Abstract
Hunger elicits diverse, yet coordinated, adaptive responses across species, but the underlying signaling mechanism remains poorly understood. Here, we report on the function and mechanism of the Drosophila insulin-like system in the central regulation of different hunger-driven behaviors. We found that overexpression of Drosophila insulin-like peptides (DILPs) in the nervous system of fasted larvae suppressed the hunger-driven increase of ingestion rate and intake of nonpreferred foods (e.g., a less accessible solid food). Moreover, up-regulation of Drosophila p70/S6 kinase activity in DILP neurons led to attenuated hunger response by fasted larvae, whereas its down-regulation triggered fed larvae to display motivated foraging and feeding. Finally, we provide evidence that neural regulation of food preference but not ingestion rate may involve direct signaling by DILPs to neurons expressing neuropeptide F receptor 1, a receptor for neuropeptide Y-like neuropeptide F. Our study reveals a prominent role of neural Drosophila p70/S6 kinase in the modulation of hunger response by insulin-like and neuropeptide Y-like signaling pathways.
Keywords: Drosophila insulin-like peptide, feeding behavior, food preference, neuropeptide F, neuroendocrine
Most animals, including humans, have evolved a highly optimized foraging strategy to cope with wide fluctuations in food availability. The state of food depletion exerts a systemic influence that initiates a broad array of adaptive behaviors. For example, deprived animals are generally more tolerant of stressful foraging conditions and less inhibited in engaging in aggressive behaviors (1, 2). They also show increased appetite and a lower threshold of food acceptance to accommodate less palatable energy sources (3, 4). In humans, feeding mechanisms evolved for protecting against starvation are now thought to be major causal factors for the widespread problem of obesity in affluent societies (5). It remains poorly understood as to what and how signaling systems are responsible for integrating hunger stimuli and organizing diverse adaptive behaviors.
The prominent feeding control mechanisms in vertebrates have been mapped to the regions of the CNS, especially the hypothalamus also known as the “feeding center” (6, 7). This notion was most strikingly demonstrated by the observation that electrical stimulation of the lateral parts of the hypothalamus was sufficient to cause self-sustained food intake (8). Pharmacological studies have implicated a variety of brain chemicals, including bioactive peptides in promoting/inhibiting food intake. Several hypothalamic neuropeptides, including agouti-related protein and neuropeptide Y (NPY) are thought to be potent feeding stimulants (9). On the other hand, peptides such as insulin, leptin, and melanocortins suppress food intake (10). However, the elucidation of the physiological roles of these molecules and their sites of action has been difficult largely because of the complexity of vertebrate models.
The Drosophila genome has seven insulin-like genes, dilp1–7, and at least one insulin receptor-like gene (dInR) (11–13). Insulin/insulin-like peptides and their receptors are widely distributed in the CNS and have been implicated in the control of body weight and reproduction, learning and memory, and axon pathfinding (14–16). An NPY-like signaling pathway, comprising neuropeptide F (NPF) and its receptor NPFR1, has also been identified in Drosophila (17, 18). We recently found that this NPY-like signaling pathway developmentally regulates larval feeding and social migratory behavior (19, 20). Interestingly, both fly NPF and mammalian NPY appear to promote feeding response in fasted animals but not those fed ad libitum (19, 21, 22).
We have used the relatively simple Drosophila larva as a genetic model to elucidate molecular and neural mechanisms underlying hunger regulation. Our main objective is to define and characterize different neuronal signaling pathways that constitute a complete central feeding apparatus. In this article, we address two fundamental questions: what enables a deprived animal to take on motivated foraging and food acquisition, and what prevents a nondeprived animal from doing so. We show that Drosophila p70/S6 kinase (dS6K) is a key organizer of different hunger-driven behaviors in Drosophila larvae. Down-regulation of the Drosophila insulin-like peptide (DILP)-neuronal dS6K activity in fed larvae is sufficient to trigger hunger-driven foraging and feeding behaviors, whereas its up-regulation suppresses the normal onset of such motivated behaviors in fasted animals. We also identified a neural pathway involving neurons expressing DILP, NPF, or NPFR1 that differentially regulates food preference but not ingestion rate. Our study reveals a molecular signaling cascade that mediates the action of DILP-neuronal dS6K in overriding a forager's normal feeding response. The conserved S6K signaling pathway may be crucial for regulating hunger-driven behaviors in diverse animals.
Methods
Flies and Media. Fly rearing and the collection of eggs and larvae have been described (19). The UAS-npfr1dsRNA flies are in a w1118 background. The UAS-shits1, UAS-dilp flies and UAS-ANF-GFP (also named UAS-preproANF-EMD containing a GFP-tagged atrial natriuretic factor fusion) were kindly provided by T. Kitamoto (University of Iowa, Iowa City), E. Hafen (Zoologisches Institute de Universitat Zurich, Zurich), and D. Deitcher (Cornell University, Ithaca, NY), respectively (23–25). The UAS-ANF-GFP, UAS-npfr1, UAS-dilp2, UAS-dilp3, and UAS-dilp4 lines are in a y w background. The elav-gal4, appl-gal4, MHC82-gal4, UAS-shits1, UAS-S6KDN, UAS-S6KACT, UAS-dInRDN, UAS-dInRACT, UAS-dp110, UAS-dPI3KDN, and UAS-dPTEN are in a w1118 background (refs. 26–31 and A. Parks, FlyBase, http://rail.bio.indiana.edu/.bin/fbidq.html?FBrf0178856). The UAS-S6KDN construct encodes a dominant negative form of S6K with the substitution of a lysine (K109) with glutamine (Q). The UAS-S6KACT encodes a constitutively active form of S6K with two substitutions: S418 to D and T422 to E (26). Other fly lines have been described (19, 32).
