Abstract
Nitric oxide (NO) donors have been shown to stimulate and inhibit the proliferation, migration, and differentiation of endothelial cells in vitro and angiogenesis in vivo. Recently, we have shown distinct thresholds for NO to regulate p53-Ser-15P, phosphorylated extracellular signal-regulated kinase (pERK), and hypoxia inducible factor 1α in tumor cells. Because these signaling pathways also promote the growth and survival of endothelial cells, we examined their roles in angiogenic responses of venous endothelial cells and vascular outgrowth of muscle explants elicited by NO. An additional protein involved in the regulation of angiogenesis is thrombospondin-1 (TSP1), a matricellular glycoprotein known to influence adhesion, migration, and proliferation of endothelial cells. Here we demonstrate a triphasic regulation of TSP1 mediated by a slow and prolonged release of NO that depends on ERK phosphorylation. Under conditions of 5% serum, a 24-h exposure of NO donor (0.1–1,000 μM) mediated a triphasic response in the expression of TSP1 protein: decreasing at 0.1 μM, rebounding at 100 μM, and decreasing again at 1,000 μM. Under the same conditions, we observed a dose-dependent increase in P53 phosphorylation and inverse biphasic responses of pERK and mitogen-activated protein kinase phosphatase-1. Both the growth-stimulating activity of low-dose NO for endothelial cells and suppression of TSP1 expression were ERK-dependent. Conversely, exogenous TSP1 suppressed NO-mediated pERK. These results suggest that dose-dependent positive- and negative-feedback loops exist between NO and TSP1. Limiting TSP1 expression by positive feedback through the ERK mitogen-activated protein kinase pathway may facilitate switching to a proangiogenic state at low doses of NO.
Keywords: guanyl cyclase, endothelial
Since its discovery, nitric oxide (NO) has acquired a reputation as both friend and foe (1). To assimilate this discordant information, it is important to consider cell/tissue specificity, redox conditions, NO concentration, as well as the duration of exposure associated with a specific response. An important question in NO research involves its role during specific stages of cancer progression (2–6). A key aspect of cancer progression as well as treatment involves the regulation of angiogenesis, which entails the induction of endothelial cell proliferation, migration, and differentiation, culminating in the sprouting of new capillaries from existing vasculature (7, 8). In addition to providing blood flow and nutrients to the tumor, angiogenesis is also involved in tumor progression and metastasis because the vasculature provides the tumor with access to distant organs, and enhanced vascularization is a marker of advanced tumors (8).
One of the many physiological functions of NO is as an important modulator of endothelial function pertaining to angiogenesis (9). Although low concentrations of NO induce an angiogenic response, high concentrations are inhibitory (10). NO mediates the proangiogenic response of several key factors including VEGF, angiopoetin-2, and estrogen (11–13). These factors mediate their effects by the phosphorylation of endothelial NO synthase (eNOS) at Ser-1179, which provides a sustained flux of NO through overcoming its calmodulin dependence and is associated with proangiogenic responses (14). Moreover, antiangiogenic agents such as endostatin and somatostatin mediate their effects through the activation of PP2A phosphatase, which is involved in the dephosphorylation of eNOS (15–17). These reports implicate eNOS and its regulation via phosphorylation/dephosphorylation events as a key modulator in angiogenesis (18, 19).
Recent studies in our laboratory have identified discrete threshold concentrations of NO required for stabilizing signaling proteins important in tumor biology (20). Low concentrations (<50 nM) of NO transiently induced extracellular signal-regulated kinase (ERK) phosphorylation, which required cGMP and is prosurvival, whereas intermediate levels (>100 nM) of NO stabilized the proangiogenic transcription factor hypoxia inducible factor 1α (HIF1α). Because angiogenesis is tightly controlled by a balance between pro- and antiangiogenic factors (21), and NO is clearly involved in the induction of proangiogenic molecules, we were interested in the elucidation of possible regulatory effects of NO on antiangiogenic proteins. Toward this end, we have identified an enhanced angiogenic response elicited by NO in explanted tissue from thrombospondin-1 (TSP1) null animals, when compared with WT controls (10). TSPs are potent antiangiogenic matricellular proteins that exert diverse effects on several angiogenic cell responses, including cell proliferation, adhesion, migration, and survival. Also, TSPs have been found to contribute to immune response and exert tumor suppressor effects (22, 23). All of these effects arise from the interaction of the whole protein or its domains with various cell surface receptors. Using the NO donor diethyltriamine NONOate (DETA/NO), which releases NO at a slow rate over a prolonged period (t1/2 ∼ 20 h), we demonstrate an NO-mediated triphasic regulatory effect on the levels of TSP1 secreted from human umbilical vein endothelial cells (HUVECs). Potent down-regulation of TSP1 by NO occurred at donor concentrations as low as 0.1 μM and was followed by an apparent reaccumulation as the donor dose increased to 100 μM. TSP1 reaccumulation paralleled increases in p53-Ser-15P as well as the induction of mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) and down-regulation of phosphorylated ERK (pERK). In addition, the NO-mediated down-regulation of TSP1 as well as the proliferative response of HUVECs was suppressed by the inhibition of ERK phosphorylation. These results implicate pERK as a mediator of regulatory effects of TSP1 during NO-induced angiogenic response of HUVECs.
