Abstract
Study Objectives
Hypocretins (HCRT-1 and HCRT-2), also known as orexins, are neuropeptides localized in neurons surrounding the perifornical region of the posterior hypothalamus. These neurons project to major arousal centers in the brain and are implicated in regulating wakefulness. In young rats and monkeys, levels of HCRT-1 are highest at the end of the wake-active period and lowest toward the end of the sleep period. However, the effects of age on the diurnal rhythm of HCRT-1 are not known.
Design
To provide such data, cerebrospinal fluid (CSF) was collected from the cisterna magna of young (2-month-old, n = 9), middle-aged (12 months, n = 10), and old (24 months, n = 10) F344 rats at 4-hour intervals, (beginning at zeitgeber [ZT]0, lights on). CSF was collected once from each rat every 4 days at 1 ZT point. After collecting the CSF at all of the time points, the rats were kept awake by gentle handling for 8 hours (ZT 0-ZT8), and the CSF was collected again at the end of the sleep-deprivation procedure. HCRT-1 levels in the CSF were determined by radioimmunoassay
Settings
Basic neuroscience research lab.
Measurements and Results
Old rats had significantly less HCRT-1 in the CSF versus young and middle-aged rats (P < .002) during the lights-on and lights-off periods and over the 24-hour period. In old rats, significantly low levels of HCRT-1 were evident at the end of the lights-off period (predominantly wake-active period). The old rats continued to have less HCRT-1 even after 8 hours of prolonged waking. Northern blot analysis did not show a difference in pre-proHCRT mRNA between age groups.
Conclusions:
In old rats there is a 10% decline in CSF HCRT-1 over the 24-hour period. Functionally, if there is less HCRT-1, which our findings indicated, and there is also a decline in HCRT receptor mRNA, as has been previously found, then the overall consequence would be diminished action of HCRT at target sites. This would diminish the waking drive, which in the elderly could contribute to the increased tendency to fall asleep during the normal wake period.
Keywords: sleep, aging, hypocretin, orexin, lateral hypothalamus
INTRODUCTION
THE NEUROPEPTIDES HYPOCRETIN-1 (HCRT-1) AND HYPOCRETIN-2 (HCRT-2) HAVE BEEN LINKED TO THE SLEEP DISORDER NARCOLEPSY,1 AND AUTOPSY REPORTS IDENTIFY EXTENSIVE LOSS OF HCRT NEURONS IN THE BRAINS OF INDIVIDUALS WITH NARCOLEPSY 2,3 Consistent with the neuronal loss, patients with narcolepsy have very low to negligible levels of HCRT in the cerebrospinal fluid (CSF).4 Mice with deletion of hypocretin5,6 or rats with extensive loss of neurons in the posterior hypothalamus where the HCRT neurons are localized7,8 display symptoms characteristic of narcolepsy.
HCRT-containing neurons are localized exclusively in the perifornical and lateral hypothalamic region of the posterior hypothalamus, and they innervate virtually the entire brain and spinal cord, providing especially heavy projections to major arousal centers.9–11 Although, no study has yet directly measured activity of HCRT neurons across different sleep stages, a recent microdialysis experiment in cats suggested that these neurons are more active during waking and rapid eye movement (REM) sleep relative to slow-wave sleep, as assessed by microdialysis measurement of HCRT levels.12 Administration of HCRT either into the ventricles or directly to target sites such as the locus coeruleus increases waking.13–15 Waking activity also increases HCRT levels in young rats16 and in narcoleptic canines in which the HCRT-2 receptor is mutated.17
HCRT neurons begin to appear on embryonic day 19 and are fully developed by postnatal day 20.18–19 There is a strong diurnal rhythm in CSF levels of HCRT, with the highest levels being reached at the end of the wake-active period and the lowest levels at the end of the sleep period in young rats16,20 and young non human primates.21 Such a rhythm of HCRT has not been determined in old rats. In this experiment, we measured CSF HCRT levels at 4-hour intervals across a 24-hour period to test the hypothesis that there is a decline in HCRT levels with age.