Molecular Cloning and Analysis. The cloning of npfr1 cDNA has been described (18), and its coding sequence was subcloned into the downstream of the UAS promoter in the pUAST vector. For making the dilp2-gal4 construct, a 2-kb fragment of the dilp2 promoter fragment was obtained through genomic PCR by using a pair of forward and reverse primers (GGCCATGGCGATGGCGATGA and GAGATCTTTACGATCAAATGGATTA) and was cloned in front of the gal4 coding sequence. More detailed information is available on request.
Food Response Assays. The quantification of intake rate, as indicated by the frequency of mouth-hook contractions, of solid and liquid foods was performed as described (19). Briefly, for the liquid food assay, larvae were transferred to a 35-mm Petri dish containing 2 g of glucose/agar paste, prepared by mixing 45 ml of 10% glucose solution with 5.5 g of agar powder (Applied Biosystems). For the solid food assay, each 35-mm Petri dish contained 2.5 ml of melted glucose agar (10 g of glucose and 2.3 g of agar per 100 ml). Solidified agar was cut into four quarters and air-dried for 1 day. The next day, multiple small cuts were made on the vertical surfaces of the agar pieces before adding larvae. Larvae displayed virtually identical feeding responses to 10% glucose, apple juice, or 10% glucose/yeast containing liquid and solid foods under deprived and nondeprived conditions (see Fig. 6, which is published as supporting information on the PNAS web site).
For those assays that involved shits1-expressing larvae, the permissive and restrictive temperatures were set at 23°C and 30°C, respectively. Larvae were reared and held at 23°C. To measure the intake of solid and liquid foods, larvae were held in 30°C water for 15 min, transferred to glucose-agar block or paste, and quantified for the frequency of mouth-hook contraction by individual larva at 30°C. Typically, the assay time was ≈20 min. The contraction frequencies of individual larva remained consistent throughout the assay. To measure the recovery of food response of larvae expressing shits1, larvae were returned from 30°C to 23°C and measured for food response at 25 and 50 min afterward. At least three separate trials were performed for each assay. Statistical analyses were performed with one-way ANOVA.
Analysis of Larva Growth and Development. For each control and experimental group, eggs collected within a 2-h interval were left on an apple juice-agar plate with yeast paste. The total numbers of hatched larvae were counted. The larvae were fed continuously on the same plate and monitored for growth. Fresh yeast paste was supplemented for third instars when necessary to ensure that they were well nourished. At the 120 h after egg laying, the number of puparia in and around the plate was recorded. The results of the analyses are presented in Table 1 and Fig. 7, which are published as supporting information on the PNAS web site.
Results
Quantitative Assessment of Larval Behavioral Responses to Prolonged Deprivation. The relatively simple Drosophila larva offers a genetically tractable model to define and characterize different neuronal signaling pathways that constitute a complete central feeding apparatus. Younger third-instar larvae forage actively and use their mouth hooks for food intake. Larvae normally feed on liquid food, and their food ingestion can be quantified by measuring the contraction rate of the mouth hooks (19). We examined how food deprivation affects larval feeding response to a liquid (e.g., 10% glucose-agar paste) and less accessible solid food (e.g., 10% glucose agar blocks). To extract embedded glucose from the solid food, larvae have to pulverize the food by scraping agar surface with mouth hooks. Unless stated otherwise, synchronized third-instar larvae (74 h after egg laying) were used for the assays.
When fed ad libitum, normal larvae (w1118) displayed significant feeding activity in the liquid food with an average mouth-hook contraction frequency of ≈30 times in a 30-s test period; in contrast, these larvae declined the solid food (Fig. 1A). However, larvae withheld from food (on a wet tissue) for 40 or 120 min displayed increased intake of both liquid and solid foods. For example, larvae fasted for 120 min showed a 100% and >500% increase in mouth-hook contraction rate in liquid and solid food, respectively. Thus, deprivation not only enhances feeding rate in a graded fashion, but also triggers motivated foraging on the less accessible food normally rejected by fed larvae. In addition, larvae display virtually identical feeding responses to liquid and solid foods containing 10% glucose, apple juice, or 10% glucose/yeast under deprived and nondeprived conditions (see Fig. 6). Therefore, these paradigms appear to provide a general assessment of larval feeding response.
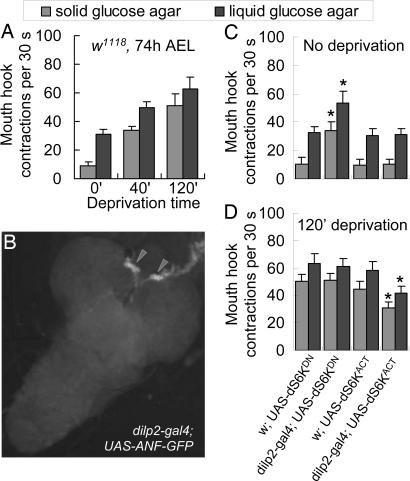
Fig. 1.
