Abstract
Current treatments for advanced prostate cancer (PCa) primarily target the androgen receptor (AR) pathway. However, the emergence of castration-resistant prostate cancer (CRPC) and resistance to AR pathway inhibitors (APPIs) remains ongoing challenges. Here, we present BSJ-5-63, a proteolysis-targeting chimera (PROTAC) targeting cyclin-dependent kinases (CDKs) CDK12, CDK7, and CDK9, offering a multipronged approach to CRPC therapy. BSJ-5-63 degrades CDK12, diminishing BRCA1 and BRCA2 expression and inducing a sustained “BRCAness” state. This sensitizes cancer cells to PARP inhibitors (PARPis) regardless of their homologous recombination repair (HRR) status. Furthermore, CDK7 and CDK9 degradation attenuates AR signaling, enhancing its therapeutic efficacy. Preclinical studies, including both in vitro and in vivo CRPC models, demonstrate that BSJ-5-63 exerts potent antitumor activity in both AR-positive and AR-negative setting. This study introduces BSJ-5-63 as a promising therapeutic agent that addresses both DNA repair and AR signaling mechanisms, with potential benefits for a board patient population.
A PROTAC induces acute BRCAness by degrading CDK12/7/9, thereby enhancing prostate cancer cell sensitivity to PARP inhibitors.
INTRODUCTION
Prostate cancer (PCa) is the second leading cause of cancer-related death in American men, trailing only behind lung cancer (1). Current primary treatment strategies for patients with advanced PCa continue to center on androgen receptor (AR)–directed therapies, which encompass androgen deprivation therapy (ADT) and AR pathway inhibitors (ARPIs), such as enzalutamide and abiraterone. However, a major challenge arises as patients undergoing ADT inevitably progress to a state known as metastatic castration-resistant prostate cancer (CRPC). Although ARPIs initially prove effective against CRPC, their therapeutic efficacy remains short-lived, often leading to the development of ARPI resistance (2, 3).
Genomic studies have revealed a variety of actionable molecular targets with underlying genomic alterations beyond the AR pathway. Notably, ~25% of metastatic CRPC cases exhibit genomic alterations of at least one gene involved in DNA damage response (DDR) with BRCA2 being the most frequently mutated (4). These alterations are associated with therapeutic vulnerabilities, particularly in the context of homologous recombination repair (HRR) deficiencies, which predict sensitivity to poly(adenosine 5′-diphosphate–ribose) polymerase (PARP) inhibitors (PARPis) (5). PARP enzymes, especially PARP1 and PARP2, play pivotal roles in various aspects of DNA damage repair. PARPis block the repair of DNA single-strand breaks and result in stalled replication forks by trapping PARP1 and PARP2 on DNA breaks (6). This, in turn, promotes the accumulation of DNA double-strand breaks (DSBs) that HRR-deficient cells cannot repair efficiently, leading to overwhelming DNA damage and cell death. In 2020, the US Food and Drug Administration (FDA) approved two PARPis, olaparib and rucaparib, as monotherapies for treating metastatic CRPC cases harboring BRCA1 and BRCA2 (BRCA1/2) mutations, capitalizing on the concept of synthetic lethality (7–10). The application of olaparib has expanded to include 12 additional DDR genes, the loss of which induces an HRR-deficient state, often referred to as “BRCAness” (11). In 2023, the FDA further approved PARPis—olaparib, niraparib, and talazoparib—for use in combination with abiraterone or enzalutamide for the treatment of metastatic CRPC cases with HRR gene mutations (12–16). The rationale for combining AR and PARP inhibition is that down-regulation of AR-targeted DDR genes induces BRCAness (17, 18), while PARP1 promotes AR-mediated transcription (19). This combination aims to enhance therapeutic efficacy, although controversy exists regarding its use as a first-line treatment for metastatic CRPC without HHR defects (20). Moreover, emerging evidence indicates that AR does not directly regulates the transcription of DDR genes, raising doubts about whether AR inhibition alone can effectively induce a BRCAness state (21).