EXPERIMENTAL PROCEDURES
Experiment 1: The Diurnal Rhythm of HCRT-1 Levels in CSF in Young and Old Rats
Subjects
Ten each, young (3 months old), middle-aged (12 months), and old (21 months) male F344 rats were obtained from the National Institutes of Health and allowed 1 month of adaptation to our research facilities. The rats were housed in Plexiglas cages and were entrained to a 12-h light-dark schedule (lights on from 7 AM to 7 PM; 150 lux) with controlled temperature (23°C ± 2°C) and food and water available ad libitum. All rats used in these experiments were free of tumors, as assessed by postmortem examination conducted at the end of the experiment. We elected not to implant the rats with electroencephalographic sleep-recording electrodes because the electrodes would have become disconnected during the 3-month course of this study, and there already exists an extensive body of literature on the sleep pattern of this age and strain of rats, including our own studies.22–24
Collection of CSF
In each rat, CSF was extracted from the cisterna magna at 4-hour intervals (ZT 0, ZT 4, ZT 8, ZT 12, ZT 16, and ZT 20), with at least 96 hours between each collection. During the lights-off period, the rat’s head was covered in heavy black cloth, and a dim red light was used during the CSF collection procedure. A random-order design was used to collect at the various ZT points so as to avoid any order effect. CSF was collected from the cisterna magna (Isofluorane anesthesia)20 with a needle (27-gauge) and immediately frozen on dry ice and maintained at −70°C until analysis. Suction was not applied to extract the CSF, but, instead, the CSF was allowed to flow freely and slowly accumulate in the hub of the syringe needle. On average, 100 μL of CSF was collected each time. Blood-tinged CSF was discarded.
HCRT-1 Radioimmunoassay
HCRT-1 was measured using commercially available 125I radioimmunoassay (RIA) kits (Phoenix Pharmaceuticals, Belmont, Calif). Twenty-five microliters of CSF was mixed with 75 μL of RIA buffer and directly applied to the RIA. The detection limits of the assay were 100 pg/mL. All comparative samples were measured in a single assay, and the intraassay of variation of the RIA was < 5 %.
Experiment 2: Effects of 8 Hours of Prolonged Waking on CSF HCRT-1 Levels
After collecting CSF at all of the ZT time points in experiment 1, the rats were kept awake for 8 hours (ZT0-ZT8) by lightly tapping the cage whenever the rats showed behavioral signs of sleep. Immediately after the sleep deprivation, the rats were anesthetized (isofluorane), and CSF was collected as described previously. This CSF represented the post–sleep-deprivation data point and was compared with CSF collected at the same ZT time point but without any sleep deprivation in experiment 1. Subsequently the rats were administered an overdose of Nembutal and perfused transcardially with 0.9% saline (50 mL) followed by 500 mL of phosphate-buffered 4% paraformaldehyde (pH 7.0). The rats were checked for tumors, and none were found.
Experiment 3: Prepro-HCRT Gene Expression in Young and Old Rats
Subjects
Five each young (3 months old) and old (21 months) male F344 rats were obtained from the National Institutes of Health and allowed 1 month of adaptation to our research facilities. Housing conditions were the same as those described for experiment 1.
RNA Isolation
The rats were anesthetized with Nembutal and sacrificed by decapitation at ZT4 (4 hours after lights on). The brains were rapidly removed and placed in a tissue slicer, and a 2-mm thick coronal slice of the posterior hypothalamus was placed on ice. With the aid of a dissecting microscope, the posterior hypothalamus and the cortex were rapidly removed (see Figure 1 for areas sampled), flash-frozen in liquid nitrogen, and stored at −70°C. The cortex was removed and served as a negative control where no HCRT mRNA would be present. Total RNA was isolated from cortex and posterior hypothalamus using the TRIzol reagent (Gibco/Invitrogen, Grand Island, NY) according to the manufacturer’s instructions. The OD260/OD280 ratio was determined to evaluate the final concentrations of the RNA. The quality of the RNA prepared from each brain region was checked by electrophoresis on a 1.2% agarose-formaldehyde gel and staining with ethidium bromide. RNA isolation was carried out with 2 other groups of 5 similar aged rats (3 and 21 months) in order to perform replicate experiments of Northern blot.
