Abstract
Background
Improving soft tissue quality and quantity around dental implants is crucial for successful outcomes. Platelet-Rich Fibrin (PRF) is showing promise in enhancing wound healing and implant stability. This systematic review aims to evaluate the clinical benefits of PRF in soft tissue regeneration around dental implants compared to standard methods.
Methods
This systematic review adhered to the PRISMA guidelines and utilizing the PICO methodology, investigated the use of Platelet-Rich Fibrin (PRF) in soft tissue augmentation during implant therapy. The primary outcomes assessed include the width of keratinized mucosa and soft tissue thickness, comparing PRF interventions to standard techniques.The study included randomized controlled trials (RCTs) and comparative studies, focusing on human patients needing implant therapy with or without PRF. An extensive search of databases and manual references was conducted; data extraction involved assessing study quality and risk of bias, but due to high heterogeneity among studies, a meta-analysis was not feasible, leading to a systematic review of the available literature.
Results
A total of 766 references were initially identified, with 29 being eligible after screening. Nine studies were included for detailed review. The findings revealed that PRF is effective in increasing the width of keratinized mucosa (KT) and soft tissue thickness (STT) around implants. Even if free gingival grafts (FGG) sometimes performed better. However, the differences between PRF and FGG were not clinically significant, and PRF offers lower cost, ease of use, and reduced morbidity. There was limited information on the esthetic outcomes of PRF, with only two studies addressing this aspect, showing mixed results.
Conclusion
Overall, PRF demonstrated positive effects on KT width and STT, but further research with rigorous methodology and larger sample sizes is needed to better understand its impact on implants health and esthetics.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-025-05922-6.
Keywords: Platelet-rich fibrin, Dental implants, Systematic review, Soft tissues, Autologous platelets concentrates
Background
It is clinically advisable to ameliorate the quantity and quality of soft tissues at sites of immediate or delayed implant placement [1–3]. In fact, despite the lack of consensus regarding the need for a minimum amount of keratinized tissue around implants, it seems clinically reasonable to aim for well-represented peri-implant mucosa. Additionally, correct management of soft tissues is very important at each surgical entry, whether it be tooth extraction, alveolar bone augmentation, the implant placement, or the implant uncovering [4–6]. In recent decades, the ad modumBränemark staged approach has been rapidly overtaken by immediate placement and immediate loading protocols [7–9]. Consequently, there has been a growing number of new surgical-prosthetic approaches aimed at promoting faster bone healing, better soft tissue sealing, and accelerating wound closure.
The local application of growth factors and biological scaffolds is believed to enhance wound healing [10–12]. Various preparations of platelets and respective fibrin‐rich matrix (PRF) have gained recognition because they provide a mature clot rich in growth factors and a protective barrier against early mechanical and microbiological insults to the surgical wound [13–16]. PRF is enriched in growth factors like platelet derived growth factor or PDGF and transformation growth factors (TGFs) alpha and beta. The enriched growth factors are released in a sustained manner for a minimum of one week, possibly extending up to 28 days [17, 18].
Platelet‐rich fibrin (PRF) is easily prepared from plasma after centrifugation of whole venous blood [19]. Plasma containing platelets undergoes spontaneous coagulation, and like a natural blood clot, activated platelets and white cells become entrapped in the fibrin‐rich matrix [20]. PRF can be processed into a PRF membrane or also mixed with grafts and biomaterials to form “sticky bone” [21, 22].
The platelet fibrin plexus is an excellent medium for proliferation as well as migration of cells, thus its regeneration potential has been increasingly investigated in different clinical scenarios. Various authors have demonstrated the efficacy of PRF in aiding bone regeneration within the maxillary sinus, and it appears to be correlated with improved implant stability [23, 24]. The 2018 systematic review by Strauss concluded that there is moderate evidence supporting the clinical benefits of PRF in ridge preservation and in the early phase of osseointegration [25]. Importantly, PRF is strongly associated with positive patient outcomes, such as reduced pain, faster healing, and lower discomfort [26, 27].
The use of PRF has been most extensively investigated in periodontology for the treatment of periodontal intrabony defects and gingival recessions, where most studies have demonstrated favorable results in soft tissue management and repair [28, 29]. It remains unclear whether PRF can improve soft tissue healing around dental implants as well. In their 2017 systematic review on the regenerative properties of PRF, Miron and colleagues highlighted that PRF’s reparative potential favors soft tissue formation and ligament regeneration [23]. The authors further recommended discussing the role of PRF in improving implant therapy outcomes.
Therefore, the purpose of the present systematic review is to report the current state of knowledge and clinical potential of PRF in regenerative soft tissue therapy around dental implants when compared to standardized controls from human clinical randomized trials, prospective comparative studies, and controlled clinical trials.
Materials and methods
Protocol development and eligibility criteria
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. The review follows the PRISMA guidelines for developing a focus question and defining inclusion and exclusion criteria. The PICOS framework was applied to guide the formulation of the research question:
- Population (P)
- Description: Humans in need of implant therapy with soft tissue augmentation requirements.
- Details: Studies involving adult patients who require or are undergoing implant therapy, specifically where there is a need for augmentation of peri-implant soft tissue, whether due to insufficient tissue quantity or quality, or to enhance esthetic outcomes.
- Intervention (I)
- Description: Platelet-Rich Fibrin (PRF) as an adjunct for soft tissue enhancement.
- Details: PRF used either alone or in combination with other graft materials, applied during soft tissue augmentation techniques associated with implant therapy. Studies utilizing any variation of PRF (e.g., L-PRF, P-PRF, T-PRF, A-PRF) were included.
- Comparison (C)
- Description: Surgical procedures without the use of PRF.
- Details: Comparison to standard soft tissue augmentation methods such as free gingival grafts (FGG), connective tissue grafts (CTG), or no intervention. This also includes cases where soft tissue procedures were performed with the sole use of traditional graft materials or non-PRF biomaterials.
Outcomes (O)
Primary outcomes
Width of KT (KT): Measurement of the increase in keratinized mucosa around the implant site, a crucial factor for implant success and esthetic outcomes. This outcome was assessed in alignment with the STA-COSM (Soft Tissue Augmentation Core Outcome Set for Implant Dentistry) framework, as proposed by Tonetti et al. in 2023 [30]. This framework highlights the importance of KT as a key metric for evaluating the effectiveness of soft tissue augmentation interventions.
Soft Tissue Thickness (STT): Assessment of the increase in soft tissue thickness around the implant site, important for tissue stability and long-term esthetic results. The measurement of STT follows the guidelines established in the STA-COSM, ensuring a standardized approach to evaluating soft tissue augmentation outcomes in implant therapy [30].
