Abstract
Modified anti-CD3 mAbs are emerging as a possible means of inducing immunologic tolerance in settings including transplantation and autoimmunity such as in type 1 diabetes. In a trial of a modified anti-CD3 mAb [hOKT3γ1(Ala-Ala)] in patients with type 1 diabetes, we identified clinical responders by an increase in the number of peripheral blood CD8+ cells following treatment with the mAb. Here we show that the anti-CD3 mAb caused activation of CD8+ T cells that was similar in vitro and in vivo and induced regulatory CD8+CD25+ T cells. These cells inhibited the responses of CD4+ cells to the mAb itself and to antigen. The regulatory CD8+CD25+ cells were CTLA4+ and Foxp3+ and required contact for inhibition. Foxp3 was also induced on CD8+ T cells in patients during mAb treatment, which suggests a potential mechanism of the anti-CD3 mAb immune modulatory effects involving induction of a subset of regulatory CD8+ T cells.
Introduction
Antibodies against T cells have been developed as means of preventing immune responses. An mAb against the CD3 molecule, the TCR, has been used successfully in the past to prevent allograft rejection but causes significant adverse events due to its activation properties in vivo. More recently, modified anti-CD3 mAbs have been engineered in which the activation properties of the mAb have been altered by changes in the Fc portions of the immunoglobulin in order to reduce the cytokine release syndrome that occurs with administration of Fc receptor–binding anti-CD3 antibody (1–5). These molecules have shown efficacy in clinical settings such as renal/pancreas and islet allograft rejection, refractory psoriatic arthritis, and type 1 diabetes mellitus (5–8). These clinical studies have found immediate effects following mAb treatment but also long-term efficacy even after the mAb was cleared and the number of circulating T cells returned to a normal level.
The mechanisms that account for these clinical effects are not understood, but much interest has focused on the role of regulatory T cells that may be involved in autoimmune responses and whose activity may control immunologic tolerance (9–16). Many studies have identified subpopulations of CD4+ T cells that do and do not express CD25 and that mediate their regulatory activity through contact-dependent mechanisms as well as soluble mediators (17–19). These cells play a role in the control of autoimmune disease in murine models of human disease and in the response to anti-CD3 therapy in these models. For example, when CD4+CD25+ T cells fail to develop, as in B7-1/B7-2–deficient NOD mice, diabetes is accelerated (20). Induction of immune tolerance to autoimmune diabetes in mice with anti-CD3 mAb induces CD4+CD25+ regulatory T cells that function in a TGF-β–dependent manner (21).
Foxp3 has emerged as a marker for T cells with regulatory activity (22–24). Deletion or mutation of Foxp3 is associated with the lymphoproliferative disorder in scurfy mice (25) and X-linked inheritance (IPEX) syndrome in humans (26, 27). It is not clear, however, whether Foxp3 expression and regulatory function are developmental features of subpopulations of T cells or whether this function and expression can be acquired. Some studies have suggested that Foxp3 expression delineates a distinct lineage of regulatory T cells (28). Other studies have shown that Foxp3 is induced by in vitro activation of CD4+CD25– human peripheral lymphocytes, which acquire the surface expression of CD25 in addition (29). More recently, CD4+ regulatory T cells have been induced by low doses of antigenic peptide, which suggests that induction of regulatory T cells may be an outcome of weak TCR stimulation (30).
In a previous report, we found that treatment of patients with new-onset type 1 diabetes with a humanized, modified anti-CD3 mAb [hOKT3γ1(Ala-Ala)] modified the loss of insulin production with effects that lasted for more than 1 year after a single-course of treatment and without chronic immune suppression (31). The clinical responses to treatment were associated with a decrease in the ratio of peripheral blood CD4 to CD8 T cells. The change in the ratio was not due to depletion of CD4 cells but instead to an absolute increase in the number of CD8+ T cells. Here, we studied the basis for these changes in CD8+ cells and have identified a population of CD8+ regulatory T cells characterized by expression of CD25 and Foxp3 induced in human peripheral blood cells by treatment with modified anti-CD3 mAb. The regulatory activity of the induced CD8+CD25+ cells is on autologous, antigen-reactive CD4+ T cells in a cell contact–dependent manner. CD8+Foxp3+ T cells can be found to be induced in the peripheral blood of patients with type 1 diabetes treated with anti-CD3 mAb. These findings provide evidence for a new mechanism for induction of immune regulation in humans involving induction of regulatory CD8+CD25+ T cells by treatment with a weak TCR agonist.
Results
MAb hOKT3 γ1(Ala-Ala) induces proliferation of CD8+ T lymphocytes.
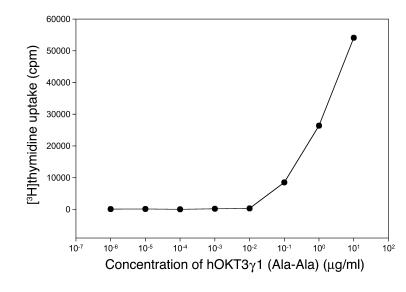
Our previously reported studies of patients with type 1 diabetes treated with hOKT3γ1(Ala-Ala) showed that the mAb induced activation of T cells in vivo based on the release of cytokines and expression of activation markers on PBMCs (32). This finding was unexpected, because earlier studies showed that the humanized and genetically modified anti-CD3 antibody hOKT3γ1(Ala-Ala) had reduced potency for inducing cell proliferation in comparison to unmodified OKT3 (1). To address the effects of the activation of T cells by the modified anti-CD3 mAb, we studied the ability of hOKT3γ1(Ala-Ala) to induce proliferation of PBMCs, measured by incorporation of [3H]thymidine in a 5-day assay (Figure 1). Based on these findings, the concentration of the antibody chosen for our in vitro study was 5 μg/ml. This concentration is higher than trough concentrations found in drug-treated subjects with type 1 diabetes (range, 150–1,200 μg/ml) but similar to concentrations achieved in other studies with this mAb and consistent with peak concentrations that were achieved in vivo (7).
Figure 1.
Anti-CD3 hOKT3γ1(Ala-Ala) antibody stimulates in vitro proliferation of human PBMCs. PBMCs were cultured for 5 days in the presence of hOKT3γ1(Ala-Ala) antibody at the indicated concentrations. Cell proliferation level was determined by [3H]thymidine uptake. Results are expressed as the mean value of duplicates and are representative of 3 independent experiments.
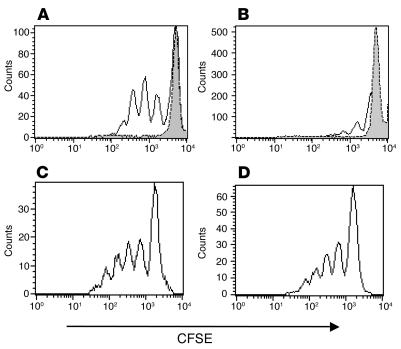
When T cell proliferation was examined by dilution of CFSE in labeled cells, we found significant differences in the pattern of proliferation among T cell subsets (Figure 2). CFSE-labeled freshly isolated PBMCs were incubated in the presence of hOKT3γ1(Ala-Ala) antibody, and the dilution of the dye was studied in subsets of T cells by flow cytometry. Over the 6–7 day culture, there was minimal proliferation of CD4+ T cells: 32% ± 5% (range, 13–48%) of the cells showed at least 1 dilution of CFSE, but generally only 1 or 2 divisions were detected (Figure 2B). However, in the same cultures, 56% ± 8% (range, 34–83%) of the CD8+ T lymphocytes underwent at least 1 and generally between 4 and 7 divisions (P < 0.03; Figure 2A). The differential effect on stimulation of T cell subset was not a general phenomenon of T cell activation, because when the same cells were activated with phytohemagglutinin (PHA), similar proportions of both CD4+ and CD8+ T cells underwent 5 divisions (Figure 2, C and D). The activation pattern of CD8+ T cells shown in Figure 2 is representative of more than 8 normal control subjects and occurred with similar frequency in patients with type 1 diabetes.
