Abstract
Redox signaling plays an important role in the positive regulation of angiogenesis by vascular endothelial growth factor, but its role in signal transduction by angiogenesis inhibitors is less clear. Using muscle explants in 3D culture, we found that explants from mice lacking the angiogenesis inhibitor thrombospondin-1 (TSP1) exhibit exaggerated angiogenic responses to an exogenous NO donor, which could be reversed by providing exogenous TSP1. To define the basis for inhibition by TSP1, we examined the effects of TSP1 on several proangiogenic responses of endothelial cells to NO. NO has a biphasic effect on endothelial cell proliferation. The positive effect at low doses of NO is sensitive to inhibition of cGMP signaling and picomolar concentrations of TSP1. NO stimulates both directed (chemotactic) and random (chemokinetic) motility of endothelial cells in a cGMP-dependent manner. TSP1 potently inhibits chemotaxis stimulated by NO. Low doses of NO also stimulate adhesion of endothelial cells on type I collagen in a cGMP-dependent manner. TSP1 potently inhibits this response both upstream and downstream of cGMP. NO-stimulated endothelial cell responses are inhibited by recombinant type 1 repeats of TSP1 and a CD36 agonist antibody but not by the N-terminal portion of TSP1, suggesting that CD36 or a related receptor mediates these effects. These results demonstrate a potent antagonism between TSP1 and proangiogenic signaling downstream of NO. Further elucidation of this inhibitory signaling pathway may identify new molecular targets to regulate pathological angiogenesis.
Keywords: angiogenesis, cell adhesion, chemotaxis, proliferation, CD36
Redox signaling plays a central role in both developmental and pathological angiogenesis (1). Low to moderate concentrations of NO act on endothelial cells to stimulate angiogenesis by activating soluble guanylyl cyclase (sGC), thereby increasing cGMP, which activates cGMP-dependent protein kinases (2) and other cGMP-dependent pathways (3, 4). These responses lead to downstream activation of the Ras-Raf-extracellular-regulated kinase (ERK) (5), vasodilator-stimulated phosphoprotein (6), and phosphatidylinositol 3-kinase-Akt pathways (7). At specific doses, NO donors induce endothelial cell chemotaxis (7, 8), permeability (9), and proliferation in vitro (10, 11) and angiogenesis in vivo (12).
Consistent with these observations, endogenous NO synthesis in endothelial cells is induced by some angiogenic factors, including vascular endothelial growth factor (VEGF) (13). VEGF receptor signaling activates Akt in a phosphatidylinositol 3-kinase- and PKC-dependent manner, which phosphorylates endothelial NO synthase (eNOS) on Ser-1177 (14, 15). This modification increases eNOS activity and NO synthesis. eNOS activation plays a crucial role in VEGF-induced angiogenesis and vascular permeability (16, 17). Conversely, NO induces VEGF transcription by inducing synthesis and stabilization of Hif-1α (18). These data suggest a positive feedback loop between NO and VEGF signaling. However, inhibitory effects of NO donors on endothelial growth, motility, adhesion, and survival have also been reported (8, 19), so this feedback signal may be complex.
Some angiogenesis inhibitors may also regulate angiogenesis at the level of NO signaling. TNP470 inhibits myristoylation of eNOS and redistributes eNOS from the plasma membrane (20). Endostatin increases activity of the phosphatase PP2A, which dephosphorylates eNOS at Ser-1177 and decreases NO synthesis (21).
Thrombospondin-1 (TSP1) is another well known angiogenesis inhibitor (reviewed in refs. 22 and 23). Several studies indicate that synthesis of TSP1 is regulated by NO signaling. Endothelial cell expression of TSP1 was induced by hypoxia or inhibition of NO synthesis by using N-nitro-l-arginine methyl ester (24). Subsequent studies in mesangial and smooth muscle cells verified that cGMP and cGMP-dependent protein kinase negatively regulate TSP1 gene expression (25, 26). It is less clear whether TSP1 can itself regulate NO signaling in endothelial cells. However, TSP1 was shown to modulate cGMP levels in monocytes (27) and melanoma cells (28). In melanoma cells, TSP1 induced a rapid, but transient, decrease in cGMP levels.
CD36 is one of several endothelial cell TSP1 receptors and is necessary for its activity to inhibit microvascular endothelial cell chemotaxis (29). CD36 is also a receptor for oxidized low-density lipoprotein (LDL), which inhibits VEGF-induced endothelial cell migration (30). Oxidized LDL inhibited Akt phosphorylation and VEGF-induced NO synthesis. TSP1 signaling through CD36 has been shown to regulate caspase-3 activation and p38 phosphorylation via a p59fyn-dependent pathway (31), but regulation of NO metabolism by TSP1 through this pathway has not been investigated.
To better define the potential cross talk between TSP1 and NO signaling, we have investigated the ability of both endogenous and exogenous TSP1 to regulate responses of endothelial cells to NO. We report here that endogenous TSP1 limits angiogenic responses to NO in a muscle explant assay. In vitro, TSP1 is a remarkably potent antagonist of NO-induced endothelial cell chemotaxis, adhesion, and proliferation. We further show that TSP1 antagonizes these cGMP-dependent endothelial responses to NO both upstream and downstream of cGMP.