The dS6K activity in DILP neurons mediates hunger regulation of both food preference and ingestion rate. Transgenic analysis of dS6K activity was performed by using the Drosophila GAL4/UAS binary expression system (40). UAS-dS6KDN and UAS-dS6KACT encode a dominant-negative and constitutively active form of dS6K, respectively. All of the fly lines used are in a w background. All of the controls in this and other figures are isogenic to the relevant experimental larvae except for the transgene tested. (A) Quantification of liquid and solid food intake by synchronized w1118 larvae (74 h after egg laying, AEL) in response to increasing food deprivation. The number of larval mouth-hook contractions within a 30-s interval was measured. We found that larvae display virtually identical feeding responses to various liquid and solid foods containing 10% glucose, apple juice, or 10% glucose/yeast under deprived and nondeprived conditions (see Fig. 6). (B) DILP neurons expressing GFP in dilp2-gal4 × UAS-GFP larvae. The dilp2-gal4 driver selectively directs a GFP reporter expression in the two clusters of medial neurosecretory cells, as indicated by arrowheads. No GFP expression was detected in cells of other tissues including the gut, imaginal discs, or salivary glands. (Magnification: ×200.) (C and D) Synchronized third-instar larvae were withheld from food for 0 or 120 min before the assay. Control larvae: w × UAS-dS6KDN; w × UAS-dS6KACT. Experimental larvae: dilp2-gal4 × UAS-dS6KDN; dilp2-gal4 × UAS-dS6KACT. At least 30 larvae per group were assayed in three separate trials. Fed larvae expressing dS6KDN in DILP neurons showed significant increases in the feeding of liquid and solid food relative to controls (P < 0.0001), whereas those overexpressing dS6KACT showed no changes in the feeding responses (P > 0.34). Fasted larvae expressing dS6KACT in DILP neurons showed attenuated feeding of the liquid and solid food relative to controls (P < 0.0001), but those overexpressing dS6KDN showed no altered feeding responses (P > 0.16). At least 20 larvae per group were assayed in three separate trials. All statistical analyses were performed by using ANOVA. Error bars are the SEM.
DILP-Neuronal dS6K Mediates Hunger Regulation of Food Response. dS6K is a cell-autonomous effector of nutrient-sensing pathways (33). We investigated a possible role of neural dS6K in coupling peripheral physiological hunger signals and neuronal activities critical for hunger-driven behaviors. The transcripts of dilp1, dilp2, dilp3, and dilp5 are predominantly expressed in two small clusters of medial neurosecretory cells that project to the ring gland, the fly heart, and the brain lobes (11, 13, 19). We generated a gal4 driver containing a 2-kb fragment from the dilp2 promoter (dilp2-gal4) that directs the specific expression of a GFP reporter in those cells (Fig. 1B and Fig. 8, which is published as supporting information on the PNAS web site). Using dilp2-gal4, we expressed two transgenes, UAS-dS6KDN, encoding a dominant negative, and UAS-dS6KACT, a constitutively active form of dS6K (26). When fed ad libitum, control larvae (w × UAS-dS6KDN or UAS-dS6KACT) behaved like w larvae (Fig. 1C). However, dilp2-gal4 × UAS-dS6KDN larvae displayed a 50% increase in the rate of liquid-food intake and significant feeding of the solid food. Conversely, fasted larvae overexpressing dS6K activity (dilp2-gal4 × UAS-dS6KACT) showed attenuated feeding response to both liquid and solid foods (Fig. 1D). These findings reveal that dS6K in DILP neurons mediates hunger regulation of approaching/consumptive behaviors, controlling both quality and quantity of food for ingestion. We also measured the body size and the developmental rate of all four groups of larvae, and no significant differences were detected (see Fig. 8 and Table 1).
Overexpression of DILPs Suppresses Hunger-Driven Behaviors. DILPs act as neurohormones in Drosophila larvae (13). Down-regulation of dS6K activity in DILP neurons may reduce DILP release, thereby promoting increased food intake that is normally triggered only by hunger. A corollary of this interpretation is that overproduction of DILPs in the nervous system should interfere with hunger response by deprived animals. To test this idea, a neural-specific elav-gal4 driver was used to direct dilp expression in the larval nervous system. Three UAS-dilp lines (UAS-dilp2, UAS-dilp3, and UAS-dilp4) were chosen for the analysis (23). The elav-gal4 × UAS-dilp2 and UAS-dilp4 larvae displayed normal feeding response when fed ad libitum (data not shown). However, the same larvae fasted for 120 min displayed significantly attenuated feeding rates, similar to those of dilp2-gal4 × UAS-dS6KACT larvae (Fig. 2). For example, the comparative analysis of the elav-gal4 × UAS-GFP control and elav-gal4 × UAS-dilp2 and UAS-dilp4 experimental larvae showed that the latter were ≈30% and 33–45% lower in the ingestion rate of the liquid and solid food, respectively; surprisingly, elav-gal4 × UAS-dilp3 and UAS-GFP larvae showed virtually identical feeding responses. Therefore, DILP2 and DILP4 negatively regulate hunger-driven feeding activities. Taken together, our results suggest that a high level of dS6K activity in DILP neurons may suppress hunger response by reducing DILP release.
Fig. 2.