Figure 2.
Diverse in vitro response of CD8+ and CD4+ subpopulations of human PBMCs to hOKT3γ1(Ala-Ala) stimulation. CFSE-labeled PBMCs were cultured in the presence of hOKT3γ1(Ala-Ala) (A and B) or PHA (C and D) for 6 days. Cells were labeled with fluorochrome-conjugated anti-CD4 and anti-CD8 and analyzed by flow cytometry. (A and C) CD8-gated cells. (B and D) CD4-gated cells. Gray histograms: cells without stimulation. Results from a single experiment representative of 6 experiments are shown.
The changes in T cell subsets observed in vitro correspond to changes observed in vivo.
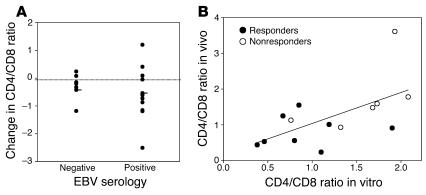
In our previous phase I/II trial, clinical responders, identified by preserved or increased C-peptide responses to a mixed-meal tolerance test 1 year after drug treatment, were found to have a decreased ratio of CD4+ to CD8+ T cells due to an increase in the absolute number of circulating CD8+ T cells (8, 31). One possible explanation for the increased number of CD8+ T cells was that the expanded CD8+ T cells were in response to activation of latent viruses, such as EBV, that may have occurred as a result of the immune suppression by the anti-CD3 mAb. The subjects in our trial included EBV-seropositive and -seronegative individuals, and therefore, if the change in T cell subsets were related to activation of EBV, we would expect to find it only in EBV-seropositive individuals. However, this was not the case (Figure 3A). Compared with the CD4+/CD8+ T cell ratio before administration of the anti-CD3 mAb, a decreased CD4+/CD8+ T cell ratio was seen at day 90 after anti-CD3 mAb treatment in EBV-seropositive and -seronegative individuals, which suggests that the cellular response to reactivation of EBV did not account for the changes that had been seen in vivo.
Figure 3.
Changes in CD4/CD8 T cell ratio in subjects with type 1 diabetes receiving hOKT3γ1(Ala-Ala) in relation to EBV status at study entry, and correlation between changes in CD4+ and CD8+ T cells in vitro during culture with anti-CD3 mAb and in vivo following treatment with anti-CD3 mAb. (A) The difference in the CD4/CD8 T cell ratio 3 months after treatment with hOKT3γ1(Ala-Ala) from the ratio before drug treatment in individuals who were EBV seropositive (n = 12) or seronegative (n = 7) at study entry was determined. The dark lines indicate the mean values for the group. A decrease in the CD4/CD8 T cell ratio (below the dotted line) occurred in both EBV-seropositive and -seronegative subjects. (B) PBMCs from patients with type 1 diabetes mellitus who received anti-CD3 mAb were studied 1.5–2 years after mAb treatment, at which time the changes in CD4/CD8 T cell ratio seen after mAb treatment had resolved. The patients were designated as clinical responders (filled circles) or nonresponders (open circles) based on their C-peptide responses at 12 months compared with baseline. The cells were cultured with hOKT3γ1(Ala-Ala), and the percentages of CD4+ and CD8+ T cells were determined after 6 days. The Pearson correlation coefficient was calculated to compare the ratio of CD4+/CD8+ T cells in vitro with the analysis of CD4+/CD8+ T cells in vivo 3 months after mAb treatment (8), and the level of significance was tested with correlation procedure SAS (r = 0.6; P = 0.024). The line indicates the relationship between the changes in vitro and in vivo [in vivo CD4/CD8 ratio = 0.174 + 0.865 (in vitro CD4/CD8 ratio)].
To determine whether the changes in T cell proliferation seen in response to the mAb in vitro were reflective of changes in cell counts in vivo, we compared the ratio of CD4+ to CD8+ T cells following culture with the anti-CD3 mAb to the CD4+/CD8+ T cell ratio in vivo following treatment with the mAb (Figure 3B). The PBMCs used in these studies were isolated from patients 1–2.5 years after mAb treatment, at a time when the changes in CD4+ /CD8+ T cell ratios that had been seen 3 months after drug treatment had resolved. There was a significant correlation between the CD4+/CD8+ T cell ratio in vitro following a 6-day culture with the anti-CD3 mAb and in vivo 3 months following the 12- or 14-day treatment course among clinical responders and nonresponders (r = 0.60; P = 0.02). These findings suggest that the changes in T cell subsets observed in vitro identify individual differences in the responses that occur in vivo.
The absence of CD4+ T cell proliferation during culture with the anti-CD3 mAb is due to the presence of CD8+ cells.
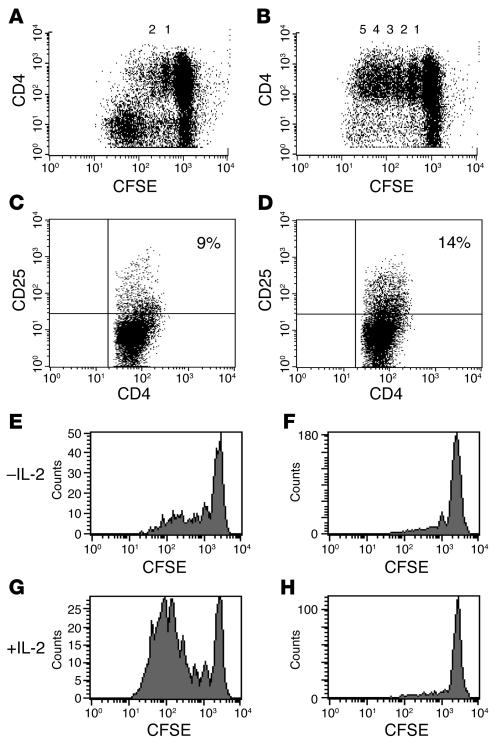
To investigate the basis for the differences in CD8+ and CD4+ T cell proliferation, we depleted CD8+ or CD4+ T cells from PBMCs and labeled the remaining cells with CFSE. These cells were then cultured with hOKT3γ1(Ala-Ala) antibody for 6 days. As seen in Figure 4A, the CD4+ T cells did not proliferate extensively in the presence of CD8+ T cells; however, in the absence of CD8+ T cells, CD4+ T cells proliferated in response to anti-CD3 mAb stimulation and underwent a number of divisions similar to CD8+ cells (Figure 4B). Likewise, in the absence of CD8+ T cells, the expression of a marker of activation, CD25, was increased on CD4+ T cells (Figure 4, C and D). In contrast, the pattern of CD8+ T cell proliferation did not change in the absence of CD4+ T cells. Proliferation of CD4+ and CD8+ T lymphocytes depended on the presence of APCs in the culture, because purified CD8+ and CD4+ T cells alone did not respond to the anti-CD3 mAb (data not shown). In addition, the failure of the CD4+ T cells to proliferate to the CD3 mAb in the presence of CD8+ T cells was not due to limiting amounts of anti-TCR mAb, because the coating and modulation of the TCR on CD4+ cells was maximal at the drug concentrations used in the presence of CD8+ cells (data not shown).