Figure 1.
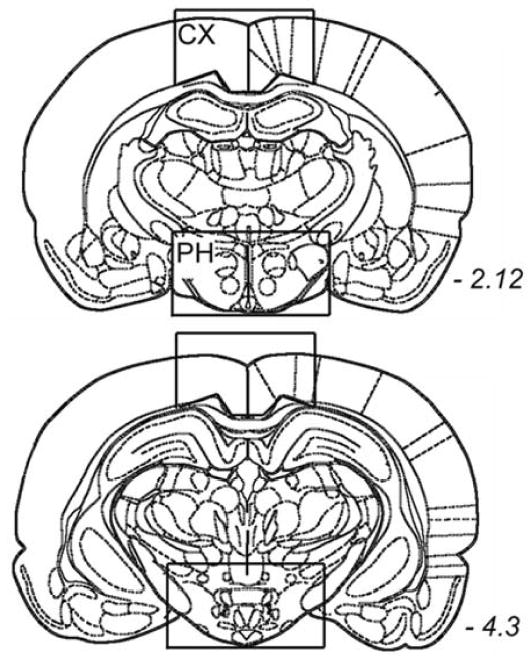
Schematic depiction of the brain regions sampled for the HCRT mRNA expression study. The schematic drawings are from the Paxinos and Watson’s rat brain atlas and the rectangles denote the area in the cortex (CX) and the posterior hypothalamus (PH) that were sampled for the detection of HCRT mRNA. The 2 drawings represent the rostral and caudal extent of the brain regions sampled (italicized numbers represent the anterior posterior bregma position).
Ten micrograms of total RNA prepared from posterior hypothalamus or cortex were separated on a 1.2% agarose-formaldehyde gel and transferred to a Nytran membrane (Schleicher and Schuell, Keene, NH). After prehybridization for 4 hours at 42°C, blots were hybridized overnight at 42ºC in 5X SSC, 50% formamide, 50 mmol sodium phosphate, pH 6.8, 1% SDS, 1 mM EDTA, 2.5X Denhart’s solution, 0.1 mg/mL milliliter salmon sperm DNA, and 5–6.105cpm/mL [32P] random prime-labeled cDNA probe for prepro-HCRT. After hybridization, membranes were washed 2 times for 20 minutes at 42°C in 1X SSC and 0.5% SDS and 2 times for 10 minutes at 55°C in 0.5X SSC and 0.5% SDS. Filters were then exposed to Kodak BioMax MS-1 film (Eastman Kodak, Rochester, NY) for 6 to 12 hours, and the autoradiographs were scanned and quantified using a Molecular Imager (Bio-Rad Laboratories, Hercules, CA). To control and normalize for variability in loading and transfer, all membranes were stripped of [32P]prepro-HCRT and probed again with a rat [32P]cyclophilin probe. Prepro-HCRT expression normalized to cyclophilin was compared between young and old rats by Student t test. Northern Blot experiments were carried out in duplicate using another set of 5 rats per age group.
RESULTS
Experiment 1
CSF levels of HCRT-1 were measured to determine whether there was a decrease in output of HCRT in old rats. Figure 2 summarizes the diurnal rhythm of CSF HCRT-1 levels in the 3 groups of rats. In agreement with previous studies in young rats,20 peak levels of HCRT-1 were found at the end of the wake-active period (ZT0), and low levels occurred toward the end of the sleep period (ZT8 and ZT12). This profile was present for each of the 3 groups of rats. However, the old rats had significantly less HCRT-1 CSF compared to the young rats during both the lights-off (Tukey posthoc test versus young rats, P < .029) and lights-on (P < .034) periods. The old rats had significantly less CSF HCRT-1 levels compared to young rats at the light-dark transition periods (F = 11.032; df = 1,15; P < .004). Over the entire 24-hour period, there was a significant difference between groups (F = 8.385; df = 2,26; P < .002), with the average CSF HCRT-1 levels in old rats being significantly less compared to young (P < .002) and middle-aged (P < .017) rats. The amplitude of the HCRT rhythm was not affected, and the acrophase was not shifted. However, to properly gauge the acrophase, many more time points would have to have been collected.