Overexpression of DILPs suppresses hunger-driven feeding activities. The elav-gal4 flies are in a w background; the UAS-dilp2, dilp3, and dilp4 lines are in a y w background. Overexpression of DILPs in the larval nervous system was directed by elav-gal4. Larvae were fasted for 120 min before the assays. The controls (elav-gal4 × UAS-GFP,w × UAS-dilp2, dilp3, and dilp4) displayed normal increases in feeding rate and motivated intake of the less preferred solid food. In contrast, the experimental larvae overexpressing dilp2 or dilp4 but not dilp3 showed significantly attenuated hunger responses (P < 0.0001 and P > 0.05, respectively). It still remains unclear why DILP3, which was shown to increase body size (23), is ineffective in attenuating feeding response. It is possible that DILP3 may be a weaker ligand to neural dInR. At least 20 larvae per group were assayed in three separate trials.
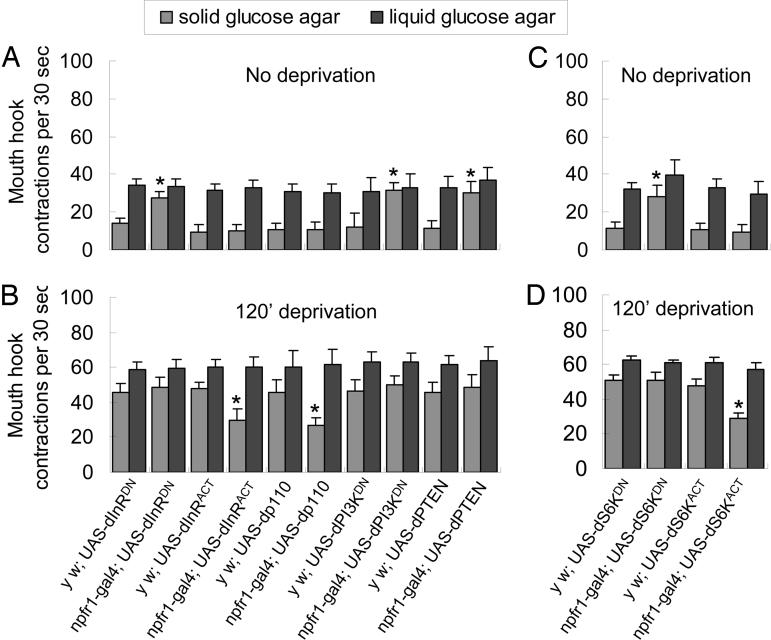
dInR and dS6K in NPFR1 Cells Differentially Regulate Food Preference. We sought to delineate the signaling mechanism that couples the dS6K activity in DILP neurons with its broad impact on hunger-driven feeding activities. Our previous study showed that fasted larvae ablated of NPF or its receptor (NPFR1) neurons are deficient in motivated feeding of the less-preferred solid food but normal in feeding of richer liquid food (17–20). We wondered whether the NPF/NPFR1 neuronal pathway might be one of the downstream effectors of the DILP pathway. To test this hypothesis, we analyzed the function of three components of the dInR signaling pathway in NPFR1 neurons: dInR, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase (dPTEN), and phosphatidylinositol 3-kinase (dPI3K) (12, 33). Five different transgenes were used: UAS-dInRACT and UAS-dInRDN encode a constitutively active and a dominant-negative form of dInR, respectively (A. Parks, FlyBase, http://rail.bio.indiana.edu/.bin/fbidq.html?FBrf0178856); UAS-Dp110 and UAS-dPI3KDN encode a catalytic subunit and a dominant-negative form of dPI3K, respectively (29); and UAS-dPTEN encodes a functional enzyme (28). When fed ad libitum, npfr1-gal4 × UAS-dInRDN, UAS-dPTEN, or UAS-dPI3KDN larvae displayed hyperactive feeding of the solid food, similar to w larvae deprived for 40 min (Fig. 3A). In contrast, fasted larvae overexpressing dInR or dPI3K (npfr1-gal4 × UAS-dInRACT or UAS-Dp110) displayed attenuated feeding response to the solid food (Fig. 3B). Importantly, larvae with up- or down-regulated dInR signaling in NPFR1 neurons did not exhibit significant changes in the intake rate of the richer liquid food relative to the paired controls. Taken together, these findings suggest that the dInR pathway negatively regulates the activity of NPFR1 neuron and mediates the DILP-regulated change in food preference but not ingestion rate. Furthermore, the results suggest that NPFR1 neurons are the direct targets of DILPs.
Fig. 3.