Secondary outcomes
Esthetic Outcome: Evaluation of visual improvements in peri-implant soft tissue, including color, contour, and papilla height.
Clinical Significance: Assessment of the practical significance of observed differences between PRF and non-PRF interventions, including long-term implant success and tissue stability.
Cost-Efficiency: Comparison of the cost-effectiveness and ease of use of PRF relative to traditional methods, considering both clinical and economic perspectives.
Morbidity: Evaluation of patient discomfort, complications, and post-operative outcomes associated with PRF versus traditional techniques. This includes measures of pain, healing time, and complication rates.
Study design (S)
Types of studies:
Randomized Controlled Clinical Trials (RCTs): High-quality studies with random assignment of participants.
Comparative Studies: Studies comparing PRF to other techniques or no treatment.
Split-Mouth Studies: Studies where one side of the mouth receives PRF, and the other receives a comparative treatment for within-subject comparisons.
Parallel Arms Studies: Studies where participants are divided into different groups, each receiving different treatments for inter-group comparisons.
Research question
Based on the PICOS framework, the following question was formulated:
Is there any additional benefit of using PRF on soft tissue healing in implant therapy when compared to traditional approaches (such as free gingival grafts or connective tissue grafts)?
Search strategy
An electronic search of three databases (MEDLINE, EMBASE, CENTRAL) was performed. Articles published from December 31, 2017 were considered. An additional hand search was carried out including the bibliographies of the selected papers and collateral papers suggested by the databases.
Search terms
The electronic search strategy included terms related to the intervention and used the following combination of keywords, MeSH and Emtree terms: (((((((((((((((((((((dental implants) OR (tooth implant)) OR (alveolar ridge augmentation)) OR (socket preservation)) OR (tooth extraction)) OR (alveolar ridge preservation)) OR (immediate implant)) OR (postextractive implant)) OR (alveolar process)) OR (soft tissue)) OR (keratinized tissue)) OR (periimplant mucosa)) AND (platelet-rich fibrin)) OR (autologous platelet concentrate)) OR (thrombocyte rich plasma)) OR (leukocyte platelet-rich fibrin)) OR (pure platelet-rich fibrin)) OR (LPRF)) OR (l prf)) OR (advanced platelet-rich fibrin)) OR (APRF)) OR (A-PRF). Cochrane search filters for RCTs and CCTs were implemented, with cohort trials also included. The results were limited to human studies.
Inclusion criteria
Randomized clinical trials (RCT) or controlled clinical trials (CCT) or comparative studies (CS) including at least 10 patients/sites per group.
Studies reporting the outcomes of soft tissue healing around dental implants in combination with the use of platelet‐rich fibrin. Long term inflammatory parameters were not considered.
Exclusion criteria
In vitro and preclinical studies, cohort studies, case series, case reports, retrospective studies, RCTs or CCTs, with less than 10 patients/sites per group, studies not reporting soft tissue outcomes, and studies not meeting all inclusion criteria.
Data extraction
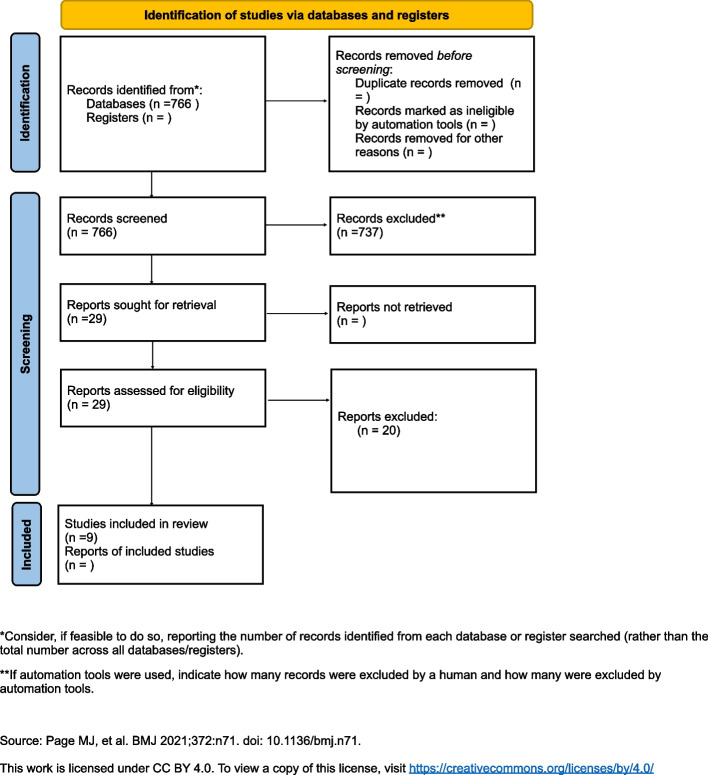
The search process is shown in Fig. 1. The included studies presented high heterogeneity regarding the timing of PRF usage, outcome measures, PRF preparation, or study duration. Therefore, a meta-analysis was not feasible. Instead, the data are reported in a systematic fashion characterizing all available literature to date. PROSPERO does not accept scoping reviews, literature reviews or mapping reviews, thus this review was not registered.
Fig. 1.
PRISMA flow-diagram
Screening and selection of studies
Publication records and titles identified by the electronic search and hand search were independently screened by two reviewers (EG and SM) based on the inclusion criteria. Discrepancies were solved by discussion. Cohen’s Kappa‐ coefficient was used as a measure of agreement between the readers. Thereafter, full texts of the selected abstracts were obtained. Where full texts could not be obtained authors and editors of the respective journal were contacted. The two reviewers independently performed the screening process, that is, from the MeSH/Emtree term search through the full‐text examination. Then, articles that met the inclusion criteria were processed for data extraction.
