Abstract
TLRs are conserved pattern recognition receptors that detect motifs of pathogens and host material released during injury. For unknown reasons, renal TLR2 mRNA is mainly expressed by tubular cells and is enhanced upon renal ischemia/reperfusion (I/R) injury. We evaluated the role of TLR2 in I/R injury using TLR2–/– and TLR2+/+ mice, TLR2 antisense oligonucleotides, and chimeric mice deficient in leukocyte or renal TLR2. Tubular cells needed TLR2 to produce significant cytokine and chemokine amounts upon ischemia in vitro. TLR2 played a proinflammatory and detrimental role in vivo after I/R injury, as reflected by a reduction in the amount of local cytokines and chemokines, leukocytes, and the level of renal injury and dysfunction in TLR2–/– mice compared with controls. Analysis of chimeric mice suggested that TLR2 expressed on renal parenchyma plays a crucial role in the induction of inflammation and injury. TLR2-antisense treatment protected mice from renal dysfunction, neutrophil influx, and tubular apoptosis after I/R injury compared with nonsense treatment. In summary, we identified renal-associated TLR2 as an important initiator of inflammatory responses leading to renal injury and dysfunction in I/R injury. These data imply that TLR2 blockade could provide a basis for therapeutic strategies to treat or prevent renal ischemic injury.
Introduction
TLRs are highly conserved receptors that have been implicated in the immune response to a variety of pathogens. TLRs detect exogenous microbial components, which can lead to the induction of innate immune responses (1) and the development of antigen-specific adaptive immunity (2). Interestingly, over the past few years, it has become clear that TLRs also recognize endogenous host material that is released during cellular injury, such as degradation products of macromolecules, products of proteolytic cascades, intracellular components of ruptured cells, and products of genes that are activated by inflammation (3). In this way, TLRs can be viewed as crucial surveillance receptors for innate and adaptive immunity to detect tissue damage, infection, and remodeling (4). Whereas TLRs are preferentially expressed on antigen-presenting cells, recent observations suggest that these receptors are also expressed on cells that are traditionally not regarded as being involved in host defense. Indeed, the majority of TLR2 mRNA in the kidney is constitutively expressed on renal tubular epithelial cells (TECs) and epithelial cells from the Bowman capsule in the murine kidney and is markedly enhanced on these cells upon renal ischemic injury (5). The functional significance of this upregulation, however, remains unknown. Interestingly, the endogenous cellular injury signals that can activate TLR2, such as heat shock proteins (6), and the contents of necrotic cells (7) are known to accumulate upon renal ischemic tissue injury (8, 9).
Following injury of TECs in renal ischemia, a cascade of proinflammatory mediators that can lead to amplification of injury either directly or indirectly is activated. The primary mechanism through which the kidney initiates this inflammatory cascade has, however, not yet been elucidated. As TECs belong to the nonimmune cells that express and upregulate TLR2 after ischemic injury, it might be hypothesized that this TEC-associated receptor plays a key role in the initiation of inflammation after renal ischemia. In support of this, the role of TLR2 in chemokine production by mouse renal TECs after stimulation with bacterial products in vitro has already been reported (10). Since renal ischemia is a major cause of acute (11) and end-stage renal failure (12), producing serious morbidity and mortality, and in addition is an important inducer of acute renal transplant rejection and delayed allograft function (13, 14), improved knowledge of the primary mechanisms involved in monitoring and mediating renal ischemia/reperfusion (I/R) injury is essential for the development of therapeutic strategies. The activation of TLR by ligands released during I/R injury may be an important link between an inflammatory immune response in the kidney and damage in this organ and in addition might trigger renal allograft rejection by activating innate and adaptive immune responses.
Together, these data prompted us to investigate the role of TLR2 in ischemic injury and repair in the kidney. For this, we induced renal I/R injury in TLR2–/– or TLR2+/+ mice, chimeric mice deficient in leukocyte or renal TLR2, and analyzed the subsequent pathophysiology. In addition, we evaluated the therapeutic potential of TLR2 antisense therapy for the treatment of renal ischemia as these nucleotides are known to be taken up mainly by the kidney, i.e., TECs (15).
Results
Reduced chemokine and cytokine production by cultured TLR2–/– TECs after simulated ischemia.
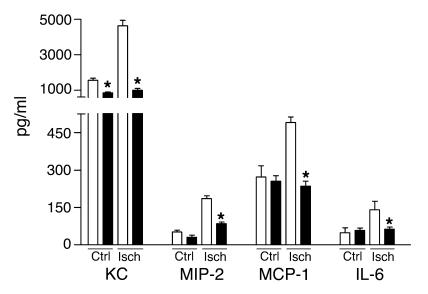
As cytokines and chemokines produced by renal TECs are critical factors in the inflammatory response during renal I/R injury (16), we measured some of the main proteins among this family that are known to be produced by TECs (17–22). As shown in Figure 1, the amount of granulocyte chemotactic keratinocyte chemoattractant (KC) and macrophage inflammatory protein-2 (MIP-2) (23, 24) and monocyte chemotactic monocyte chemoattractant protein-1 (MCP-1) (25) was significantly lower in supernatants of TLR2–/– TECs when subjected to simulated ischemia as compared with TLR2+/+ TECs. Furthermore, TLR2–/– TECs produced significantly less proinflammatory IL-6 after simulated ischemia than TLR2+/+ TECs, while the production of IL-1β and TNF-α could not be detected. In addition, when TLR2–/– TECs were stimulated with kidney homogenates of mice subjected for 1 day to renal I/R injury, significantly less KC (5791.5 ± 176.0 [TLR2+/+] vs. 3404.0 ± 208.0 [TLR2–/–] pg/ml; P < 0.05), MIP-2 (293.0 ± 11.9 [TLR2+/+] vs. 239.0 ± 8.3 [TLR2–/–]; P < 0.05), and MCP-1 (472.0 ± 13.5 [TLR2+/+] vs. 265.7 ± 265.7 [TLR2–/–]; P < 0.05) was produced than with TLR2+/+ TECs. IL-6, IL-1β, and TNF-α could not be detected.
Figure 1.
Cytokine and chemokine production by primary murine TECs from TLR2+/+ (white bars) and TLR2–/– (black bars) mice subjected to simulated ischemia (Isch). TLR2–/– TECs produce less KC, MIP-2, MCP-1, and IL-6 as compared with TECs from TLR2+/+ mice when subjected to simulated ischemia. The presence of protein was measured in supernatants by specific ELISA. Data are mean ± SEM of 4 mice per group, measured in duplicate. *P < 0.05. Ctrl, control.
Reduced chemokine and cytokine levels in TLR2–/– mice subjected to renal I/R injury.
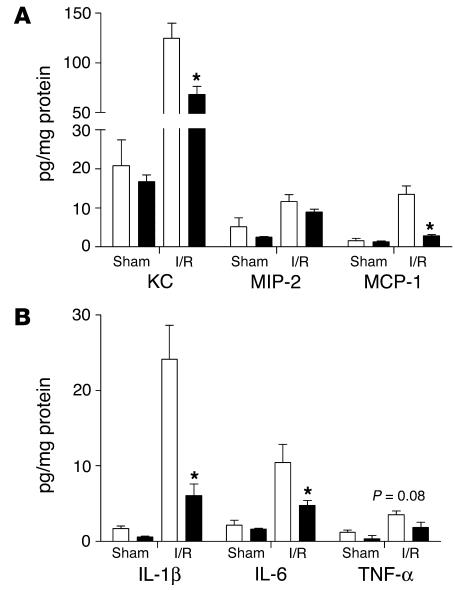
We next investigated whether TLR2 deficiency also influenced early chemokine and cytokine production in vivo after renal I/R injury. For this we measured KC, MIP-2, MCP-1, TNF-α, IL-1β, and IL-6 protein in kidney homogenates of TLR2–/– mice and TLR2+/+ mice 1 day after renal I/R injury. This revealed that early KC and MCP-1 levels were significantly lower in kidney homogenates from TLR2–/– mice compared with those in TLR2+/+ mice, while renal MIP-2 levels were similar in both groups of animals (Figure 2). In addition, kidneys from TLR2–/– mice contained significantly less IL-1β and IL-6 compared with kidneys from TLR2+/+ mice (Figure 2). Although renal TNF-α levels followed a similar trend, this difference did not reach statistical significance (P = 0.08, Figure 2). As a previous study implied that TNF-α levels were maximal 1 to 2 hours after the induction of I/R injury (26), we additionally measured this cytokine 2 hours after reperfusion. Although higher TNF-α levels were found at this early stage after the induction of I/R injury as compared with the 1-day time point, no significant differences could be found between TLR2–/– mice (19.7 ± 2.9) and TLR2+/+ mice (25.9 ± 2.4; P = 0.1).
