Abstract
Mutations underlying hereditary cataracts in two families impair the function of an enzyme that synthesizes the lens molecule lanosterol. The finding may lead to non-surgical prevention and treatment of cataracts.
In this issue, Zhao et al.1 (page 607) identify a mutation in the gene encoding the enzyme lanosterol synthase (LSS) as the cause of inherited cataracts in two families. LSS, which is produced in the lens, synthesizes lanosterol, a molecule that is amphipathic (that is, it has both hydrophilic and hydrophobic properties). The authors show that lanosterol can dissolve the precipitates, and even the amyloid-like fibril structures, of mutant lens crystallin proteins that are the cause of cataracts in some individuals. Furthermore, lanosterol effectively treated naturally occurring cataracts in rabbit lenses and in dogs in vivo. In addition to elucidating the visual process, this work promises to continue in the tradition of lens research by expanding scientific insight into broad and often seemingly unrelated areas of enquiry.
The eye lens has been intensively studied for almost two centuries. In 1833, optics scientist David Brewster deduced the fine structure of the cod lens, calculating that it contained 5 million fibre cells, each 4.8 millimetres long, using only a candle and a finely ruled steel bar2. In 1901, embryologist Hans Spemann’s study of lens development resulted in the concept of inductive cellular interactions during embryonic development3. Studies of lens biochemistry began in the late nineteenth century with descriptions of the high concentrations of heterogeneous structural proteins now known as crystallins4. Subsequently, one of the first genetic locations on a non-sex chromosome to be associated with disease was linked to cataract susceptibility5, and messenger RNA molecules encoding chicken lens δ-crystallins were among the first mRNAs to be isolated, cloned and studied6.
The function of the eye lens is to transmit light and focus it on the retina. The lens accomplishes this through a single cell type that follows a developmental pattern, beginning as a member of the germinative zone in the single layer of anterior epithelial cells overlaying a mass of fibre cells. The epithelial cells then migrate laterally towards the lens equator, where they elongate and invert to form secondary fibre cells, arranged in a curved, onion-like configuration. As they do this, the cells synthesize large amounts of crystallins, such that they contain perhaps the highest concentration of proteins found in any tissue. They also degrade organelles, minimize extracellular space and increase the density of their cell membranes to levels approaching that of the cell’s cytoplasm, all of which decrease light scattering7. Thus, transparency is accomplished largely through a combination of the microarchitecture of the lens and, on a molecular level, the densely packed lens crystallins (Fig. 1).
Figure 1 |. The eye lens.
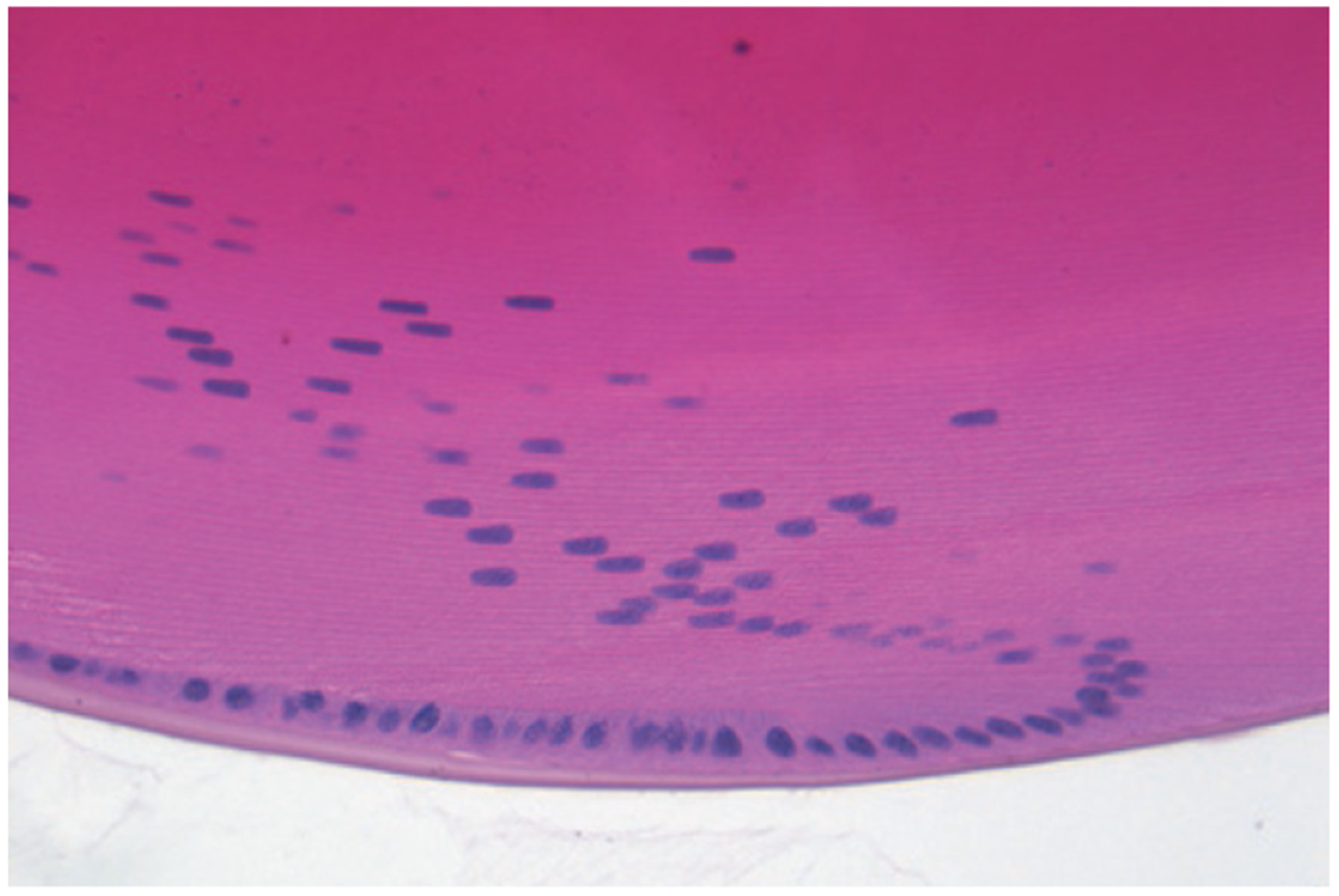
This cross-section of a mouse eye lens shows the curved, onion-like configuration of fibre cells, which are packed close together and lose subcellular structures such as nuclei (coloured blue) as they mature and move to the centre of the lens. Fibre cells contain highly ordered crystallin proteins, the intracellular concentration of which increases towards the interior of the lens (seen as darkening pink). This combined cellular and intracellular structure gives transparency to the lens. Denaturation and aggregation of crystallin proteins can result in the lens opacity known as a cataract. Zhao et al.1 show that the molecule lanosterol can redissolve crystallin aggregates and alleviate cataracts.
