The sequencing of the human and mouse genomes has been heralded as one of the most important recent achievements in biological science. Attention has now turned to the functional annotation of the 25,000 or so genes encoded by the mammalian genome. Although there are many experimental approaches that address gene function, the most relevant approach for extrapolation to human development, physiology, and disease is to analyze the phenotype of mutations for each gene in a whole mammalian model, the mouse. Many phenotypes relevant to congenital traits and abnormal pathologies in humans have emerged from gene knockout studies in the mouse, providing a strong justification for expanding the collection of mouse mutants to include all genes. Recent discussions between funding agencies and the mouse genetics community (1, 2) have garnered support for an international, concerted effort to generate a resource of mutations in every gene in the mouse genome. This effort will use a combination of gene targeting and gene trapping in ES cells, two well established technologies that were developed in parallel in the late 1980s and gradually perfected over the years (3, 4). In this issue of PNAS, Friedel et al. (5) ingeniously combine gene targeting and gene trapping to mutate genes expressed in ES cells at a high efficiency. It may surprise many to learn that this hybrid method of “targeted trapping” is applicable to a majority of genes in the mouse.
Gene targeting has been widely used over the past 15 years to engineer precise modifications in the mouse genome. The collective efforts of many laboratories have thus far produced targeted mutations in ≈3,600 genes, or just <15% of the genome (a list of targeted gene mutations is maintained by The Jackson Laboratory, www.informatics.jax.org). Gene targeting relies on rare homologous recombination events between an exogenous DNA construct introduced into cells and its cognate genomic locus, to engineer precise modifications in the genome. Typically, targeting constructs contain several kilobases of genomic DNA flanking a drug selection marker driven by a heterologous promoter. Improvements in molecular cloning methods, specifically recombineering of bacterial artificial chromosomes (6–8), have reduced the effort and increased the precision for building targeting vectors. Despite these advances, gene targeting remains a labor-intensive undertaking that is not easily scalable because of the effort required to screen ES cell colonies for a small fraction of correctly targeted events (9).
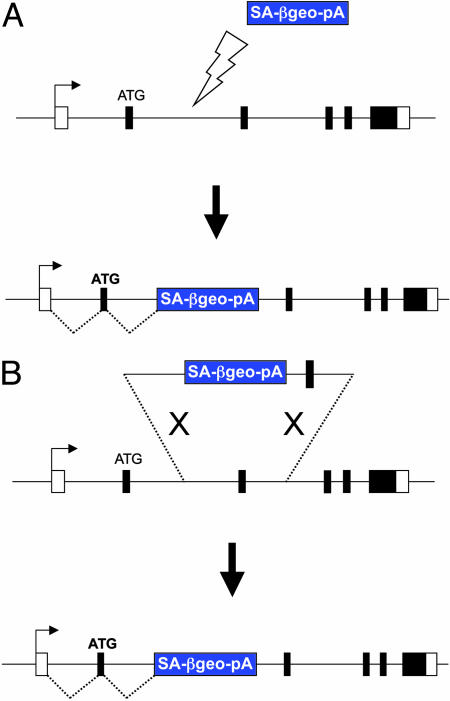
Gene trapping, by contrast, relies on random integration of a promoterless reporter construct (10, 11) and is limited to genes expressed in ES cells. The most widely used vectors contain a splice acceptor and polyadenylation signal flanking the βgeo reporter gene (11), such that the reporter is activated upon insertion into introns of genes (Fig. 1A). To identify the target gene, PCR-based strategies, such as 5′ RACE, are used to generate a sequence tag for each insertion (12). Gene trapping has gained prominence in recent years as a high-throughput method for the isolation of random insertional mutations in ES cells (13). Both privately and publicly funded efforts have generated large libraries of ES cells harboring gene-trap insertions (the International Gene Trap Consortium maintains a database of gene-trap cell lines available from the public resource at www.genetrap.org). Combined, these resources are estimated to contain mutations in >60% of genes in mice (14). Gene-trap libraries have become an important source of new mutations in mice for the pharmaceutical industry and academic investigators. The National Institutes of Health-funded BayGenomics program (http://baygenomics.ucsf.edu), for example, has distributed >2,000 gene-trap ES cell lines to academic laboratories. Clearly, there is a high demand for mutant ES cell resources within the mouse genetics community, and the impact of gene-trap resources is beginning to emerge in the published literature (15–18).
Fig. 1.