Figure 2.
Early chemokine (A) and proinflammatory cytokine (B) levels in kidneys from TLR2+/+ (white bars) and TLR2–/– (black bars) mice subjected for 1 day to renal I/R injury. TLR2–/– mice have significantly reduced KC, MCP-1, IL-1β, and IL-6 levels in their kidneys as compared with kidneys from TLR2+/+ mice. The presence of protein was measured in kidney homogenates by specific ELISA. Data are mean ± SEM of 8 mice per group. *P < 0.05.
Reduced influx of interstitial granulocytes and macrophages in TLR2–/– mice.
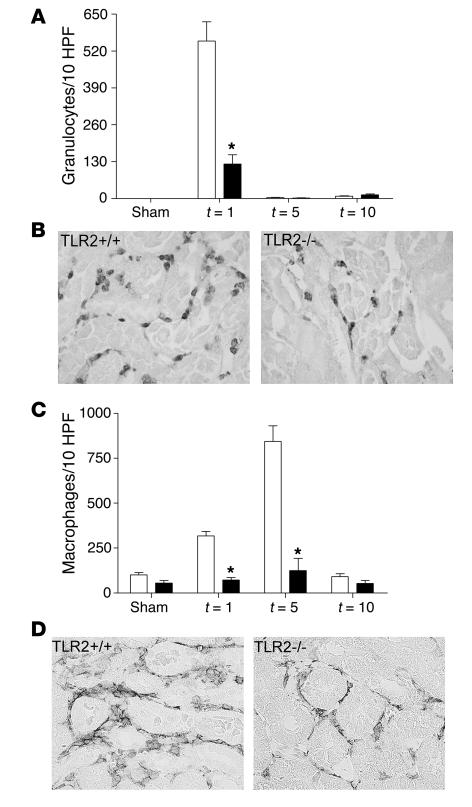
To evaluate the effect of chemokine and cytokine production on in vivo leukocyte influx, we analyzed the amount of granulocytes and macrophages in the injured kidneys of TLR2+/+ and TLR2–/– mice. Interestingly, significantly fewer interstitial infiltrated granulocytes were found in TLR2–/– mice 1 day after renal I/R as compared with TLR2+/+ mice (Figure 3, A and B). Five and 10 days after I/R injury, levels of granulocyte infiltration went back to control levels and did not differ between TLR2+/+ and TLR2–/– mice (Figure 3A). Macrophages mainly infiltrated the outer medullas of kidneys from TLR2+/+ mice 1 day after I/R injury with a maximum on day 5. Importantly, a significant decrease was observed in the number of interstitial infiltrated macrophages in the TLR2–/– mice 1 and 5 days after I/R when compared with TLR2+/+ mice (Figure 3, C and D). No significant differences in the number of interstitial infiltrated macrophages were found 10 days after I/R injury between TLR2–/– mice and TLR2+/+ mice (Figure 3C) when injury was already repaired (Figures 4 and 5). Together, these data suggest an important role for TLR2 in the induction of a proinflammatory immune response during renal I/R injury.
Figure 3.
Leukocyte influx in kidneys from TLR2+/+ (white bars) and TLR2–/– (black bars) mice 1, 5, and 10 days (t = 1, 5, and 10) after renal I/R or sham operation. The number of granulocytes (A; t = 1) and macrophages (C; t = 1, 5) were significantly lower in TLR2–/– mice compared with TLR2+/+ animals after renal I/R as counted in 10 randomly selected high-power fields (HPFs) on outer medulla. Leukocytes from 8 mice per group were counted on renal tissue sections stained for Ly-6G (B; t = 1) and F4/80 (D; t = 5). Magnification, ×400. *P < 0.05.
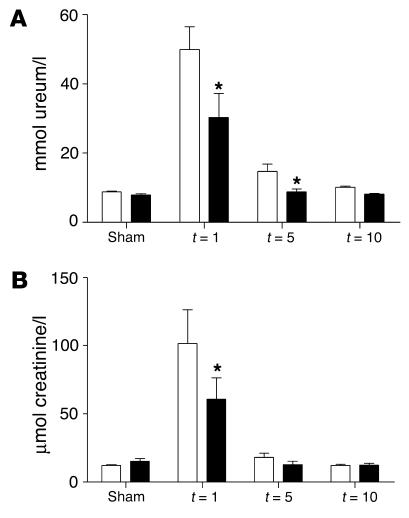
Figure 4.
Renal function of TLR2–/– mice (black bars) was improved compared with that of TLR2+/+ mice (white bars) 1 and 5 days after renal I/R as reflected by reduced ureum (A) and creatinine (B) values in plasma. Data are mean ± SEM of 8 mice per group. *P < 0.05.
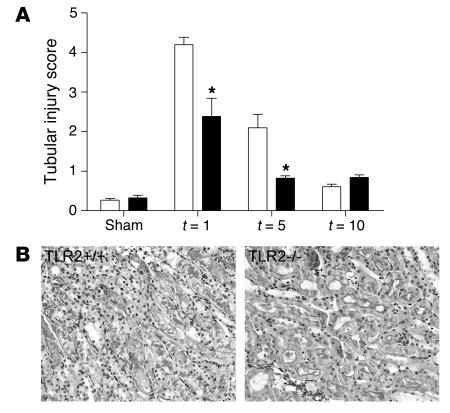
Figure 5.
Score for characteristic histologic signs of renal injury (A) using PAS-D–stained renal tissue sections (B; t = 1). Tubular damage in TLR2–/– mice (black bars) was significantly lower 1 and 5 days after I/R injury in the outer medulla of the kidney than in kidneys from TLR2+/+ mice (white bars). Data are mean ± SEM of 8 mice per group. Magnification, ×400. *P < 0.05.
TLR2 deficiency reduced renal dysfunction and the extent of acute tubular damage.
To study the effect of TLR2 deficiency on renal function after I/R injury, we measured levels of serum creatinine and ureum at different time points in TLR2–/– and TLR2+/+ mice. TLR2+/+ mice showed a transient renal dysfunction (peak serum creatinine and ureum on day 1) with almost complete recovery by day 10 as compared with sham-operated animals (Figure 4). TLR2–/– mice, however, were partially protected against acute renal failure as reflected by significantly lower serum creatinine and ureum levels as compared with TLR2+/+ mice. To confirm that renal injury was present and to investigate the impact of TLR2 deficiency on tubular injury, we next evaluated renal histology. After 1 day of reperfusion, ischemic kidneys of TLR2+/+ mice showed widespread tubular necrosis, tubular dilation, brush border loss, and cast formation in the outer medullas of the kidneys (Figure 5B). Kidneys from TLR2–/– mice displayed, however, significantly less tubular damage when compared with those of TLR2+/+ mice 1 and 5 days after renal I/R injury (Figure 5A). We found an almost complete recovery of tubular cells by day 10 as compared with sham-operated control mice. Thus, TLR2 deficiency had a profound protective effect on renal function and damage after renal I/R induction.
TLR2–/– mice show less tubular epithelial apoptosis and regeneration in I/R renal injury.