Figure 4.
Reduced proliferative response of CD4+ cells to hOKT3γ1(Ala-Ala) stimulation occurs only in the presence of CD8+ cells but is not due to lack of IL-2. CFSE-labeled cells were cultured in the presence of hOKT3γ1(Ala-Ala) for 6 days. Cells were stained with PE-conjugated anti-CD4 and anti-CD25 mAbs and analyzed on a FACSCalibur flow cytometer. (A and C) Bulk PBMCs. (B and D) PBMCs depleted of CD8+ T cells. Representative results of 6 independent experiments are shown. The numbers over the dots in A and B represent the number of cell divisions. The percentages in C and D represent the percentage of CD4+ cells that were CD25+. PBMCs were cultured with the anti-CD3 mAb in the absence (E and F) or presence (G and H) of recombinant IL-2 (50 U/ml). The addition of IL-2 to the cultures enhanced proliferation of CD8+ T cells (E and G) but did not have an effect on CD4+ T cell proliferation in the PBMCs (F and H). Representative results of 4 independent experiments are shown.
It is possible that the lack of proliferation of CD4+ T lymphocytes was due to consumption of IL-2 by the proliferating CD8+ cells. It has been shown, for example, that addition of exogenous IL-2 can restore proliferative function of certain “anergized” cells (33). We therefore tested whether IL-2 would cause CD4+ T cell proliferation when added to a culture of PBMCs together with hOKT3γ1(Ala-Ala) (Figure 4, E–H). However, there was no increase in proliferation with the addition of IL-2 (50 U/ml), which indicated that CD4+ cells were unresponsive to IL-2 when cultured with the mAb in the presence of CD8+ T cells (Figure 4, F and H). In contrast, CD8+ cells from the same cultures responded with even greater rates of proliferation when IL-2 was added to cultures with the anti-CD3 mAb (Figure 4, E and G).
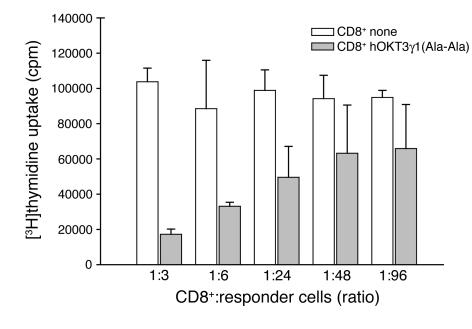
Following culture with mAb, hOKT3 γ1(Ala-Ala) CD8+ lymphocytes suppress antigen-specific responses.
These studies showed that CD8+ T lymphocytes from the PBMC cultures stimulated with hOKT3γ1(Ala-Ala) exhibit suppressive activity against the autologous CD4+ T cells present in the same culture. To determine whether the CD8+ cells could also regulate CD4+ cells responding to other antigens, we tested the ability of the activated CD8+ T cells to affect proliferative responses of CD4+ T cells to tetanus toxoid. CD8+ T cells were isolated from PBMCs cultured in the presence or absence of hOKT3γ1(Ala-Ala) for 6 days, irradiated, and added to CD8+ T cell–depleted PBMCs from the same donor that were pulsed with tetanus toxoid. Cells cultured for 3 days in the presence of CD8+ T cells from cultures activated with anti-CD3 mAb showed a 60% reduction in responses to antigen compared with the cells cultured with CD8+ T cells that had not been cultured with the antibody (Figure 5) The suppression of CD4+ T cell responses was dependent on the number of CD8+ T cells in the culture and was still noticeable at ratio of 1 CD8+ T cell per 96 responding cells.
Figure 5.
CD8+ lymphocytes from PBMC cultures stimulated with hOKT3γ1(Ala-Ala) suppress tetanus-specific response. CD8 cells isolated from fresh PBMCs (CD8 none; white bars) or from PBMCs cultured for 6 days in the presence of hOKT3γ1(Ala-Ala) (gray bars) were irradiated and mixed with fresh PBMCs depleted of CD8+ T cells at the indicated ratios. Cells were cultured with or without the presence of tetanus toxoid for 3 days. Cell proliferation was determined by [3H]thymidine uptake, and the data are expressed as the difference (in counts per minute) between cultures with and without antigen. The background counts (responder cells with CD8+ cells in the absence of antigen) ranged between 542 and 1,796 cpm. Representative results of 3 independent experiments are shown.
Regulation of CD4+ T cells by CD8+ T cells in the presence of anti-CD3 hOKT3 γ1(Ala-Ala) mAb depends on cell-cell contact.
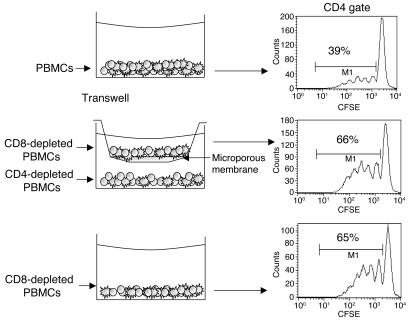
To determine whether suppression of CD4+ proliferation by CD8+ cells is mediated by soluble factors, we added neutralizing mAbs against IL-10 or TNF-α to the cultures of PBMCs and mAb hOKT3γ1(Ala-Ala). None of these mAbs reversed the inhibition of CD4+ cells cultured in the presence of CD8+ cells and the anti-CD3 mAb. TGF-β was not required for inhibition of CD4+ T cells, because in 2 separate experiments, the level of proliferating CD4+ T cells (29.4% of the total CD4+ cells) was not increased when anti–TGF-β mAb was added to the cultures (28.5% when 67% of the CD8+ T cells were proliferating; data not shown). Likewise, addition of CTLA4-Ig did not increase proliferation of CD4+ T cells to the anti-CD3 mAb, even when anti-CD28 mAb was added, which suggests that signaling by CD28 and/or CTLA4 by B7 ligands did not mediate the inhibition (data not shown). In addition, there was no evidence of increased death of CD4+ cells analyzed by staining with annexin V (data not shown). We tested whether cell-cell contact was required by separating CD4+ T and CD8+ T cells by a microporous Transwell membrane, which prevented cell contact (Figure 6) but allowed for medium exchange. CFSE-labeled PBMCs depleted of CD8+ T cells in the upper chamber were separated from PBMCs depleted of CD4+ T cells in the lower chamber and cultured in the medium in the presence of hOKT3γ1(Ala-Ala). The CD4+ T cells proliferated in response to hOKT3γ1(Ala-Ala) when separated from CD8+ T cells at a rate similar to cells cultured in the absence of CD8+ cells (Figure 6, middle row), which indicates that cell-cell contact was required for regulation.