Materials and Methods
HUVECs and endothelial growth media (EGM) media bullet kits were purchased from Cambrex Biotechnology (Walkersville, MD). Antiserum recognizing denatured TSP1 was prepared by immunizing rabbits with reduced and alkylated TSP1. HIF1α mAb was purchased from Transduction Laboratories (Lexington, KY) polyclonal Abs recognizing pERK, p53-Ser-15P were obtained from Cell Signaling Technology (Beverly, MA), and monoclonal actin and polyclonal MKP-1 Abs were purchased from Santa Cruz Biotechnology. ECF Western blotting kits and Hybond P membrane were purchased from Amersham Pharmacia. U0126 MAP kinase/ERK kinase [1/2] (MEK[1/2]) inhibitor was from Cell Signaling Technology, and the guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) was purchased from Sigma. Fluorescent immunoreactive proteins were visualized on a Typhoon 8600 variable mode imager (Molecular Dynamics, Piscataway, NJ). Equal loading was verified by Coomassie-stained gels. Protein levels were quantified by using imagequant software and normalized to actin.
Cell Culture. HUVECs were routinely cultured in EGM media supplemented with 5% FBS, epidermal growth factor, bovine brain extract, cortisone, gentamycin, penicillin, and streptomycin and maintained at 37°C in an atmosphere of 5% CO2 and room air. For experimental purposes, the cells were trypsinized and plated at a density of one million cells per 100-mm tissue culture dish and grown for 72 h. The cells were then washed with PBS and incubated in phenol red-free EGM media containing 5% FBS, bovine brain extract, heparin, and penicillin streptomycin and exposed to the NO donor DETA/NO (0.1–1,000 μM) for times ranging from 15 min to 24 h in the presence and absence of 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one, U0126, or exogenous TSP1. The cells were harvested in lysis buffer containing protease and phosphatase inhibitors and stored at –70°C until further use.
Animals. C57B16 mice were housed five per cage in a pathogen-free environment. Handling and care of animals was in compliance with the guidelines established by the Animal Care and Use Committee of the National Cancer Institute.
Muscle Explant Assay. Pectoralis major muscle biopsies were harvested from 8- to 10-wk-old C57B16 mice and explanted in type I collagen as described (24). Explants were incubated in EGM media containing FBS and treatment agents for 7 days. Migration of cells through the extracellular matrix was then measured. Results are presented as mean ± SD, n ≥ 3.
Cell Proliferation. Proliferation of endothelial cells was quantified by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide colorimetric assay as described (25). Results are presented as mean ± SD, n = 3.
Concentration of TSP1 Protein from Media. Media was collected from control and treated cells and centrifuged at 180 × g to remove floating cells and then concentrated on heparin columns. In brief, the columns were prepared in borosilicate Pasteur pipettes packed with glass wool and 400 μl of heparin-agarose beads (Sigma). The columns were washed with 1 ml of affinity column buffer (10 mM Tris/150 mM NaCl/1 mM CaCl2, pH 7.5), conditioned with 1 ml of the same buffer containing 1% BSA, 1 mM N-ethylmaleimide, 1 mM PMSF, and protease inhibitor mixture (Calbiochem), and then washed again. All media samples were pH-adjusted to 7.5 and applied to the columns, and the TSP1 was eluted with 0.6 M NaCl. Protein concentrations of the conditioned media were measured spectrophotometrically at 280 nm.
Real-Time PCR. Control and NO-treated HUVECs were harvested in TRIzol reagent, and RNA was extracted by chloroform extraction. cDNA was prepared and real-time PCR was performed as described (26).
Western Blotting. Heat-denatured protein (40 μg) from cell lysates or 0.5–1 μg of protein from conditioned media was electrophoresed on 4–20% SDS-polyacrylamide gels and then transferred onto Hybond P membranes. Transferred protein was blocked overnight in 5% milk and then incubated with the desired primary Abs followed by FITC-conjugated secondary Abs. The blots were washed and exposed to ECF substrate. Fluorescent immunoreactive protein was visualized on a Typhoon 8600 variable mode imager. Equal loading was verified by Coomassie-stained gel, and protein levels were quantified by using imagequant software and normalized to actin.
