Interleukin-1β Contributes to the Generation of Experimental Febrile Seizures
Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ
Ann Neurol 2005;57:152–155
Fever can provoke “febrile” seizures (FSs). Because complex FSs may promote development of temporal lobe epilepsy, understanding their mechanisms is clinically important. By using an immature rodent model and transgenic technology, we examined the role of interleukin-1β, (IL-1β), a pyrogenic, proinflammatory cytokine, in FSs. IL-1β receptor–deficient mice were resistant to experimental FSs. This resistance appeared independent of genetic background and was attributed to lack of IL-1β signaling, because exogenous cytokine reduced the seizure threshold in wild-type but not receptor-deficient mice, independent of strain. In addition, high IL-1β doses induced seizures only in IL-1β receptor-expressing mice. These data indicate that IL-1β signaling contributes critically to fever-induced hyperexcitability underlying FS, constituting a potential target for their prevention.
COMMENTARY
A question central to understanding the mechanisms of febrile seizures is whether they have unique features that differentiate them from other seizure syndromes. Fever is a typical response to a variety of exogenous pyrogens, with infection being the most common cause. In this regard, fever induced by systemic injection of lipopolysaccharide, an endotoxin from the cell membrane of gram-negative bacteria, enhances susceptibility to seizures provoked by both pentylenetetrazole and kainic acid (1,2). This finding suggests that exogenous pyrogens can facilitate seizures with different underlying mechanisms.
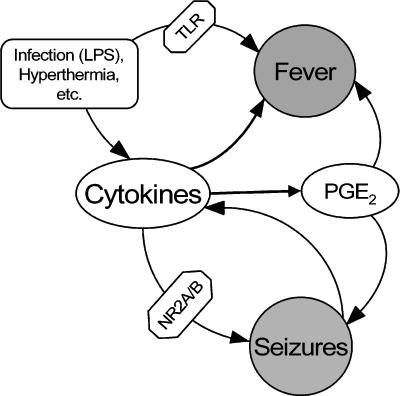
Exogenous pyrogens induce fever through interactions with cytokines, which are referred to as endogenous pyrogens. These include interleukins 1β, 6, and 8 (IL-1β, IL-6, IL-8), tumor necrosis factor-α, macrophage inflammatory protein-1, and others (3). Furthermore, exogenous pyrogens may induce fever independent of cytokines, by acting at Toll-like receptor, which belongs to the IL-1 receptor family (4) (Figure 1).
FIGURE 1.
Cytokines are a concurrent component of fever and seizures Exogenous pyrogenic factors (e.g., lipopolysaccharide) or hyperthermia lead to fever by acting at Toll-like receptors and through the induction of cytokines. Cytokines facilitate seizures through phosphorylation of the NR2A/B subunit of N-methyl-d-aspartate receptors and via prostaglandin E2, an endogenous cytokine-induced pyrogen. Seizures, in turn, lead to the expression of cytokines in the brain, thus forming a vicious circle that contributes to seizure progression. PGE2, Prostaglandin E2; LPS, lipopolysaccharide.
It is conceivable that both exogenous pyrogens and cytokines induce or at least enhance propensity to febrile seizures concurrent with inducing fever. To evaluate this possibility, Dube and collaborators carried out a series of experiments to determine whether febrile convulsions can be induced in the absence of the endogenous pyrogen IL-1β or its target IL-1 receptor type I. The authors used an earlier observation that immature animals developed convulsions when fever was mimicked by a sustained, prolonged physical hyperthermia (5). Dube et al. attempted to provoke similar febrile-like seizures in IL-1 receptor type I knockout mice. The authors found that whereas IL-1 receptor type I knockouts were capable of developing seizures in response to hyperthermia, a higher temperature was required for the convulsions to evolve. Furthermore, exogenous administration of recombinant IL-1β to wild-type mice both decreased temperature threshold for febrile seizures and, at high doses, was sufficient for inducing seizures in normothermic animals.