Figure 6.
Suppression of CD4+ cell proliferation by CD8+ lymphocytes is not mediated by soluble factors. CFSE-labeled cells were cultured in the presence of hOKT3γ1(Ala-Ala) for 6 days. Cells were labeled with fluorochrome-conjugated anti-surface marker mAb and analyzed by flow cytometry. Histograms were gated on CD4+ lymphocytes. Shown are PBMCs (top panel), CD8-depleted PBMCs separated by Transwell membrane from CD4-depleted PBMCs (middle panel), and CD8-depleted PBMCs (bottom panel). The numbers in each histogram represent the percentage of CD4+ cells with dilution of CFSE. Results are representative of 4 independent experiments.
Phenotype and cytokine production profile of CD8+ T cells following stimulation with hOKT3γ1(Ala-Ala).
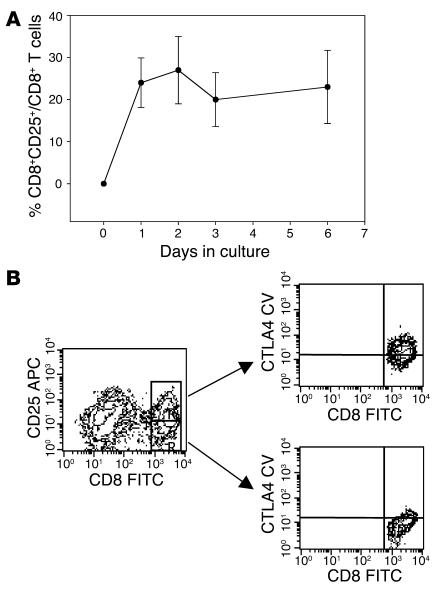
We studied the expression of activation and other cell-surface markers, as well as cytokines produced by subsets of T cells that were cultured with hOKT3γ1(Ala-Ala). In the absence of the antibody, the CD8+ T cells did not express activation markers CD25 or CD69. The majority of these unstimulated CD8+ T cells expressed CD28, 60–80% were of a naive phenotype (CD45RA only), and about 15% expressed only CD45RO. Following culture with anti-CD3 mAb, the expression of CD25 increased on CD8+ cells within 24 hours, and peak expression occurred by day 2 (Figure 7A). CD25 expression was seen on both proliferating (27.3% ± 13.3%) and nonproliferating (15.6% ± 5.3%) CD8+ T cells (n = 3 separate experiments). The CD8+ T cells lost the naive phenotype, and most of the cells (60–80%) expressed CD45RO. The CD8+ T cells that were stimulated with anti-CD3 mAb showed enhanced expression of intracellular CTLA4, particularly among the CD25+CD8+ cells (Figure 7B). As assessed by intracellular staining, the CD8+ T cells did not produce IL-2 or IFN-γ, but IL-10 was detected in about 2% of CD8 cells after a 6-day culture with anti-CD3 mAb. However, when cytokine gene transcripts were analyzed by RT-PCR, increased IL-10 expression was found in some but not all experiments, even when sorted subsets of CD8+ cells were studied. Thus, increased cytokine gene expression was not a consistent finding among the activated CD8+ T cells.
Figure 7.
Induction of CD25+ population in CD8 cells stimulated with hOKT3γ1(Ala-Ala). (A) Freshly isolated PBMCs were cultured in the presence of hOKT3γ1(Ala-Ala) for 6 days. For analysis of CD25 expression, cells were collected on days 0 (before stimulation), 1, 2, 3, and 6 of the culture, labeled with flourochrome-conjugated anti-CD8 and anti-CD25, and analyzed by flow cytometry. The results are presented as a ratio of CD8+CD25+-expressing cells to total CD8+ cells. Mean values (± SEM) of 4 independent experiments are shown. (B) Intracellular expression of CTLA4 analyzed by flow cytometry. Left: gated CD8 lymphocytes; upper right: gated CD8+CD25+ cells; lower right: gated CD8+CD25– cells. Representative results from 3 independent experiments are shown. CTLA4CV, CTLA4 CyChrome.
CD8+CD25+ cells are responsible for inhibition of antigen-specific response of CD4+ T cells.
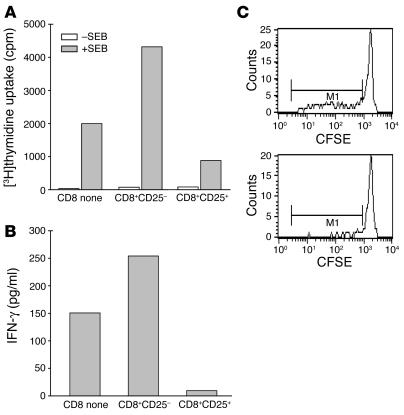
We sought to identify markers that designate the CD8+ T cells that were responsible for regulation of antigen-reactive CD4+ T cells. The correlation between intracellular expression of CTLA4 and upregulation of CD25 molecules on the surface of CD8 cells in our initial experiments directed our interest to study the function of this population. Therefore, CD8+CD25+ and CD8+CD25– populations of CD8+ T cells were sorted from PBMCs after culture with anti-CD3 mAb for 6 days and tested for their ability to inhibit antigen-specific responses to staphylococcal enterotoxin B (SEB) (Figure 8, A and B). As control, CD8+ T cells from autologous PBMCs were added to the cultures. There was a 6-fold inhibition of proliferation in response to the superantigen when irradiated CD8+CD25+ but not CD8+CD25– cells were added (Figure 8A) to the cultures, and the release of IFN-γ was lower in culture supernatants in the presence of CD8+CD25+ T compared with CD8+CD25– T cells (Figure 8B). Inhibition of SEB-specific CD4 cell expansion by CD8+CD25+ cells was also seen at the clonal level. We analyzed expansion of Vβ3-positive CD4 cells in the 6-day cocultures. There was a greater than 50% reduction in the number of Vβ3-positive CD4 cells in the cultures with CD8+CD25+ compared with the culture with nonactivated CD8 cells (Figure 8C). Thus, these results suggest that the induced CD8+CD25+ T cells can regulate antigen-specific responses.
Figure 8.
The CD8+CD25+ cells induced with hOKT3γ1(Ala-Ala) suppress CD4 cell response to SEB and IFN-γ secretion. (A) CD8+CD25+ or CD8+CD25– cells sorted from PBMCs stimulated for 6 days with hOKT3γ1(Ala-Ala) or CD8+ cells from fresh PBMCs were irradiated and cocultured for 3 days with fresh PBMCs depleted of CD8 in the presence of SEB. Proliferative response was assessed by [3H]thymidine uptake. (B) The levels of IFN-γ in the supernatants from the same cultures were measured by Luminex system using Th1/Th2 multiplex microspheres. Representative results of 2 independent experiments are shown. (C) Sorted CD8+CD25+ (bottom panel) or untreated CD8 cells (upper panel) were cocultured for 6 days with CFSE-labeled, CD8-depleted PBMCs at 1:2 ratio in the presence of SEB. SEB-specific clonal expansion was analyzed by flow cytometry. The expansion of Vβ3-positive CD4 (M1) lymphocytes was reduced from 46.1% to 15.5% of Vβ3+ T cells. Similar results were obtained in 2 additional studies.