Results
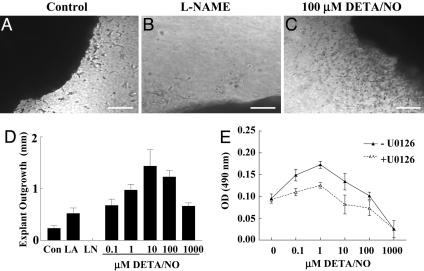
To investigate the signaling pathways associated with NO-mediated regulation of angiogenesis, an NO-induced proangiogenic response was first characterized in a tissue explant model to demonstrate the effect of NO on vascular cell outgrowth. The NO-releasing compound DETA/NO, which releases NO at a slower rate over a prolonged period (t1/2 ∼ 24 h), was used. Enhanced cellular outgrowth away from the tissue border is clearly seen when comparing control (Fig. 1A) with tissue exposed to 100 μM DETA/NO (Fig. 1C). Conversely, outgrowth was undetectable in tissue treated with the NOS inhibitor N-nitro-l-Arg methyl ester (l-NAME) (Fig. 1 B and D). DETA/NO had a biphasic effect on outgrowth (Fig. 1D), because low doses (0.1 μM) enhanced the outgrowth distance, which peaked at 10 μM and then began to decrease as the concentration approached 1,000 μM. Moreover, when compared with control, the migration distance of tissue supplemented with l-Arg, to stimulate endogenous production of NO by eNOS, was similar to that of tissue treated with 0.1 μM DETA/NO (Fig. 1D), suggesting that this dose of DETA/NO produces concentrations similar to those generated endogenously by eNOS in this model. Consistent with the explant responses, NO induced a biphasic proangiogenic response in HUVECs under full growth conditions (EGM media supplemented with 5% FBS, bovine brain extract, heparin, and penicillin streptomycin) to mimic a cancer and/or wound-healing environment (27) (Fig. 1E). NO-induced HUVEC proliferation at doses as low as 0.1 μM, which peaked at 1 μM and then decreased toward control levels as the concentration approached 100 μM, whereas doses at 1,000 μM failed to demonstrate a proliferative response, most likely due to growth arrest and/or cell death.
Fig. 1.
NO-mediated proangiogenic response as characterized by vascular cell outgrowth and endothelial cell proliferation. C57B16 WT mouse muscle fragments were embedded in 3D collagen matrices and exposed to control, 1 mM l-Arg, 500 μM l-NAME, or 0.1–1,000 μM DETA/NO and migration distance evaluated. [Bar, 500 μm(A–C).] (D) Vascular cell invasion of collagen matrices was quantified in each of four quadrants as the distance of farthest cell invasion from the muscle border in response to l-Arg (LA), l-NAME (LN), or DETA/NO. Results are presented as mean ± SD, n ≥ 3. (E) Proliferative effects of chronic exposure to NO were determined in HUVECs exposed to 0.1–1,000 μM DETA/NO in the presence and absence of the MEK[1/2] inhibitor U0126. Proliferation was quantified by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay, and results are presented as mean ± SD, n ≥ 3.
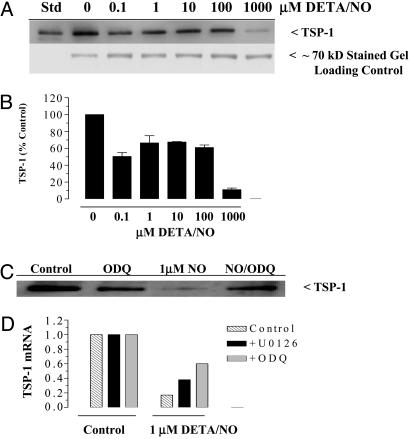
The complex responses of HUVEC and explant cultures to NO suggest that both pro-and antiangiogenic signaling pathways are regulated downstream of NO. TSP1 is a potent antiangiogenic factor known to regulate both cell migration and proliferation (22, 23). Moreover, the companion paper (10) shows an enhanced NO-mediated proangiogenic response in TSP1-null animals when compared with WT control and a potent activity of TSP1 to inhibit NO-induced angiogenic response. Also, NO was previously shown to down-regulate TSP1 in a cGMP-dependent manner in mesangial cells (28), suggesting that NO may similarly regulate TSP1 expression in endothelial cells. Toward this end, Fig. 2 A and B demonstrates an NO-mediated triphasic effect on the levels of TSP1 secreted into the media by HUVECs. At 0.1 μM DETA/NO, secreted TSP1 levels decreased ≈50%, which returned to ≈75% of control as DETA/NO doses increased up to 100 μM and then fell to barely detectable levels at 1,000 μM DETA/NO. The low-dose NO-mediated reduction in TSP1 protein was apparent by 8–10 h of exposure to DETA/NO (data not shown) and was cGMP-dependent (Fig. 2C). In addition, cGMP-dependent NO down-regulation of TSP1 occurred at the level of mRNA and was suppressed by U0126 as well (Fig. 2D). These results demonstrate that proangiogenic low doses of NO function, at least in part, by down-regulation of TSP1 mRNA and protein in a manner involving both cGMP and pERK.
Fig. 2.