Figure 2.
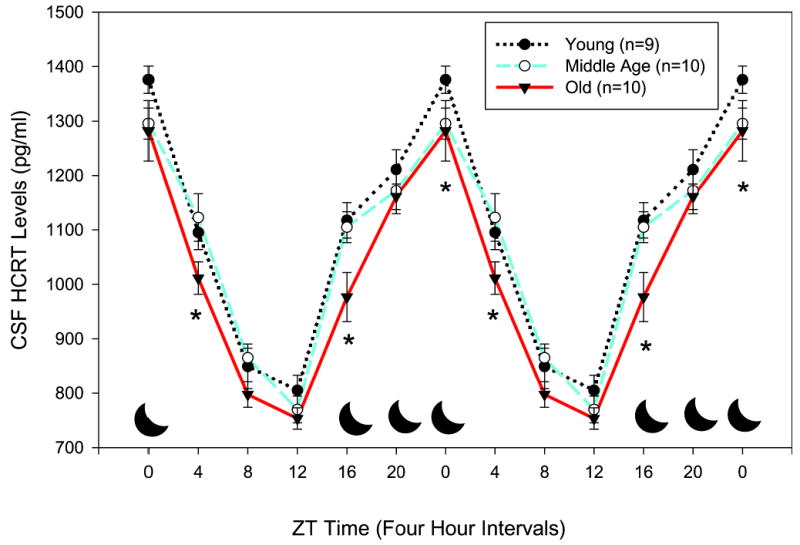
Diurnal rhythm of cerebrospinal fluid (CSF) hypocretin (HCRT)-1 concentrations in young, middle-aged, and old F344 rats. Rats were entrained to a 12-hour lights-on/lights-off cycle. In each rat, CSF was extracted at 4-hour intervals, and radioimmunoassay measured HCRT-1. The data represent average (± SEM) HCRT-1 concentrations at 4-hour intervals for each group of rats. Each data point represents HCRT-1 levels during the preceding 4-hour interval. Asterisks denote significant difference compared to young rats. The black moon icon represents the lights-off period. One young rat died during the CSF extraction and was excluded from study.
Experiment 2
Because CSF HCRT-1 levels are increased in response to prolonged waking, rats were kept awake for 8 hours to drive the activity of the HCRT neurons, and CSF was measured. All 3 groups of rats had significantly increased CSF HCRT-1 levels in response to 8 hours of prolonged waking (P < .001), a finding that is consistent with other studies.16,17 However, the overall CSF HCRT-1 levels after 8 hours of prolonged waking were still lower in old compared to young rats (P < .015) (Figure3).
Figure 3.
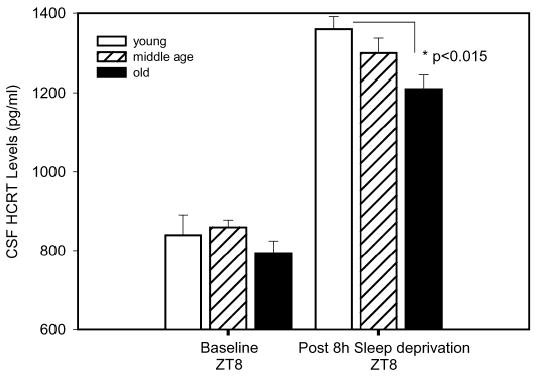
Changes in hypocretin (HCRT)-1 cerebrospinal (CSF) fluid concentrations in young (n = 9), middle-aged (n = 10), and old (n = 10) F344 rats after 8 hours of prolonged waking. The rats were kept awake for 8 hours (ZT0-ZT8) by lightly tapping the cage to keep them active or whenever they showed behavioral signs of sleep. Electroencephalographic sleep recordings were not obtained because the patency of the sleep electrodes would not be maintained during the 3-month period of this study. Immediately after the sleep deprivation, the rats were anesthetized and CSF was collected as described in the materials and methods section. Old F344 rats had significantly less CSF HCRT-1 compared to young F344 rats (P < .05).