Data extraction and quality assessment
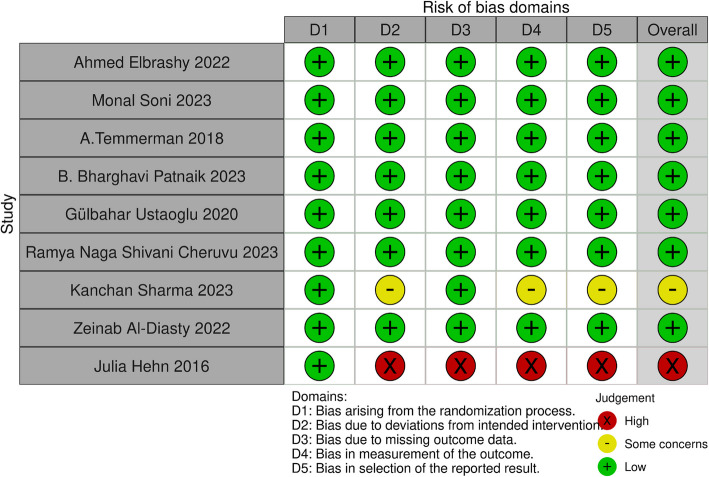
The inclusion criteria were applied for data extraction. The studies were classified according to study design and type of intervention. Then, outcomes were compiled in tables. All extracted data were double‐checked, and any questions that came up during the screening and the data extraction were discussed among the authors to reach consensus. Two reviewers (EG and SM) independently evaluated the methodological quality of all included studies using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials [31]. All included studies were assessed based on the following criteria: (a) sequence generation, (b) allocation concealment, (c) blinding of participants and personnel, (d) blinding of outcome assessment, (e) incomplete outcome data, (f) selective reporting, and (g) other potential biases. Any disagreements were resolved through discussion until consensus was reached. Each study was categorized into one of the following groups: low risk of bias if all quality criteria were rated as"present,"moderate risk of bias if one or more key domains were"unclear,"and high risk of bias if one or more key domains were deemed"not present"(Fig. 2).
Fig. 2.
Plot of the risk of bias domains for each included study
Results
Selection of studies
The literature search identified 766 potential references in Medline, of which 29 were eligible after title and abstract screening (inter‐reviewer agreement κ > 0.90). Of the 29 full‐ text articles, 20 articles were excluded based on their lack of controls or appropriate endpoints matching the search criteria. Nine studies did meet the inclusion criteria and therefore were assessed, eight randomized clinical trials and one prospective comparative study. The included studies are summarized in Table 1 [32–40].
Table 1.
Included studies
| Author (year) | Study design | Sample size | Test treatment | Control treatment | Follow up | PRF preparation | Outcome measures | Conclusion |
|---|---|---|---|---|---|---|---|---|
|
Ahmed Elbrashy 2022 [32] |
Randomized clinical trial |
20 | The PRF plug was packed into the buccal gap space the PRF plug was maintained in place with approximating sutures using 4–0 resorbable suture material | Xenograft | 6 months | 700 RPM for 12 min | Pink esthetic score (PES) |
There was no statistically significant difference between control and the test groups |
| Monal Soni 2023 [33] |
Randomized controlled clinical trial |
18 |
The L-PRF membrane was placed to fill the gap between the implant and the socket wall |
Natural healing | 6 months |
2700 RPM for 12 min |
Tissue biotype assessment |
L-PRF does offer benefits when placed in the extraction sockets for immediate implant placement sites in terms of a thicker tissue biotype |
|
A.Temmerman (2018) [34] |
Randomized controlled clinical trial |
8 |
The L-PRF membranes were fixed using resorbable sutures over a superficial, split-thickness flap preparation at the moment of implant uncovering |
Free gingival graft | 6 weeks | 2700 rpm for 12 min |
KT, shrinkage of KT |
L-PRF can increase the width of KM around implants. Furthermore, the use of LPRF results in a lower surgical time with less postoperative discomfort and pain for the patients in comparison to the FGG |
| B. Bharghavi Patnaik (2023) [35] |
Randomized controlled clinical trial |
10 |
The L-PRF was placed over the surface in the form of a poncho and the rest of the surrounding membrane was tucked in to the surrounding mucosa |
Natural healing | 6 weeks | NA |
KT, thickness of peri-implant mucosa STT (biotype), healing |
Sohn’s poncho technique in combination with L-PRF has the poten- tial to improve the thickness of peri-implant mucosa and the width of keratinized mucosa around implants |
|
Gülbahar Ustaoglu (2020) [36] |
Randomized controlled clinical trial |
30 |
The titanium prepared (T- PRF) membrane was inserted in the prepared mucoperiosteal flap at the facial site and secured with horizontal mattresses |
Connective tissue graft | 3 months |
2,700 rpm for 12 min Titanium PRF |
KT, STT |
Both groups experienced a greater increase in peri-implant STT at the OAC level, and T-PRF can be considered as an autogenous alternative to CTG |
| Included studies- 2 | ||||||||
|
Ramya Naga Shivani Cheruvu (2023) [37] |
Randomized controlled clinical trial |
40 | The PRF membrane was placed along with the healing abutment, using the poncho technique to cover the implant | Natural healing | 6 months |
2,700 rpm for 12 min |
width of keratinized tissue (WKT), and the thickness of keratinized tissue (TKT) | The PRF membrane enhances periimplant tissue wound healing, with gains in soft tissue width and thickness around nonsubmerged implants |
|
Kanchan Sharma (2023) [40] |
Comparative study | 15 |
PRF membrane in the osteotomy site and over it |
Natural healing | 9 months | 10-min centrifugation at 3000 RPM | Jemt papilla index | Statistically significant differences for the papilla index score at baseline and six and nine months of loading |
|
Zeinab Al- Diasty 2022 [38] |
Split mouth randomized clinical study |
15 |
At the moment of implant exposure, a partial thickness flap was reflected and apically displaced to receive either a PRF membrane or a FGG |
Free gingival graft | 3 months |
3,000 rpm for 10 min |
KT, graft shrinkage |
The Onlay PRF membrane increased the KT width with the advantages of a lower surgical time and less postoperative pain in comparison to the FGG. FGG had a significantly higher ability to augment and increase KT |
| Julia Hehn 2016 [39] |
Randomized controlled clinical trial |
31 |
PRF membrane over a recipient bed created with splitthickness flap |
Natural healing | 6 months | NA | Tissue thickness |
Soft tissue augmentation with PRF performed with a split-flap technique cannot be recommended for thickening thin mucosa |
The effect of PRF on keratinized mucosa width (KT)
The evaluation of PRF on the keratinized soft tissue regeneration and/or healing has been investigated in eight studies. In all studies, PRF was associated with an increase of the width of peri-implant keratinize tissue, the increase was significantly greater when compared with a negative control group, but smaller when compared with a free gingival graft (FGG). Studies were divided according to the timing of implant placement, either immediate or delayed.