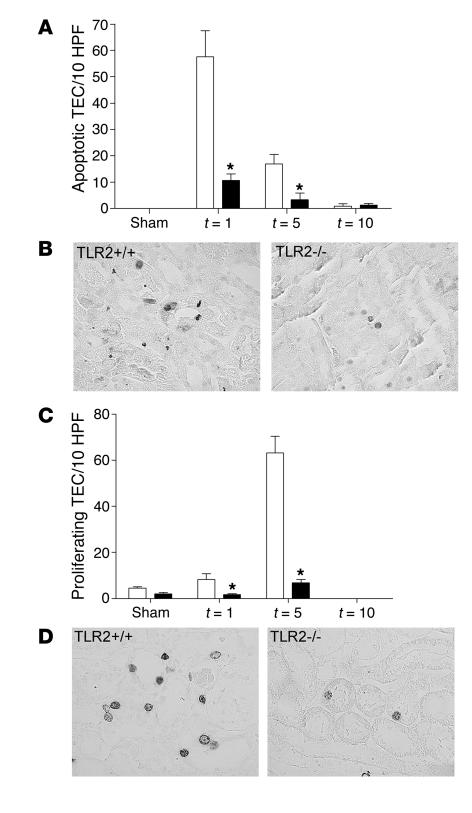
Next to cell infiltration, I/R injury in the kidney is characterized by TEC apoptosis. As TLR2 is identified as a novel “death receptor” that is connected to the apoptotic machinery (27), we assessed the amount of tubular apoptosis during renal I/R injury by immunohistochemical staining for active-caspase 3 (Figure 6B). Compared with sham-operated animals, mice subjected to I/R injury demonstrated significant TEC apoptosis within 1 day (Figure 6A). Notably, the number of apoptotic TECs in kidneys from TLR2–/– mice was markedly lower than that in kidneys from TLR2+/+ mice 1 and 5 days after renal I/R injury (Figure 6A). Next, we investigated regeneration of injured tubular cells after ischemic renal injury by measuring DNA synthesis via immunohistochemical staining for BrdU (Figure 6D). One day after ischemic renal injury, proliferation of tubular cells began, reaching a maximum on day 5. The number of BrdU-positive TECs in kidneys from TLR2–/– mice was significantly lower than that in kidneys from TLR2+/+ mice 1 and 5 days after injury (Figure 6C).
Figure 6.
Apoptotic and proliferating tubular cells in kidneys from TLR2+/+ (white bars) and TLR2–/– (black bars) mice 1, 5, and 10 days after renal I/R or sham operation. The number of apoptotic (A) and proliferating (C) tubular cells was significantly lower in kidneys from TLR2–/– mice than in kidneys from TLR2+/+ animals 1 and 5 days after I/R as counted in 10 randomly selected high-power fields on outer medulla. Tubular cells from 8 mice per group were counted on renal tissue sections stained for active caspase-3 (B; apoptosis, t = 1) and BrdU (D; proliferation, t = 5). Data are mean ± SEM of 8 mice per group. Magnification, ×400. *P < 0.05.
Generation of chimeric mice.
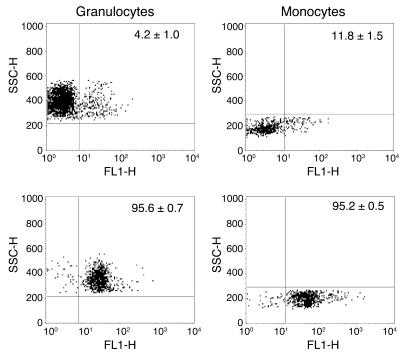
Since TLR2 is expressed on both leukocytes and renal parenchyma, we next examined the contribution of these cell types to the outcome of renal I/R injury. We utilized bone marrow transplantation in irradiated TLR2–/– and TLR2+/+ mice in order to generate chimeric mice expressing TLR2 on leukocytes but not on renal parenchyma cells (kidney-TLR2–/– mice, n = 10) as well as mice expressing TLR2 on renal parenchyma cells but not on leukocytes (leukocyte-TLR2–/– mice, n = 10). We determined the expression of TLR2 on blood neutrophils and monocytes by flow cytometric analysis, as these cells express TLR2 (28) and are central mediators of renal tissue injury (29–31). This analysis revealed that the neutrophil and monocyte compartments of all mice consisted of at least 95.6% (TLR2+ granulocytes) and 88.2% (TLR2– monocytes) donor-derived cells, indicating extensive engraftment 6 weeks after transplantation (Figure 7). No differences were found in the total number of white blood cells between both groups of animals prior to the induction of renal I/R injury (leukocyte-TLR2–/– mice: 7.4 ± 0.6 × 106/ml vs. kidney-TLR2–/– mice: 6.2 ± 0.8 × 106/ml; P = 0.19).
Figure 7.
Donor recipient chimerism of the hematopoietic system 6 weeks after bone marrow transplantation in TLR2+/+ and TLR2–/– mice. Peripheral blood neutrophils and monocytes collected from TLR2+/+ mice transplanted with TLR2–/– bone marrow (leukocyte-TLR2–/– mice, upper panels) and TLR2–/– mice transplanted with TLR2+/+ bone marrow (kidney-TLR2–/– mice, lower panels) were subjected to FACS analysis of TLR2 expression. At least 94% of the neutrophil and 82% of the monocyte populations of all mice consisted of donor-derived cells indicating extensive engraftment. Data are mean ± SEM of 10 mice per group. SSC-H, side scatter height; FL1-H, fluorescence intensity.
Increased renal dysfunction, granulocyte influx, and TEC apoptosis in TLR2+/+ mice is mediated by renal-associated TLR2.
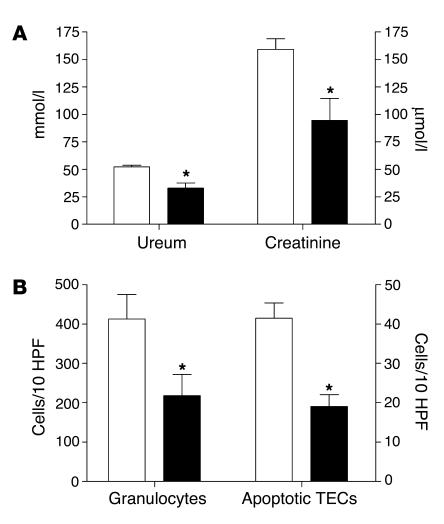
To study whether TLR2 expressed on the kidney and/or on infiltrated leukocytes contributes to renal I/R injury, we compared serum creatinine and ureum levels of kidney-TLR2–/– mice and leukocyte-TLR2–/– mice. Interestingly, we found that kidney-TLR2–/– mice were partly protected against renal failure as reflected by significantly lower serum creatinine and ureum levels as compared with leukocyte-TLR2–/– mice 1 day after reperfusion (Figure 8A). Further analysis revealed that the number of granulocytes and apoptotic tubular cells in kidneys from kidney-TLR2–/– mice was significantly lower 1 day after I/R injury than that in kidneys from leukocyte-TLR2–/– mice (Figure 8B). Together, these data suggest that TLR2 expressed on the renal parenchyma plays a primary role in the early induction of inflammation and I/R injury.
Figure 8.
Renal function of kidney-TLR2–/– mice (black bars) was enhanced compared with that of leukocyte-TLR2–/– mice (white bars) 1 day after renal I/R as shown by reduced ureum and creatinine values in plasma (A). The amount of granulocytes and apoptotic tubular cells was significantly lower in kidney-TLR2–/– mice than in leukocyte-TLR2–/– animals 1 day after I/R as counted in 10 randomly selected high-power fields on outer medulla (B). Data are mean ± SEM of 10 mice per group. *P < 0.05.
Distribution and effect of antisense oligonucleotides in vivo.