Cyclin-dependent kinase 12 (CDK12) is among the genes associated with BRCAness (11). Unlike other BRCAness genes directly involved in HRR, CDK12, a kinase linked to phosphorylation of the C-terminal domain (CTD) of RNA polymerase II (Pol II), is essential for the transcriptional processivity of long mRNA transcripts, including those from key HRR genes, such as BRCA1/2 (22, 23). CDK12 alterations, predominantly biallelic and truncating mutations, have been identified in 5 to 7% of PCa cases (24, 25). Given that CDK12 loss of function mirrors the effects of BRCA1/2 mutations, CDK12 deficiency has emerged as a viable biomarker for PARPi sensitivity (26). Nevertheless, clinical studies have shown a lack of HRR defects and limited efficacy of PARPis in patients with PCa with somatic CDK12 mutations (24, 27–29). In contrast, CDK12 inhibition sensitizes various cancers to PARPis by disrupting HRR processes (30–34), suggesting that acute CDK12 suppression through inhibitors or degraders could rapidly diminish CDK12’s modulation of HRR genes, creating a transient BRCAness state that enhances PARPi sensitivity. This approach minimizes the likelihood of cancer cells establishing feedback modulation signals to restore HRR after CDK12 genomic alterations (35). Consequently, a combinatorial approach involving CDK12 and PARP inhibition holds potential benefits for patients with CRPC beyond their BRCA1/2 mutational status.
In addition to CDK12, CDK7 and CDK9 also have central roles in regulating Pol II by phosphorylating its CTD (36). CDK7 and CDK9 are crucial for the transcriptional activation of AR. CDK7 activates MED1 by phosphorylating T1457, whereas CDK9 phosphorylates AR at Ser81, both promoting AR-mediated oncogenic transcription (37–41). As a master regulator of transcription, CDK7 also phosphorylates and activates CDK9 within the positive transcription elongation factor (P-TEFb) complex (42). The AR remains a major therapeutic target in CRPC, with MED1 serving as an intermediator between the AR and Pol II to facilitate the transcription of AR-targeted genes. Inhibition of CDK7/9 may attenuate AR signaling when direct AR targeted therapies fail, potentially overcoming enzalutamide and abiraterone resistance. Given AR’s influence on a range of DDR genes (17, 18), including some HRR genes, targeting CDK7/9 to inhibit AR could suppress AR-controlled HRR gene expression. This strategy may further promote the BRCAness state and increase CRPC susceptibility to PARP inhibition (43).
This study introduces BSJ-5-63, a proteolysis-targeting chimera (PROTAC) that degrades CDK12, CDK7, and CDK9, leading to the down-regulation of BRCA1/2 and inhibition of AR signaling. BSJ-5-63 demonstrates potent antitumor effects and significantly enhances CRPC sensitivity to PARPis. The induced BRCAness state provides a therapeutic window for combining BSJ-5-63 with PARPis, offering a strategy to minimize toxicity and prevent the emergence of resistance.
RESULTS
BSJ-5-63 degrades CDK12, CDK7, and CDK9
We have previously developed a selective CDK12 degrader, BSJ-4-116 (fig. S1A), using the ligand of the E3 ubiquitin ligase cereblon (CRBN) (34). BSJ-4-116 preferentially down-regulates long DDR genes such as BRCA1/2 by enhancing intronic polyadenylation and inducing premature transcriptional termination. Consequently, BSJ-4-116 increases the sensitivity of T cell acute lymphoblastic leukemia cells to PARPis. However, BSJ-4-116 exhibited poor metabolic stability with a half-life of 2.1 min and intrinsic clearance of 333 μl/min per milligram in hepatic microsomes, compared to the control compound, the FDA-approved multi-targeted receptor kinase inhibitor sunitinib, with a half-life of 10 min and intrinsic clearance of 69 μl/min per milligram (table S1). Simultaneously, we have expanded our efforts by generating a series of degrader molecules. By substituting the CRBN ligand with the Von Hippel–Lindau (VHL) ligand via distinct linkers, we introduced a CDK12 degrader, BSJ-5-63 (Fig. 1A), which showed a longer half-life of 11.1 min in hepatic microsomes and a lower intrinsic clearance of 62 μl/min per milligram.
Fig. 1. BSJ-5-63 degrades CDK12/7/9.