Materials and Methods
Cells and Reagents. Human umbilical vein endothelial cells (HUVECs) (Cambrex, Walkersville, MD) were maintained in endothelial growth medium (EGM; Cambrex) and 5% FCS in 5% CO2 at 37°C. Cells were used at passages 4–8. Purity of cultures was monitored by immunochemical staining with monoclonal human anti-CD31 antibody (Sigma). Bovine aortic endothelial cells (BAECs) were cultured in DMEM with 5% FCS. Human dermal microvascular endothelial cells (HDMVECs; Cambrex) were maintained in DMEM with 2.5% FCS or EGM-2 MV (Cambrex) with 5% FCS and used within passages 4–9. 8-Bromo (8Br)-cGMP and 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) were obtained from Sigma. Diethylamine NONOate (DEA/NO) and diethyltriamine NONOate (DETA/NO) were kindly provided by Joseph Saavedra and Larry Keefer (National Cancer Institute, Frederick). TSP1 was prepared from human platelets obtained from the National Institutes of Health blood bank as described (32). Recombinant proteins expressed in insect cells containing the N-terminal region (NoC1) or the 3 type 1 repeats of TSP1 (3TSR) were generously provided by Deane Mosher (University of Wisconsin, Madison) and Jack Lawler (Harvard Medical School, Boston). Murine anti-human CD36 antibody (clone SMΦ) was purchased from Chemicon International (Temecula, CA). UO126 was from Cell Signaling Technology (Beverly, MA). Phosphodiesterase (PDE) inhibitors were from Calbiochem.
Animals. C57B16 WT and TSP1 null mice (33) were housed in a pathogen-free environment. Handling and care of animals was in compliance with the guidelines established by the Animal Care and Use Committee of the National Cancer Institute.
Muscle Explant Assay. Pectoralis major muscle biopsies were harvested from 8- to 10-week-old WT and TSP1 null mice and explanted in type I collagen as described (34). Explants were incubated in the presence of EGM with FCS and treatment agents for 7 days, and cell migration through the extracellular matrix was measured. Results represent the mean ± SD of at least three separate experiments.
Endothelial Cell Assays. Proliferation of endothelial cells was measured with a nonradioactive colorimetric assay (CellTiter 96, Promega) as described (35). Chemotaxis in modified Boyden chambers and adhesion assays were performed as described (35).
Intracellular cGMP Measurement. HUVECs (104 cells per well) grown overnight in 96-well culture plates were pretreated for 24 h with medium containing 2.5% FCS before treatment with NO donors and other agents in serum-free medium. Intracellular cGMP levels were determined by using an enzyme immunoassay (Amersham Pharmacia Biosciences).
Statistics. All studies were repeated at least three times, and results are presented as the mean ± SD, with analysis of significance done by using Student's t test and P < 0.05 taken as significant.
Additional methods are provided in Supporting Text, which is published as supporting information on the PNAS web site.
Results
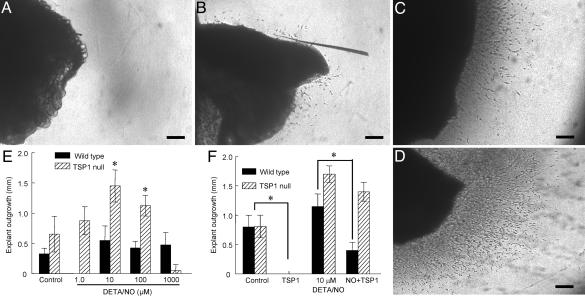
Endogenous TSP1 Inhibits NO-Stimulated Vascular Cell Outgrowth. To define the relationship between endogenous TSP1 and NO during the process of angiogenesis, an ex vivo model of neovessel formation was developed wherein biopsies of pectoralis major muscle from WT and TSP1 null mice were embedded in 3D type I collagen matrixes. As shown previously (34, 35), basal vascular outgrowth from TSP-1 null muscle explants was substantially faster than that from WT explants (Fig. 1 A and B). Addition of the NO donor DETA/NO significantly increased responses in the null explants but to a lesser extent in WT explants (Fig. 1 C–E). Maximal stimulation was obtained at 10 μM DETA/NO, but 1,000 μM DETA/NO inhibited cell invasion and tube formation to or below untreated levels. Addition of exogenous TSP1 abrogated basal outgrowth from null and WT explants and inhibited NO-driven outgrowth in WT more than in null explants (Fig. 1F). Thus, endogenous TSP1 antagonizes the angiogenic activity of NO in the explant assay.
Fig. 1.
Extracellular matrix invasion by vascular cells is modulated by NO and endogenous TSP1. (A–D) WT (A and C) and TSP1–/– (B and D) mouse muscle fragments were embedded in 3D collagen matrices and examined after 10 d either untreated (A and B) or treated with 100 μM DETA/NO (C and D). (Scale bars: 250 μm.) (E and F) Explants were treated with 1–1,000 μM DETA/NO (E) or 10 μM DETA/NO in the presence or absence of exogenous TSP1 (2.2 nM) (F). Vascular cell invasion of collagen matrices was quantified as the distance of farthest cell invasion from the muscle border in each of four quadrants. All experiments represent the mean ± SD of at least three separate experiments.