Gene trapping versus targeted trapping. (A) Gene trapping depends on random insertions of a promoterless reporter gene, such as βgeo, equipped with splice acceptor (SA) and polyadenylation (pA) signals. The reporter is activated after insertions into introns of expressed genes to generate a fusion mRNA that can be characterized by 5′ RACE. (B) Targeted trapping relies on homologous recombination to introduce a promoterless gene-trap cassette into predefined loci. The trapping cassette is flanked by genomic sequences of the target locus that do not contain the promoter of the target gene.
The targeted trapping approach described by Friedel et al. (5) represents a highly efficient method for inactivating genes expressed in ES cells. By simply flanking a gene-trap cassette with genomic sequences, gene-trap insertions can be directed with precision into introns of genes by homologous recombination (Fig. 1B). The only requirement for the construction of targeted trapping vectors is to avoid including the promoter of the target gene in the arms of homology. Like random gene trapping, activation of the βgeo-selectable marker requires splicing to exons of genes expressed in ES cells. Thus, correctly targeted events are greatly enriched among the drug-resistant colonies, and random integrations elsewhere in the genome are suppressed. A further degree of enrichment is gained by the use of gene-trap cassettes that lack a translation initiation signal and therefore require the production of an in-frame fusion protein. The use of promoterless drug selection markers for gene targeting is not new (19, 20) but has been used to target only a handful of genes. Curiously, despite the early success of promoterless targeting, this approach was never embraced by researchers.
Friedel et al. (5) chose a set of 24 genes for targeted trapping, focusing on cell surface proteins that may play a role in axon guidance. For this purpose, a secretory trap cassette (21) was flanked by the arms of homology to the target gene. An astonishingly high frequency of correctly targeted events was observed for 16 of the 24 loci, averaging >50%. Importantly, Friedel et al. establish the threshold of expression above which targeted trapping is effective. Semiquantitative RT-PCR was used to measure steady-state mRNA levels of selected target genes relative to transferrin receptor, a gene expressed at low levels in ES cells. Efficient targeting was observed for most genes expressed above 1% the level of transferrin receptor. A retrospective analysis showed that 97% of genes previously trapped by random gene trapping are in fact expressed above this threshold. Thus, genes accessible to random trapping appear to be equally good substrates for targeted trapping. A survey of randomly selected genes predicts that more than half of all genes are expressed above this threshold and, therefore, are likely to be accessible to random and targeted trapping. This result agrees well with the fraction of genes that is thought to be accessible to gene trapping, estimated to be ≈60% of all mouse genes (14). Therefore, most of the genes accessible to targeted trapping are already represented in the existing gene-trap libraries, and a list of trapped genes in public and private resources (available from the International Gene Trap Consortium, www.genetrap.org) serves as a useful guide for identifying genes for targeted trapping.
The work of Friedel et al. (5) comes at a propitious time and has important strategic implications for international efforts aimed at generating a complete collection of reporter-tagged null mutations in mice. The public gene-trap resources contain mutations in ≈40% of genes; however, the efficiency of trapping new genes has dropped to ≈10% (one new gene is trapped for every 10 colonies isolated) and will continue to diminish well before saturation is achieved. The efficiency of targeted trapping exceeds the current efficiency of random gene trapping; therefore, targeted trapping could be put to good use to systematically target the remaining 20% of trappable genes that are not currently represented in the public resource. Furthermore, a significant fraction of trapped genes are represented by single events and thus the generation of additional mutant cell lines for these loci will be beneficial. Although most gene-trap mutations studied in mice effectively mutate the endogenous gene at the site of insertion, the generation of null alleles is not always guaranteed. Hypomorphic mutations (16) or insertions that disrupt only a subset of mRNA isoforms (22) are possible. The rapid generation of additional null alleles by targeted trapping will be valuable in these cases. Finally, the mouse genetics community has expressed a strong desire for reporter-tagged, conditional null alleles to enable temporal or tissue-specific ablation of gene function (1, 2). Targeted trapping can be readily adapted for conditional mutagenesis by, for example, incorporating conditional gene-trap cassettes (23).
The efficiency of targeted trapping exceeds the current efficiency of random gene trapping.
Gene targeting in ES cells will continue to be the workhorse for the functional analysis of genes in mice for many years to come. The detailed analysis of genes will require all manner of alleles beyond the generation of null mutations. Targeted trapping is a welcome addition to the arsenal of molecular tools with which to address gene function in the mouse.
Author contributions: W.C.S. wrote the paper.
See companion article on page 13188.