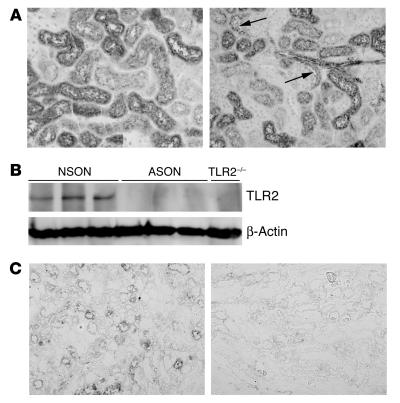
To confirm our in vivo data and to determine whether TLR2 antisense oligonucleotide (ASON) treatment could affect clinical outcome of renal disease, we evaluated TLR2 ASON treatment in mice after renal I/R injury. First, we analyzed the distribution of ASON uptake using FITC-labeled ASONs. This revealed that oligonucleotide accumulation was greatest in kidney and liver, intermediate in spleen, and very low in lung (data not shown). ASONs were predominantly targeted to renal tubular cells (Figure 9A, left panel) and epithelial cells from the Bowman capsule (Figure 9A, right panel), even when renal arteries were clamped for 45 minutes (data not shown). FITC was not detected within the glomerular tuft (Figure 9A, right panel). To assess whether systemic administration of TLR2 ASONs inhibited the amount of TLR2 protein, we also analyzed TLR2 in kidney from mice subjected for 1 day to renal I/R injury by immunoblotting and immunohistochemistry. As shown in Figure 9, B and C, TLR2 ASONs were able to reduce the amount of TLR2 protein in the kidney as compared with nonsense oligonucleotides (NSONs).
Figure 9.
Distribution and effect of ASONs in the kidneys of mice after i.p. injection of ASONs. (A) FITC-labeled ASONs were primarily targeted to the renal tubular cells (left panel) and epithelial cells from the Bowman capsule (right panel, arrows) as demonstrated by immunohistochemical analysis of FITC on renal tissue sections. Magnification, ×200. TLR2 ASON treatment reduced the amount of TLR2 protein present in kidneys from mice subjected for 1 day to renal I/R injury when compared with NSON treatment as shown by immunoblotting (B; TLR2 is detected at ∼97 kDa) and immunohistochemistry (C; left panel NSON, right panel TLR2 ASON). Magnification, ×200.
Reduction in renal dysfunction, granulocyte influx, and TEC apoptosis after TLR2 ASON treatment.
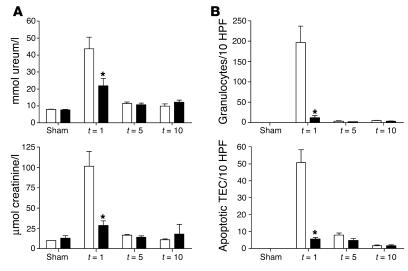
To evaluate whether TLR2 ASON treatment could influence clinical outcome of renal ischemic injury, TLR2 ASONs and NSONs were delivered i.p. to mice before and during the induction of I/R injury. Most interestingly, we found that mice treated with TLR2 ASONs had a markedly improved renal function 1 day after I/R injury compared with mice treated with control NSONs, as reflected by significantly lower serum creatinine and ureum levels (Figure 10A). We found an almost complete recovery of renal function by days 5 and 10 as compared with sham-operated mice. Accordingly, the number of apoptotic TECs and granulocytes in kidneys from TLR2 ASON-treated mice was significantly lower 1 day after I/R injury than that in kidneys from NSON-treated animals (Figure 10B). No significant differences were found in the number of neutrophils and apoptotic TECs 5 and 10 days after I/R injury between TLR2–/– mice and TLR2+/+ mice (Figure 10B) when injury was already repaired (Figure 10A).
Figure 10.
Renal function of mice treated with TLR2 ASONs (black bars) was improved compared with that of mice treated with TLR2 NSONs (white bars) 1 day after renal I/R as reflected by reduced ureum and creatinine values in plasma (A). The number of apoptotic tubular cells and granulocytes was significantly lower in TLR2 ASON–treated mice compared with NSON-treated animals 1 day after I/R as counted in 10 randomly selected high-power fields on the outer medulla (B). Data are mean ± SEM of 8 mice per group. *P < 0.05.
Discussion
The pathogenesis of I/R renal injury involves a complex interaction among renal hemodynamics, tubular injury, and subsequent inflammatory responses. In the latter processes, TECs are central players, i.e., injury of these cells can result in a cascade of responses that lead to the local production of proinflammatory mediators, which in turn can increase renal injury and dysfunction by, for instance, causing increased migration of proinflammatory cells into the renal parenchyma (32). The precise mechanisms through which TECs initiate these proinflammatory responses in vivo have not been elucidated completely. In the present study, we identified TLR2 expressed on renal parenchyma as an important player in the initiation of an exaggerated inflammatory response leading to renal injury and dysfunction in I/R injury of the kidney in vivo. Moreover, we found that TLR2 ASON treatment decreased dysfunction of the kidney after renal I/R injury in vivo.
Renal TECs from mice constitutively express mRNA for TLR2 both in vitro (10) and in vivo (5), which is upregulated after ischemic stress in vivo (5). The functional implication of this upregulation has remained, however, unidentified. The current study demonstrates that endogenous TLR2 plays a proinflammatory role in vivo after renal I/R injury, as reflected by reduced cytokine and chemokine production as well as reduced leukocyte infiltration in TLR2–/– mice as compared with TLR2+/+ animals. These observations are compatible with our in vitro data, in which we found that primary murine TECs need TLR2 in order to produce significant amounts of chemokines and cytokines after simulated ischemia and stimulation with ischemic kidney homogenate. Together, these observations suggest that renal cells are able to produce cellular injury signals in response to ischemia that can induce an inflammatory response via TLR2. TLR2 has previously been shown to be required for chemokine production of murine TECs that are exposed to bacterial components in vitro (10). Moreover, oxidative stress in neonatal rat cardiomyocytes has activated the potent inducer of proinflammatory cytokine gene expression NF-κB via TLR2 (33, 34). Apparently, TLR2 is capable of monitoring cellular injury caused by ischemic stress and is subsequently crucial for the initiation of a proinflammatory immune response during ischemia. Possible endogenous ligands for TLR2 that are known to accumulate during ischemic stress (8, 9) are heat shock protein-70 (6) and the contents of necrotic cells (7). It can be postulated that the activation of TLR by ligands released during renal I/R injury may be a key link between a proinflammatory immune response in the kidney and renal damage and dysfunction. Indeed, mice deficient for TLR2 had less severe renal damage and dysfunction than wild-type mice as well as an early impaired leukocyte influx. These data emphasize the concept of Anders et al. suggesting that family members of TLRs might play an important role in the pathogenesis of a number of renal diseases (35). Although the role of neutrophils in I/R injury is controversial (36, 37), many studies suggest that these cells mediate tissue injury and contribute to the development of renal failure (29, 30, 38, 39). For that reason, the impaired neutrophil influx in kidneys from TLR2–/– mice, presumably as a consequence of reduced renal proinflammatory cytokine and chemokine levels, would explain the lower degree of renal damage and dysfunction in these animals compared with controls. In addition, diminished macrophage numbers in TLR2–/– mice could also contribute to the reduced renal damage in these animals. Indeed, activated macrophages are able to release soluble molecules that are known to cause renal damage (40), and depletion of these cells appears protective against ischemic acute renal failure (31). The impaired macrophage influx in TLR2–/– mice compared with TLR2+/+ animals is probably due to reduced MCP-1 levels in the kidney and may reflect the reduced need for these cells to phagocytose and remove the lower amount of necrotic and apoptotic TECs. An alternative and attractive explanation for the preserved renal function in TLR2–/– mice could also be the reduction in TEC apoptosis we found after ischemic injury. Indeed, it is suggested that apoptosis in the kidney, either directly or indirectly, significantly contributes to I/R-induced inflammation as well as to the consequent tissue damage (41). The reduction in TEC apoptosis in TLR2–/– mice can be explained by previous work implying a proapoptotic function of TLR2. That is, stimulation of TLR2 by bacterial lipoproteins induces apoptosis in monocytic cells by caspase activation (27, 42). Besides the reduction in death of TECs, we also found significantly less regeneration of these cells in TLR2–/– mice as compared with TLR2+/+ animals. The latter could reflect the reduced tubular damage in these animals. Conversely, the decrease in TEC proliferation could indicate that TLR2 is necessary for tissue repair. This is in agreement with findings of Lee et al. demonstrating that necrotic cells can directly enhance the expression of genes that play a role in inflammatory and tissue-repair responses through activation of the NF-κB and TLR2 pathways (7).