In their split-mouth, randomized, controlled pilot clinical study, Temmerman and colleagues tested L-PRF membranes against a FGG harvested from the palate in eight patients in need for bilateral widening of the KT around implants in the lower jaw presenting less than 2 mm buccal KT [34]. In both groups, a buccal split-thickness flap was raised and repositioned apically in order to receive either PRF or the FGG. The authors set the cut-off from clinical efficacy at ≥ 2 mm gain in buccal KT. The total bucco-lingual width of KT was significantly increased in both groups; the mean gain of KT varied from 6.0 mm ± 0.8 for the test group to 7.3 mm ± 1.2 for the control group, with 1.3 mm ± 0.9 extra gain (P < 0.05) for the FGG sites. The mean amount of KT vestibular for the implant at the test site was 3.3 mm ± 0.9 and 3.8 mm ± 1.0 at the control site. Shrinkage of the augmented sites 6 weeks later were slightly higher at the test site (32.1%) than at the control side (23.6%). However, this difference was not statistically significant.
In their split mouth randomized controlled clinical trial, Patnaik and colleagues tested the efficacy of the L-PRF membrane using Sohn’s poncho technique over implants placed in the mandibular posterior region in peri-implant mucosal enhancement at second stage surgery [35]. At control sites, a conventional healing abutment was placed. While there was a consistent increase in the width of KT from baseline (2.25 ± 0.44 mm), 4 weeks (3.52 ± 0.62 mm) to 6 weeks (3.65 ± 0.64 mm) in the test group, there was a slight and non-significant decline from the 4 weeks’ to 6 weeks’ time point in the control group. The comparative analysis of healing scores between test and control groups led to a significant difference in healing at 1 week and 2 weeks’ time points, with L-PRF group denoting faster healing.
Ustaoglu and al compared Titanium-Prepared PRF (T-PRF) versus connective tissue graft (CTG) contextual with implant placement in thin soft tissue areas [36]. Both the CTG and the T-PRF were inserted in the prepared mucoperiosteal flap at the facial site and secured with horizontal mattresses. Comparison of the baseline and 3-month measurements from the 2 groups showed a significant increase in KTW. Compared with the test group, the control group performed better.
Cheruvu et al. reported the outcomes of a randomized controlled clinical trial comparing the effects of PRF around non submerged implants against a negative control [37]. The PRF membrane was placed along with the healing abutment, using the poncho technique to cover the implant. The authors reported a gain in KT w in the test group vs the negative control at 6 months post-op: on average, 1.25 ± 0.67 mm were gained in the test group and 0.59 ± 0.35 mm in the control group.
Zeinab Al-Diasty and colleagues compared the use of PRF versus the FGG during the second stage to increase the amount of KT split-mouth randomized clinical study with fifteen patients [38]. In both groups, a partial thickness flap was reflected and apically displaced to achieve an adequate recipient site. There was a significant increase in KT after 1 and 3 months in both groups. The KT in the FGG group was significantly higher than that in the PRF group after 1 and 3 months and PRF group showed a significantly higher mean shrinkage percentage in the PRF group compared to the FGG group.
In summary, the collected RCTs have demonstrated that the use of PRF leads to significant KT gain around both submerged and non-submerged implants. While free gingival grafts performed better, the difference was not clinically relevant as per the a priori success parameters defined by the authors (KT ≥ 2 mm and peri-implant soft tissue health or bone levels). Within 3- to 6-month observation period, the shrinkage of KT in PRF group was higher than FGG group, but eventually became moot thereafter.
The effect of PRF on soft tissue thickness (STT)
The evaluation of PRF on soft tissue thickness has been investigated in six studies. In all studies, PRF was associated with a significant increase of the soft tissue thickness. Only CTG placed under the muco-periosteal flap outperformed PRF, but the difference was not clinically relevant.
The RCCT by Soni and colleagues explored the outcomes of a PRF membrane secured over immediate implants when compared with a negative control [33]. The study included tissue biotype or STT assessment amongst primary outcome measures. The tissue thickness was measured using an endodontic reamer and, on average, PRF group outperformed the negative control group.
Patnaik and colleagues, in their split mouth RCCT exploring outcomes for PRF with Sohn’s poncho technique, showed a statistically significant increase in soft tissue thickness at 4 weeks (3.73 ± 0.34 mm vs 2.21 ± 0.37 mm) and 6 weeks (4.20 ± 0.37 mm vs 2.4 ± 0.48 mm) at the buccal site for the test group [35].
In the study by Ustaoglu and colleagues comparing titanium-prepared PRF (T-PRF) and CTG, both groups experienced an increase in peri-implant STT at the crestal level, still, the CTG group achieved better results, but the difference was not significant [36].
In the RCCT by Cheruvu and colleagues on non-submerged implants comparing a PRF poncho over the healing abutment with a negative control, the mean facial STT scores between the 2 groups, significant gains were observed in test group vs control group at 3 and 6 months post-op: on average, 0.97 ± 0.12 mm were gained in the test group and 0.59 ± 0.57 mm in the control group [37]. Thus, compared to natural healing, in the case of non-submerged implants, the use of a PRF poncho led to a double the thickness of facial soft tissues.
In their studies, Hehn and colleagues randomized 31 patients in the lower mandible using a split-flap technique [39]. In the test group, mucosa was treated with a PRF membrane. In the control group, implantation was realized without soft tissue augmentation. Soft tissue augmentation with PRF led to a significant tissue loss. In the test group, the crestal tissue thickness dropped from 2.20 mm ± 0.48 SD at baseline to 0.9 mm ± 1.02 SD at reentry, whereas crestal mucosa in the control group showed higher stability (2.64 mm ± 0.48 SD at baseline to 2.62 mm ± 0.61 SD at reentry). The authors decided to interrupt test group allocations.
In summary, with the exception of Hehn’s study, the collected RCTs have demonstrated that the use of PRF leads to a significant increase of soft tissue thickness around implants. While in one study the CTG performed better, the difference was not clinically relevant.
The effect of PRF on the esthetic outcome
Only two studies included the esthetic outcome in the analysis of PRF performance around implants.
In their RCT, Ahmed Elbrashy and colleagues compared the effect of xenograft or PRF to graft the jumping gap in immediate implant placement in the maxillary premolar region [32]. No significant differences in pink esthetic score could be observed by the authors.
In their comparative study, Kanchan Sharma and colleagues compared implant placement with or without the use of PRF as an osteotomy site healing enhancer [40]. The authors reported significant better outcomes for the test group in terms of interdental papilla representation (measured with the Jemt index).
In summary there is little information regarding the effect of PRF on the esthetic outcome of implants. The lack of information seems to be related to lack of reporting from authors.
A graphic summary of the results is presented in Fig. 3 (R version 4.3.2 (2023–10–31) –"Eye Holes").
Fig. 3.