The dInR and dS6K activities in NPFR1 neurons selectively regulate food selection. The npfr1-gal4 is in a y w background, whereas all of the UAS line are in a w background. (A and B) UAS-dInRDN and UAS-dInRACT each encodes a dominant-negative and a constitutively active form of dInR. UAS-dp110 and UAS-dPI3KDN encode a catalytic subunit and a dominant-negative form of dPI3K, respectively. UAS-dPTEN encodes WT dPTEN. Control larvae: yw × UAS-dInRDN, UAS-dInRACT, UAS-dp110, UAS-dPI3KDN, and UAS-dPTEN. Experimental larvae: npfr1-gal4 × UAS-dInRDN, UAS-dInRACT, UAS-dp110, UAS-dPI3KDN, and UAS-dPTEN. At least 20 larvae per group were assayed in three separate trials. Fed larvae expressing transgenes (dInRDN, dPI3KDN, or dPTEN) that suppress dInR signaling in NPFR1 neurons showed significant feeding of the solid food (P < 0.0001), whereas deprived larvae expressing transgenes (dInRACT or Dp110) that enhance dInR signaling displayed attenuated feeding of the solid but not liquid food (P < 0.0001). (C and D) Control larvae: yw × UAS-dS6KDN, yw × UAS-dS6KACT. Experimental larvae: npfr1-gal4 × UAS-dS6KDN, npfr1-gal4 × UAS-dS6KACT. Larvae expressing dS6KDN in NPFR1 cells showed significant feeding of the solid but not liquid food (P < 0.0001) without deprivation, whereas fasted larvae expressing dS6KACT displayed attenuated feeding of the solid but not liquid food (P < 0.0001).
We also evaluated a possible role of dS6K in hunger regulation of the functioning of NPFR1 neurons, by expressing UAS-dS6KDN and UAS-dS6KACT using npfr1-gal4 (19). When fed ad libitum, npfr1-gal4 × UAS-dS6KDN larvae displayed hyperactive feeding of the solid food, similar to npfr1-gal4 × UAS-dInRDN larvae (Fig. 3C). However, these larvae, unlike dilp2-gal4 × UAS-dS6KDN animals, displayed no increases in the ingestion rate of the richer liquid food. Conversely, fasted larvae overexpressing dS6K (npfr1-gal4 × UAS-dS6KACT) displayed attenuated feeding response to the solid food (Fig. 3D). These findings suggest that dS6K also negatively regulates the activity of NPFR1 neurons in food preference, but does not mediate the regulation of feeding rate by DILP signaling.
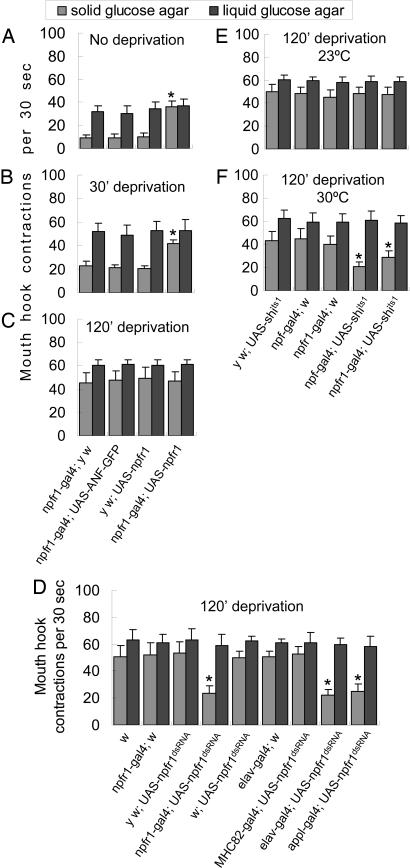
Overexpression of NPFR1 Promotes Hunger-Adaptive Change in Food Preference. We also examined the food response of the solid and liquid food by larvae overexpressing an npfr1 cDNA under the control of an npfr1-gal4 driver. In the presence of the liquid food, both experimental (npfr1-gal4 × UAS-npfr1) and control larvae (e.g., npfr1-gal4 × UAS-ANF-GFP), fed or fasted, showed similar intake rates and comparable increases in feeding response to hunger (Fig. 4 A–C, P > 0.25). However, when forced to feed on the solid food, fed experimental larvae exhibited significant intake of the solid food (30 times per 30 s, P < 0.001), whereas fed controls rejected the same food. Thus, NPFR1 overexpression selectively promotes change in food preference without increasing ingestion rate. We also observed that the feeding responses of NPFR1-overexpressing larvae and controls fasted for 120 min were indistinguishable (Fig. 4C). Thus, the effect of NPFR1 overexpression on food preference is detectable only in fed or mildly fasted larvae, suggesting hunger-activated NPFR1 signaling approaches to a plateau in severely fasted animals.
Fig. 4.
The NPF/NPFR1 pathway acutely mediates hunger regulation of food preference. The UAS-npfr1 and UAS-ANF-GFP are in the y w background, whereas the elav-gal4, appl-gal4, MHC82-gal4, UAS-shits1, and UAS-npfr1dsRNA are in a w background. (A–C) NPFR1-overexpressing larvae fasted for 0, 30, or 120 min were assayed. The rate of mouth-hook contractions was scored individually (n = 15–20 per group for each of three trials). Among the fed larvae, the experimental group (npfr1-gal4 × UAS-npfr1) showed significantly higher activity of extracting agar-embedded glucose relative to controls (npfr1-gal4 × y w, npfr1-gal4 × UAS-ANF-GFP, and y w × UAS-npfr1; P < 0.0001), whereas the ingestion rate of the liquid food remained unchanged (P > 0.25). The enhancement of motivated intake of the solid food was also observed in NPFR1-overexpressing larvae fasted for 30 min (P < 0.001), but the difference became insignificant after 120 min (P > 0.86). (D) Larvae expressing npfr1 dsRNA in NPFR1 cells and the nervous system were tested for their feeding responses to liquid and solid food. Larvae were fasted for 120 min before the assay. The control larvae (w1118, npfr1-gal4 × w, y w × npfr1dsRNA, w × UAS-npfr1dsRNA, elav-gal4 × w, and MHC-82-gal4 × UAS-npfr1dsRNA) showed enhanced feeding responses, whereas the experimental larvae (npfr1-gal4, elav-gal4, and appl-gal4 × UAS-npfr1dsRNA) were deficient in feeding response to the solid but not liquid food (P < 0.0001; n > 20 per group). The UAS-npfr1dsRNA line was previously shown to reduce npfr1 transcript levels and NPFR1 signaling activity (32). (E and F) UAS-shits1 encodes a semidominant-negative form of dynamin that blocks neurotransmitter release at a restrictive temperature (>29°C). Larvae were fasted for 120 min before the assay (n > 20 per group for each of three trials). At 23°C, both experimental larvae (npf-gal4 and npfr1-gal4 × UAS-shits1) and control larvae (y w × UAS-shits1, npf-gal4 × w, and npfr1-gal4 × w) displayed normal food response (P > 0.45). At 30°C, the experimental larvae showed deficits in motivated feeding response to the solid food but not liquid food (P < 0.0001), whereas the controls remained normal. The feeding behaviors of control larvae at both temperatures were also similar (P > 0.08).