To investigate whether the impaired renal function and increased inflammatory response in TLR2+/+ mice was due to renal-associated and/or leukocyte-associated TLR2, we next generated chimeric mice by means of bone marrow transplantation in irradiated mice. This study showed that during I/R injury, TLR2 on renal parenchyma plays a crucial role in the early development of acute renal failure, as reflected by a lower amount of serum ureum/creatinine in kidney-TLR2–/– mice as compared with in leukocyte-TLR2–/– mice. Renal TLR2 could be involved in the induction of renal failure by participating in an exaggerated proinflammatory response. Indeed, we found an impaired granulocyte influx in kidneys from kidney-TLR2–/– mice as compared with leukocyte-TLR2–/– mice. This is consistent with our in vitro data indicating that tubular cells need TLR2 to produce significant amounts of neutrophil chemokines during ischemic conditions. In addition, we show a reduction in apoptotic tubular cells in kidneys from kidney-TLR2–/– mice as compared with leukocyte-TLR2–/– mice, again stressing a possible proapoptotic role for TLR2 on tubular cells. Evidently, TLR2 expressed on renal cells plays a crucial role in renal I/R pathogenesis, presumably by inducing a proinflammatory response.
To determine whether the proinflammatory and detrimental effects of TLR2 we found were due to a compensatory mechanism in the knockout animals and to determine whether TLR2 ASON treatment could affect the clinical outcome of renal disease, we next studied the effect of TLR2 ASON treatment on mice after renal I/R injury. In line with previous studies (15, 43), we found that the kidney is the major site of ASON uptake, with the highest accumulation in TECs as well as epithelial cells from the Bowman capsule and with much lower uptake by cells in other segments of the nephron. Therefore, the kidney may be an ideal target organ for application of TLR2 ASONs in vivo. Indeed, we found that TLR2 ASON treatment reduced renal dysfunction after I/R injury as reflected by lower serum ureum and creatinine levels as compared with NSON treatment. Similar to our previous results, we found that the increased renal function seems to be correlated to an inhibition of the exaggerated proinflammatory response as shown by an impaired granulocyte influx in kidneys from mice treated with TLR2 ASONs as compared with NSONs. In addition, we found a reduction in apoptotic TECs in kidneys from mice treated with TLR2 ASONs as compared with NSON-treated animals. The use of antisense technology has already been successfully applied to treat renal ischemic injury in animals, i.e., ICAM-1 antisense ASONs, which block adhesion of inflammatory cells, prevented renal I/R injury and enhanced immediate graft function in renal transplantation in rats in a more downstream manner (44, 45). That blockade of TLR2 signaling might be promising in the prevention of ischemia-induced renal allograft rejection and delayed allograft function is supported by a study demonstrating that mice deficient in TLR2 had a delay in skin allograft rejection (46). Taken together, these data suggest that blockade of an inappropriate immune response with specific TLR2 signaling antagonists such as antisense could provide a theoretical basis for the development of therapeutic strategies meant to slow down or prevent renal ischemic injury. A possible effect of ASONs outside the kidney should, however, be taken into account.
Although TLR2 is ultimately disadvantageous for the development of I/R injury in the kidney, it should be noted that this receptor plays a beneficial role in the immune response against invading pathogens (47), which might explain the presence of this highly conserved receptor in the kidney. Indeed, TLR2 is critical for resistance to many invading pathogens in different organ systems by induction of a proinflammatory response, as reviewed by Takeda and Akira (47). To date, 3 studies of the urinary system describe an important and beneficial role for TLR4 and TLR11 in the defense against Escherichia coli (48–50).
This study is, to our knowledge, the first to show that endogenous TLR2 plays a proinflammatory and detrimental role in the kidney after renal ischemic injury in vivo, as reflected by reduced cytokine and chemokine production, reduced leukocyte infiltration, and a lower degree of renal dysfunction and tubular damage in mice deficient for TLR2. Most importantly, the induction of I/R-induced inflammation and injury seems to be mediated by TLR2 that is expressed on renal cells. This study strongly supports the idea that the function of TLR2 extends beyond host defence against invading pathogens. TLR2 appears to be capable of monitoring ischemic damaged kidney and as a result is crucial for activating primary immune responses in this organ. These results provide new insights into the primary mechanism by which the kidney regulates inflammatory responses and the way renal ischemic injury is monitored. They will also assist us in evaluating the therapeutic potential of TLR2 antisense therapy for the treatment of renal ischemia. As no effective treatment is available for renal injury following ischemia (51), blockade of an inappropriate/detrimental immune response with specific TLR signaling antagonists such as antisense could represent a new approach for treating or preventing renal failure and renal transplant rejection and dysfunction caused by renal ischemia.
Methods
Mice.
Pathogen-free 9- to 12-week-old male C57BL/6 wild-type mice were purchased from Charles River Laboratories. TLR2–/– mice were generated as described previously (52), backcrossed to C57BL/6 background 6 times, and bred in the animal facility of the Academic Medical Center in Amsterdam, The Netherlands. Age- and sex-matched mice were used in all experiments. The Animal Care and Use Committee of the University of Amsterdam approved all experiments.
Primary culture of mouse renal TECs.
Primary mouse renal TECs were generated following the method described by Wuthrich et al. (53). Briefly, the renal capsule was removed, and tissue from the outer cortex was cut into pieces of approximately 1 mm3. Then single cortical tubular cell suspensions of freshly dissected kidney cortices from TLR2+/+ and TLR2–/– mice (4 mice per group) were prepared by collagenase type 1A (Sigma-Aldrich) dispersion at 37°C for 1 hour and washed in RPMI 1640 medium (Gibco BRL; Invitrogen Corp.). TECs were subsequently grown in duplicate to confluence on 6-well plates for 10 days in RPMI 1640 medium supplemented with 10% FCS (Integro), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen Corp.). TECs were identified by characteristic polygonal or cobblestone-shaped morphology.
Simulated ischemia in renal TECs in vitro.
Ischemia in renal TECs was simulated by immersing the cellular monolayer in mineral oil according to the protocol of Meldrum et al. (54). This method has also been applied for the induction of ischemia in cultured myocytes (55, 56). This immersion simulated ischemia by depriving cells of oxygen and nutrients as well as by limiting metabolite washout (56, 57). In brief, cells were serum starved for 24 hours, washed twice with PBS, and immersed in mineral oil for 60 minutes at 37°C. After extensive washing, cells were incubated for 24 hours in medium. Intracellular adenosine triphosphate (ATP) measurements were performed with the ATP Bioluminescence Assay Kit HS II (Roche Diagnostics Corp.) according to the manufacturer’s protocol. Cell lysates were analyzed for ATP levels using a Lumat luminometer (Berthold Technologies). To correct for possible loss of cells due to hypoxic stress, the protein content of the lysates was measured with the Bradford Protein Assay (Bio-Rad Laboratories). Measurements showed marked ATP depletion consistent with ischemic conditions. Moreover, no differences in ATP depletion were found between TLR2+/+ (71.0% ± 5.8% of control) and TLR2–/– TECs (60.1% ± 3.5 % of control), which could indicate a differential effect of mineral oil on KO versus wild-type TECs (P = 0.34). In a separate experiment, TECs were serum starved overnight and stimulated for 24 hours with kidney homogenate (obtained as described hereafter) of mice that had been subjected to renal I/R and sacrificed after 1 day. Supernatants were subsequently collected and stored at –70°C until ELISAs were performed. The number of cells present in the wells after injury/stimulation was determined by treating cells with trypsine-EDTA after which suspended cells were counted using a hemacytometer. This revealed no statistically significant differences in the number of TLR2+/+ and TLR2–/– TECs. Cytokine levels present in the kidney homogenate used to stimulate TECs were subsequently substracted from the level of cytokines detected in supernatants 24 hours after stimulation.
Renal I/R injury model.