Experiment 3
The decline in HCRT-1 in old rats could reflect a decrease in prepro-HCRT gene expression across the aging process. To test this hypothesis, we measured prepro-HCRT mRNA levels in the posterior hypothalamus (see Figure 1 for region that was dissected) of young and old rats by Northern blot analysis (Figure 4). Prepro-HCRT expression was limited to the posterior hypothalamus, consistent with previously published results,9 and we found no difference in mRNA between age groups.
Figure 4.
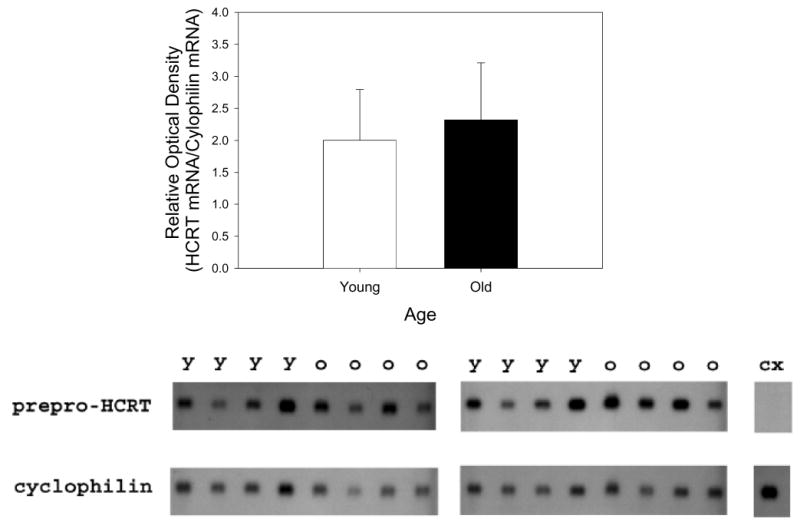
Northern blot analysis of preprohypocretin (HCRT) expression in the posterior hypothalamus of young F344 rats (Y; n=10) and old F344 rats (O; n = 10). Northern blots of total RNA from the posterior hypothalamus was hybridized to [32P]-labeled prepro-HCRT and cyclophilin probes as described in the materials and methods section. The bar graph summarizes the results of the ratio of optical measurement readings of [32P]-labeled prepro-HCRT and cyclophilin labeling. No prepro-HCRT expression was detected in the cortex (CX), since the HCRT somata are found only in the posterior hypothalamus. Each lane corresponds to an individual rat, and each blot could accommodate only 8 lanes. The third blot where the mRNA from 2 each young and old rats was run is not shown.
DISCUSSION
The results indicate that old (21-month-old) rats have significantly less HCRT-1 in the CSF, especially at the end of the dark wake-active period and over the 24-hour period. The pattern of the diurnal rhythm of HCRT in this study is consistent with those reported previously by others in young rats16 and young nonhuman primate.21 The overall diurnal pattern of CSF HCRT in the old rats was aligned with that of the other younger rats, and there was no change in phase position.
It is not yet known whether the HCRT rhythm is phase advanced in elderly humans, although elderly humans have an earlier wake time and also have a 1-hour phase advance of the core body temperature and melatonin rhythms.25 However, in elderly humans, CSF HCRT-1 assayed at a single time point is not different compared to young subjects.26 In the present study, we also found that at some time points there were no differences in CSF levels of HCRT. However, at specific times of day, old rats had significantly less CSF HCRT compared to young rats, indicating that it is necessary to observe the overall diurnal rhythm of HCRT rather than to measure a single time point. In humans, the lack of a change in CSF HCRT levels at a single time point may also be due to variability associated with physical activity of the individuals and pathology.