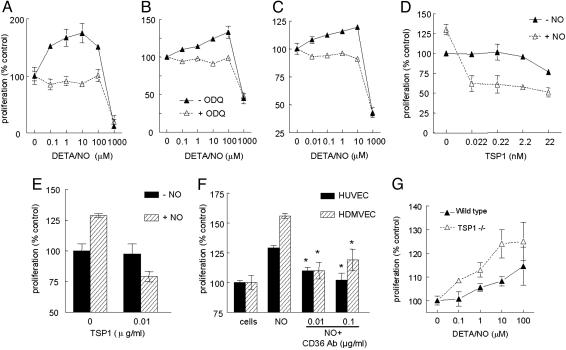
NO Is a Biphasic Modulator of Endothelial Cell Proliferation. BAECs showed a biphasic proliferative response to DETA/NO, with low doses promoting proliferation and high doses inhibiting proliferation (Fig. 2A). HUVECs (Fig. 2B) and HDMVECs (Fig. 2C) had similar biphasic proliferative responses to NO. The positive effects of NO may be less pronounced in the human cells because of the additional growth factors required in the media for these cells. The stimulatory effect of low NO doses may depend on cGMP signaling because treatment with NO donor in the presence of 10 μM ODQ, a specific inhibitor of sGC, abrogated NO-stimulated cell proliferation in all types of endothelial cells but had no effect on the inhibition of proliferation at the highest dose of NO (Fig. 2 A–C).
Fig. 2.
Biphasic effects of NO on proliferation of endothelial cells are modulated by exogenous and endogenous TSP1. (A) BAECs at 1,500 cells per well in DMEM supplemented with 2.5% FCS were incubated for 72 h in the presence of the indicated concentrations of DETA/NO with or without 10 μM ODQ. (B and C) HUVECs (B) or HDMVECs (C) were cultured in EGM or EGM-2MV, respectively, with 5% FCS and the indicated concentrations of DETA/NO with or without 10 μM ODQ. (D) BAECs were cultured with the indicated concentrations of TSP1 and 10 μM DETA/NO. (E and F) HDMVECs (E) and HUVECs (F) were incubated in the presence of TSP1 with or without DETA/NO or 10 μM DETA/NO with or without CD36 antibody (0.01–0.1 μg/ml). (G) Proliferation of WT and TSP1 null mouse lung endothelial cells was determined in the presence of the indicated concentrations of DETA/NO. Results represent the mean ± SD of three separate experiments.
TSP1 Inhibits NO-Stimulated Proliferation. TSP1 inhibits proliferation of endothelial cells, with reported IC50 values of 5–70 nM, depending on the growth medium (36–38). Consistent with those studies, 22 nM TSP1 was required to significantly inhibit BAEC proliferation stimulated by serum in the absence of NO donor, but proliferation stimulated by 10 μM DETA/NO was inhibited by 50% at <22 pM TSP1 (Fig. 2D). Comparable inhibition of NO-induced but not basal HDMVEC proliferation was observed at 22 pM TSP1 (Fig. 2E). The inhibitory responses to TSP1 in HUVECs suggested that the TSP1 receptor CD36, which is expressed selectively in microvascular cells, does not mediate this activity. However, the CD36 antibody SMΦ, an agonist of CD36 signaling (29), significantly inhibited NO-stimulated proliferation of both HUVECs and HDMVECs (Fig. 2F). Thus, the low-level expression of CD36 in HUVECs is sufficient for this response (39, 40).
To confirm that endogenous TSP1 levels are sufficient to explain the differences in NO responses shown in Fig. 1, proliferation of lung-derived endothelial cells from TSP1 null and WT mice was assessed in the presence of DETA/NO (Fig. 2G). Low-dose NO stimulated proliferation of TSP1 null cells significantly more than WT cells.
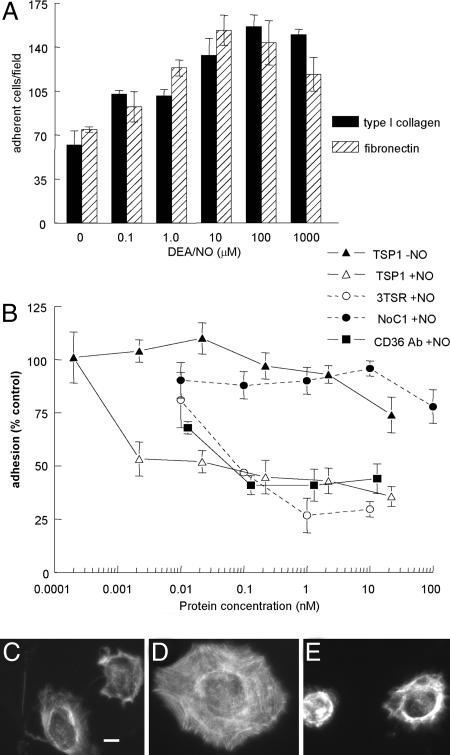
TSP1 Inhibits NO-Stimulated Endothelial Cell Adhesion. DEA/NO was used to investigate effects of NO on short-term adhesion assays due to its rapid release kinetics (t1/2 2–5 min; ref. 41). Adhesion of HUVECs on a type I collagen substrate was stimulated by low or intermediate doses of DEA/NO but inhibited above 100 μM (Fig. 3A). Cells also showed increased spreading and assembly of actin stress fibers at stimulatory NO doses (Fig. 3 C and D). As found for stimulation of proliferation, the stimulatory effect of NO on adhesion was blocked by 10 μM ODQ (Fig. 6A, which is published as supporting information on the PNAS web site), suggesting that both stimulatory responses occur via activation of sGC. Treatment of cells with decomposed DEA/NO did not increase matrix adhesion, verifying that NO, rather than a decomposition product, is responsible for its effects (Fig. 6B). Stimulation was also seen on a fibronectin substrate (Fig. 3A), showing that this response is not restricted to α2β1 integrin-mediated adhesion.
Fig. 3.