Radar graphic summary of the systematic review on the effectiveness of PRF against CTG and FGG. Values closer to 2 describe more desirable outcomes. 0: Indicates the least desirable outcome or no observed benefit. 1: Represents an intermediate or moderate level of benefit. 2: Denotes the most desirable or optimal outcome
Discussion
In this systematic review, randomized clinical trials and comparative studies assessing the role of PRF in peri-implant soft tissue healing were analyzed. PRF's performance was compared either to a negative control or to conventional treatments, such as connective tissue grafts and free gingival grafts. The studies revealed considerable heterogeneity in surgical techniques; however, the overall evidence supported PRF's effectiveness in enhancing the amount of keratinized tissue and the thickness of the peri-implant mucosa, which were the primary outcomes of this review. Despite the clear need for further research, PRF shows potential for regenerating and improving soft tissues around dental implants. To our knowledge, this is the first systematic review investigating the benefits of PRF for peri-implant soft tissues.
PRF’s ability to enhance soft tissue healing has already been demonstrated in other clinical contexts, such as tooth extractions, management of oro-antral communications, and osteonecrotic lesions of the jaws [41–43]. A recent review evaluated the use of autologous platelet concentrates as adjuvant therapies for the surgical management of medication-related osteonecrosis of the jaws (MRONJ), analyzing 58 articles [44]. Compared to surgical treatment alone, PRP and L-PRF applications appear to improve healing outcomes in MRONJ management.
Despite its versatile and promising results, there is still a lack of consensus on the optimal use of PRF, particularly regarding the type of PRF (P-PRF, L-PRF, A-PRF, T-PRF, H-PRF). Future research should categorize results based on these PRF variants to standardize applications and improve comparative analyses.
PRF offers a biotechnological solution capable of releasing growth factors (GFs) essential for tissue repair over time. Recent studies have also explored PRF's potential in cancer treatment. Indirect PRF treatment of tumor cells led to a significant reduction in viable cancer cells, particularly with low-RCF PRF, suggesting potential future applications in localized tumor management [45].
However, the histoconductive properties of PRF and other natural biomaterials warrant critical examination, as they may be ineffective or even inhibitory for keratinized tissue promotion at the implant level. The studies included in the present review consistently demonstrated that PRF significantly increased the width of peri-implant keratinized tissue compared to negative control groups, although the increase was less pronounced when compared to results from free gingival grafts (FGG). The positive effect of PRF was evident regardless of whether implant placement was immediate or delayed.
These clinical outcomes align with numerous proof-of-concept, in vitro, and preclinical studies that have demonstrated PRF's efficacy in enhancing soft tissue regeneration. L-PRF can accelerate wound healing by stimulating fibroblast wound closure in vitro and enhancing fibroblasts'ability to promote endothelial tube formation [46, 47]. It has also been shown to boost cell proliferation in various cells involved in soft tissue repair, stimulate the mitogenic activity of endothelial cells vital for angiogenesis, and release a range of growth factors into the surrounding microenvironment [48, 49]. These growth factors serve as chemotactic agents for various cell types, such as monocytes, fibroblasts, endothelial cells, and stem cells, creating favorable tissue microenvironment and directly influencing the proliferation and differentiation of progenitor cells [50]. This is primarily due to the increased angiogenesis at defect sites, driven by enhanced microvascularization [28]. For this reasons, clinically, L-PRF has been used to protect resorbable barrier membranes in guided bone regeneration (GBR) procedures in cases of flap dehiscence after bone augmentation. Talon et al. reported higher cell adhesion and spreading on expanded polytetrafluoroethylene (e-PTFE) membranes when coated with L-PRF [51]. This is significant for this type of membrane due to the high rate of flap dehiscence or perforation. The same principle applies when L-PRF is used at the palatal donor site after harvesting a free gingival graft or connective tissue graft [52]. Various studies have shown faster wound healing and less postoperative discomfort when applying L-PRF to wounds [53, 54].
It is important to notice that its ease of use, combined with its low cost and low morbidity, and the autologous source, makes PRF an ideal biomaterial for peri-implant soft tissue establishment and enhancement. Thus, studies reporting no differences with the standard comparison, FGG or CTG, are actually testifying the potential of PRF even more, as both FGG and CTG are associated with greater patients’ morbidity. Also, the same studies reporting greater outcomes in terms of soft tissue keratinization and thickening in FGG and CTG groups are also disclosing the absence of a real clinical differential between the techniques, as 6 months after surgeries, no patient displayed signs of tissue inflammation or crestal bone loss.
The greater thickening of peri-implant soft tissues with CTG could be related to the significant histoconduction of this biomaterial once implanted in the recipient area [55]. The same could be inferred for tissue keratinization and FGG; nevertheless, studies have been pointing out the ability of PRF to induce keratinization and this phenomenon is consistently reported in the case of second intention healing scenarios [56]. All studies that implemented a PRF membrane versus a negative control denoted better results in terms of both KT and STT. The PRF group also demonstrated faster healing of the wound.
In contradiction with the rest of the studies, the RCT by Hehn and colleagues found that PRF led to significant tissue loss, prompting them to halt PRF allocations due to better stability in the control group [39]. The authors themselves recognized that the possible explanation for their failure relied in the flap design, which was a split thickness flap. It is likely that flap release maneuvers may have masked the final outcome as they are poorly forgiving when compared to a negative control. Also, the PRF membrane might perform better when left exposed and induce granulation tissue formation and cells diapedesis and differentiation [57, 58]. Biological information for tissue thickening is associated with periosteal detachment and following granulation tissue accumulation [59].
While aesthetic outcomes are important, they have not been widely studied in relation to PRF in peri-implant tissues. Future research should prioritize uniformity in aesthetic measurements and follow-up periods to draw more definitive conclusions on PRF’s impact on esthetics.
Most authors failed to assess the esthetic outcome, so it is likely that the evidence is already there, but only a few cared to share.
The authors of 2024 Consensus Statements and Recommended Clinical Procedures Regarding Optimizing Esthetic Outcomes in Implant Dentistry made a general observation, that the available data on esthetic outcomes were predominantly represented by case series studies [60]. The authors belief is that given the importance of the prevention of esthetic complications, it is difficult to construct a RCT around a technique that may increase the risk of adverse esthetic outcomes. In the specific case of soft tissue augmentation procedures, the authors conclude that future research in soft tissue augmentation should focus on standardized outcomes and long-term studies to assess the stability and esthetic impact of various techniques, including innovative materials like PRF. Comparative trials and patient-centered metrics are essential to refine protocols and improve predictability, ensuring optimal esthetic results in implant-supported therapies.