Neural NPFR1 Is Responsible for Hunger-Regulated Food Selection. We also selectively knocked down npfr1 activity by expressing npfr1 dsRNA in the nervous system. The UAS-npfr1dsRNA lines were previously used to functionally disrupt npfr1 activity (32). We found that 120-min fasted larvae expressing npfr1 dsRNA in NPFR1 or the nervous system (npfr1-gal4, elav-gal4, or appl-gal4 × UAS-npfr1dsRNA) were deficient in motivated feeding of the solid but not liquid food (Fig. 4D). In contrast, all control larvae, including those expressing npfr1dsRNA in muscle cells (MHC82-gal4 × UAS-npfr1dsRNA), showed normal feeding responses. These results indicate that neural NPFR1 mediates hunger regulation of food selection.
The NPF/NPFR1 Pathway Acutely Regulates Food Selection. A potential problem of the previous transgenic studies is that NPF/NPFR1 signaling is likely to be disrupted in a relatively early stage of larval development. Conceivably, the NPF/NPFR1 neuronal pathway could be essential for ad libitum or hunger-driven feeding of richer liquid foods, but such an activity might be masked by some yet-unidentified compensatory mechanism triggered by its early loss. To test this idea, we attempted to disrupt NPF/NPFR1 neuronal signaling in a temporally controlled manner by expressing a temperature-sensitive allele of shibire (shits1) driven by npf-gal4 or npfr1-gal4. The shits1 allele encodes a semidominant-negative form of dynamin that blocks neurotransmitter release at a restrictive temperature (>29°C) (34). At the permissive temperature of 23°C, 120-min-fasted experimental larvae (npf-gal4 and npfr1-gal4 × UAS-shits1) and paired controls (y w × UAS-shits1 and npf-gal4 and npfr1-gal4 × w1118) displayed normal feeding responses to both liquid and solid foods (Fig. 4E). However, if larvae were incubated at 30°C for 15 min, controls still displayed normal feeding activities, whereas the experimental larvae showed attenuated feeding response to the solid but not liquid food (Fig. 4F). Therefore, there was no detectable developmental or physiological compensation for the loss of NPF signaling in Drosophila larvae. These results also suggest that the NPF/NPFR1 neuronal pathway is acutely required to initiate and maintain larval hunger response. We found that the foraging activity of the experimental larvae was completely restored when the assay temperature was reduced to 23°C (see Fig. 9, which is published as supporting information on the PNAS web site), suggesting that the NPF system can modulate the intensity and duration of feeding response.
Discussion
We have shown that dS6K regulates different, yet coordinated, behaviors controlling quantitative and qualitative aspects of hunger-adaptive food response. We provide evidence that dS6K mediates hunger regulation of two opposing insulin- and NPY-like signaling activities, dynamically modifying larval food preference and feeding rate based on the nutritional state (Fig. 5). For example, hunger stimuli may cause a reduction of dS6K activity in DILP neurons, resulting in the suppression of DILP signaling that negatively regulates a downstream NPF/NPFR1-dependent and another NPF-independent neuronal pathway. The DILP/NPFR1 neuronal pathway selectively mediates hunger-adaptive change in food preference, possibly by overriding the high threshold of food acceptance set by a separate default pathway, enabling hungry animals to be receptive to less preferred foods. The NPF/NPFR1-independent pathway promotes a general increase in the ingestion rate of preferred/less preferred foods, enabling animals to compete effectively for limited food sources. Our study also implicates the presence of a separate default pathway for mediating the selective intake of preferred foods (baseline feeding) in larvae fed ad libitum. This default pathway may be largely insensitive to DILP or NPF signaling, because overexpression of dS6K, DILPs, or NPFR1 in nondeprived larvae did not affect ad libitum feeding in the liquid food (see Figs. 1, 2, 3 and data not shown). We suggest that the conserved S6K pathway may be critical for regulating behavioral adaptation to hunger in diverse organisms, including humans, and its components are potential drug targets for appetite control.
Fig. 5.