NO-stimulated endothelial cell adhesion to extracellular matrix is sGC-dependent and abrogated by TSP1. (A) HUVEC (2 × 105 cells per ml) attachment was determined on surfaces coated with type I collagen (5 μg/ml) or fibronectin (10 μg/ml). After incubating 1 h in the presence of indicated concentrations of DEA/NO, cells were fixed, stained, and counted. (B) Adhesion of DEA/NO-treated endothelial cells was analyzed in the presence of the indicated concentrations of exogenous TSP1, 3TSR, NoC1, and CD36 antibody with results normalized to maximum response under DEA/NO stimulation (10 μM). All results are presented as the mean ± SD of at least three separate experiments. (C–E) Untreated HUVECs (C) or HUVECs in the presence of 10 μM DEA/NO (D) or DEA/NO plus 220 pM TSP1 (E) were incubated on a collagen substrate for 60 min and then fixed and stained with phalloidin to visualize F actin. (Scale bar: 10 μm.)
Exogenous TSP1 at <2.2 pM abrogated the stimulation by NO of cell adhesion to collagen (Fig. 3B) and reversed the effect of NO on cell spreading (Fig. 3E and Fig. 7, which is published as supporting information on the PNAS web site). In contrast, 22 nM TSP1 only minimally inhibited basal adhesion on collagen. Inhibition by TSP1 was not caused by direct effects on NO autooxidation as assessed by two methods (Fig. 6 C and D).
An antiadhesive activity of TSP1 was previously localized to its N-domain (42), but 100 nM of NoC1 containing this sequence was required to significantly inhibit NO-stimulated HUVEC adhesion on type I collagen (Fig. 3B). The primary antiadhesive activity instead localized to the TSRs, which inhibited NO-stimulated adhesion with an IC50 of ≈100 pM (Fig. 3B). The lower activity of this monomeric fragment relative to intact TSP1 may be caused by trivalent presentation of this sequence in native TSP1. As found for native TSP1, 3TSR did not inhibit adhesion of unstimulated HUVECs on collagen.
CD36 is one of several cell surface receptors for the TSRs, and the CD36 agonist antibody inhibited NO-induced adhesion at concentrations comparable to TSP1 (Fig. 3B). Therefore, CD36 ligation is sufficient to inhibit NO-stimulated adhesion, suggesting that CD36 mediates the antiadhesive activities of TSP1 and 3TSR in the context of NO signaling.
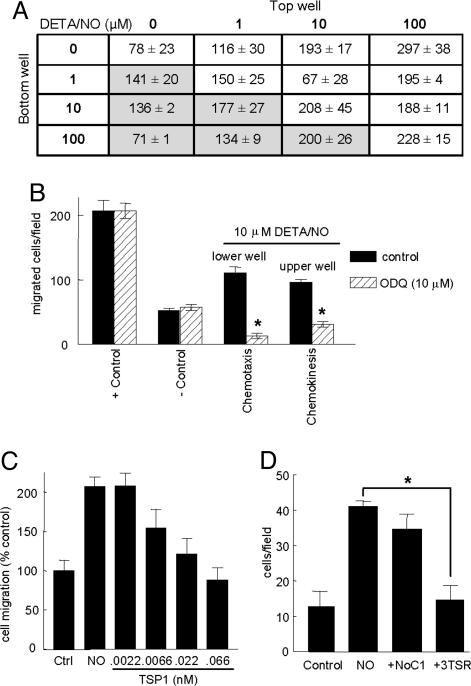
NO Is Both Chemotactic and Chemokinetic for Endothelial Cells. NO has been reported to either stimulate or inhibit endothelial cell migration across a gradient (chemotaxis) (7, 8). We observed a biphasic chemotactic response in HUVECs, with enhancement at low doses of NO and inhibition at high doses (Fig. 4A). Based on a checkerboard analysis, NO also stimulates endothelial cell motility in the absence of a gradient (chemokinesis, unshaded areas of Fig. 4A), which was maximal at dosages of NO donor 10-fold higher than those maximal for chemotaxis. Based on inhibition by ODQ, both motility responses of endothelial cells to NO depend on sGC (Fig. 4B). This inhibition was specific in that neither basal nor serum-stimulated chemotaxis (plus control) was inhibited by ODQ (Fig. 4B).
Fig. 4.
TSP1 inhibits endothelial cell migration to NO via its type 1 repeats. (A) HUVEC chemotaxis and chemokinesis were assessed in modified Boyden chambers. Migration was induced by DETA/NO at the indicated concentrations in the lower or upper chamber. Cells (0.3–0.5 × 105 per well) added to the upper chamber were allowed to migrate for 5.5 h at 37°C in 5% CO2. Results are presented as the number of cells migrated per field ± SD. (B) Cells were treated under chemotactic (10 μM DETA/NO in lower well) or chemokinetic conditions (100 μM in upper well) with or without 10 μM ODQ. (C) Chemotaxis of unstimulated cells (control) or cells toward 10 μM DEA/NO in the lower chamber was assessed in the presence of the indicated concentrations of TSP1 added in the upper chamber. (D) Cells were treated with 1 nM of the recombinant TSP1 fragments NoC1 and 3TSR, and cell migration to 10 μM DETA/NO was determined. Results are from at least three separate experiments.
The TSRs of TSP1 Inhibit NO-Stimulated Chemotaxis. TSP1 inhibited HUVEC chemotaxis stimulated by 10 μM DETA/NO with an IC50 of 7 pM (Fig. 4C). This inhibitory activity of TSP1 also localized to its TSRs, because NO-driven chemotaxis in HUVECs was inhibited to background levels by the recombinant 3TSR at 1 nM but not by the trimeric N-terminal fragment NoC1 at the same concentration (Fig. 4D).