When comparing all the articles included in this review, there were biases related to the study type, patient characteristics, and variations in medical and surgical protocols. These factors can potentially confound the evaluation of treatment outcomes. Typical confounding factors included the type of jaw involved, location of the implant within the jaw, PRF method of preparation, the position of the PRF membrane/plug within the implant site -over it, around it, secured to the flap, in a poncho fashion-). Another confounding factor affecting treatment outcomes, that is the moment for soft tissue augmentation—either at the time of implant insertion or during the second surgical procedure -, was not homogeneous among studies. Factors that may have affected soft tissue outcomes such as the vertical bone height, the number of implants, and muscle hyperactivity (level of muscle attachment) were not considered by most studies.
The present review has a few specific limitations: studies were not categorized based on the type of PRF used. Also, the nine studies differed for the implant placement protocol (immediate post-extraction or delayed), as well as for the implant healing approach (submerged or tissue-level). The heterogeneity of the included studies prevented a meta-analysis of the results for any of the explored soft tissue outcome measures; thus, a quantitative analysis was not feasible. Also, the included studies differed in the type of indexes and success criteria applied, making a comparison difficult.
Future research directions
Standardization of surgical procedures aimed at ameliorating peri-implant soft tissue outcomes with PRF membrane/plug is needed to facilitate clinical implementation of the technique and the comparison between studies. There is need of randomized clinical trials with long follow-up period assessing the soft tissue outcomes of PRF in combination with implant therapy. Future studies on PRF should standardize methodologies to enable meta-analysis. Well-designed randomized clinical trials or prospective case series studies of consecutively enrolled subjects with clearly defined inclusion and exclusion criteria could provide important information to validate surgical procedures and materials associated with PRF preparation and use.
Conclusion
Within the limitation of this systematic review, the following conclusions can be drawn:
PRF significantly enhances the width of keratinized mucosa () and soft tissue thickness (STT) around implants compared to negative controls.
While PRF shows promising results, free gingival grafts (FGG) often yield slightly better outcomes, though these differences are not clinically significant.
Clinically, PRF stands out due to its ease of use, low cost, low morbidity, and autologous nature. Even studies reporting no significant differences between PRF and traditional grafts (e.g., FGG, CTG) emphasize PRF's potential, as traditional methods are associated with higher morbidity. At six months post-surgery, studies revealed no signs of tissue inflammation or crestal bone loss in either group, supporting PRF as a viable alternative.
The impact of PRF on esthetic outcomes remains underreported and requires further investigation.
Overall, PRF appears to be a beneficial adjunct in soft tissue management for implant therapy, with further research needed to fully elucidate its effects on esthetic results.
Supplementary Information
Acknowledgments
Clinical trial number
Not applicable.
Abbreviations
- A-PRF
Advanced Platelet-Rich Fibrin
- CCT
Controlled Clinical Trials
- CTG
Connective Tissue Graft
- FGG
Free Gingival Graft
- ID-COSM
Implant Dentistry Core Outcome Set and Measurement
- KT
Keratinized Tissue
- L-PRF
Leukocyte-Platelet-Rich Fibrin
- PICO
Population, Intervention, Comparison, Outcome
- PICOS
Population, Intervention, Comparison, Outcome, Study Design
- PRF
Platelet-Rich Fibrin
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- RCT
Randomized Controlled Trial
- RCCT
Randomized Controlled Clinical Trial
- STA-COSM
Soft Tissue Augmentation Core Outcome Set for Implant Dentistry
- STT
Soft Tissue Thickness
- T-PRF
Titanium-Prepared Platelet-Rich Fibrin
Authors’ contributions
EG: Concept/Design, Data analysis/interpretation, Drafting article, Critical revision of article, Approval of article, Data collection; NB: Drafting article, Critical revision of article, Approval of article; UC: Drafting article, Critical revision of article, Approval of article; MM: Drafting article, Critical revision of article, Approval of article; PP: Data analysis/interpretation, Drafting article, Critical revision of article, Approval of article; SM: Concept/Design, Data analysis/interpretation, Drafting article, Critical revision of article, Approval of article, Data collection.
Funding
The author’s declare no funding.
Data availability
All the data that supports the findings of this study are available in the cited publications. No new data were generated for this study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thoma DS, Naenni N, Figuero E, Hämmerle CHF, Schwarz F, Jung RE, Sanz-Sánchez I. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 15):32–49. 10.1111/clr.13114. [DOI] [PubMed] [Google Scholar]
- 2.Suárez-López Del Amo F, Lin GH, Monje A, Galindo-Moreno P, Wang HL. Influence of soft tissue thickness on peri-implant marginal bone loss: a systematic review and meta-analysis. J Periodontol. 2016;87(6):690–9. 10.1902/jop.2016.150571. [DOI] [PubMed] [Google Scholar]
- 3.Giannobile WV, Jung RE, Schwarz F; Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 1—Effects of soft tissue augmentation procedures on the maintenance of peri-implant soft tissue health. Clin Oral Implants Res. 2018;29(Suppl 15):7–10. 10.1111/clr.13110 [DOI] [PubMed]
- 4.Schwarz F, Ramanauskaite A. It is all about peri-implant tissue health. Periodontol 2000. 2022;88(1):9–12. 10.1111/prd.12407. [DOI] [PubMed] [Google Scholar]
- 5.Roccuzzo M, Roccuzzo A, Marruganti C, Fickl S. The importance of soft tissue condition in bone regenerative procedures to ensure long-term peri-implant health. Periodontol 2000. 2023;93(1):129–38. 10.1111/prd.12496. [DOI] [PubMed] [Google Scholar]
- 6.Canullo L, Menini M, Covani U, Pesce P. Clinical outcomes of using a prosthetic protocol to rehabilitate tissue-level implants with a convergent collar in the esthetic zone: a 3-year prospective study. J Prosthet Dent. 2020;123:246–51. 10.1016/j.prosdent.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Hämmerle CHF, Chen ST, Wilson TG Jr. Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. Int J Oral Maxillofac Implants. 2004;19(Suppl):26–8. [PubMed] [Google Scholar]