NO-mediated down-regulation of the antiangiogenic factor TSP1. HUVECs were plated as described in Materials and Methods and grown to 70% confluence (≈2 × 106 cells). (A and C) Cells were exposed to 0.1–1,000 μM DETA/NO for 24 h and the sample media concentrated on heparin columns as described in Materials and Methods and then immunoblotted for TSP1 levels vs. μM DETA/NO. (B) Quantification of the protein bands in A. (D) Real-time PCR was performed on cDNA prepared from cells exposed to 1 μM DETA/NO for 2 h.
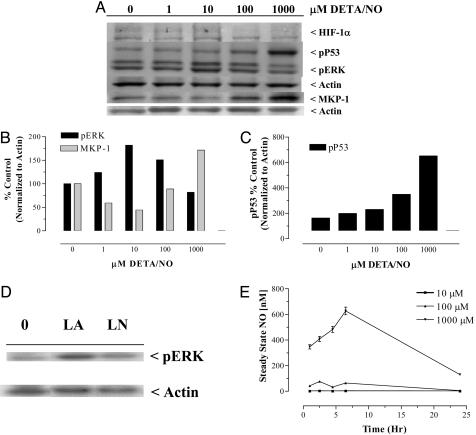
Our laboratory has shown (20) distinct threshold effects of NO on HIF1α stabilization as well as both p53-Ser-15P and ERK phosphorylation in MCF-7 breast cancer cells. We were interested in the roles of these signaling proteins, as well as MKP-1 phosphatase, another regulatory enzyme in the MAP kinase pathway, in NO regulation of TSP1. Although NO released by DETA/NO had no effect on HIF1α in this model, the levels of p53-Ser-15P increased in a dose-response manner with the highest induction (≈6-fold above control) occurring at 1,000 μM DETA/NO (Fig. 3 A and C). The enhanced levels of p53-Ser-15P exhibited at 1–100 μM DETA/NO may be associated with the modest reaccumulation of TSP1 occurring at the same doses (Fig. 2 A and B), which is consistent with the known positive regulation of TSP1 by P53 (23, 29–31). Steady-state nanomolar levels of NO associated with dose-response concentrations of DETA/NO were determined by using a NO analyzer and compared with a standard curve generated by the reduction of NO2– and are shown in Fig. 3E. Although the steady-state levels (≈500 nM) from 1,000 μM DETA/NO in this study were similar to previously reported values (20), 100 μM donor concentrations corresponded to ≈57 nM NO, and 10 μM DETA/NO yielded ≈2 nM, which is near the limit of detection of the instrument. These results suggest that the proangiogenic effects associated with chronic low fluxes of NO generated by 1–10 μM DETA/NO correspond to steady-state levels of NO, which remarkably are at or below 2 nM.
Fig. 3.
NO modulation of molecular target proteins in HUVECs. (A) Representative immunoblot of protein from cells exposed to 1–1,000 μM DETA/NO for 24 h (n = 3). (B and C) Quantification of the NO-induced changes in target protein levels shown in A. (D) Modulation of the levels of pERK by endogenous NO after a 24-h exposure of HUVECs to 1 mM l-Arg or 5 mM l-NAME. (E) Steady-state nanomolar levels of NO generated by 10–1,000 μM DETA/NO in treatments media.
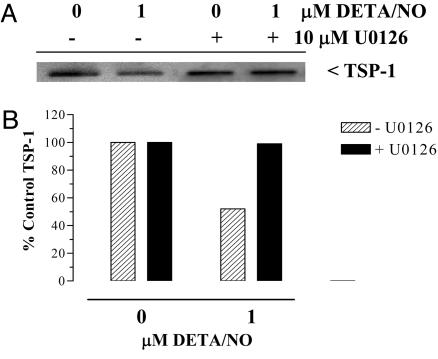
Modulation of MAP kinase signaling was also observed in HUVECs after exposure to DETA/NO. pERK and MKP-1 demonstrated inverse biphasic regulatory responses to increasing doses of DETA/NO. When compared with the untreated control, pERK increased maximally at 10 μM DETA/NO, whereas MKP-1 levels decreased at 1–10 μM DETA/NO and then rebounded at 100 and 1,000 μM DETA/NO (Fig. 3 A and B). Similar increases in MKP-1 have been observed in human breast cancer cells (32). This pattern of enhanced pERK correlates with the proangiogenic response at low doses of NO, as shown in Fig. 1E, as inhibition of pERK by U0126 suppressed NO-induced angiogenic response. Time course studies demonstrated a transient pattern of ERK phosphorylation, which occurred at 4 and 10 h of exposure to DETA/NO (data not shown). Interestingly, the reduction in detectable levels of secreted TSP1 was apparent by 8–10 h of exposure to DETA/NO (data not shown). To test the involvement of pERK in the effect of NO on TSP1 regulation, HUVECs were treated with 1 μM DETA/NO for 10 h in the presence and absence of the MEK[1/2] inhibitor U0126. Inhibition of pERK abolished NO-induced down-regulation of TSP1 (Fig. 4).