CD8+CD25+ cells express Foxp3.
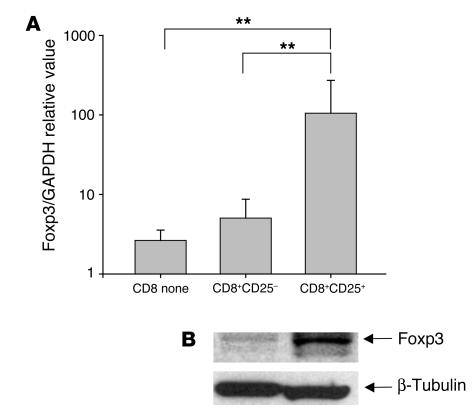
Our studies indicated that the regulatory function of CD8+ cells following anti-CD3 mAb stimulation involved a subpopulation of the CD8+CD25+ T cells that were induced during culture with the anti-CD3 mAb. We therefore asked whether the induction of regulatory CD8+CD25+ T cells was associated with expression of Foxp3 in these cells. We cultured PBMCs with anti-CD3 mAb for 6 days and sorted CD8+ cells into CD25+ and CD25– subpopulations. Foxp3 expression was analyzed by real-time PCR (Figure 9A) and Western blotting (Figure 9B) (29). Foxp3 expression was increased between 10- and 40-fold on CD8+CD25+ T cells compared with CD8+CD25– or nonactivated CD8+ cells (P ≤ 0.02). In pilot studies, we found that the expression of Foxp3 transcripts in CD8+CD25+ T cells was approximately one-ninth of the expression of Foxp3, relative to GAPDH, in CD4+CD25+ T cells sorted from the same cultures after culture with the anti-CD3 mAb for 6 days (CD8+CD25+, 102 ± 56 arbitrary units vs. CD4+CD25+, 931 ± 98 arbitrary units; P < 0.05). These studies indicated that the CD8+CD25+ T cells that are induced have a functional inhibitory role and express Foxp3, a transcription factor associated with immune regulatory T cells.
Figure 9.
Increased expression of Foxp3 in CD8+CD25+ cells induced with hOKT3γ1(Ala-Ala). (A) PBMCs were cultured for 6 days with hOKT3γ1(Ala-Ala) and sorted based on the expression of CD25. Foxp3 expression was measured by quantitative real-time PCR. Results are presented as Foxp3 gene expression normalized to GAPDH expression, and the results for CD8+CD25+ or CD8+CD25– cells were compared with those for freshly isolated CD8+ T cells from the same subject. Mean values (±SD) of 4 independent experiments are shown. **P ≤ 0.02. (B) Expression of Foxp3 was studied in CD8+CD25+ and CD8+CD25– cells from the same cultures by Western blotting and was detected at higher levels in CD8+CD25+ cells.
Induction of Foxp3+CD8+ T cells in vivo by treatment with hOKT3γ1(Ala-Ala).
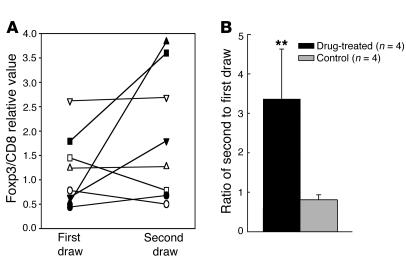
To determine whether treatment with anti-CD3 mAb also induced Foxp3 in CD8+ T cells in patients, we isolated CD8+ T cells from subjects with type 1 diabetes who were treated with hOKT3γ1(Ala-Ala) and measured the expression of Foxp3 RNA by real-time PCR. The samples were taken from subjects before and at the conclusion of the 12-day drug treatment or, in the case of one subject, 10 weeks after treatment. The changes in the expression of Foxp3 were compared with those in contemporaneous samples drawn at approximately the same intervals from control subjects with type 1 diabetes (Figure 10A). The Foxp3 expression in the control group was similar in the first and second samples (the ratio of the second to the first sample was 0.81 ± 0.13), whereas in the drug-treated group, the Foxp3 levels were 3.36 ± 1.27–fold higher after anti-CD3 mAb treatment compared with before treatment (P = 0.02; Figure 10B). CD8+ T cells from all 4 drug-treated subjects showed increased expression of Foxp3 after drug treatment (range, 54–608% increase), whereas the levels of Foxp3 in CD8+ T cells in the 2 samples from each of the control subjects were within 10% (Figure 10A). These data indicate that treatment with anti-CD3 mAb expands CD8+ T cells in vivo and induces cells with markers of regulatory T cells in patients.
Figure 10.
Changes in Foxp3 expression in vivo in CD8+ PBMCs following treatment with anti-CD3 mAb. CD8+ T cells were isolated from the same individual before (first draw) and after (second draw) treatment with anti-CD3 mAb (filled symbols; n = 4) or in control subjects with type 1 diabetes (open symbols) on 2 (n = 3) or 3 (n = 1) occasions. The expression of Foxp3 relative to CD8 (×100) for each individual is plotted (A), and the average ratio of the second/first sampling in each group (± SEM) is shown (B). **P = 0.02.
Discussion
In a trial of hOKT3γ1(Ala-Ala) for treatment of new-onset type 1 diabetes, we found that clinical response (reflected by preservation or increase in C-peptide responses to a mixed meal) was associated with a decrease in the ratio of CD4+ to CD8+ T cells due to an increase in the number of circulating CD8+ T cells (8, 31). We report here our study of the basis for this change and identification of a previously unrecognized population of regulatory CD8+ T cells that are induced in peripheral blood cells by a modified anti-CD3 mAb and that may be involved in the persistent immunologic effects of the mAb. The regulatory activity of the induced CD8+ cells was directed toward autologous CD4 lymphocytes and influenced their proliferative responses to the anti-CD3 antibody and other antigen-specific responses. Like other humanized modified anti-CD3 antibodies, hOKT3γ1(Ala-Ala) was initially thought to be nonactivating, but our findings and previous studies in patients indicate that the drug does deliver an activation signal and even induces proliferation of CD8+ T cells (1, 32). Unlike stimulation with conventional mitogen, such as PHA, significant CD4+ T cell proliferation was not seen in response to the mAb in vitro.
In our studies, instead of activation, we found that CD4+ T cells cultured in the presence of CD8+ T lymphocytes and modified anti-CD3 mAb were unresponsive to antibody stimulation. The preferential expansion of CD8+ T cells in response to anti-TCR antibodies has been described previously, but these studies suggested that CD4+ and CD8+ T cells were intrinsically different in their ability to undergo limited and extensive proliferation, respectively, to fulfill their role as regulatory and effector cells (34). In our studies, the lack of CD4+ T proliferation was completely reversed when CD8+ T cells were removed from the culture. Moreover, other signs of CD4+ T cell activation occurred when CD8+ T cells were removed, including increased expression of CD25. The inhibitory effect of the CD8+ T cells was not due to the consumption of IL-2 by the proliferating cells and involved cell-cell contact, because the inhibition was reversed when CD8+ and CD4+ T cells were separated, and CD4 T cell proliferation did not occur when IL-2 was added to the cultures.