- 8.Bassir SH, El Kholy K, Chen CY, Lee KH, Intini G. Outcome of early dental implant placement versus other dental implant placement protocols: A systematic review and meta-analysis. J Periodontol. 2019;90(5):493–506. 10.1002/JPER.18-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitman J, Seyssens L, Christiaens V, Cosyn J. Immediate implant placement with or without immediate provisionalization: a systematic review and meta-analysis. J Clin Periodontol. 2022;49(10):1012–23. 10.1111/jcpe.13686. [DOI] [PubMed] [Google Scholar]
- 10.Phipps MC, Xu Y, Bellis SL. Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. PLoS ONE. 2012;7:e40831. 10.1371/journal.pone.0040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nevins M, Kao RT, McGuire MK, et al. Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial. J Periodontol. 2013;84:456–64. 10.1902/jop.2012.120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavelli L, Ravidà A, Barootchi S, Chambrone L, Giannobile WV. Recombinant human platelet-derived growth factor: a systematic review of clinical findings in oral regenerative procedures. JDR Clin Trans Res. 2021;6(2):161–73. 10.1177/2380084420938960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borzini P, Mazzucco L. Platelet gels and releasates. Curr Opin Hematol. 2005;12(6):473–9. 10.1097/01.moh.0000177831.70657.e8. [DOI] [PubMed] [Google Scholar]
- 14.Egierska D, Perszke M, Mazur M, Duś-Ilnicka I. Platelet-rich plasma and platelet-rich fibrin in oral surgery: a narrative review. Dent Med Probl. 2023;60(1):177–86. 10.17219/dmp/147298. [DOI] [PubMed] [Google Scholar]
- 15.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–67. 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353–60. 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 17.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun’s Platelet-Rich Fibrin (PRF) clot and membrane. J Periodontol. 2010;81(4):546–55. 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 18.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):707–13. 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie. 2001;42:55–62. [Google Scholar]
- 20.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 21.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–44. 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Cirmeni M, Fedele O, Giammarinaro E, Marconcini S, Covani U, Caso G. Immediate implant and socket preservation using sticky bone and leukocyte-platelet-rich fibrin in the anterior maxilla: a 3-year case report. Clin Adv Periodontics. 2023;13(3):144–8. 10.1002/cap.10202. [DOI] [PubMed] [Google Scholar]
- 23.Miron RJ, Zucchelli G, Pikos MA, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21(6):1913–27. 10.1007/s00784-017-2133-z. [DOI] [PubMed] [Google Scholar]
- 24.Zwittnig K, Mukaddam K, Vegh D, et al. Platelet-rich fibrin in oral surgery and implantology: a narrative review. Transfus Med Hemother. 2022;50(4):348–59. 10.1159/000527526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss FJ, Stähli A, Gruber R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: a systematic review. Clin Oral Implants Res. 2018;29(Suppl 18):6–19. 10.1111/clr.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakur V, Mittal S, Tewari S, et al. Comparative histological evaluation of two PRF formulations (PRF High and PRF Medium) on quality of life and healing outcome of apicomarginal defects: A randomized clinical trial. J Craniomaxillofac Surg. 2023;51(3):166–77. 10.1016/j.jcms.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e56–60. 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Singh N, Kashyap M. Is autologous sticky bone better than a simple mixture of autologous PRF and bioactive glass in the regeneration of human periodontal intrabony defects? An extensive clinical and CBCT study. Int J Periodontics Restorative Dent. 2023;43(7):s264–82. 10.11607/prd.6152. [DOI] [PubMed] [Google Scholar]
- 29.Silva FFVE, Chauca-Bajaña L, Caponio VCA, et al. Regeneration of periodontal intrabony defects using platelet-rich fibrin (PRF): A systematic review and network meta-analysis. Odontology. Published online May 21, 2024. 10.1007/s10266-024-00949-7. [DOI] [PMC free article] [PubMed]
- 30.Tonetti MS, Sanz M, Avila-Ortiz G, Berglundh T, Cairo F, Derks J, Figuero E, Graziani F, Guerra F, Heitz-Mayfield L, Jung RE, Lai H, Needleman I, Papapanou PN, Sailer I, Sanz-Sanchez I, Schwarz F, Shi J, Thoma D. Relevant domains, core outcome sets and measurements for implant dentistry clinical trials: The Implant Dentistry Core Outcome Set and Measurement (ID-COSM) international consensus report. Clin Oral Implants Res. 2023;34(Suppl 25):4–21. 10.1111/clr.14074. PMID: 37232121. [DOI] [PubMed] [Google Scholar]
- 31.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. 10.1136/bmj.l4898. [DOI] [PubMed]
- 32.Elbrashy A, Osman AH, Shawky M, Askar N, Atef M. Immediate implant placement with platelet rich fibrin as space filling material versus deproteinized bovine bone in maxillary premolars: a randomized clinical trial. Clin Implant Dent Relat Res. 2022;24(3):320–8. 10.1111/cid.13075. [DOI] [PubMed] [Google Scholar]
- 33.Soni M, Gugnani S, Pandit N, Bali D, Sharma M. Efficacy of leukocyte-platelet-rich fibrin membrane in immediate postextraction implant placement: a randomized controlled trial. J Indian Soc Periodontol. 2023;27(1):63–9. 10.4103/jisp.jisp_219_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Temmerman A, Cleeren GJ, Castro AB, Teughels W, Quirynen M. L-PRF for increasing the width of keratinized mucosa around implants: a split-mouth, randomized, controlled pilot clinical trial. J Periodontal Res. 2018;53(5):793–800. 10.1111/jre.12568. [DOI] [PubMed] [Google Scholar]
- 35.Patnaik BB, Penmetsa GS, Raju MS, et al. Peri-implant mucosal enhancement using leukocyte platelet rich fibrin under Sohn’s poncho technique: a randomized controlled clinical trial. Clin Adv Periodontics. 2024;14(2):134–41. 10.1002/cap.10259. [DOI] [PubMed] [Google Scholar]
- 36.Ustaoğlu G, Paksoy T, Gümüş KÇ. Titanium-prepared platelet-rich fibrin versus connective tissue graft on peri-implant soft tissue thickening and keratinized mucosa width: a randomized. Controlled Trial J Oral Maxillofac Surg. 2020;78(7):1112–23. 10.1016/j.joms.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Cheruvu RNS, Katuri KK, Dhulipalla R, et al. Evaluation of soft tissue and crestal bone changes around non-submerged implants with and without a platelet-rich fibrin membrane: a randomized controlled clinical trial. Dent Med Probl. 2023;60(3):437–43. 10.17219/dmp/150408. [DOI] [PubMed] [Google Scholar]