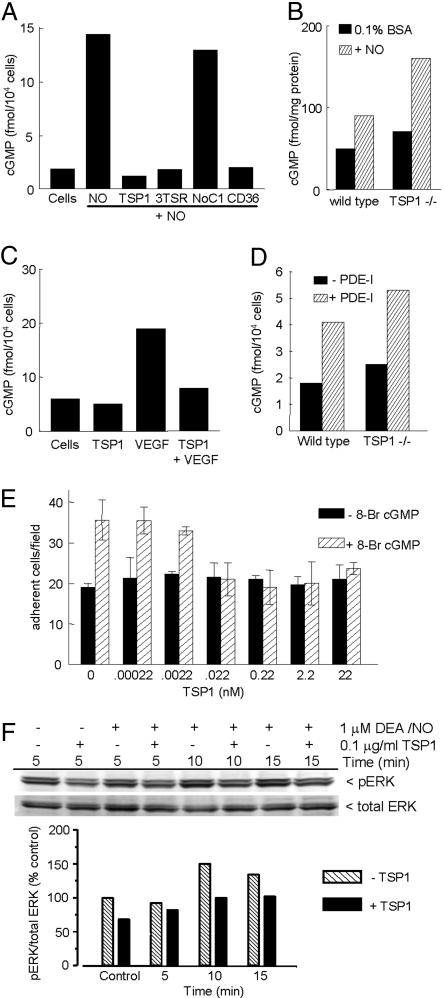
TSP1 Inhibits NO Signaling Upstream and Downstream of cGMP. The similar activities of TSP1 and ODQ to inhibit NO-stimulated endothelial cell responses, and previous reports that TSP1 modulates cGMP in other cell types (27, 28), suggested that TSP1 may inhibit angiogenic responses at the level of this second messenger. DEA/NO transiently induced cGMP levels in HUVECs that were maximal at 5–15 min and returned to near-basal levels by 30 min (Fig. 8, which is published as supporting information on the PNAS web site). Addition of 100 pM TSP1 prevented the NO-stimulated increase in cGMP (Fig. 5A). Similar inhibition of cGMP accumulation was observed in the presence of 3TSR or the agonist CD36 antibody (Fig. 5A), consistent with the inhibitory activities of these molecules for NO-stimulated responses. In contrast, NoC1 only minimally inhibited cGMP formation (Fig. 5A). Basal cGMP levels were moderately but consistently higher in TSP1 null endothelial cells, indicating that basal TSP1 is sufficient to regulate this pathway (Fig. 5B). Consistent with preferential enhancement of explant outgrowth by NO in the TSP1 nulls (Fig. 1), addition of DEA/NO enhanced cGMP to a greater extent in TSP1 null endothelial cells.
Fig. 5.
TSP1 inhibits NO-induced cGMP synthesis and signaling downstream of cGMP in endothelial cells. (A) HUVECs (5 × 103 per well) were weaned from EGM plus 5% FCS to 0.1% BSA as described in Supporting Text and treated with 10 μM DEA/NO for 5 min in the absence or presence of 100 pM of TSP1, 3TSR, NoC1, or CD36 antibody. Mean intracellular cGMP levels from duplicate determinations are presented for a representative experiment. (B) Intracellular cGMP was determined for weaned unstimulated TSP1 null and WT lung endothelial cells and cells treated for 5 min with 10 μM DEA/NO. (C) Intracellular cGMP was analyzed in weaned HUVECs after treatment with 1 μg/ml TSP1 with or without VEGF (30 ng/ml) for 10 min. (D) WT and TSP1 null lung-derived endothelial cells (5,000 cells per well) were incubated for 30 min in EGM plus 0.1% BSA with or without inhibitors of cGMP PDEs [PDE1 (25 μM 8-methoxymethyl-3-isobutyl-1-methylxanthine], PDE2 (20 nM erythro-9-[3-(2-hydroxynonyl)]adenine·HCl), and PDE5 [1 μM 4-{[3′,4′-(methylenedioxy)-benzyl}amoni)-6-methoxyquinazoline)], and intracellular cGMP levels were determined. (E) Modulation of endothelial cell adhesion to 5 μg/ml type I collagen was tested with 10 μM 8Br-cGMP stimulation with or without TSP1. Results are from three separate experiments. (F) HUVECs were harvested 0, 5, and 15 min after addition of 1 μM DEA/NO in medium containing 5% serum, brain extract, and heparin. Cell lysates were resolved on SDS gels and blotted with anti-ERK and phospho-ERK (pERK).
Because VEGF signaling stimulates sGC by increasing NO production (13–15), cGMP may be an intersection point between TSP1 signaling and this proangiogenic pathway. Consistent with these results, addition of TSP1 reversed the increase in cGMP stimulated by VEGF (Fig. 5C).