The phenotype of the activated CD8+ T cells suggested a number of possible markers of regulatory T cells. There was increased expression of CTLA4 and CD25 on the activated CD8+ T cells, which was reminiscent of CD4+CD25+ regulatory T cells (20, 21). Our studies of Foxp3 expression on CD8+ T cells following activation with anti-CD3 mAb showed that this transcription factor was expressed on CD8+CD25+ cells rather than CD8+CD25– cells. Murine studies (22, 35, 36) have shown that Foxp3 was expressed specifically in CD4+CD25+ T cells, was not expressed in CD4+CD25– or CD8+ T cells, and was responsible for the suppressor activity of the CD4+CD25+ cells, because introduction of Foxp3 into CD4+CD25– cells by retroviral vector was shown to induce regulatory function (22). Recent studies have questioned the significance of Foxp3 expression as a marker of regulatory T cells on human cells, suggesting instead that unlike murine cells, Foxp3 may identify activated T cells in humans (37). However, our functional studies of the CD8+CD25+ and CD8+CD25– subsets confirmed that regulatory properties were limited to the former subpopulation, although all cells had been activated with anti-CD3 mAb.
The CD8+CD25+Foxp3+ regulatory T cells were induced from CD25– cells by TCR stimulation with the modified anti-CD3 mAb. CD25 was not detected on the PBMCs before the start of cultures, and similarly, in patients, CD8+CD25+ T cells were not detected before anti-CD3 mAb treatment. Our studies do not resolve whether the Foxp3+ cells represent a separate lineage of CD8+ cells that may have lost their expression of CD25 in the periphery but reacquired this marker with TCR activation (28). Cosmi et al. reported CD8+CD25+Foxp3+ cells with regulatory activity in the thymus, suggesting the possibility that the effect of the anti-CD3 mAb is to stimulate the expansion of these cells in the periphery rather than their de novo induction (38, 39). Walker et al. demonstrated that CD4+CD25+ regulatory cells can be generated from human CD4+CD25– cells that are found in peripheral blood by activation with plate-bound anti-CD3 and costimulation with anti-CD28 antibody, and Apostolou et al. showed that regulatory cells can be induced by antigen stimulation (29, 30).
The subpopulation of regulatory CD8+ cells that we have identified has similarities to and differences from other previously described regulatory subpopulations. CD4+ cells, some of which express CD25, regulate T cell responses through cell contact–dependent mechanisms as well as production of soluble mediators including IL-10 and TGF-β (15, 17–19, 40, 41). Subpopulations of CD8+ regulatory T cells have been described that are restricted by the class I molecule HLA-E on activated CD4+ T cells and lyse their CD4+ target, whereas the CD8+CD25+ cells that we have identified do not cause death of CD4+ cells (42–44). Likewise, the cells we identified expressed activation markers and increased levels of CD28; this is different from the activity of the regulatory T cells described by Chang et al. that interact with APCs via immunoglobin-like transcript 3 (ILT3) and ILT4, which in turn induce CD4+CD25+ regulatory cells (45–47). The CD8+CD25+ thymocytes described by Annunziato et al. shared a number of functional and phenotypic similarities with the cells we have identified in the peripheral blood after culture with anti-CD3 mAb, including expression of high levels of mRNA of Foxp3 and glucocorticoid-induced TNF receptor and a contact-dependent mechanism of regulation (48). However, CTLA4 was not constitutively expressed in the cytoplasm of our cells but was also induced by anti-CD3, and antibodies against CTLA4 neutralized the regulatory activity of the thymocytes but not the cells we have identified in the peripheral blood.
The subpopulation of CD8+ T regulatory cells requires activation by the anti-CD3 mAb. The drug concentrations used in vitro were higher than those routinely achieved in vivo but may be similar to the peak concentrations that are achieved following the drug administration for 12 days. Our studies with patients’ samples showed that the expansion of CD8+ T cells that were seen in vitro were related to the changes that were observed in vivo in drug-treated patients. Although the number of subjects included in this analysis was relatively small, this finding raises the possibility that the in vitro responses might be used to predict clinical responses if these observations are confirmed in a prospective study involving a larger number of subjects. In addition, we found that treatment with anti-CD3 mAb hOKT3γ1(Ala-Ala) increased the expression of Foxp3 in CD8+ T cells in vivo. The levels of Foxp3 expression were similar in control subjects over 2 or 3 samplings but increased an average of 3.4-fold in patients treated with the anti-CD3 mAb. The total duration of expression of Foxp3 in the CD8+ T cells is not known at this point, nor is the trafficking of these cells to the lymphoid tissue or antigen sites, but even 10 weeks after drug treatment, we found a 54% increase in the expression of Foxp3 compared with that before treatment. Nonetheless, these observations suggest that our findings in vitro also occur in patients treated with the mAb (39).
Induction of regulatory CD8+ T cells may be a common feature of immune inhibitory therapies that successfully modulates immunity in humans. Lange et al. found that the ratio of CD4 to CD8 T cells decreased following treatment with anti-thymocyte globulin, which was attributed to a decrease in the number of CD4+ cells and expansion of the CD8+ peripheral T cells, and Bas et al. found that acquisition of donor unresponsiveness was correlated with increased numbers of CD8+CD45RA+ T cells in the peripheral blood (49, 50). An antigen-nonspecific CD8+ suppressor population has also been described that inhibits T cell proliferation and CTL function (51). Finally, CD8+CD28– regulatory cells have been shown to participate in the induction of tolerance to heart allografts (45).
In summary, we have identified a new population of human regulatory T cells that is induced in vitro in peripheral blood CD8+ cells by antigen receptor stimulation and in vivo in patients treated with anti-CD3 mAb. Our findings of activation and proliferation of CD8+ cells are consistent with previous observations in vivo of an increase in the number of circulating CD8+ cells following treatment with the anti-CD3 mAb (31). Our findings are also consistent with the postulated role of regulatory T cells following treatment with anti-CD3 mAb, and the findings presented here show that the regulatory cells can be identified by expression of CD25 and Foxp3+. The relationship between activation and expansion of CD8+ T cells and clinical response to treatment with the modified anti-CD3 mAb suggests that these regulatory cells, induced by anti-CD3 mAb treatment, may play a role in the effects of immune therapy.
Methods
Antibody and reagents.
The humanized anti-CD3 mAb hOKT3γ1(Ala-Ala) was developed and produced as previously described (1); OKT3 was a gift from Ortho Pharmaceuticals. Annexin V–APC, anti-human CD3, CD8, CD4, CD25, CD28, CTLA4, and isotype-matched negative control mAbs conjugated with FITC, PE, APC, peridinin chlorophyll-a protein, or CyChrome were obtained from BD Biosciences. Human recombinant IL-2, purified neutralizing human anti-FasL, anti–TNF–α, anti–TGF-β antibodies were obtained from R&D Systems; CTLA4-Ig, anti–IL-10 and Th1/Th2 multiplex microspheres (Luminex Corp.) from BioSource International. Tetanus toxoid was purchased from Biologic Laboratories (University of Massachusetts); PHA and SEB were obtained from Sigma-Aldrich. Polyclonal rabbit anti-human Foxp3 antibody was a gift from Steven Ziegler (Benaroya Research Institute) (29), and polyclonal rabbit anti-human β-tubulin antibody was obtained from SouthernBiotech.