Fig. 4.
Inhibition of ERK phosphorylation reverses NO-mediated down-regulation of TSP1. (A) Cells were plated as described in Fig. 1A and exposed to 1 μM DETA/NO for 10 h in the presence and absence of the MEK[1/2] inhibitor U0126. The sample media were concentrated and immunoblotted for TSP1 levels as described in the Fig. 2 legend. Data are representative of two experiments. (B) Quantification of the immunoblot shown in A.
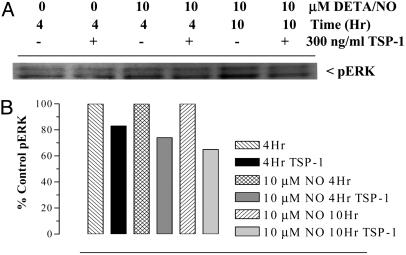
Because NO-mediated TSP1 down-regulation was suppressed by inhibiting pERK, and because TSP1 inhibits NO-induced angiogenic response (10), we examined the possible involvement of TSP1 in NO-induced ERK phosphorylation. In this experiment, HUVECs were exposed to 10 μM DETA/NO in the presence and absence of exogenous TSP1 (300 ng/ml), and the cells were harvested at 4 and 10 h of NO to evaluate pERK levels. Fig. 5 shows suppressive effects of exogenous TSP1 on the levels of NO-induced pERK. Similar suppressive effects of exogenous TSP1 on NO-induced ERK phosphorylation by the NO donor DEA/NO were also reported in the companion manuscript (10). Because NO-induced proliferation of HUVECs was suppressed by U0126 (Fig. 1E), these results demonstrate bidirectional crosstalk between NO and TSP1, which involves at least in part ERK phosphorylation and modulation of the MAP kinase pathway.
Fig. 5.
Exogenous TSP1 suppresses NO-mediated ERK phosphorylation. (A) Cells were exposed to 10 μM DETA/NO for 4 and 10 h in the presence and absence of 300 ng/ml TSP1 and then immunoblotted for pERK. (B) Quantification of immunoreactive protein levels demonstrated TSP1 suppression of pERK.
Discussion
The role of NO in cancer depends on temporal and spatial aspects of exposure to NO and changing cellular responses to NO during disease progression, which combine to yield either positive or negative outcomes for the tumor and host (4). Despite this complexity, a rationale for the duality of NO is beginning to emerge from elucidating the NO dose dependencies for specific cellular and molecular targets in tumors (20). Angiogenesis is a critical component in cancer progression and an attractive treatment target for cancer as well as wound healing. This report demonstrates an NO-mediated proliferative response in HUVECs, which occurred concomitantly with down-regulation of the antiangiogenic protein TSP1. This proangiogenic response was induced by low sustained fluxes of NO and required a progrowth signal mediated by ERK phosphorylation, which was suppressed by exogenous TSP1. Higher sustained doses of DETA/NO (1,000 μM) induced up-regulation or phosphorylation of signaling molecules (p53-Ser-15P and MKP-1) that arrest growth and induce cell death. Intermediate concentrations (100 μM) also up-regulated p53-Ser-15P and MKP-1, down-regulated pERK, and were associated with a modest reaccumulation of TSP1 in the media. This observation is consistent with other reports demonstrating P53-mediated up-regulation of TSP1 (23, 29–31). In addition, this pattern of differential signaling and protein expression is supportive of bidirectional crosstalk between NO and TSP1 during the NO-induced angiogenic response (10).
Endothelial cells are known to produce low levels of NO via eNOS. Under our experimental conditions, basal NO production in HUVECs was insufficient to modulate TSP1, because treatment with l-NAME resulted in insignificant changes in the levels of TSP1 when compared with the untreated control (data not shown) and is consistent with the effect of l-NAME on pERK levels when compared with the untreated control shown in Fig. 3D. In the tissue environment, inducible NOS may be another important source of NO from either endothelial cells (33) or neighboring inflammatory cells. Furthermore, macrophages produce high and low NO fluxes depending on specific cytokines involved in inducible NOS induction (34). These NO fluxes produced by inducible NOS could dictate TSP1 levels. Under our experimental conditions, the NO flux produced by 10 μM DETA/NO was ≈2 nM NO (Fig. 3E), which stimulated guanylyl cyclase in endothelial cells (10). As reported, cGMP-mediated ERK phosphorylation was purported to provide a progrowth pathway (20, 35–37). In this study, NO mediated a cGMP-dependent down-regulation of TSP1 in association with enhanced proliferation. ERK phosphorylation is also an important mediator of the bidirectional crosstalk between NO and TSP1, because its inhibition by U0126 suppressed the NO-mediated down-regulation of TSP1 as well as the proliferative response of HUVECs. Similarly, exogenous TSP1 suppressed NO-induced ERK phosphorylation as well as angiogenic responses shown in the companion paper (10). These results are supported by other published reports showing a requirement of pERK during angiogenic response. A similar dose-dependent proliferative response to the NO donor SNAP involving ERK phosphorylation has been reported in microvascular endothelial cells, because low doses (0.1–0.3 mM) significantly enhanced cell migration, adhesion, and ERK phosphorylation, whereas higher doses (0.5–4 mM) attenuated these responses (38). Estradiol, which increases eNOS activity and is a known mediator of tumor angiogenesis induced both proliferative and migration responses of HUVECs, which required ERK phosphorylation and TSP1 suppression (39, 40). In an in vivo model, Raf-1 inhibition resulted in reduced tumor growth and a significant inhibition of neovascularization in colon, breast, and nonsmall-cell lung cancer xenografts, which was also associated with the inhibition of ERK phosphorylation (41). Tumor angiogenic response of human myeloma cells by VEGF was also suppressed by the inhibition of pERK (42).