Because NO directly activates sGC, this enzyme may be a target of the TSP1/CD36 inhibitory signal. Alternatively, TSP1 may lower cGMP by activating a cGMP-PDE. In the latter case, inhibiting these PDEs should eliminate the distinction between WT and TSP1 null endothelial cells. Although a combination of PDE1, PDE3, and PDE5 inhibitors increased basal cGMP in WT cells as expected (Fig. 5D), cGMP in treated TSP1 null cells remained higher than in treated WT cells. Therefore, a PDE is unlikely to be the direct target of the TSP1 inhibitory signal.
sGC may not be the only target, however, because TSP1 also inhibited endothelial cell adhesion stimulated by 8Br-cGMP (Fig. 5E). The dose dependence for inhibition of 8Br-cGMP-stimulated adhesion resembled that for NO-stimulated adhesion. One target of cGMP signaling is the ERK cascade. Addition of TSP1 inhibited the transient induction of ERK phosphorylation by DEA/NO at 5 min (Fig. 5F). Therefore, TSP1 also acts downstream of cGMP to inhibit endothelial cell responses to NO, including adhesion and ERK phosphorylation.
Discussion
The present data rationalize apparently contradictory reports concerning the effects of NO on endothelial cell adhesion, chemotaxis, and proliferation. With different NO donors and assay conditions, stimulatory and inhibitory effects of NO donors on each response have been reported (e.g., ref. 8 and references therein). We observed biphasic dose responses to NO for each of these end-points, which we propose can be explained by different thresholds for specific NO-dependent signaling pathways, analogous to breast carcinoma cell responses to NO (41). The cGMP pathway is activated by the lowest doses of NO and mediates stimulatory effects of NO on adhesion, chemotaxis, and proliferation. In contrast, high-dose NO inhibits each response independent of sGC. TSP1 is a potent inhibitor of the former cGMP-dependent endothelial cell responses. cGMP signaling plays a central role in both acute responses to NO (5 min to 1 h) and long-term proliferation responses. TSP1 inhibits both NO-induced cGMP synthesis and signaling downstream of cGMP.
Consistent with a previous report (19), we find that inhibition of proliferation by high-dose NO is independent of cGMP signaling. By analogy to NO signaling in breast carcinoma cells (41), high-dose NO may inhibit these endothelial cell responses through activation of p53 and MKP1 expression (47). Further work is required to define these inhibitory signaling pathways.
NO donors are known chemotactic factors for endothelial cells (7, 8), but NO-stimulated chemokinesis has not been demonstrated previously to our knowledge. The chemokinetic activity of NO could be important to facilitate migration of endothelial cells in the absence of an NO gradient. Notably, the chemokinetic activity is maximal at ≈10-fold higher NO donor concentrations than are optimal for chemotactic migration. Both chemotaxis and chemokinesis require cGMP signaling, but a second signal initi-ated by higher concentrations of NO may also be required for chemokinesis.
The consistently high potency of TSP1 for inhibiting NO responses was unexpected. A comparable potency was reported for TSP1 to inhibit endothelial cell chemotaxis stimulated by fibroblast growth factor 2 (FGF2) (IC50 = 22 pM) (36), but others obtained a much higher IC50 of 700 pM (43). In contrast, 50% inhibition of endothelial cell proliferation stimulated by FGF2 typically requires 5–70 nM TSP1 (36–38). TSP1 inhibits endothelial cell adhesion, as assessed by focal adhesion disruption, with a reported IC50 of 2–4 nM (44). The corresponding NO-stimulated endothelial cell responses are much more sensitive to TSP1 inhibition. The markedly higher potency for inhibition of cGMP-dependent NO-stimulated responses may involve signaling pathways distinct from those previous identified for inhibition by TSP1 of the same endothelial cell responses driven by other agonists. Activation of p38 in endothelial cells downstream of CD36 required at least 5 nM TSP1 (31). Based on the sensitivity of both NO-stimulated cGMP synthesis and 8Br-cGMP-stimulated adhesion to pM doses of TSP1, this inhibitory signaling affects targets both upstream and downstream of cGMP. Because NO directly activates sGC, TSP1 signaling may inhibit sGC or prevent its activation by increasing cellular metabolism of NO, but does not selectively activate a cGMP PDE.
The high potency of TSP1 for inhibiting NO signaling may contribute to its pathophysiological role as a circulating angiogenesis inhibitor. Circulating plasma TSP1 levels were 0.3–15 nM in tumor-bearing animals (45), and mean values of 0.6–2.3 nM were reported in patients with colon carcinomas (46). These are clearly adequate concentrations to completely inhibit NO-stimulated endothelial responses.
An antiadhesive activity of TSP1 was previously mapped to a peptide, Hep1, derived from residues 17–35 of the N-module (42). However, we found a more potent antiadhesive activity for the TSRs of TSP1. Thus, TSP1 possesses two independent antiadhesive domains that use different signaling pathways. cGMP-dependent protein kinase activity is necessary but not sufficient for the antiadhesive activity of Hep1 (42). In contrast, TSP1 and its TSRs inhibit endothelial cell adhesion stimulated by the cGMP pathway.
TSP1 inhibits microvascular but not HUVEC chemotaxis stimulated by FGF2 (29). This differential activity was ascribed to selective expression of CD36 on microvascular endothelial cells (29). However, others have reported (39, 40) and we have confirmed low-level expression of CD36 in HUVECs. Because we see potent inhibition of NO-induced adhesion, proliferation, and cGMP accumulation in HUVECs by both TSRs and a CD36 agonist antibody, CD36 presumably mediates the corresponding responses to TSP1. However, we cannot exclude roles of other receptors that interact with TSRs, including β1 integrins (23), TGF-β, or proteoglycans.
Our data distinguish TSP1 from the angiogenesis inhibitors endostatin and TNP470 with respect to the mechanism by which it modulates NO signaling. Endostatin and TNP470 both regulate eNOS (20, 21), which is upstream of the two TSP1-sensitive steps that we identified in this signaling cascade. Our evidence that TSP1 inhibits at least two targets downstream of eNOS implicates the eNOS–sGC–cGMP pathway as a convergence point for proangiogenic and antiangiogenic signaling. Further definition of the targets and mechanism of inhibition by TSP1 signaling may reveal new therapeutic targets to modulate pathological angiogenic responses.