A model for the hunger regulation of adaptive feeding behaviors in Drosophila larvae. Our results suggest that DILP2 neurons, and possibly together with DILP4 neurons, negatively regulate two downstream hunger-responsive feeding systems: an NPF/NPFR1-dependent pathway specialized for motivated feeding and an NPF/NPFR1-independent pathway for general enhancement of feeding rate. In DILP neurons, dS6K activity is likely to positively regulate DILP synthesis and/or release. In fed larvae, a relatively high level of DILP signaling suppresses the two downstream pathways. In fasted animals, hunger stimuli down-regulate dS6K activity in DILP neurons, which in turn leads to decreased DILP signaling and therefore disinhibition of the two pathways. The former overrides the high threshold set by the default pathway, enabling hungry animals to engage in motivated foraging and food selection. The latter enhances feeding rate, allowing animals to compete effectively for limited food sources. DILPs negatively regulate the NPFR1 pathway through the dInR/dPI3K/dS6K pathway in NPFR1 neurons. Our data also implicate the presence of a separate default pathway for ad libitum feeding of higher-quality foods (baseline feeding) by fed larvae. This default pathway may be largely insensitive to DILP or NPF signaling, because overexpression of dS6K, DILPs, or NPFR1 in fed larvae did not affect baseline feeding in the presence of the liquid food (see Figs. 1, 2, 3 and data not shown).
The functional differences of DILP1–7 had not been reported previously. In this study, we show that dilp2, dilp3, and dilp4 are functionally distinct. DILP2 and DILP3 both are produced in the same medial neurosecretory cells. However, unlike DILP2, DILP3 is apparently not involved in suppressing deprivation-motivated feeding. It is still unclear whether the differential activities of DILP2 and DILP3 reflect their structural divergence or are caused by the presence of yet-unidentified dInR isoforms. DILP4 is not expressed within the two medial clusters of DILP neurons (11). Under acute deprivation, the level of dilp4 transcripts showed a 5-fold reduction in the larval CNS (data not shown). Thus, it is possible that DILP4 may play a localized role in promoting feeding response inside the CNS.
Feeding is a reward-seeking behavior, and deprivation strengthens the reinforcing effect (reward value) of food (35). Our studies suggest a previously uncharacterized role of the DILP/dInR signaling pathway in regulating an animal's perception of food quality. The DILP/NPF neural network may regulate an animal's incentive to acquire lower-quality foods by modifying the reward circuit. This hypothesis is interesting in light of the findings that foods and abused substances may act on the same reward circuit, and highly palatable foods can reduce drug-seeking behaviors (36–38). It is also possible that the DILP/NPF system might represent a specialized neural circuit that positively alters the reward value of lesser-quality foods. Conceivably, a better understanding of the action of this signaling system may provide fresh insights into neural mechanisms for controlling eating and drug-seeking behaviors.
Given its prominent role in behavioral adaptation to hunger, the insulin/NPY-like neural network is likely of primary importance to animal evolution. In addition, insulin and NPY family molecules have been found in a wide range of animals from humans to worms (39). Therefore, the insulin/NPY-like network may be a useful model for studying comparatively how diverse animals have evolved distinct ways of adapting an ancestral neural system to suit their respective lifestyles.
Supplementary Material
Acknowledgments
We thank E. Hafen, T. Kitamoto, U. Heberlein (University of California, San Francisco), D. Pan (University of Texas Southwestern Medical Center, Dallas), J. H. Park (University of Tennessee, Knoxville), and the Bloomington Fly Stock Center (Indiana University, Bloomington) for fly lines and J. S. Willis for critical reading of the manuscript. This work was funded by National Institutes of Health Grant DK-58348 (to P.S.).
Author contributions: P.S. designed research; Q.W., Y.Z., and J.X. performed research; P.S. contributed new reagents/analytic tools; Q.W. and P.S. analyzed data; and Q.W. and P.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: dS6k, Drosophila p70/S6 kinase; dInR, Drosophila insulin receptor; NPY, neuropeptide Y; NPF, neuropeptide F; NPFR, NPF receptor; DILP, Drosophila insulin-like peptide; PTEN, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase; PI3K, phosphatidylinositol 3-kinase.
References
- 1.Cabanac, M. (1985) Physiol. Behav. 35, 701-709. [DOI] [PubMed] [Google Scholar]
- 2.Dethier, V. G. (1976) The Hungry Fly (Harvard Univ. Press, Cambridge, MA).
- 3.Carr, K. D. (1996) Neurochem. Res. 21, 1455-1467. [DOI] [PubMed] [Google Scholar]
- 4.Wong, R. (1995) Biological Perspectives on Motivated Activities (Ablex, Norwood, NJ).
- 5.Berthoud, H. R. (2002) Neurosci. Biobehav. Rev. 26, 393-428. [DOI] [PubMed] [Google Scholar]
- 6.Anand, B. K. & Brobeck, J. R. (1951) Proc. Soc. Exp. Biol. Med. 77, 323-324. [DOI] [PubMed] [Google Scholar]
- 7.Hillebrand, J. J., de Wied, D. & Adan, R. A. (2002) Peptides 23, 2283-2306. [DOI] [PubMed] [Google Scholar]
- 8.Hess, W. R. (1954) Diencephalon: Autonomic and Extrapyramidal Functions (Grune & Stratton, New York).