This report and others clearly identify ERK as a molecular target in angiogenic response; therefore, the identification of molecules capable of targeting ERK exclusively within the tumor may be therapeutically beneficial. Toward this end, the interrelationship between NO and TSP1 described herein, as well as the companion report (10), provides evidence that TSP1 mediates its antiangiogenic effect in part through suppression of ERK phosphorylation (Figs. 1E and 5) and may therefore be a promising therapeutic agent. Indeed, multiple approaches are currently being developed for the application of both TSP1 and TSP2 in cancer therapy (reviewed in ref. 43). These therapeutic designs include cell-based gene therapy, low-dose chemotherapy, combination therapies, and systemic delivery of recombinant proteins or synthetic peptides (43). Low-dose chemotherapy, also known as antiangiogenic chemotherapy or metronomic dosing, involves the optimization of the effects of cytotoxic drugs by administering them continuously at low nontoxic doses (43, 44). Low-dose chemotherapy appears to provide a promising new approach, because the targeted endothelial cells within the tumor bed are genetically stable and are therefore at a reduced risk of developing drug resistance, and low dosage produces significantly fewer side effects due to selectivity of endothelial cells (43). Using a Lewis lung carcinoma tumor model, a recent report has shown that TSP1 secreted from the tumor microenvironment, which colocalized with epidermal growth factor receptor and fibroblast-specific protein (markers for tumor cells and perivascular cells, respectively), mediated the antiangiogenic and tumor growth-suppressive effects of low-dose cyclophosphamide (44). Evidence of endothelial cell selectivity was demonstrated by a reduction in CD31-positive vasculature (endothelial cell marker) in the tumors of cyclophosphamide-treated animals (44). Moreover, these antiangiogenic and tumor suppressive responses did not occur with tumor cells that did not express TSP1 (44). Similarly, the combined use of TSP1 with curative radiation therapy resulted in enhanced radiation response of D12 melanoma tumors characterized by multiple responses, including inhibited angiogenesis, increased apoptosis of microvascular endothelial cells within the tumor, enhanced radiation-induced tumor growth delay, decreased fraction of hypoxic cells, and increased radiosensitization of endothelial cells (45, 46). Furthermore, D12 melanoma tumors suppressed metastatic growth at distant organ sites by secreting TSP1 into the blood of the tumor-bearing animal (47). The surgical resection of these tumors resulted in enhanced neovascularization and accelerated growth of pulmonary micrometastases (47). These studies provide direct evidence of a selective nontoxic beneficial role of TSP1 in cancer therapy.
Conclusion
This paper and the companion report (10) have identified a dose-dependent interrelationship between NO and TSP1 to regulate endothelial cell signal transduction at the levels of cGMP and ERK phosphorylation. These results explain, at least in part, the duality of NO by demonstrating the existence of dose-dependent positive- and negative-feedback loops between NO and TSP1, which may be exploited to identify unique therapeutic approaches for controlling pathological angiogenesis.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Author contributions: L.A.R., J.S.I., M.G.E., D.D.T., D.D.R., and D.A.W. designed research; L.A.R., J.S.I., M.G.E., D.D.T., and D.D.R. performed research; and L.A.R., D.D.R., and D.A.W. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HUVEC, human vascular endothelial cells; DETA/NO, diethyltriamine NONOate; eNOS, endothelial NO synthase; l-NAME, N-nitro-l-Arg methyl ester; TSP1, thrombospondin-1; ERK, extracellular signal-regulated kinase; MAP, mitogen-activated protein; MEK[1/2], MAP kinase/ERK kinase [1/2]; MKP-1, MAP kinase phosphatase-1; pERK, phosphorylated ERK; HIF1α, hypoxia inducible factor 1α; EGM, endothelial growth media.