Cell isolation and T cell culture conditions.
Human PBMCs were isolated from buffy coats, from heparinized whole blood from volunteer donors (Bergen Community Regional Blood Center) or participants in a clinical trial of hOKT3γ1(Ala-Ala). The design and preliminary data from this clinical trial has previously been published (8, 31). An analysis of CD4+ and CD8+ T cell subsets was done in drug-treated subjects in the study at baseline and at 1 and 3 months after mAb treatment. EBV serostatus was not an entry criteria for the study but was determined retrospectively in 19/21 subjects by ELISA: 12 drug-treated subjects were found to be EBV IgG+, and 7 subjects were found to be EBV IgG– at the time of enrollment. None of the drug-treated subjects developed clinical signs or symptoms of reactivation of EBV following treatment with the anti-CD3 mAb. This study was approved by the Institutional Review Board at Columbia University and collaborating institutions. Approval for use of patient samples was obtained from the Columbia University Institutional Review Board.
PBMCs from study participants were isolated and frozen before and on the final day of the 12-day course of treatment with the anti-CD3 mAb (n = 3) or, in the case of 1 individual, before and 10 weeks after drug treatment. PBMCs were also collected at the same time from control patients with type 1 diabetes on 2 (n = 3) or 3 (n = 1) occasions and frozen.
Positive isolation or depletion of CD8 or CD4 cells was performed using magnetic beads (Dynal Biotech) according to the manufacturer’s instruction. We found that 96–100% of CD4 or CD8 cells were depleted from the PBMCs with this method by staining after cell depletion. For isolation of CD8+ T cells from patients and the control subjects, the PBMCs were thawed, and T cells were first negatively selected, which was followed by positive selection of CD8+ cells with magnetic beads. The CD8+ cells isolated in this manner were placed in TRIzol (Invitrogen Corp.) for use in real-time analysis of Foxp3 expression.
PBMCs, PBMCs depleted of CD8+ cell, or PBMCs depleted of CD4+ cells resuspended in serum-free AIM-V medium (Invitrogen Corp.) (2 × 106 cells/ml) were cultured in the presence of hOKT3γ1(Ala-Ala) at the indicated concentrations for 5–7 days at 37°C and 5% CO2. In certain experiments, neutralizing mAbs to IL-10, TNF-α, or FasL (10 μg/ml) or rIL-2 (50 U/ml) were added to cultures.
CFSE labeling.
PBMCs were stained with 5 μM CFSE (Invitrogen Corp.) in PBS for 15 minutes at 37°C, than washed, resuspended in AIM-V medium and incubated for additional 30 minutes. Labeled cells were washed, counted, and resuspended in AIM-V medium for cell culture.
Flow cytometry.
Cells (1 × 105/sample) were washed with FACS staining buffer (PBS, 2% FBS or 1% BSA, 0.1% sodium azide) and stained for 30 minutes at 4°C with fluorochrome-conjugated mAbs at the concentration recommended by the manufacturer. Cells were washed and fixed with 1% paraformaldehyde prior to analysis in a FACSCalibur flow cytometer using CellQuest version 3.3 software (BD Biosciences). For the intracellular staining of IL-2, IL-10, IFN-γ, and CTLA4 expression, stimulated cells were cultured in the presence of Golgi Stop (BD Biosciences). Surface-labeled and fixed cells were permeabilized with Perm Buffer (BD Biosciences) for 15 minutes at room temperature. Cells were incubated for 30 minutes at 4°C with fluorochrome-conjugated mAbs, washed, and analyzed in a flow cytometer.
Cell sorting.
Cells were labeled with fluorochrome-conjugated anti–cell surface molecule antibody in sterile FACS staining buffer (PBS, 2% FBS) without sodium azide, washed, and sorted using FACSAria (BD Biosciences). RNA was isolated from freshly sorted cells, or the cells were irradiated and added to functional assays.
Transwell experiments.
Transwell experiments were performed in 6-well plates (0.4-μm pore size; Costar, Corning Inc.). CFSE-labeled PBMCs depleted of CD4+ cells were placed in the lower chamber, and PBMCs depleted of CD8+ cells were placed in the upper chamber in serum-free medium for 5–6 days in the presence of 5 μg/ml hOKT3γ1(Ala-Ala). In parallel, control nondepleted PBMCs and PBMCs depleted of CD8+ T cells stimulated the same way as described above were cultured in separate wells. At the end of the culture, cells were labeled with fluorochrome-conjugated anti-CD4 mAb, and flow cytometric analysis was performed.
[3H]thymidine incorporation assay.
Proliferation assay was performed in 96-well round-bottom plates. Cells (1 × 105/well) were cultured in the presence of tetanus toxoid (10 μg/ml), SEB (1 μg/ml), or in other assays with the indicated concentrations of antibody for 3–5 days. [3H]thymidine (1 μCi/well; PerkinElmer) was added 18 hours before the end of incubation. Cells were harvested, and incorporated radioactivity was counted in the Wallac MicroBeta Counter (PerkinElmer).
Western blot analysis of Foxp3 expression.
Isolated T cells were washed in PBS, lysed in NuPAGE LDS sample buffer (Invitrogen Corp.), and separated on 10% Bis-Tris polyacrylamide gel (Invitrogen Corp.) in denaturing conditions at 5 × 105 of cells per lane. Proteins were transferred to nitrocellulose membranes (Invitrogen Corp.), which, after blocking in TBS with 0.1% Tween-20 and 3% nonfat dry milk (Sigma-Aldrich), were probed with polyclonal rabbit-anti human Foxp3 (1:2,000) followed by HRP-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories Inc.). Specific signal was detected by ECL chemiluminescent system (Amersham Biosciences). Blots were washed and reprobed with anti–β-tubulin antibody.
Isolation of total RNA and real-time PCR analysis of Foxp3.
Analysis of Foxp3 expression by real-time PCR was performed as described previously (29). Briefly, RNA was isolated from fresh PBMCs, CD8+ T cells isolated from PBMCs, or subpopulations that were sorted from hOKT3γ1(Ala-Ala)–stimulated cells using an RNeasy Mini Kit (QIAGEN). Primers used for real-time PCR were synthesized by Invitrogen Corp. according to the published sequence (29): GAPDH: 5′-CCACATCGCTCAGACACCAT-3′ and 5′-GGCAACAATATCCACTTTACCAGAGT-3′; CD8: 5′-CCCTGAGCAACTCCATCATGT-3′ and 5′-GTGGGCTTCGCTGGCA-3′; Foxp3: 5′-GAAACAGCACATTCCCAGAGTTC-3′ and 5′-ATGGCCCAGCGGATGAG-3′. Results are expressed as a fold or percent change of relative values of Foxp3 expression normalized to GAPDH or CD8, as described previously for activated CD8+ T cells (52, 53).
Statistics.