To identify a possible mechanism that might produce the decline in CSF HCRT-1 levels, we assessed, in individual rats, the levels of HCRT mRNA in the posterior hypothalamus where the HCRT somata are located and found no change between age groups. However, using the in-situ hybridization method, a more sensitive method compared to Northern blot analysis and which enables the precise localization and identification of HCRT mRNA in individual cells, Porkka-Heiskanen et al27 have discovered that there is a decline in HCRT mRNA in the more caudal regions of the lateral hypothalamus. Their work showed that this region also has fewer HCRT mRNA-containing neurons. Such a regional decline in mRNA and cell number could have produced an overall decline in CSF HCRT levels detected in the present study. However, to conclusively determine that there is HCRT neuronal loss with aging, it is necessary to perform immunocytochemistry to label HCRT because it is possible that synthesis and degradation of the peptide might be such that even though old rats express less HCRT mRNA, there is a sufficient amount of peptide present in the cytoplasm to give a robust signal. One way to resolve this would be to detect both HCRT mRNA and peptide in the same neuron by combining in-situ hybridization with immunocytochemistry. In mice, a regional loss of prepro-HCRT mRNA has not been not found,28 although Terao et al did find declines in HCRT receptor-2 mRNA. In whole brain homogenates of mouse brains, previous work has not identified changes in HCRT-1 levels with age or with circadian time.29
Porkka-Heiskanen et al27 measured both HCRT-1 (orexin A) and HCRT-2 (orexin B) in punches of the hypothalamic region where the HCRT somata are located and found declines in both peptides in 12- and 24-month-old rats relative to young (3-month-old) rats. We measured only HCRT-1 in CSF because it is a more stable peptide30 that does not degrade easily relative to HCRT-2 and found that it was lower in 21-month-old rats; HCRT-1 levels in 12-month-old rats were not significantly different compared to young (3-month-old) rats. One possibility is that older (21-month-old) rats compared to 12- or 3-month-old rats have a greater deterioration in activity, release, flow dynamics, clearance, and loss of terminal field of HCRT neurons such that in the CSF, where the HCRT of the overall brain would accumulate, the levels are lower.
To determine whether the decline in CSF HCRT in old rats might result from reduced activation of the HCRT neurons, we kept the rats awake for 8 hours and measured the CSF HCRT levels. Prolonged waking16 and exercise17 increase CSF levels of HCRT, and we hypothesized that when challenged by prolonged waking, there would not be a difference in CSF HCRT levels between young and old rats. The basal levels of HCRT at ZT8 were not different between the age groups of rats (Figure 3). However, after 8 hours of prolonged waking, old rats had significantly lower CSF levels of HCRT compared to young rats (P < .015), suggesting that HCRT neurons in old rats are not as activated in response to 8 hours of prolonged waking, resulting in less overall output of HCRT from these neurons.
In the present study, we found a 10% decline in HCRT levels in the old rats. We suggest that although a severe loss of HCRT leads to narcolepsy, a more modest decline in HCRT could decrease wakefulness and lead to sleepiness during the normal wake-active periods. Indeed, if there were a more severe decline in CSF HCRT, then one would expect the old rats to show obvious symptoms of narcolepsy, which they do not. Functionally, what is important are extracellular levels of HCRT acting on its receptor. If there is less HCRT, which our findings indicate, and there is also a decline in HCRT receptor mRNA as found by Kilduff’s group,28 then the overall consequence would be diminished action of HCRT at target sites. Because HCRT has been implicated in regulating arousal, the physiologic consequences of a diminished action of HCRT due to lower circulating levels of HCRT and fewer HCRT receptors (since the HCRT receptor mRNA is less in old mice28) might be to decrease arousal at certain times of day and fragmenting normal sleep-wake architecture. Indeed, a deterioration in sleep architecture with aging has been found in mice,31 rats,32–35 cats,36–37 monkeys,38 and humans.39 Our studies22–24 have shown that old rats (similar in age to those in the present study) demonstrate significant alterations in sleep architecture consistent with data from other studies.