References
- 1.Blaise, G. A., Gauvin, D., Gangal, M. & Authier, S. (2005) Toxicology 208, 177–192. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins, D. C., Charles, I. G., Thomsen, L. L., Moss, D. W., Holmes, L. S., Baylis, S. A., Rhodes, P., Westmore, K., Emson, P. C. & Moncada, S. (1995) Proc. Natl. Acad. Sci. USA 92, 4392–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dachs, G. U. & Tozer, G. M. (2000) Eur. J. Cancer 36, 1649–1660. [DOI] [PubMed] [Google Scholar]
- 4.Wink, D. A., Vodovotz, Y., Laval, J., Laval, F., Dewhirst, M. W. & Mitchell, J. B. (1998) Carcinogenesis 19, 711–721. [DOI] [PubMed] [Google Scholar]
- 5.Hofseth, L. J., Perwez Hussain, S., Wogan, G. N. & Harris, C. C. (2003) Free Radical Biol. Med. 34, 955–968. [DOI] [PubMed] [Google Scholar]
- 6.Xie, K. & Huang, S. (2003) Free Radical Biol. Med. 34, 969–986. [DOI] [PubMed] [Google Scholar]
- 7.Calzada, M. J. & Roberts, D. D. (2005) Curr. Pharmacol. Des. 11, 849–866. [DOI] [PubMed] [Google Scholar]
- 8.Morabito, A., Sarmiento, R., Bonginelli, P. & Gasparini, G. (2004) Crit. Rev. Oncol. Hematol. 49, 91–107. [DOI] [PubMed] [Google Scholar]
- 9.Marinos, R. S., Zhang, W., Wu, G., Kelly, K. A. & Meininger, C. J. (2001) Am. J. Physiol. 281, H482–H489. [DOI] [PubMed] [Google Scholar]
- 10.Isenberg, J. S., Ridnour, L. A., Perruccio, E. M., Espey, M. G., Wink, D. A. & Roberts, D. D. (2005) Proc. Natl. Acad. Sci. USA 102, 13141–13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papapetropoulos, A., Garcia-Cardena, G., Madri, J. A. & Sessa, W. C. (1997) J. Clin. Invest. 100, 3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papapetropoulos, A., Garcia-Cardena, G., Dengler, T. J., Maisonpierre, P. C., Yancopoulos, G. D. & Sessa, W. C. (1999) Lab. Invest. 79, 213–223. [PubMed] [Google Scholar]
- 13.Xu, W., Liu, L. Z., Loizidou, M., Ahmed, M. & Charles, I. G. (2002) Cell. Res. 12, 311–320. [DOI] [PubMed] [Google Scholar]
- 14.Scotland, R. S., Morales-Ruiz, M., Chen, Y., Yu, J., Rudic, R. D., Fulton, D., Gratton, J. P. & Sessa, W. C. (2002) Circ. Res. 90, 904–910. [DOI] [PubMed] [Google Scholar]
- 15.Urbich, C., Reissner, A., Chavakis, E., Dernbach, E., Haendeler, J., Fleming, I., Zeiher, A. M., Kaszkin, M. & Dimmeler, S. (2002) FASEB J. 16, 706–708. [DOI] [PubMed] [Google Scholar]
- 16.Le Romancer, M., Reyl-Desmars, F., Cherifi, Y., Pigeon, C., Bottari, S., Meyer, O. & Lewin, M. J. (1994) J. Biol. Chem. 269, 17464–17468. [PubMed] [Google Scholar]
- 17.Greif, D. M., Kou, R. & Michel, T. (2002) Biochemistry 41, 15845–15853. [DOI] [PubMed] [Google Scholar]
- 18.Duda, D. G., Fukumura, D. & Jain, R. K. (2004) Trends Mol. Med. 10, 143–145. [DOI] [PubMed] [Google Scholar]
- 19.Bernatchez, P. N, Bauer, P. M., Yu, J., Prendergast, J. S., He, P. & Sessa, W. C. (2005) Proc. Natl. Acad. Sci. USA 102, 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas, D. D., Espey, M. G., Ridnour, L. A., Hofseth, L. J., Mancardi, D., Harris, C. C. & Wink, D. A. (2004) Proc. Natl. Acad. Sci. USA 101, 8894–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkman, J. (2003) Curr. Mol. Med. 3, 643–651. [DOI] [PubMed] [Google Scholar]
- 22.Lawler, J. (2002) J. Cell. Mol. Med. 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts, D. D. (1996) FASEB J. 10, 1183–1191. [PubMed] [Google Scholar]
- 24.Isenberg, J. S., Calzada, M. J., Zhou, L., Guo, N., Lawler, J., Wang, X., Frazier, W. A. & Roberts, D. D. (2005) Matrix Biol. 24, 110–123. [DOI] [PubMed] [Google Scholar]