Renal I/R injury was induced as described previously (58). Briefly, renal arteries were clamped for 45 minutes using microaneurysm clamps through a midline abdominal incision under general anesthesia (0.07 ml/10 g mouse of fentanyl citrate fluanisone midazolam mixture containing: 1.25 mg/ml midazolam [Roche Diagnostics Corp.], 0.08 mg/ml fentanyl citrate, and 2.5 mg/ml fluanisone [Janssen Pharmaceutica]). After clamp removal, kidneys were inspected for restoration of blood flow. The abdomen was closed in 2 layers, and all mice received a subcutaneous injection of 50 μg/kg buprenorphin (Temgesic; Schering-Plough) for analgetic purposes and were allowed to recover from surgery for 12 hours at 32°C in a ventilated stove. Sham-operated mice (n = 8 per group) underwent the same procedure without clamping and were sacrificed 1 day after surgery. To maintain fluid balance and volume status, mice were supplemented with 1 ml sterile 0.9% NaCl i.p. To mark proliferating cells, 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) was injected i.p. (50 mg/kg body wt) 1 hour before sacrifice. Mice (n = 8 per group) were sacrificed 1, 5, and 10 days after surgery. To measure early TNF-α levels, 6 mice per group were sacrificed 2 hours after reperfusion. At the time of sacrifice, mice were weighed, blood was collected by heart puncture in heparin-containing tubes and stored at –70°C, and kidneys were harvested for further analysis. No significant differences were found between weights of TLR2+/+ and TLR2–/– mice at all time points (24.1 ± 0.27 and 24.7 ± 1.0 g, respectively; P = 0.74 for t = 1, the time point with the highest level of renal dysfunction).
Bone marrow transplantation.
Total bone marrow was collected from female TLR2+/+ or TLR2–/– mice by flushing femurs and tibiae with sterile PBS containing 10% FCS, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Corp.). To ensure short-term survival of the recipients (59), single cell suspension of a syngeneic spleen from a female TLR2+/+ or TLR2–/– mice was obtained by crushing spleens in PBS containing 10% FCS, penicillin, and streptomycin through a 40-μm cell strainer (BD). Male TLR2+/+ (n = 12) and TLR2–/– (n = 12) mice were lethally irradiated with 2 doses of 5 Gy, separated by 3 hours, using a 137Cs irradiator (CIS bio international; SCHERING S.A.). After the last irradiation dose, 5 × 106 TLR2+/+ or TLR2–/– bone marrow cells and 2 × 105 TLR2+/+ or TLR2–/– spleen cells in sterile PBS were injected into the tail vein of recipient irradiated mice. The mice were kept in microisolator cages for 6 weeks to complete engraftment with donor bone marrow, after which renal I/R injury was induced as described above. One week prior and 4 weeks following transplantation, mice received sterile acidified tap water (12 × 10–3 M HCl) containing 0.16% neomycin sulphate (Sigma-Aldrich) to prevent the immunocompromised recipients from infections.
FACS analysis.
To assay bone marrow reconstitution, whole blood collected from tails was harvested from mice prior to I/R surgery and analyzed by flow cytometry to determine donor/recipient chimerism of the hematopoietic compartment. Total white blood cell counts were performed on a Coulter ACT diff2 (Beckman Coulter). Subsequently, erythrocytes were lysed for 10 minutes at 4°C in 160 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA (pH 7.4). Remaining leukocytes were brought to a concentration of 4 × 106 cells/ml FACS buffer (PBS supplemented with 0.5% BSA, 0.01% NaN3, and 100 mM EDTA) and stained for TLR2 (IgG1κ anti-TLR2 mAb named T2.5; ref. 60) and a secondary goat anti-mouse IgG1-FITC (SouthernBiotech) for 30 minutes at 4°C. TLR2 was analyzed on forward- and side-angle scatter–gated neutrophils and monocytes known to mediate tissue injury and to express TLR2. The number of positive cells was determined by setting a quadrant marker for nonspecific staining. To correct for background staining, unlabeled cells were used. All analyses were performed on a FACSCalibur (BD).
Distribution of FITC-ASONs in vivo.
To examine the in vivo distribution of ASONs, TLR2+/+ mice were treated twice i.p. with 4 nmol FITC-labeled ASONs (ACTACTACACTAGACTAC) 29 hours and 5 hours before sacrifice. To evaluate whether FITC-ASONs could also reach the kidney when renal arteries are clamped, we gave this compound 24 hours before and directly after the induction of renal I/R injury. These oligonucleotides were designed and manufactured by Biognostik. Kidney, liver, lung, and spleen were removed, fixed for 24 hours in 10% formalin, and embedded in paraffin. Sections of 4 μm were deparaffinized and boiled for 10 minutes in 10 mM sodium citrate buffer (pH 6.0). Subsequently, endogenous peroxidase activity and nonspecific binding were blocked with 0.3% H2O2 in 100% methanol and 10% normal goat serum (DakoCytomation). Sections were then exposed to rabbit anti-FITC (DakoCytomation), and this was followed by a further incubation with HRP-conjugated goat-anti rabbit IgG (PowerVision; ImmunoVision Technologies Co.). Slides were developed using 1% H2O2 and 3,3′-diaminobenzidin-tetra-hydrochloride (DAB) (Sigma-Aldrich) in 0.05 M Tris-HCl (pH 7.9). The slides were counterstained with Methyl Green (Sigma-Aldrich).
TLR2 ASON treatment of mice.
Mice were injected twice i.p. with 4 nmol TLR2 ASONs (GAGCTCGTAGCATCCTCT) or 4 nmol NSONs (GCTCTATGACTCCCAG) (both designed and manufactured by Biognostik) 24 hours before, during, and 2 days after the induction of renal I/R injury and sacrificed after 1, 5, and 10 days as described above. Sham-operated mice underwent the same procedure without clamping and were sacrificed 1 day after surgery. No significant differences were found between the weights of NSON- and ASON-treated mice at all time points (26.1 ± 0.3 and 25.2 ± 0.5 g, respectively; P = 0.19 for t = 1, the time point with the highest level of renal dysfunction).
Immunoprecipitation and immunoblotting.
Kidney homogenates from ASON- and NSON-treated animals subjected for 1 day to renal I/R injury were diluted in lysis buffer containing 300 mM NaCl, 30 mM Tris, 2 mM MgCl2, 2 mM CaCl2, 1% Triton X-100, and 1% protease inhibitor cocktail II (Sigma-Aldrich), homogenized, sonicated, and incubated at 4°C for 30 minutes. Renal homogenates (40 μg protein) were centrifuged, after which supernatants (cell lysates) were cleared by addition of Protein G-Sepharose beads for 1 hour at 4°C and incubated with 2 μg anti-TLR2 (IgG1κ anti-TLR2 mAb T2.5) for 1 hour at 4°C. Immune complexes were collected on Protein G-Sepharose beads during 1 hour incubation at 4°C and eluted by incubation in lysis buffer (20 mM Tris-HCl, 300 mM NaCl, 2% NP-40, 20% glycerol, 10 mM EDTA, 4 mM Na3VO4, 10 mM NaF, and 1% protease inhibitor cocktail II). Fractions before and after immunoprecipitation (to detect β-actin and TLR2, respectively) were separated by SDS-PAGE and transferred onto activated PVDF membranes (Millipore). Subsequently, membranes were blocked with 5% (w/v) nonfat dry milk in Tris-buffered saline containing 0.1% Tween (TBS-T) and incubated with primary antibody (affinity purified rabbit polyclonal anti-murine TLR2, monoclonal anti–β-actin; Sigma-Aldrich), followed by incubation with goat anti-rabbit HRP (DakoCytomation) or goat anti-mouse HRP (BioSource International), respectively, and visualized with ECL reagent (Amersham Pharmacia Biotech).
Preparation of renal tissue for cytokine measurements.
For cytokine measurements, snap-frozen kidneys were diluted in lysis buffer and homogenized and incubated at 4°C for 30 minutes. Homogenates were subsequently centrifuged at 1500 g at 4°C for 15 minutes, and supernatants were stored at –70°C until assays were performed.
ELISA.
Cytokines and chemokines (TNF-α, IL-1β, IL-6, KC, MIP-2, and MCP-1) were measured using specific ELISAs (R&D Systems) according to manufacturer instructions. The detection limits were 31 pg/ml for TNF-α, 8 pg/ml for IL-1, 16 pg/ml for IL-6, 12 pg/ml for KC, 94 pg/ml for MIP-2, and 7 pg/ml for MCP-1. Cytokine levels were corrected for the amount of protein present using the Bio-Rad protein assay (Bio-Rad Laboratories) with IgG as standard.