Supplementary Material
Acknowledgments
We thank Drs. Larry Keefer, Jack Lawler, and Deane Mosher for providing reagents and mice. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Author contributions: J.S.I., M.G.E., D.A.W., and D.D.R. designed research; J.S.I., L.A.R., E.M.P., and M.G.E. performed research; J.S.I., L.A.R., E.M.P., M.G.E., D.A.W., and D.D.R. analyzed data; and J.S.I., L.A.R., E.M.P., D.A.W., and D.D.R. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BAEC, bovine aortic endothelial cell; DETA/NO, diethyltriamine NONOate; DEA/NO, diethylamine NONOate; EGM, endothelial growth medium; eNOS, endothelial NO synthase; HDMVEC, human dermal microvascular endothelial cell; HUVEC, human umbilical vein endothelial cell; ODQ, 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one; PDE, phosphodiesterase; sGC, soluble guanylyl cyclase; TSP1, thrombospondin-1; TSR, TSP1 repeat; VEGF, vascular endothelial growth factor; 8Br, 8-bromo; ERK, extracellular-regulated kinase.
References
- 1.Maulik, N. (2002) Antioxid. Redox Signal. 4, 783–784. [DOI] [PubMed] [Google Scholar]
- 2.Morbidelli, L., Donnini, S., Chillemi, F., Giachetti, A. & Ziche, M. (2003) Clin. Cancer Res. 9, 5358–5369. [PubMed] [Google Scholar]
- 3.Zaragoza, C., Soria, E., Lopez, E., Browning, D., Balbin, M., Lopez-Otin, C. & Lamas, S. (2002) Mol. Pharmacol. 62, 927–935. [DOI] [PubMed] [Google Scholar]
- 4.Raj, U. & Shimoda, L. (2002) Am. J. Physiol. 283, L671–L677. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira, C. J., Schindler, F., Ventura, A. M., Morais, M. S., Arai, R. J., Debbas, V., Stern, A. & Monteiro, H. P. (2003) Free Radical Biol. Med. 35, 381–396. [DOI] [PubMed] [Google Scholar]
- 6.Smolenski, A., Poller, W., Walter, U. & Lohmann, S. M. (2000) J. Biol. Chem. 275, 25723–25732. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki, K., Smith, R. S., Jr., Hsieh, C. M., Sun, J., Chao, J. & Liao, J. K. (2003) Mol. Cell. Biol. 23, 5726–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, M. K., Tsugawa, K., Tarnawski, A. S. & Baatar, D. (2004) Biochem. Biophys. Res. Commun. 318, 520–528. [DOI] [PubMed] [Google Scholar]
- 9.Breslin, J. W., Pappas, P. J., Cerveira, J. J., Hobson, R. W., II, & Duran, W. N. (2003) Am. J. Physiol. 284, H92–H100. [DOI] [PubMed] [Google Scholar]
- 10.Hood, J. & Granger, H. J. (1998) J. Biol. Chem. 273, 23504–23508. [DOI] [PubMed] [Google Scholar]
- 11.Meininger, C. J. & Wu, G. (2002) Methods Enzymol. 352, 280–295. [DOI] [PubMed] [Google Scholar]
- 12.Fulton, D., Gratton, J. P. & Sessa, W. C. (2001) J. Pharmacol. Exp. Ther. 299, 818–824. [PubMed] [Google Scholar]
- 13.Papapetropoulos, A., Garcia-Cardena, G., Madri, J. A. & Sessa, W. C. (1997) J. Clin. Invest. 100, 3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton, D., Gratton, J. P., McCabe, T. J., Fontana, J., Fujio, Y., Walsh, K., Franke, T. F., Papapetropoulos, A. & Sessa, W. C. (1999) Nature 399, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimmeler, S., Fleming, I., Fisslthaler, B., Hermann, C., Busse, R. & Zeiher, A. M. (1999) Nature 399, 601–605. [DOI] [PubMed] [Google Scholar]
- 16.Murohara, T., Asahara, T., Silver, M., Bauters, C., Masuda, H., Kalka, C., Kearney, M., Chen, D., Symes, J. F., Fishman, M. C., et al. (1998) J. Clin. Invest. 101, 2567–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukumura, D., Gohongi, T., Kadambi, A., Izumi, Y., Ang, J., Yun, C. O., Buerk, D. G., Huang, P. L. & Jain, R. K. (2001) Proc. Natl. Acad. Sci. USA 98, 2604–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasuno, K., Takabuchi, S., Fukuda, K., Kizaka-Kondoh, S., Yodoi, J., Adachi, T., Semenza, G. L. & Hirota, K. (2004) J. Biol. Chem. 279, 2550–2558. [DOI] [PubMed] [Google Scholar]
- 19.Heller, R., Polack, T., Grabner, R. & Till, U. (1999) Atherosclerosis 144, 49–57. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida, T., Kaneko, Y., Tsukamoto, A., Han, K., Ichinose, M. & Kimura, S. (1998) Cancer Res. 58, 3751–3756. [PubMed] [Google Scholar]