Certainly the fact that IL-1 receptor type I knockout mice did not exhibit absolute resistance to hyperthermia-induced convulsions indicates that IL-1β is not the only factor involved in the generation of febrile seizures. Indeed, other mediators of fever, such as IL-6, TNF-α, and prostaglandin E2, all affect seizure susceptibility. IL-1β, however, may have an intriguing clinical implication, because a polymorphism of the IL-1β gene promoter is associated with febrile seizures in children (6).
Seizure regulation by IL-1β is not limited to febrile convulsions. For example, IL-1 receptor type I knockout animals, of the same type as used by Dube et al., exhibited enhanced resistance to seizures induced by bicuculline (7). Furthermore, intrahippocampal injection of recombinant IL-1β in normal animals enhanced the severity of seizures provoked by both bicuculline (7) and kainic acid (8). In contrast, transgenic animals that overexpressed IL-1ra, an endogenous antagonist of IL-1 receptor type I, as well as wild-type animals that received an injection of recombinant IL-1ra receptor antagonist, showed enhanced resistance to bicuculline seizures (7). Interestingly, nonfebrile seizures themselves led to the expression of IL-1β in microglia (8), suggesting that IL-1β induced by seizures may in turn exacerbate ongoing seizures, apparently acting at its neuronal receptor (again, see Figure 1).
A possible mechanism by which IL-1 receptor type I activation leads to seizure involves increased phosphorylation of the NR2A/B subunit of N-methyl-d-aspartate receptor through activation of tyrosine kinases, which results in N-methyl-d-aspartate receptor–mediated Ca2+ influx (9). Thus, cytokines would appear to be endogenous proconvulsant factors that contribute to seizures of various origins, including febrile convulsions. In turn, the expression and release of cytokines may be triggered by a variety of conditions, including infection, noninfectious fever, stress (10), and seizures themselves.
It will be important to assess whether IL-1β and its receptor play a role in seizures associated with infection, given that lipopolysaccharide-induced fever has both cytokine-dependent and cytokine-independent components (3,4). It will be interesting to know in particular whether lipopolysaccharide facilitates seizures in IL-1 receptor type I knockout mice. An additional question worth addressing is whether IL-1β/IL-1 receptor type I only regulates the threshold for hyperthermia-induced seizures or whether it enhances their severity and duration as well—and thus, possibly affects long-term outcome of febrile convulsions.
References
- 1.Heida JG, Boisse L, Pittman QJ. Lipopolysaccharide-induced febrile convulsions in the rat: Short-term sequelae. Epilepsia. 2004;45:1317–1329. doi: 10.1111/j.0013-9580.2004.13704.x. [DOI] [PubMed] [Google Scholar]
- 2.Sayyah M, Javad-Pour M, Ghazi-Khansari M. The bacterial endotoxin lipopolysaccharide enhances seizure susceptibility in mice: Involvement of proinflammatory factors nitric oxide and prostaglandins. Neuroscience. 2003;122:1073–1080. doi: 10.1016/j.neuroscience.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Roth J, de Souza GEP. Fever-induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res. 2001;34:301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J Endotoxin Res. 2004;10:301–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 5.Bender RA, Dube C, Baram TZ. Febrile seizures and mechanisms of epileptogenesis: insights from an animal model. Adv Exp Med Biol. 2004;548:213–225. doi: 10.1007/978-1-4757-6376-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virta M, Hurme M, Helminen M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr Neurol. 2002;26:192–195. doi: 10.1016/s0887-8994(01)00380-0. [DOI] [PubMed] [Google Scholar]
- 7.Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, DeLuigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, Iverfeldt K, Bartfai T. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: Functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8670. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haveman J, Geerdink AG, Rodermond HM. Cytokine production after whole body and localized hyperthermia. Int J Hyperthermia. 1997;13:337–339. doi: 10.3109/02656739609027685. [DOI] [PubMed] [Google Scholar]