, Dahl AL, Mugnaini E. The length of parallel fibers in the cat cerebellar cortex. An experimental light and electron microscopic study. Exp Brain Res. 1976;26:39–58. doi: 10.1007/BF00235248. [DOI] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. The recurrent collaterals of Purkinje cell axons: a correlated study of the rat’s cerebellar cortex with electron microscopy and the Golgi method. Z Anat Entwicklungsgesch. 1971;134:200–234. doi: 10.1007/BF00519300. [DOI] [PubMed] [Google Scholar]
- Dieudonné S, Dumoulin A. Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J Neurosci. 2000;20:1837–1848. doi: 10.1523/JNEUROSCI.20-05-01837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles, J.C., Ito, M. & Szentágothai, J. (1967) The Cerebellum as a Neuronal Machine Springer-Verlag, Berlin.
- Fox, C.A., Siegesmund, K.A. & Dutta, C.R. (1964) The Purkinje cell dendritic branchlets and their relation with the parallel fibers: light and electron microscopic observations. In Cohen, M.M. & Snider, R.S. (Eds), Morphological and Biochemical Correlates of Neural Activity Harper & Row, New York, pp. 112–141.
- Gardner-Medwin AR. An extreme supernormal period in cerebellar parallel fibres. J Physiol (Lond) 1972;222:357–371. doi: 10.1113/jphysiol.1972.sp009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Napper RMA. Quantitative studies on the mammalian cerebellum. Prog Neurobiol. 1991;36:437–463. doi: 10.1016/0301-0082(91)90012-p. [DOI] [PubMed] [Google Scholar]
- Hirano, A. & Llena, J.F. (1995) Morphology of central nervous system axons. In Waxman, S.G., Kocsis, J.D. & Stys, P.K. (Eds), The Axon: Structure, Function and Pathophysiology Oxford University Press, New York, pp. 49–67.
- Hoffmeister B, Janig W, Lisney SJ. A proposed relationship between circumference and conduction velocity of unmyelinated axons from normal and regenerated cat hindlimb cutaneous nerves. Neuroscience. 1991;42:603–611. doi: 10.1016/0306-4522(91)90402-a. [DOI] [PubMed] [Google Scholar]
- Huang CM, Liu G. Electrophysiological mapping of the auditory areas in the cerebellum of the cat. Brain Res. 1985;335:121–129. doi: 10.1016/0006-8993(85)90282-3. [DOI] [PubMed] [Google Scholar]
- Hursh JB. Conduction velocity and diameter of nerve fibers. Am J Physiol. 1939;127:131–139. [Google Scholar]
- Jakob, A. (1928) Das Kleinhirn Springer, Berlin.
- Korn H, Axelrad H. Electrical inhibition of Purkinje cells in the cerebellum of the rat. Proc Natl Acad Sci USA. 1980;77:6244–6247. doi: 10.1073/pnas.77.10.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainé J, Axelrad H. Morphology of the Golgi-impregnated Lugaro cell in the rat cerebellar cortex: a reappraisal with a description of its axon. J Comp Neurol. 1996;375:618–640. doi: 10.1002/(SICI)1096-9861(19961125)375:4<618::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lainé J, Axelrad H. Extending the cerebellar Lugaro cell class. Neuroscience. 2002;115:363–374. doi: 10.1016/s0306-4522(02)00421-9. [DOI] [PubMed] [Google Scholar]
- Lange W. The myelinated parallel fibers of the cerebellar cortex and their regional distribution. Cell Tissue Res. 1976;166:489–496. [PubMed] [Google Scholar]
- Lange W. The myelination of the cerebellar cortex in the cat. Cell Tissue Res. 1978;188:509–520. doi: 10.1007/BF00219788. [DOI] [PubMed] [Google Scholar]
- Lennie P. The cost of cortical computation. Curr Biol. 2003;13:493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Llinás, R.R. (1982) General discussion: radial connectivity in the cerebellar cortex: a novel view regarding the functional organization of the molecular layer. In Palay, S.L. & Chan-Palay, V. (Eds), The Cerebellum: New Vistas Exp. Brain Res. (Suppl.)Vol, 6. Springer-Verlag, New York, pp. 189–194.