Detection of granulocytes and macrophages.
Renal tissues were fixed for 24 hours in 10% formalin and embedded in paraffin. Sections of 4 μm were deparaffinized and digested with a solution of 0.25% pepsine (Sigma-Aldrich) in 0.01M HCl for granulocyte detection or boiled for 10 minutes in 10 mM sodium citrate buffer (pH 6.0) for macrophage detection. Subsequently, endogenous peroxidase activity and nonspecific binding were blocked as described, and sections were exposed to FITC-labeled anti-mouse Ly-6G mAb (BD Biosciences — Pharmingen) or rat anti-mouse F4/80 IgG2b mAb (Serotec), respectively. For staining of granulocytes, slides were incubated with rabbit anti-FITC antibody (DakoCytomation), which was followed by a further incubation with HRP-conjugated goat anti-rabbit IgG (ImmunoVision Technologies Co.) For macrophage staining, slides were incubated with rabbit anti-rat biotin (DakoCytomation), which was followed by streptavidin-ABC solution (DakoCytomation). The slides were developed using 1% H2O2 and DAB (Sigma-Aldrich) in 0.05M Tris-HCl (pH 7.9) and counterstained with Methyl Green (Sigma-Aldrich); the number of positive cells was counted in 10 nonoverlapping fields (×400).
Detection of apoptosis and proliferation.
Tissue sections of kidneys were deparaffinized and boiled for 10 minutes in 10 mM sodium citrate buffer (pH 6.0). Nonspecific binding and endogenous peroxidase activity were blocked as described, followed by incubation with rabbit anti-human active caspase 3 polyclonal antibody (Cell Signaling Technology), followed by a further incubation with HRP-conjugated goat-anti rabbit IgG (ImmunoVision Technologies Co.), and further processed as described above. To detect BrdU, DNA was denatured in 2 M HCl, and antigen retrieval was performed by 0.4% pepsin (Sigma-Aldrich) in 0.01M HCl. Sections were then incubated in 5% normal goat serum (DakoCytomation), 0.1% bovine serum albumin (Sigma-Aldrich), and 0.1% Tween-20 (Sigma-Aldrich) and exposed to mouse IgG1 anti-BrdU antibodies (Sigma-Aldrich). This was followed by a further incubation with goat anti-mouse IgG1-HRP (SouthernBiotech). Slides were finally developed using 1% H2O2 and DAB (Sigma-Aldrich) in 0.05M Tris-HCl (pH 7.9). The slides were counterstained with Methyl Green (Sigma-Aldrich), and the number of positive TECs were counted in 10 nonoverlapping fields (×400).
Detection of TLR2.
Kidneys were snap frozen in liquid nitrogen and stored at –70°C prior to use. For histological examination of TLR2, kidneys were cut into 6-μm sections, fixed for 10 minutes with ice-cold acetone, and air dried. The sections were incubated in 5% normal goat serum (DakoCytomation) for 10 minutes and then exposed overnight to anti-TLR2 mAb T2.5 in PBS. Endogenous peroxidase activity was quenched by a solution of 10% NaN3/0.03% H2O2 (Merck) in PBS. Slides were incubated with goat anti-mouse IgG-1-HRP (Southern Biotechnology Associates) with 5% normal human serum, rinsed again, and developed using NovaRed (Vector Laboratories).
Histopathological scoring.
All histopathological scorings were made in the cortex using PAS-D–stained renal tissue sections and performed on coded slides. The percentage of damaged tubules in the corticomedullary junction was estimated using a 5-point scale according to the following criteria: tubular dilatation, cast deposition, brush border loss, and necrosis in 10 randomly chosen, nonoverlapping fields (×400 magnification). Lesions were graded on a scale from 0 to 5: 0 = normal; 1 = mild, involvement of less than 10% of the cortex; 2 = moderate, involvement of 10 to 25% of the cortex; 3 = severe, involvement of 25 to 50% of the cortex; 4 = very severe, involvement of 50–75% of cortex; 5 = extensive damage, involvement of more than 75% of the cortex.
Plasma biochemical analysis.
The recovery of renal function was determined by measuring creatinine and ureum in plasma samples obtained after intervention by enzyme reactions involving urease and creatinase and using standard autoanalyzer methods by our hospital research services.
Statistics.
Differences between groups were analyzed using Mann-Whitney U test. Values are expressed as mean ± SEM. A value of P < 0.05 was considered statistically significant.
Acknowledgments
The authors wish to thank Joris Roelofs and Guangxun Meng for expert technical assistance. This work was supported by a grant from The Netherlands Organization for Scientific Research to J.C. Leemans, G. Stokman, G.J.D. Teske, and S. Florquin.
Footnotes
Nonstandard abbreviations used: ASON, antisense oligonucleotide; ATP, adenosine triphosphate; DAB, 3,3′-diaminobenzidin-tetra-hydrochloride; I/R, ischemia/reperfusion; KC, keratinocyte chemoattractant; MCP-1, monocyte chemoattractant protein-1; MIP-2, macrophage inflammatory protein-2; NSON, nonsense oligonucleotide; TEC, tubular epithelial cell.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson GB, Brunn GJ, Platt JL. Activation of mammalian Toll-like receptors by endogenous agonists. Crit. Rev. Immunol. 2003;23:15–44. doi: 10.1615/critrevimmunol.v23.i12.20. [DOI] [PubMed] [Google Scholar]
- 4.Johnson GB, Brunn GJ, Tang AH, Platt JL. Evolutionary clues to the functions of the Toll-like family as surveillance receptors. Trends Immunol. 2003;24:19–24. doi: 10.1016/s1471-4906(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 5.Wolfs TG, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J. Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 6.Asea A, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 7.Li M, et al. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 2001;166:7128–7135. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 8.Van Why SK, et al. Induction and intracellular localization of HSP-72 after renal ischemia. Am. J. Physiol. 1992;263:F769–F775. doi: 10.1152/ajprenal.1992.263.5.F769. [DOI] [PubMed] [Google Scholar]
- 9.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am. J. Physiol. Renal Physiol. 2003;284:F608–F627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi N, et al. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J. Immunol. 2002;169:2026–2033. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal M, Swartz R. Acute renal failure. Am. Fam. Physician. 2000;61:2077–2088. [PubMed] [Google Scholar]
- 12.Preston RA, Epstein M. Ischemic renal disease: an emerging cause of chronic renal failure and end-stage renal disease. J. Hypertens. 1997;15:1365–1377. doi: 10.1097/00004872-199715120-00001. [DOI] [PubMed] [Google Scholar]
- 13.Halloran PF, et al. The “injury response”: a concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant. Proc. 1997;29:79–81. doi: 10.1016/s0041-1345(96)00015-2. [DOI] [PubMed] [Google Scholar]
- 14.Shoskes DA, Halloran PF. Delayed graft function in renal transplantation: etiology, management and long-term significance. J. Urol. 1996;155:1831–1840. doi: 10.1016/s0022-5347(01)66023-3. [DOI] [PubMed] [Google Scholar]
- 15.Carome MA, et al. Distribution of the cellular uptake of phosphorothioate oligodeoxynucleotides in the rat kidney in vivo. Nephron. 1997;75:82–87. doi: 10.1159/000189504. [DOI] [PubMed] [Google Scholar]
- 16.Furuichi K, Wada T, Yokoyama H, Kobayashi KI. Role of cytokines and chemokines in renal ischemia-reperfusion injury. Drug News Perspect. 2002;15:477–482. doi: 10.1358/dnp.2002.15.8.840067. [DOI] [PubMed] [Google Scholar]
- 17.Brauner A, et al. Escherichia coli-induced expression of IL-1 alpha, IL-1 beta, IL-6 and IL-8 in normal human renal tubular epithelial cells. Clin. Exp. Immunol. 2001;124:423–428. doi: 10.1046/j.1365-2249.2001.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boswell RN, et al. Interleukin 6 production by human proximal tubular epithelial cells in vitro: analysis of the effects of interleukin-1 alpha (IL-1 alpha) and other cytokines. Nephrol. Dial. Transplant. 1994;9:599–606. doi: 10.1093/ndt/9.6.599. [DOI] [PubMed] [Google Scholar]