Human crystallins are divided into two families, α- and βγ-crystallins; together, these make up 90% of the water-soluble proteins in lens cells8. They are extremely stable, highly ordered and provide a relatively constant refractive index, which allows lens transparency9. Because differentiated lens fibre cells lack the synthetic apparatus to produce new proteins, crystallins are not turned over, and those in the centre of the lens are among the oldest proteins in the body. Preserving crystallin structure and function is therefore crucial for prevention of lens opacities. Other biological activities of the lens serve primarily to protect the complementary systems of crystallin packing and fibre-cell arrangement from disruption and damage by age and external insults, especially ultraviolet light, oxidative stress and glycation.
The genes that cause cataracts when mutated tend to encode proteins that are involved in one of these biological pathways or functional groupings of proteins that are critical for lens homeostasis. In families at risk of congenital cataracts for which the mutant gene is known, slightly less than half have mutations in lens crystallins, with others having mutations in growth or transcription factors, membrane proteins, chaperone proteins and proteins involved in protein degradation, among others10. Zhao and colleagues’ identification of LSS mutations as a cause of congenital cataracts adds a new pathway.
The catastrophic structural changes in crystallins seen in many hereditary cataracts can overwhelm the defensive systems of the lens, and might also be refractory to the solubilizing activity of lanosterol identified by the authors. Nevertheless, this agent might be more therapeutically applicable to the slow progressive denaturation of crystallins seen in age-related cataracts. In age-related cataracts, damaged βγ-crystallin proteins are bound by α-crystallins, which act like chaperones — proteins that assist the folding or unfolding of other proteins — except that, instead of refolding the denatured βγ-crystallins, α-crystallins solubilize them11, thereby reducing light scattering. However, as more crystallins are damaged and bound over time, the protein complexes themselves become large enough to scatter light11,12. Eventually, the complexes precipitate, forming the insoluble protein fraction (termed high molecular weight aggregates) that increases with normal ageing and especially in cataractous lenses. This identifies cataracts, in at least some cases, as a protein-misfolding disease13.
Although surgery to remove cataracts is efficacious and safe, ageing populations around the world are predicted to require a doubling of cataract surgery in the next 20 years14. The same population demographics suggest that, if development of age-related cataracts in susceptible individuals could be delayed by even ten years, the need for surgery could be reduced by almost half15. Pre-symptomatic screening of age-related cataracts is easy, and the eye is easily accessible for topical application of drugs. Zhao and colleagues show that eye drops containing lanosterol successfully treated natural cataracts in dogs. The potential for this finding to be translated into the first practical pharmacological prevention, or even treatment, of human cataracts could not come at a more opportune time. Furthermore, this approach might serve as a model for other protein-misfolding diseases affecting a variety of tissues and organ systems. ■
References
- 1.Zhao L et al. Nature 523, 607–611 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Brewster D Phil. Trans. R. Soc. Lond 123, 323–332 (1833). [Google Scholar]
- 3.Spemann H Vehr. Anat. Ges 15, 61–79 (1901). [Google Scholar]
- 4.Zhang T et al. Hum. Mutat 30, E603–E611 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Renwick JH & Lawler SD Ann. Hum. Genet 27, 67–84 (1963). [DOI] [PubMed] [Google Scholar]
- 6.Zelenka PS & Piatigorsky J Proc. Natl Acad. Sci. USA 71, 1896–1900 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michael R, van Marle J, Vrensen GF & van den Berg TJ Exp. Eye Res 77, 93–99 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Bloemendal H et al. Prog. Biophys. Mol. Biol 86, 407–485 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Benedek GB Appl. Optics 10, 459–473 (1971). [DOI] [PubMed] [Google Scholar]
- 10.Shiels A & Hejtmancik JF Clin. Genet 84, 120–127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao PV, Huang Q-L, Horwitz J & Zigler JS Jr Biochim. Biophys. Acta 1245, 439–447 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Datiles MB III et al. Arch. Ophthalmol 126, 1687–1693 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreau KL & King JA Trends Mol. Med 18, 273–282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor HR Br. J. Ophthalmol 84, 1–2 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kupfer C Invest. Ophthalmol. Vis. Sci 28, 2–8 (1987). [PubMed] [Google Scholar]
- 16.Sun N, Shibata B, Hess JF & FitzGerald PG Mol. Vis 21, 428–442 (2015). [PMC free article] [PubMed] [Google Scholar]