All results are presented as mean ± SEM. The change in the CD4/CD8 T cell ratio was calculated as the difference in the ratio of CD4+ to CD8+ cells at day 90 and before drug treatment. Comparisons between groups were made using the Mann-Whitney U test. The Pearson correlation coefficient was calculated and tested for statistical significance to compare the changes in CD4/CD8 T cells in vitro and in vivo following treatment with the anti-CD3 mAb. A simple regression analysis was also done to describe this relationship. P < 0.05 was considered to be of statistical significance. Calculations were performed with StatView version 5.0 and SAS software version 9 (SAS Institute Inc.).
Acknowledgments
This work was supported by NIH grants DK57846, AI-98-010, DK063608, and RR00645.
Footnotes
Nonstandard abbreviations used: PHA, phytohemagglutinin; SEB, staphylococcal enterotoxin B.
Conflict of interest: J.A. Bluestone has a patent application for the anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala).
References
- 1.Xu D, et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter PA, Tso JY, Press OW, Yu X, Anasetti C. Non-FcR-binding, humanized anti-CD3 antibody Hu291 induces apoptosis of human T cells more effectively than OKT3 and is immunosuppressive in vivo. Transplant. Proc. 2000;32:1545–1546. doi: 10.1016/s0041-1345(00)01343-9. [DOI] [PubMed] [Google Scholar]
- 3.Bolt S, et al. The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur. J. Immunol. 1993;23:403–411. doi: 10.1002/eji.1830230216. [DOI] [PubMed] [Google Scholar]
- 4.Smith JA, Tso JY, Clark MR, Cole MS, Bluestone JA. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J. Exp. Med. 1997;185:1413–1422. doi: 10.1084/jem.185.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Utset TO, et al. Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J. Rheumatol. 2002;29:1907–1913. [PubMed] [Google Scholar]
- 6.Hering BJ, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am. J. Transplant. 2004;4:390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 7.Woodle ES, et al. Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3gamma1(Ala-Ala) in the treatment of acute renal allograft rejection. Transplantation. 1999;68:608–616. doi: 10.1097/00007890-199909150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Herold KC, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 9.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 10.Bach JF. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 2003;3:189–198. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM. Regulatory T cells in autoimmmunity* [review] Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 12.Chatenoud L, Salomon B, Bluestone JA. Suppressor T cells — they’re back and critical for regulation of autoimmunity! [review] Immunol. Rev. 2001;182:149–163. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- 13.Cobbold SP, et al. Regulatory T cells and dendritic cells in transplantation tolerance: molecular markers and mechanisms [review] Immunol. Rev. 2003;196:109–124. doi: 10.1046/j.1600-065x.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 14.Wood KJ, Luo S, Akl A. Regulatory T cells: potential in organ transplantation. Transplantation. 2004;77:S6–S8. doi: 10.1097/01.TP.0000106477.70852.29. [DOI] [PubMed] [Google Scholar]
- 15.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol. Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 16.Battaglia M, Blazar BR, Roncarolo MG. The puzzling world of murine T regulatory cells. Microbes Infect. 2002;4:559–566. doi: 10.1016/s1286-4579(02)01573-3. [DOI] [PubMed] [Google Scholar]
- 17.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 1-producing, contact-independent type 1-like regulatory T cells. J. Exp. Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen ZM, et al. IL-10 and TGF-beta induce alloreactive CD4+CD25- T cells to acquire regulatory cell function. Blood. 2003;101:5076–5083. doi: 10.1182/blood-2002-09-2798. [DOI] [PubMed] [Google Scholar]
- 19.O’Garra A, Barrat FJ. In vitro generation of IL-10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by Th1- and Th2-inducing cytokines. Immunol. Lett. 2003;85:135–139. doi: 10.1016/s0165-2478(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 20.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 21.Belghith M, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 22.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 23.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? [review] Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J. Clin. Invest. 2003;112:1310–1312. doi:10.1172/JCI200320274. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 27.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 28.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25- subpopulations. J. Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 29.Walker MR, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J. Clin. Invest. 2003;112:1437–1443. doi:10.1172/JCI200319441. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herold KC, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herold KC, et al. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3γ1(Ala-Ala) J. Clin. Invest. 2003;111:409–418. doi:10.1172/JCI200316090. doi: 10.1172/JCI16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int. Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 34.Foulds KE, et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 35.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 36.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 37.Morgan ME, et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum. Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Cosmi L, et al. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004;103:3117–3121. doi: 10.1182/blood-2003-09-3302. [DOI] [PubMed] [Google Scholar]
- 39.Cosmi L, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura K, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 41.Roncarolo MG, Gregori S, Levings M. Type 1 T regulatory cells and their relationship with CD4+CD25+ T regulatory cells [review] Novartis Found. Symp. 2003;252:115–127; discussion 127–131, 203–210. [PubMed] [Google Scholar]
- 42.Ware R, et al. Human CD8+ T lymphocyte clones specific for T cell receptor V beta families expressed on autologous CD4+ T cells. Immunity. 1995;2:177–184. doi: 10.1016/s1074-7613(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Chess L. The specific regulation of immune responses by CD8+ T cells restricted by the MHC class Ib molecule, Qa-1. Annu. Rev. Immunol. 2000;18:185–216. doi: 10.1146/annurev.immunol.18.1.185. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Goldstein I, Glickman-Nir E, Jiang H, Chess L. Induction of TCR Vbeta-specific CD8+ CTLs by TCR Vbeta-derived peptides bound to HLA-E. J. Immunol. 2001;167:3800–3808. doi: 10.4049/jimmunol.167.7.3800. [DOI] [PubMed] [Google Scholar]
- 45.Chang CC, et al. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat. Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 46.Colovai AI, Ciubotariu R, Liu Z, Cortesini R, Suciu-Foca N. CD8(+)CD28(-) T suppressor cells represent a distinct subset in a heterogeneous population. Transplant. Proc. 2001;33:104–107. doi: 10.1016/s0041-1345(00)01927-8. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Yu B, Fan J, Cortesini R, Suciu-Foca N. CD8(+)CD28(-) T cells suppress alloresponse of CD4(+) T cells both in primates and rodents. Transplant. Proc. 2001;33:82–83. doi: 10.1016/s0041-1345(00)01914-x. [DOI] [PubMed] [Google Scholar]
- 48.Annunziato F, et al. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J. Exp. Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lange H, et al. Immediate and long-term results of ATG induction therapy for delayed graft function compared to conventional therapy for immediate graft function. Transpl. Int. 1999;12:2–9. doi: 10.1007/s001470050178. [DOI] [PubMed] [Google Scholar]
- 50.Bas J, et al. In vitro donor-specific hyporesponsiveness and T cell subsets in renal allograft recipients. Allergol. Immunopathol. (Madr.). 1993;21:136–140. [PubMed] [Google Scholar]
- 51.Filaci G, et al. Nonantigen specific CD8+ T suppressor lymphocytes originate from CD8+CD28- T cells and inhibit both T-cell proliferation and CTL function. Hum. Immunol. 2004;65:142–156. doi: 10.1016/j.humimm.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Marshall DR, et al. Effector CD8+ T cells recovered from an influenza pneumonia differentiate to a state of focused gene expression. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6074–6079. doi: 10.1073/pnas.0501960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen MB, et al. Status of activation of circulating vaccine-elicited CD8+ T cells. J. Immunol. 2000;165:2287–2296. doi: 10.4049/jimmunol.165.4.2287. [DOI] [PubMed] [Google Scholar]