- 19.Jevnikar AM, et al. Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int. 1991;40:203–211. doi: 10.1038/ki.1991.201. [DOI] [PubMed] [Google Scholar]
- 20.Daemen MA, de Vries B, van’t Veer C, Wolfs TG, Buurman WA. Apoptosis and chemokine induction after renal ischemia-reperfusion. Transplantation. 2001;71:1007–1011. doi: 10.1097/00007890-200104150-00032. [DOI] [PubMed] [Google Scholar]
- 21.Rangan GK, Wang Y, Tay YC, Harris DC. Inhibition of NFkappaB activation with antioxidants is correlated with reduced cytokine transcription in PTC. Am. J. Physiol. 1999;277:F779–F789. doi: 10.1152/ajprenal.1999.277.5.F779. [DOI] [PubMed] [Google Scholar]
- 22.Ueland JM, Gwira J, Liu ZX, Cantley LG. The chemokine KC regulates HGF-stimulated epithelial cell morphogenesis. Am. J. Physiol. Renal Physiol. 2004;286:F581–F589. doi: 10.1152/ajprenal.00289.2003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XW, Liu Q, Wang Y, Thorlacius H. CXC chemokines, MIP-2 and KC, induce P-selectin-dependent neutrophil rolling and extravascular migration in vivo. Br. J. Pharmacol. 2001;133:413–421. doi: 10.1038/sj.bjp.0704087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XW, Wang Y, Liu Q, Thorlacius H. Redundant function of macrophage inflammatory protein-2 and KC in tumor necrosis factor-alpha-induced extravasation of neutrophils in vivo. Eur. J. Pharmacol. 2001;427:277–283. doi: 10.1016/s0014-2999(01)01235-3. [DOI] [PubMed] [Google Scholar]
- 25.Rice JC, Spence JS, Yetman DL, Safirstein RL. Monocyte chemoattractant protein-1 expression correlates with monocyte infiltration in the post-ischemic kidney. Ren. Fail. 2002;24:703–723. doi: 10.1081/jdi-120015673. [DOI] [PubMed] [Google Scholar]
- 26.Meldrum KK, Meldrum DR, Meng X, Ao L, Harken AH. TNF-alpha-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H540–H546. doi: 10.1152/ajpheart.00072.2001. [DOI] [PubMed] [Google Scholar]
- 27.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–3336. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirschning CJ, Schumann RR. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr. Top. Microbiol. Immunol. 2002;270:121–144. doi: 10.1007/978-3-642-59430-4_8. [DOI] [PubMed] [Google Scholar]
- 29.Lauriat S, Linas SL. The role of neutrophils in acute renal failure. Semin. Nephrol. 1998;18:498–504. [PubMed] [Google Scholar]
- 30.Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am. J. Kidney Dis. 1999;34:384–399. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- 31.Dursun B, He Z, Ljubanovic D, Faubel S, Edelstein CL. Macrophage depletion is protective against ischemic ARF in the mouse. October 27–November 1, 2004. St. Louis, Missouri, USA. J. Am. Soc. Nephrol. 2004;15:713. [Google Scholar]
- 32.Daha MR, van Kooten C. Is the proximal tubular cell a proinflammatory cell? Nephrol. Dial. Transplant. 2000;15(Suppl. 6):41–43. doi: 10.1093/ndt/15.suppl_6.41. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 2001;1:625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 34.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J. Biol. Chem. 2001;276:5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 35.Anders HJ, Banas B, Schlondorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J. Am. Soc. Nephrol. 2004;15:854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 36.Paller MS. Effect of neutrophil depletion on ischemic renal injury in the rat. J. Lab. Clin. Med. 1989;113:379–386. [PubMed] [Google Scholar]
- 37.Thornton MA, Winn R, Alpers CE, Zager RA. An evaluation of the neutrophil as a mediator of in vivo renal ischemic-reperfusion injury. Am. J. Pathol. 1989;135:509–515. [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss SJ. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 39.Horl WH, Schafer RM, Horl M, Heidland A. Neutrophil activation in acute renal failure and sepsis. Arch. Surg. 1990;125:651–654. doi: 10.1001/archsurg.1990.01410170099021. [DOI] [PubMed] [Google Scholar]
- 40.Nikolic-Paterson DJ, Atkins RC. The role of macrophages in glomerulonephritis. Nephrol. Dial. Transplant. 2001;16(Suppl. 5):3–7. doi: 10.1093/ndt/16.suppl_5.3. [DOI] [PubMed] [Google Scholar]
- 41.Daemen MA, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J. Clin. Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aliprantis AO, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 43.Rifai A, Brysch W, Fadden K, Clark J, Schlingensiepen KH. Clearance kinetics, biodistribution, and organ saturability of phosphorothioate oligodeoxynucleotides in mice. Am. J. Pathol. 1996;149:717–725. [PMC free article] [PubMed] [Google Scholar]
- 44.Haller H, et al. Antisense oligonucleotides for ICAM-1 attenuate reperfusion injury and renal failure in the rat. Kidney Int. 1996;50:473–480. doi: 10.1038/ki.1996.338. [DOI] [PubMed] [Google Scholar]
- 45.Dragun D, et al. ICAM-1 antisense oligodesoxynucleotides prevent reperfusion injury and enhance immediate graft function in renal transplantation. Kidney Int. 1998;54:590–602. doi: 10.1046/j.1523-1755.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J. Clin. Invest. 2003;111:1571–1578. doi:10.1172/JCI200317573. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell. Microbiol. 2003;5:143–153. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 48.Shahin RD, Engberg I, Hagberg L, Svanborg Eden C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J. Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- 49.Zhang D, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 50.Schilling JD, Martin SM, Hung CS, Lorenz RG, Hultgren SJ. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4203–4208. doi: 10.1073/pnas.0736473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weight SC, Bell PR, Nicholson ML. Renal ischaemia–reperfusion injury. Br. J. Surg. 1996;83:162–170. [PubMed] [Google Scholar]
- 52.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 53.Wuthrich RP, et al. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990;37:783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- 54.Meldrum KK, Meldrum DR, Hile KL, Burnett AL, Harken AH. A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J. Surg. Res. 2001;99:288–293. doi: 10.1006/jsre.2001.6201. [DOI] [PubMed] [Google Scholar]
- 55.Vander Heide RS, Rim D, Hohl CM, Ganote CE. An in vitro model of myocardial ischemia utilizing isolated adult rat myocytes. J. Mol. Cell. Cardiol. 1990;22:165–181. doi: 10.1016/0022-2828(90)91113-l. [DOI] [PubMed] [Google Scholar]
- 56.Henry P, Popescu A, Puceat M, Hinescu ME, Escande D. Acute simulated ischaemia produces both inhibition and activation of K+ currents in isolated ventricular myocytes. Cardiovasc. Res. 1996;32:930–939. [PubMed] [Google Scholar]
- 57.Vanheel B, Leybaert L, De Hemptinne A, Leusen I. Simulated ischemia and intracellular pH in isolated ventricular muscle. Am. J. Physiol. 1989;257:C365–C376. doi: 10.1152/ajpcell.1989.257.2.C365. [DOI] [PubMed] [Google Scholar]
- 58.Stokman G, Leemans JC, Claessen N, Weening JJ, Florquin S. Hematopoietic stem cell mobilization therapy accelerates recovery of renal function independent of stem cell contribution. J. Am. Soc. Nephrol. 2005;16:1684–1692. doi: 10.1681/ASN.2004080678. [DOI] [PubMed] [Google Scholar]
- 59.Schoenmakers SH, Groot AP, Florquin S, Reitsma PH, Spek CA. Blood cell-derived tissue factor influences host response during murine endotoxemia. Blood Cells Mol. Dis. 2004;32:325–333. doi: 10.1016/j.bcmd.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Meng G, et al. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J. Clin. Invest. 2004;113:1473–1481. doi:10.1172/JCI200420762. doi: 10.1172/JCI20762. [DOI] [PMC free article] [PubMed] [Google Scholar]