References
- 1.Austin, C. P., Battey, J. F., Bradley, A., Bucan, M., Capecchi, M., Collins, F. S., Dove, W. F., Duyk, G., Dymecki, S., Eppig, J. T., et al. (2004) Nat. Genet. 36, 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auwerx, J., Avner, P., Baldock, R., Ballabio, A., Balling, R., Barbacid, M., Berns, A., Bradley, A., Brown, S., Carmeliet, P., et al. (2004) Nat. Genet. 36, 925–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Weyden, L., Adams, D. J. & Bradley, A. (2002) Physiol. Genomics 11, 133–164. [DOI] [PubMed] [Google Scholar]
- 4.Stanford, W. L., Cohn, J. B. & Cordes, S. P. (2001) Nat. Rev. Genet. 2, 756–768. [DOI] [PubMed] [Google Scholar]
- 5.Friedel, R. H., Plump, A., Lu, X., Spilker, K., Jolicoeur, C., Wong, K., Venkatesh, T. R., Yaron, A., Hynes, M., Chen, B., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 13188–13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang, Y., Muyrers, J. P., Testa, G. & Stewart, A. F. (2000) Nat. Biotechnol. 18, 1314–1317. [DOI] [PubMed] [Google Scholar]
- 7.Yu, D., Ellis, H. M., Lee, E. C., Jenkins, N. A., Copeland, N. G. & Court, D. L. (2000) Proc. Natl. Acad. Sci. USA 97, 5978–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, E. C., Yu, D., Martinez de Velasco, J., Tessarollo, L., Swing, D. A., Court, D. L., Jenkins, N. A. & Copeland, N. G. (2001) Genomics 73, 56–65. [DOI] [PubMed] [Google Scholar]
- 9.Valenzuela, D. M., Murphy, A. J., Frendewey, D., Gale, N. W., Economides, A. N., Auerbach, W., Poueymirou, W. T., Adams, N. C., Rojas, J., Yasenchak, J., et al. (2003) Nat. Biotechnol. 21, 652–659. [DOI] [PubMed] [Google Scholar]
- 10.Gossler, A., Joyner, A. L., Rossant, J. & Skarnes, W. C. (1989) Science 244, 463–465. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich, G. & Soriano, P. (1991) Genes Dev. 5, 1513–1523. [DOI] [PubMed] [Google Scholar]
- 12.Skarnes, W. C., Auerbach, B. A. & Joyner, A. L. (1992) Genes. Dev. 6, 903–918. [DOI] [PubMed] [Google Scholar]
- 13.Zambrowicz, B. P., Friedrich, G. A., Buxton, E. C., Lilleberg, S. L., Person, C. & Sands, A. T. (1998) Nature 392, 608–611. [DOI] [PubMed] [Google Scholar]
- 14.Skarnes, W. C., von Melchner, H., Wurst, W., Hicks, G., Nord, A. S., Cox, T., Young, S. G., Ruiz, P., Soriano, P., Tessier-Lavigne, M., et al. (2004) Nat. Genet. 36, 543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leighton, P. A., Mitchell, K. J., Goodrich, L. V., Lu, X., Pinson, K., Scherz, P., Skarnes, W. C. & Tessier-Lavigne, M. (2001) Nature 410, 174–179. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell, K. J., Pinson, K. I., Kelly, O. G., Brennan, J., Zupicich, J., Scherz, P., Leighton, P. A., Goodrich, L. V., Lu, X., Avery, B. J., et al. (2001) Nat. Genet. 28, 241–249. [DOI] [PubMed] [Google Scholar]
- 17.Hansen, J., Floss, T., Van Sloun, P., Fuchtbauer, E. M., Vauti, F., Arnold, H. H., Schnutgen, F., Wurst, W., von Melchner, H. & Ruiz, P. (2003) Proc. Natl. Acad. Sci. USA 100, 9918–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zambrowicz, B. P., Abuin, A., Ramirez-Solis, R., Richter, L. J., Piggott, J., BeltrandelRio, H., Buxton, E. C., Edwards, J., Finch, R. A., Friddle, C. J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 14109–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartzberg, P. L., Goff, S. P. & Robertson, E. J. (1989) Science 246, 799–803. [DOI] [PubMed] [Google Scholar]
- 20.Sedivy, J. M. & Sharp, P. A. (1989) Proc. Natl. Acad. Sci. USA 86, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skarnes, W. C., Moss, J. E., Hurtley, S. M. & Beddington, R. S. (1995) Proc. Natl. Acad. Sci. USA 92, 6592–6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess, R. W., Skarnes, W. C. & Sanes, J. R. (2000) J. Cell. Biol. 151, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnutgen, F., De-Zolt, S., Van Sloun, P., Hollatz, M., Floss, T., Hansen, J., Altschmied, J., Seisenberger, C., Ghyselinck, N. B., Ruiz, P., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 7221–7226. [DOI] [PMC free article] [PubMed] [Google Scholar]