- 38.Al-Diasty Z, El-Meadawy S, Salem AS, Mowafey B. Onlay platelet-rich fibrin membrane versus free gingival graft in increasing the width of keratinized mucosa around dental implants: a split-mouth randomized clinical study. J Adv Periodontol Implant Dent. 2022;14(2):53–61. 10.34172/japid.2022.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hehn J, Schwenk T, Striegel M, Schlee M. The effect of PRF (platelet-rich fibrin) inserted with a split-flap technique on soft tissue thickening and initial marginal bone loss around implants: results of a randomized, controlled clinical trial. Int J Implant Dent. 2016;2(1):13. 10.1186/s40729-016-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma K, Roy S, Kumari A, et al. A comparative evaluation of soft and hard tissue changes around dental implants placed with and without platelet-rich fibrin. Cureus. 2023;15(3):e36908. 10.7759/cureus.36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lahham C, Ta'a MA, Lahham E, Michael S, Zarif W. The effect of recurrent application of concentrated platelet-rich fibrin inside the extraction socket on the hard and soft tissues. a randomized controlled trial. BMC Oral Health. 2023;23(1):677. 10.1186/s12903-023-03400-5. PMID: 37726689; PMCID: PMC10507883. [DOI] [PMC free article] [PubMed]
- 42.Salgado-Peralvo AO, Mateos-Moreno MV, Uribarri A, Kewalramani N, Peña-Cardelles JF, Velasco-Ortega E. Treatment of oroantral communication with Platelet-Rich Fibrin: a systematic review. J Stomatol Oral Maxillofac Surg. 2022;123(5):e367–75. 10.1016/j.jormas.2022.03.014. Epub 2022 Mar 19 PMID: 35318134. [DOI] [PubMed] [Google Scholar]
- 43.Asfour MAR, Aljoujou AA, Saifo MS, Jabban HAL. The use of advanced-platelet rich fibrin (A-PRF) in the management of medication-related osteonecrosis of the jaw (MRONJ): A case report. Clin Case Rep. 2023;11(11):e8259. 10.1002/ccr3.8259.PMID:38028038;PMCID:PMC10675097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennardo F, Barone S, Antonelli A, Giudice A. Autologous platelet concentrates as adjuvant in the surgical management of medication-related osteonecrosis of the jaw. Periodontol 2000. 2024. 10.1111/prd.12608. Epub ahead of print. PMID: 39345044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dohle E, Parkhoo K, Bennardo F, Schmeinck L, Sader R, Ghanaati S. Immunomodulation of cancer cells using autologous blood concentrates as a patient-specific cell culture system: a comparative study on osteosarcoma and fibrosarcoma cell lines. Bioengineering (Basel). 2024;11(4):303. 10.3390/bioengineering11040303. PMID:38671725; PMCID: PMC11048113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanco J, García Alonso A, Hermida-Nogueira L, Castro AB. How to explain the beneficial effects of leukocyte- and platelet-rich fibrin. Periodontol 2000. Published online June 24, 2024. 10.1111/prd.12570. [DOI] [PMC free article] [PubMed]
- 47.Ratajczak J, Vangansewinkel T, Gervois P, et al. Angiogenic properties of ‘leukocyte- and platelet-rich fibrin.’ Sci Rep. 2018;8(1):14632. 10.1038/s41598-018-32929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pitzurra L, Jansen IDC, de Vries TJ, Hoogenkamp MA, Loos BG. Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J Periodontal Res. 2020;55(2):287–95. 10.1111/jre.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miron RJ, Fujioka-Kobayashi M, Bishara M, et al. Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue Eng Part B Rev. 2017;23(1):83–99. 10.1089/ten.teb.2016.0233. [DOI] [PubMed] [Google Scholar]
- 50.Sclafani AP, Romo T 3rd, Ukrainsky G, et al. Modulation of wound response and soft tissue ingrowth in synthetic and allogeneic implants with platelet concentrate. Arch Facial Plast Surg. 2005;7(3):163–9. 10.1001/archfaci.7.3.163. [DOI] [PubMed] [Google Scholar]
- 51.Talon I, Schneider A, Ball V, Hemmerlé J. Functionalization of PTFE materials using a combination of polydopamine and platelet-rich fibrin. J Surg Res. 2020;251:254–61. 10.1016/j.jss.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 52.Torumtay Cin G, Lektemur Alpan A, Açikgöz G, Özlü UG. Ultrasonographic analysis of palatal donor site healing accelerated with platelet-rich fibrin following subepithelial connective tissue harvesting. J Appl Oral Sci. 2024;32:e20230448. 10.1590/1678-7757-2023-0448. [DOI] [PubMed] [Google Scholar]
- 53.de Almeida MCL, Rocha RGG, Magno MB, Lima RR, Saito MT. Performance of multiple therapeutic approaches for palatal wound healing after soft tissue graft removal - an overview of systematic reviews. Clin Oral Investig. 2024;28(6):347. 10.1007/s00784-024-05733-z. [DOI] [PubMed] [Google Scholar]
- 54.Lektemur Alpan A, Torumtay CG. PRF improves wound healing and postoperative discomfort after harvesting subepithelial connective tissue graft from palate: a randomized controlled trial. Clin Oral Investig. 2020;24(1):425–36. 10.1007/s00784-019-02934-9. [DOI] [PubMed] [Google Scholar]
- 55.Tavelli L, Barootchi S, Stefanini M, Zucchelli G, Giannobile WV, Wang HL. Wound healing dynamics, morbidity, and complications of palatal soft-tissue harvesting. Periodontol 2000. 2023;92(1):90–119. 10.1111/prd.12466. [DOI] [PubMed] [Google Scholar]
- 56.Cui A, Zhou J, Mudalal M, et al. Soft tissue regeneration around immediate implant placement utilizing a platelet-rich fibrin membrane and without tightly flap closure: Two case reports. Medicine (Baltimore). 2020;99(40):e22507. 10.1097/MD.0000000000022507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salgado-Peralvo AO, Uribarri A, Kewalramani N, Peña-Cardelles JF, Liñares A. The use of platelet-rich fibrin in vestibuloplasty: a 36-month follow-up technique report. Clin Adv Periodontics. 2023;13(1):33–7. 10.1002/cap.10201. [DOI] [PubMed] [Google Scholar]
- 58.Boutros S, Bernard RW, Galiano RD, Addona T, Stokes B, McCarthy JG. The temporal sequence of periosteal attachment after elevation. Plast Reconstr Surg. 2003;111(6):1942–7. 10.1097/01.PRS.0000055045.08656.D0. [DOI] [PubMed] [Google Scholar]
- 59.Harrison JW, Jurosky KA. Wound healing in the tissues of the periodontium following periradicular surgery. 2. The dissectional wound. J Endod. 1991;17(11):544–52. 10.1016/s0099-2399(06)81720-6. [DOI] [PubMed] [Google Scholar]
- 60.Morton D, Chen ST, Martin WC, Levine RA, Buser D. Consensus statements and recommended clinical procedures regarding optimizing esthetic outcomes in implant dentistry. Int J Oral Maxillofac Implants. 2014;29(Suppl):216–20. 10.11607/jomi.2013.g3. PMID: 24660199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data that supports the findings of this study are available in the cited publications. No new data were generated for this study.