- 9.Beck, B. (2001) Neurosci. Biobehav. Rev. 25, 143-158. [DOI] [PubMed] [Google Scholar]
- 10.Porte, D., Jr., Baskin, D. G. & Schwartz, M. W. (2002) Nutr. Rev. 60, S20-S29. [DOI] [PubMed] [Google Scholar]
- 11.Brogiolo, W., Stocker, H., Ikeya, T., Rintelen, F., Fernandez, R. & Hafen, E. (2001) Curr. Biol. 11, 213-221. [DOI] [PubMed] [Google Scholar]
- 12.Oldham, S. & Hafen, E. (2003) Trends Cell Biol. 13, 79-85. [DOI] [PubMed] [Google Scholar]
- 13.Rulifson, E. J., Kim, S. K. & Nusse, R. (2002) Science 296, 1118-1120. [DOI] [PubMed] [Google Scholar]
- 14.Bruning, J. C., Gautam, D., Burks, D. J., Gillette, J., Schubert, M., Orban, P. C., Klein, R., Krone, W., Muller-Wieland, D. & Kahn, C. R. (2000) Science 289, 2122-2125. [DOI] [PubMed] [Google Scholar]
- 15.Gerozissis, K. (2003) Cell Mol. Neurobiol. 23, 1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song, J., Wu, L., Chen, Z., Kohanski, R. A. & Pick, L. (2003) Science 300, 502-505. [DOI] [PubMed] [Google Scholar]
- 17.Brown, M. R., Crim, J. W., Arata, R. C., Cai, H. N., Chun, C. & Shen, P. (1999) Peptides 20, 1035-1042. [DOI] [PubMed] [Google Scholar]
- 18.Garczynski, S. F., Brown, M. R., Shen, P., Murray, T. F. & Crim, J. W. (2002) Peptides 23, 773-780. [DOI] [PubMed] [Google Scholar]
- 19.Wu, Q., Wen, T., Lee, G., Park, J. H., Cai, H. N. & Shen, P. (2003) Neuron 39, 147-161. [DOI] [PubMed] [Google Scholar]
- 20.Shen, P. & Cai, H. N. (2001) J. Neurobiol. 47, 16-25. [DOI] [PubMed] [Google Scholar]
- 21.Bannon, A. W., Seda, J., Carmouche, M., Francis, J. M., Norman, M. H., Karbon, B. & McCaleb, M. L. (2000) Brain Res. 868, 79-87. [DOI] [PubMed] [Google Scholar]
- 22.Segal-Lieberman, G., Trombly, D. J., Juthani, V., Wang, X. & Maratos-Flier, E. (2003) Am. J. Physiol. 284, E1131-E1139. [DOI] [PubMed] [Google Scholar]
- 23.Ikeya, T., Galic, M., Belawat, P., Nairz, K. & Hafen, E. (2002) Curr. Biol. 12, 1293-1300. [DOI] [PubMed] [Google Scholar]
- 24.Kitamoto, T. (2002) Proc. Natl. Acad. Sci. USA 99, 13232-13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao, S., Lang, C., Levitan, E. S. & Deitcher, D. L. (2001) J. Neurobiol. 49, 159-172. [DOI] [PubMed] [Google Scholar]
- 26.Barcelo, H. & Stewart, M. J. (2002) Genesis 34, 83-85. [DOI] [PubMed] [Google Scholar]
- 27.Davis, G. W., DiAntonio, A., Petersen, S. A. & Goodman, C. S. (1998) Neuron 20, 305-315. [DOI] [PubMed] [Google Scholar]
- 28.Gao, X., Neufeld, T. P. & Pan, D. (2000) Dev. Biol. 221, 404-418. [DOI] [PubMed] [Google Scholar]
- 29.Leevers, S. J., Weinkove, D., MacDougall, L. K., Hafen, E. & Waterfield, M. D. (1996) EMBO J. 15, 6584-6594. [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, D. M. & Goodman, C. S. (1994) Neuron 13, 507-523. [DOI] [PubMed] [Google Scholar]
- 31.Torroja, L., Chu, H., Kotovsky, I. & White, K. (1999) Curr. Biol. 9, 489-492. [DOI] [PubMed] [Google Scholar]
- 32.Wen, T., Parrish, C. A., Xu, D., Wu, Q. & Shen, P. (2005) Proc. Natl. Acad. Sci. USA 102, 2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, G. (2002) Biol. Res. 35, 305-313. [DOI] [PubMed] [Google Scholar]
- 34.Kitamoto, T. (2002) J. Neurogenet. 16, 205-228. [DOI] [PubMed] [Google Scholar]
- 35.Saper, C. B., Chou, T. C. & Elmquist, J. K. (2002) Neuron 36, 199-211. [DOI] [PubMed] [Google Scholar]
- 36.Hajnal, A. & Norgren, R. (2001) Brain Res. 904, 76-84. [DOI] [PubMed] [Google Scholar]
- 37.Levine, A. S., Kotz, C. M. & Gosnell, B. A. (2003) J. Nutr. 133, 831S-834S. [DOI] [PubMed] [Google Scholar]
- 38.Martel, P. & Fantino, M. (1996) Pharmacol. Biochem. Behav. 53, 221-226. [DOI] [PubMed] [Google Scholar]
- 39.Larhammar, D. (1996) Regul. Pept. 62, 1-11. [DOI] [PubMed] [Google Scholar]
- 40.Brand, A. H. & Perrimon, N. (1993) Development (Cambridge, U.K.) 118, 401-415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.