The New Anticonvulsant Retigabine Favors Voltage-dependent Opening of the Kv7.2 (KCNQ2) Channel by Binding to Its Activation Gate
Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H
Mol Pharmacol 2005;67:1009–1017
Retigabine (RTG) is an anticonvulsant drug with a novel mechanism of action. It activates neuronal KCNQ-type K+ channels by inducing a large hyperpolarizing shift of steady-state activation. To identify the structural determinants of KCNQ channel activation by RTG, we constructed a set of chimeras by using the neuronal KV7.2 (KCNQ2) channel, which is activated by RTG, and the cardiac KV7.1 (KCNQ1) channel, which is not affected by this drug. Substitution of either the S5 or the S6 segment in KV7.2 by the respective parts of KV7.1 led to a complete loss of activation by RTG. Trp236 in the cytoplasmic part of S5 and the conserved Gly301 in S6 (KV7.2), considered as the gating hinge (Ala336 in KV7.1), were found to be crucial for the RTG effect: mutation of these residues could either knock out the effect in KV7.2 or restore it partially in KV7.1/KV7.2 chimeras. We propose that RTG binds to a hydrophobic pocket formed upon channel opening between the cytoplasmic parts of S5 and S6 involving Trp236 and the channel's gate, which could well explain the strong shift in voltage-dependent activation.
COMMENTARY
Voltage-gated ion channels are important determinants of neuronal activity. Numerous ion-channel mutations have been linked to epilepsy, and many antiepileptic medications modulate sodium or calcium channels. Potassium channels are probably as important in regulating membrane potential, yet little has been done to develop therapeutic modulators of this protein. The article by Wuttke et al. explores the mechanism whereby a novel antiepileptic medication, retigabine (RTG), enhances a subset of voltage-gated potassium channels.
The canonical structure for voltage-gated ion channels is a combination of four cassettes, each made of six transmembrane (TM) domains. Whereas this structure is typical of sodium and calcium channels, which have all four cassettes contained within a single large protein, the arrangement is much more complicated for potassium channels. More than 80 human potassium channel–related genes have been identified, many with multiple splice variants and accessory proteins (1). This diversity allows a wide range of channels, some of which are voltage sensitive, whereas others respond to many different stimuli, including calcium, G proteins, ATP, and changes in pH. Fortunately, the multitude of potassium channels all are based on a common structural motif, two TM domains (M1 and M2) and an intervening P loop. Voltage-gated K+ channels (Kv) contain six TM domains, with TM1–4 serving as the voltage sensor (2) and TM5–6 being analogous to M1-P–M2 in other K+ channel families.
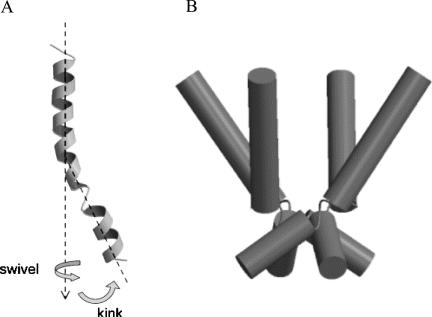
A combination of four of these motifs is required to form functional potassium channels. A key feature of potassium channels is their ability to allow the selective passage of K+ but not of other cations. A pore is formed at the interface of the four TM6 (M2 in non-Kv channels) segments. However, the selectivity filter also includes a section of the extracellular P loop, which folds back down and lines the extracellular portion of the pore. Beyond the selectivity filter, closer to the intracellular membrane, the four TM6 domains of a resting potassium channel are closely juxtaposed, forming a barrier to ion flow. This region, known as the gate, is the putative site of retigabine action. With channel activation, TM6 is thought to bend at a “hinge” near its midsection, which contains a relatively conserved glycine. As depicted in Figure 1, this event allows the intracellular portion of the pore to widen, permitting passage of ions (3).
FIGURE 1.
Model of potassium channel gating. A: The TM6 domain of KV is relatively linear at rest. Activation involves a swivel and bending motion proximate to a “hinge” region near the middle of TM6. B: In the intact channel, four linear TM6 domains are closely opposed, resulting in a severe narrowing of the pore (i.e., the gate). As pictured, activation induces a bend in each subunit, moving them slightly apart and thereby opening the gate, which allows the passage of potassium ions. Adapted with permission from Sansom MS, Shrivastava IH, Bright JN. Potassium channels: Structures, models, simulations [Review]. Biochim Biophys Acta 2002;1565:294–307.