- 25.Calzada, M. J., Zhou, L, Sipes, J. M., Zhang, J., Krutzsch, H. C., Iruela-Arispe, M. L., Annis, D. S., Mosher, D. F. & Roberts, D. D. (2004) Circ. Res. 94, 462–470. [DOI] [PubMed] [Google Scholar]
- 26.Pendrak, M. L., Yan, S. S. & Roberts, D. D. (2004) Eukaryot. Cell. 3, 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuxhorn, J. A., Ayala, G. E. & Rowley, D. R. (2001) J. Urol. 166, 2472–2483. [PubMed] [Google Scholar]
- 28.Wang, S., Shiva, S., Poczatek, M. H., Darley-Usmar, V. & Murphy-Ullrich, J. E. (2002) J. Biol. Chem. 277, 9880–9888. [DOI] [PubMed] [Google Scholar]
- 29.Dameron, K. M, Volpert, O. V., Tainsky, M. A. & Bouck, N. (1994) Science 265, 1582–1584. [DOI] [PubMed] [Google Scholar]
- 30.Gautam, A., Waldrep, J. C., Densmore, C. L., Koshkina, N., Melton, S., Roberts, L., Gilbert, B. & Knight, V. (2002) Gene Ther. 9, 353–357. [DOI] [PubMed] [Google Scholar]
- 31.Klein-Soyer, C., Ceraline, J., Orvain, C., de la Salle, C., Bergerat, J. P. & Cazenave, J. P. (1997) Biol. Cell 89, 295–307. [PubMed] [Google Scholar]
- 32.Pervin, S., Singh, R., Freije, W. A. & Chaudhuri, G. (2003) Cancer Res. 63, 8853–8860. [PubMed] [Google Scholar]
- 33.de Assis, C. M., Plotkowski, C. M., Fierro, I. M., Barja-Fidalgo, C. & de Freitas, M. S. (2002) Nitric Oxide 7, 254–261. [DOI] [PubMed] [Google Scholar]
- 34.Espey, M. G., Mirand, K. M., Pluta, R. M. & Wink, D. A. (2000) J. Biol. Chem. 275, 11341–11347. [DOI] [PubMed] [Google Scholar]
- 35.Ko, G. Y., Ko, M. L. & Dryer, S. E. (2001) J. Neurosci. 21, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hess, A., Labbe, D., Michel, O., Teranishi, M. A., Orzechowska, O., Schmidt, A., Addicks, K. & Bloch, W. (2002) Brain Res. 956, 236–245. [DOI] [PubMed] [Google Scholar]
- 37.Kim, T. W., Lee, C. H., Choi, C. Y., Kwon, N. S., Baek, K. J., Kim, Y. G. & Yun, H. Y. (2003) Neurosci. Lett. 344, 209–211. [DOI] [PubMed] [Google Scholar]
- 38.Jones, M. K., Tsugawa, K., Tarnawski, A. S. & Baatar, D. (2004) Biochem. Biophys. Res. Comm. 318, 520–528. [DOI] [PubMed] [Google Scholar]
- 39.Weiner, C. P., Lizasoain, I., Baylis, S. A., Knowles, R. G., Charles, I. G. & Moncada, S. (1994) Proc. Natl. Acad. Sci. USA 91, 5212–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sengupta, K., Banerjee, S., Saxena, N. K. & Banerjee, S. K. (2004) Mol. Cancer Res. 2, 150–158. [PubMed] [Google Scholar]
- 41.Wilhelm, S. M., Carter, C., Tang, L., Wilkie, D., McNabola, A., Rong, H., Chen, C., Zhang, X., Vincent, P., McHugh, M., et al. (2004) Cancer Res. 64, 7099–7109. [DOI] [PubMed] [Google Scholar]
- 42.Giuliani, N., Lunghi, P., Morandi, F., Colla, S., Bonomini, S., Hojden, M., Rizzoli, V. & Bonati, A. (2004) Leukemia 18, 628–635. [DOI] [PubMed] [Google Scholar]
- 43.Lawler, J. & Detmar, M. (2004) Int. J. Biochem. Cell Biol. 36, 1038–1045. [DOI] [PubMed] [Google Scholar]
- 44.Hamano, Y., Sugimoto, H., Soubasakos, M. A., Kieran, M., Olsen, B. R., Lawler, J., Sudhakar, A. & Kalluri, R. (2004) Cancer Res. 64, 1570–1574. [DOI] [PubMed] [Google Scholar]
- 45.Rofstad, E. K., Henriksen, K., Galappathi, K. & Mathiesen, B. (2003) Cancer Res. 63, 4055–4061. [PubMed] [Google Scholar]
- 46.Rofstad, E. K., Galappathi, K. & Mathiesen, B. (2004) Int. J. Rad. Oncol. Biol. Phys. 58, 493–499. [DOI] [PubMed] [Google Scholar]
- 47.Rofstad, E. K. & Graff, B. A. (2001) J. Invest. Dermatol. 117, 1042–1049. [DOI] [PubMed] [Google Scholar]