- 21.Urbich, C., Reissner, A., Chavakis, E., Dernbach, E., Haendeler, J., Fleming, I., Zeiher, A. M., Kaszkin, M. & Dimmeler, S. (2002) FASEB J. 16, 706–708. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong, L. C. & Bornstein, P. (2003) Matrix Biol. 22, 63–71. [DOI] [PubMed] [Google Scholar]
- 23.Calzada, M. J. & Roberts, D. D. (2005) Curr. Pharm. Des. 11, 849–866. [DOI] [PubMed] [Google Scholar]
- 24.Phelan, M. W., Forman, L. W., Perrine, S. P. & Faller, D. V. (1998) J. Lab. Clin. Med. 132, 519–529. [DOI] [PubMed] [Google Scholar]
- 25.Dey, N. B., Boerth, N. J., Murphy-Ullrich, J. E., Chang, P. L., Prince, C. W. & Lincoln, T. M. (1998) Circ. Res. 82, 139–146. [DOI] [PubMed] [Google Scholar]
- 26.Wang, S., Skorczewski, J., Feng, X., Mei, L. & Murphy-Ullrich, J. E. (2004) J. Biol. Chem. 279, 34311–34322. [DOI] [PubMed] [Google Scholar]
- 27.Suchard, S. J. & Mansfield, P. J. (1996) J. Cell Physiol. 168, 217–227. [DOI] [PubMed] [Google Scholar]
- 28.Guo, N., Zabrenetzky, V. S., Chandrasekaran, L., Sipes, J. M., Lawler, J., Krutzsch, H. C. & Roberts, D. D. (1998) Cancer Res. 58, 3154–3162. [PubMed] [Google Scholar]
- 29.Dawson, D. W., Pearce, S. F., Zhong, R., Silverstein, R. L., Frazier, W. A. & Bouck, N. P. (1997) J. Cell Biol. 138, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavakis, E., Dernbach, E., Hermann, C., Mondorf, U. F., Zeiher, A. M. & Dimmeler, S. (2001) Circulation 103, 2102–2107. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez, B., Volpert, O. V., Crawford, S. E., Febbraio, M., Silverstein, R. L. & Bouck, N. (2000) Nat. Med. 6, 41–48. [DOI] [PubMed] [Google Scholar]
- 32.Roberts, D. D., Cashel, J. & Guo, N. (1994) J. Tissue Cult. Methods 16, 217–222. [Google Scholar]
- 33.Lawler, J., Sunday, M., Thibert, V., Duquette, M., George, E. L., Rayburn, H. & Hynes, R. O. (1998) J. Clin. Invest. 101, 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isenberg, J. S., Calzada, M. J., Zhou, L., Guo, N., Lawler, J., Wang, X. Q., Frazier, W. A. & Roberts, D. D. (2005) Matrix Biol. 24, 110–123. [DOI] [PubMed] [Google Scholar]
- 35.Calzada, M. J., Zhou, L., Sipes, J. M., Zhang, J., Krutzsch, H. C., Iruela-Arispe, M. L., Annis, D. S., Mosher, D. F. & Roberts, D. D. (2004) Circ. Res. 94, 462–470. [DOI] [PubMed] [Google Scholar]
- 36.Taraboletti, G., Roberts, D., Liotta, L. A. & Giavazzi, R. (1990) J. Cell Biol. 111, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel, T., Guo, N. H., Krutzsch, H. C., Blake, D. A., Hartman, J., Mendelovitz, S., Panet, A. & Roberts, D. D. (1993) J. Cell Biochem. 53, 74–84. [DOI] [PubMed] [Google Scholar]
- 38.Guo, N. H., Krutzsch, H. C., Inman, J. K., Shannon, C. S. & Roberts, D. D. (1997) J. Pept. Res. 50, 210–221. [DOI] [PubMed] [Google Scholar]
- 39.Liu, Y., Zhu, Y., Rannou, F., Lee, T. S., Formentin, K., Zeng, L., Yuan, X., Wang, N., Chien, S., Forman, B. M. & Shyy, J. Y. (2004) Circulation 110, 1128–1133. [DOI] [PubMed] [Google Scholar]
- 40.Kopprasch, S., Pietzsch, J., Westendorf, T., Kruse, H. J. & Grassler, J. (2004) Int. J. Biochem. Cell Biol. 36, 460–471. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, D. D., Espey, M. G., Ridnour, L. A., Hofseth, L. J., Mancardi, D., Harris, C. C. & Wink, D. A. (2004) Proc. Natl. Acad. Sci. USA 101, 8894–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy-Ullrich, J. E., Pallero, M. A., Boerth, N., Greenwood, J. A., Lincoln, T. M. & Cornwell, T. L. (1996) J. Cell Sci. 109, 2499–2508. [DOI] [PubMed] [Google Scholar]
- 43.Tolsma, S. S., Volpert, O. V., Good, D. J., Frazier, W. A., Polverini, P. J. & Bouck, N. (1993) J. Cell Biol. 122, 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy-Ullrich, J. E. & Höök, M. (1989) J. Cell Biol. 109, 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volpert, O. V., Lawler, J. & Bouck, N. P. (1998) Proc. Natl. Acad. Sci. USA 95, 6343–6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita, Y., Kurohiji, T., Tuszynski, G. P., Sakai, T. & Shirakusa, T. (1998) Cancer 82, 632–638. [DOI] [PubMed] [Google Scholar]
- 47.Ridnour, L. A., Isenberg, J. S., Espey, M. G., Thomas, D. D., Roberts, D. D. & Wink, D. A. (2005) Proc. Natl. Acad. Sci. USA 102, 13147–13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.