RTG is an antiepileptic medication undergoing clinical trials. It is effective for a wide range of seizure models and has few drug interactions (4). RTG has several pharmacologic actions, but an interesting and unique action is its ability to enhance KCNQ2/KCNQ3 potassium channels (which under the new nomenclature also are designated KV7.2 and KV7.3). KCNQ2/KCNQ3 heterotetramers form the voltage-gated ion channel that mediates the M-current, which regulates the subthreshold excitability and responsiveness to neurotransmitters of many neurons. Interestingly, mutations in the genes encoding KCNQ2 and KCNQ3 are associated with the epilepsy syndrome, benign familial neonatal convulsions. The M-current is somewhat active at resting membrane potential and then slowly increases activity during depolarization. Its slow kinetics probably preclude an involvement in shaping individual action potentials but allow the M-current to alter spike frequency during bursts of neuronal firing. RTG affects the M-current in multiple ways, including accelerating activation, slowing deactivation, and shifting the voltage activation curve by 30 mV toward more hyperpolarized potentials. How RTG causes these changes is the focus of the present article.
To help elucidate the mechanism of potassium channel modulation, the authors took advantage of the fact that whereas KCNQ2-5 respond to RTG, the closely related cardiac channel KCNQ1 does not. Chimeric proteins were generated, and segments of KCNQ1were transplant into KCNQ2 proteins and vice versa. Electrophysiological recordings from oocytes expressing these recombinant proteins demonstrated that potassium-current enhancement by RTG involves TM5 and TM6. The TM5 and TM6 regions of the KCNQ1 and KCNQ2 proteins differ by only 13 amino acids. KCNQ2 channels lost RTG sensitivity when a single tryptophan in TM5 was mutated to leucine, as found in KCNQ1 proteins. To investigate the role of TM6 in RTG action, the authors made use of the fact that KCNQ1 is one of the few potassium channels without glycine in the putative hinge segment. As previously mentioned, this site is important in channel opening; thus, mutating the glycine in most potassium channels results in a nonfunctional protein. How KCNQ1 proteins can function without the glycine is unknown, but the phenomenon may suggest some differences in channel activation for this particular Kv channel. Nonetheless, as might be expected, mutating glycine in KCNQ2 to alanine resulted in a channel that could not open. Conversely, a KCNQ2 protein containing the KCNQ1 TM6 sequence produces a channel that is functional but insensitive to RTG. However, when that single glycine is reintroduced into the homologous position of TM6, the channel regains RTG sensitivity. The authors propose a model in which RTG binds in a pocket between TM5 and TM6, thus stabilizing the bend in TM6 that is thought to occur on opening. This event would theoretically reduce the energy required for activation (so less depolarization is needed to open the channel) and explain the faster activation and slower deactivation induced by RTG. The authors' theory fits well with single-channel data that RTG prolongs both open states by two- to fourfold (5). A more recent article by Schenzer et al. (6) found similar structural requirements for RTG sensitivity by using Kv7.3 proteins.
Chimera and point mutation studies come with several important caveats. First, although these techniques can help identify residues that are important in RTG action, it is often difficult to tell whether the sites involve binding, transduction, or some other process distant from the binding site. This issue is made particularly evident by the number of nonfunctional chimeras and mutants the authors screened. Use of this screening technique does not make it possible to assess whether the mutants would have retained RTG sensitivity if they had been able to open at all. The authors did not determine whether the RTG-insensitive mutants had lost RTG binding or the ability of RTG to alter gating. It is likely that none of these residues acts alone; the process of activation probably involves many interactions among many residues. To fully understand residue functions, mutating several points at once—to either abolish or restore drug effect—is often required (7). Despite the limitations of the studies by Wuttke and colleagues, they provide important clues about the mechanism for a potentially unique and effective tool to modulate potassium channels in epilepsy.
References
- 1.Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. Review. [DOI] [PubMed] [Google Scholar]
- 2.Ahern CA, Horn R. Stirring up controversy with a voltage sensor paddle. Trends Neurosci. 2004;27:303–307. doi: 10.1016/j.tins.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Sansom MS, Shrivastava IH, Bright JN. Potassium channels: Structures, models, simulations. Biochim Biophys Acta. 2002;1565:294–307. doi: 10.1016/s0005-2736(02)00576-x. Review. [DOI] [PubMed] [Google Scholar]
- 4.Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs: A summary of the Seventh Eilat Conference (EILAT VII). Epilepsy Res. 2004;61:1–48. doi: 10.1016/j.eplepsyres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Tatulian L, Brown DA. Effect of the KCNQ potassium channel opener retigabine on single KCNQ2/3 channels expressed in CHO cells. J Physiol. 2003;549:57–63. doi: 10.1113/jphysiol.2003.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenzer A, Friedrich T, Pusch M, Saftig P, Jentsch TJ, Grötzinger J, Schwake M. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci. 2005;25:5051–5060. doi: 10.1523/JNEUROSCI.0128-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson G, Karlin A. Acetylcholine receptor channel structure in the resting, open, and desensitized states probed with the substituted-cysteine-accessibility method. Proc Natl Acad Sci U S A. 2001;98:1241–1248. doi: 10.1073/pnas.031567798. [DOI] [PMC free article] [PubMed] [Google Scholar]