In this study, we focused on the HCRT system because it is implicated in waking. Other neurotransmitter systems implicated in waking also decline with aging,40–41 including the wake-induced expression of immediate-early genes such as c-Fos.42 The sleep-promoting aspect of the network might be less active as a result of a decline in number or sensitivity of adenosine receptors.23 In addition, the output molecules from the suprachiasmatic nucleus, the brain region responsible for the timing of sleep and waking, might also decline. These output molecules include transforming growth factor α43 and prokineticin,44 which have been found to have an effect on sleep and locomotor activity. It is not known whether these suprachiasmatic nucleus factors decline with aging, but a reduction would disrupt circadian sleep-wake cycles.
Acknowledgments
We thank Thomas S. Kilduff for the generous gift of prepro-HCRT cDNA. We also thank Jill Winston for providing technical assistance. EM is an HHMI investigator.
Footnotes
Disclosure Statement
Research supported by NIH grants NS30140, AG15853, AG09975, MH55772, MH01600, NS23724 and by the Medical Research Service of the Department of Veterans Affairs.
References
- 1.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 2.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 3.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 5.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 6.Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 7.Gerashchenko D, Kohls MD, Greco M, et al. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–83. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerashchenko D, Blanco-Centurion C, Greco MA, Shiromani PJ. Effects of lateral hypothalamic lesion with the neurotoxin hypocretin2-saporin on sleep in long Evans rats. Neuroscience. 2003;116:223–35. doi: 10.1016/s0306-4522(02)00575-4. [DOI] [PubMed] [Google Scholar]
- 9.Peyron C, Tighe DK, Van Den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and g protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 11.De Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiyashchenko LI, Mileykovskiy BY, Maidment N, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–6. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourgin P, Huitron-Resendiz S, Spier AD, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–5. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvath TL, Peyron C, Diano S, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–59. [PubMed] [Google Scholar]
- 15.Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida Y, Fujiki N, Nakajima T, et al. Fluctuation of extracellular hypocretin-1 (orexin a) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–81. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1079–86. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Den Pol AN, Patrylo PR, Ghosh PK, Gao XB. Lateral hypothalamus: early developmental expression and response to hypocretin (orexin) J Comp Neurol. 2001;433:349–63. doi: 10.1002/cne.1144. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Ueta Y, Hara Y, et al. Postnatal development of orexin/hypocretin in rats. Brain Res Mol Brain Res. 2000;78:108–19. doi: 10.1016/s0169-328x(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 20.Fujiki N, Yoshida Y, Ripley B, Honda K, Mignot E, Nishino S. Changes in CSF hypocretin-1 (orexin a) levels in rats across 24 hours and in response to food deprivation. Neuroreport. 2001;12:993–7. doi: 10.1097/00001756-200104170-00026. [DOI] [PubMed] [Google Scholar]
- 21.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–60. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiromani PJ, Lu J, Wagner D, et al. Compensatory sleep response to 12 h wakefulness in young and old rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R125–33. doi: 10.1152/ajpregu.2000.278.1.R125. [DOI] [PubMed] [Google Scholar]