- Marr D. A theory of cerebellar cortex. J Physiol (Lond) 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally KJ, Peters A. A new method for intense staining of myelin. J Histochem Cytochem. 1998;46:541–545. doi: 10.1177/002215549804600415. [DOI] [PubMed] [Google Scholar]
- Mittmann W, Koch U, Häusser M. Feed-forward inhibition shapes the spike output of cerebellar Purkinje neurons. J Physiol (Lond) 2004;563:369–378. doi: 10.1113/jphysiol.2004.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JA. Cerebellar responses to teleceptive stimuli in alert monkeys. Brain Res. 1975;83:369–390. doi: 10.1016/0006-8993(75)90831-8. [DOI] [PubMed] [Google Scholar]
- Mugnaini, E. (1972) The histology and cytology of the cerebellar cortex. In Larsell, O. & Jansen, J. (Eds), The Comparative Anatomy and Histology of the Cerebellum University of Minnesota Press, Minneapolis, pp. 201–264.
- Mugnaini E. The length of cerebellar parallel fibers in chicken and rhesus monkey. J Comp Neurol. 1983;220:7–15. doi: 10.1002/cne.902200103. [DOI] [PubMed] [Google Scholar]
- Napper RMA, Harvey RJ. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J Comp Neurol. 1988;274:168–177. doi: 10.1002/cne.902740204. [DOI] [PubMed] [Google Scholar]
- Palay, S.L. & Chan-Palay, V. (1974) Cerebellar Cortex: Cytology and Organization Springer, Berlin.
- Palkovits M, Magyar P, Szentágothai J. Quantitative histological analysis of the cerebellar cortex in the cat. III Structural organization of the molecular layer. Brain Res. 1971;34:1–18. doi: 10.1016/0006-8993(71)90347-7. [DOI] [PubMed] [Google Scholar]
- Pichitpornchai C, Rawson JA, Rees S. Morphology of parallel fibres in the cerebellar cortex of the rat: an experimental light and electron microscopic study with biocytin. J Comp Neurol. 1994;342:206–220. doi: 10.1002/cne.903420205. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal, S. (1911) Histologie Du Systeme Nerveux de L’homme at Des Vertebres Maloine, Paris.
- Ritchie, J.M. (1995) Physiology of axons. In Waxman, S.G., Kocsis, J.D. & Stys, P.K. (Eds), The Axon: Structure, Function and Pathophysiology Oxford University Press, New York, pp. 68–96.
- Shultz JR, Harrison KH, Wagers MW, Burish MJ, Wang SS-H. Speed limits in mammalian brains: scaling constraints from biophysics. Soc. Neurosci Abstr. 2003:43.8. [Google Scholar]
- Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemishperes revealed by micromapping. Brain Behav Evol. 1978;15:94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- Soha JM, Kim S, Crandall JE, Vogel MW. Rapid growth of parallel fibers in the cerebella of normal and staggerer mutant mice. J Comp Neurol. 1997;389:642–654. doi: 10.1002/(sici)1096-9861(19971229)389:4<642::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Mapping local functional activity by measurement of local cerebral glucose utilization in the central nervous system of animals and man. Harvey Lecture. 1983;79:77–143. [PubMed] [Google Scholar]
- Sultan F. Exploring a critical parameter of timing in the mouse cerebellar microcircuitry: the parallel fiber diameter. Neurosci Lett. 2000;280:41–44. doi: 10.1016/s0304-3940(99)00984-2. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG. Variations in conduction velocity and excitability following single and multiple impulses of visual callosal axons in the rabbit. Exp Neurol. 1976;53:128–150. doi: 10.1016/0014-4886(76)90288-0. [DOI] [PubMed] [Google Scholar]