- 23.Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–70. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Salin-Pascual RJ, Upadhyay U, Shiromani PJ. Effects of hypocaloric diet on sleep in young and old rats. Neurobiol Aging. 2002;23:771–6. doi: 10.1016/s0197-4580(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 25.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 26.Kanbayashi T, Yano T, Ishiguro H, et al. Hypocretin-1 (orexin-a) levels in human lumbar csf in different age groups: infants to elderly persons. Sleep. 2002;25:337–9. doi: 10.1093/sleep/25.3.337. [DOI] [PubMed] [Google Scholar]
- 27.Porkka-Heiskanen T, Alanko L, Kalinchuk A, Heiskanen S, Stenberg D. The effect of age on prepro-orexin gene expression and contents of orexin a and b in the rat brain. Neurobiol Aging. 2004;25:231–8. doi: 10.1016/s0197-4580(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 28.Terao A, Apte-Deshpande A, Morairty S, Freund YR, Kilduff TS. Age-related decline in hypocretin (orexin) receptor 2 messenger rna levels in the mouse brain. Neurosci Lett. 2002;332:190–4. doi: 10.1016/s0304-3940(02)00953-9. [DOI] [PubMed] [Google Scholar]
- 29.Lin L, Wisor J, Shiba T, et al. Measurement of hypocretin/orexin content in the mouse brain using an enzyme immunoassay: the effect of circadian time, age and genetic background. Peptides. 2002;23:2203–11. doi: 10.1016/s0196-9781(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida Y, Fujiki N, Maki RA, Schwarz D, Nishino S. Differential kinetics of hypocretins in the cerebrospinal fluid after intracerebroventricular administration in Rats. Neurosci Lett. 2003;346:182–6. doi: 10.1016/s0304-3940(03)00571-8. [DOI] [PubMed] [Google Scholar]
- 31.Eleftheriou BE, Zolovick AJ, Elias MF. Electroencephalographic changes with age in male mice. Gerontologia. 1975;21:21–30. doi: 10.1159/000212027. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg RS, Zepelin H, Rechtschaffen A. Sleep in young and old rats. J Gerontol. 1979;4:525–32. doi: 10.1093/geronj/34.4.525. [DOI] [PubMed] [Google Scholar]
- 33.Van Gool WA, Mirmiran M. Age-related changes in the sleep pattern of male adult rats. Brain Res. 1983;279:394–8. doi: 10.1016/0006-8993(83)90217-2. [DOI] [PubMed] [Google Scholar]
- 34.Zepelin H, Whitehead WE, Rechtschaffen A. Aging and sleep in the albino rat. Behav Biol. 1972;7:65–74. doi: 10.1016/s0091-6773(72)80189-5. [DOI] [PubMed] [Google Scholar]
- 35.Zepelin H, Rechtschaffen A. Mammalian sleep, longevity, and energy metabolism. Brain Behav Evol. 1974;10:425–70. doi: 10.1159/000124330. [DOI] [PubMed] [Google Scholar]
- 36.Bowersox SS, Floyd T, Dement WC. Electroencephalogram during sleep in the cat. Sleep. 1983;7:380–5. doi: 10.1093/sleep/7.4.380. [DOI] [PubMed] [Google Scholar]
- 37.Bowersox SS, Baker T, Dement WC. Sleep-wakefulness patterns in the aged cat. Electroencephalogr Clin Neurophysiol. 1984;58:240–52. doi: 10.1016/0013-4694(84)90110-x. [DOI] [PubMed] [Google Scholar]
- 38.Pegram V, Bert J, Naquet R. The ontogeny of EEG sleep patterns in the baboon. Psychophysiology. 1969;6:228. [Google Scholar]
- 39.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 40.Lee JJ, Chang CK, Liu IM, Chi TC, Yu HJ, Cheng JT. Changes in endogenous monoamines in aged rats. Clin Exper Pharmacol Physiol. 2001;28:285–9. doi: 10.1046/j.1440-1681.2001.03439.x. [DOI] [PubMed] [Google Scholar]
- 41.Miguez JM, Aldegunde M, Paz-Valinas L, Recio J, Sanchez-Barcelo E. Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging. J Of Neural Transmission. 1999;106:1089–98. doi: 10.1007/s007020050225. [DOI] [PubMed] [Google Scholar]
- 42.Basheer R, Shiromani PJ. Effects of prolonged wakefulness on c-fos and Ap1 activity in young and old rats. Brain Res Mol Brain Res. 2001:89153–7. doi: 10.1016/s0169-328x(01)00045-6. [DOI] [PubMed] [Google Scholar]
- 43.Kramer A, Yang FC, Snodgrass P, et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–5. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 44.Cheng MY, Bullock CM, Li C, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–10. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]