(A) Chemical structures of BSJ-5-63 and its negative control analog BSJ-5-63-NC. (B) Proteome-wide selectivity of BSJ-5-63. Quantitative proteomics showing the relative abundance of proteins measured by multiplexed quantitative mass spectrometry (MS)–based proteomic analysis in 22Rv1 cells treated with BSJ-5-63 (100 nM) or dimethyl sulfoxide (DMSO) for 8 hours. The treatment with BSJ-5-63-NC (250 nM) was used as a control. CDK12, CDK7, and CDK9 are marked. Dotted lines indicate the threshold for statistically significant degradation of proteins. (C) Western blots of the indicated proteins in LNCaP, C4-2B, and 22Rv1 cells after 8 hours of treatment with BSJ-5-63 or BSJ-5-63-NC in a dose-dependent manner. Representative blots from three independent experiments are shown. (D) Western blots of the indicated proteins in LNCaP and 22Rv1 cells after treatment with 500 nM BSJ-5-63 in a time-course manner. Representative blots from three independent experiments are shown.
To assess the specificity of BSJ-5-63 degradation, we conducted a comprehensive proteome-wide analysis using CRPC 22Rv1 cells treated with BSJ-5-63 (100 nM) for 8 hours, in parallel with the negative control BSJ-5-63-NC (250 nM), in which the stereocenter on the hydroxyproline moiety of the VHL ligand was inversed to block binding to VHL. Proteomic analysis demonstrated that BSJ-5-63 specifically degrades CDK12 and its associated partner proteins cyclin K (CCNK) while sparing CDK13 (Fig. 1B and data S1 and S2), suggesting a potential therapeutic strategy akin to BSJ-4-116 through the suppression of DDR genes. BSJ-5-63 also degraded CDK7 and CDK9. This finding is important in the context of PCa as CDK7 and CDK9 play pivotal roles in AR-mediated transcription and have been considered as therapeutic targets in PCa (36, 37, 39). Therefore, BSJ-5-63 shows potential as a treatment for PCa by targeting multiple pathways. To compare the distinct effects of the ligands CRBN and VHL in PCa cells, we examined BSJ-4-116–mediated protein degradation in 22Rv1 cells using the same proteomic analysis. Our observations revealed a notable contrast in the protein degradation profiles. While BSJ-5-63 degraded a few other proteins, including CCND1, BSJ-4-116 exhibited an increased capacity for inducing protein degradation (fig. S1B and data S1). This raises concerns about more off-target effects when CRBN is used as a ligand in PCa cells. In addition, we did not observe degradation of CDK7 and CCNK, indicating potential differences in the therapeutic effects.
Western blot analysis further validated that BSJ-5-63 effectively induces CDK12 and CCNK degradation in PCa cell lines 22Rv1, LNCaP, and LNCaP-derived C4-2B in a dose- and time-dependent manner, whereas CDK13 protein levels remained unchanged (Fig. 1, C and D). Degradation of CDK7 and CDK9 was also observed. CDK7 facilitates transcription initiation through phosphorylation of the CTD of RNA Pol II at Ser5 and Ser7, whereas both CDK9 and CDK12 promote phosphorylation at Ser2, a modification essential for transcript elongation (44). As expected, BSJ-5-63 markedly reduced the phosphorylation of RNA Pol II at Ser2, Ser5, and Ser7. In contrast, the negative control compound, BSJ-5-63-NC, had no impact on the protein levels of CDK12/7/9 or the phosphorylation of RNA Pol II. Western blot analysis of 22Rv1 cells confirmed that BSJ-4-116 induced the degradation of CDK12 and CDK9, without affecting CDK7. This resulted in a reduction of the phosphorylation of RNA Pol II at Ser2 and Ser7, while Ser5 phosphorylation remained unaffected (fig. S1C). Last, we demonstrated that pretreatment with the proteasome inhibitor MG132 rescued the BSJ-5-63–induced degradation of CDK12/7/9 (fig. S2).
BSJ-5-63 induces a BRACness state and impairs HRR activity
To investigate the impact of BSJ-5-63 on transcription, we conducted RNA-sequencing (RNA-seq) analysis of 22Rv1 cells following BSJ-5-63 treatment for 8 hours. We identified profound alterations in the expression levels of 1026 genes with BSJ-5-63 treatment as opposed to only 207 genes with the control BSJ-5-63-NC treatment (Fig. 2A and data S3). These results indicate a substantial influence on transcription as a consequence of CDK12/7/9 degradation. Consistently, a pathway analysis revealed regulation of transcription from the RNA Pol II promoter as the most enriched down-regulated pathway (Fig. 2B). This was followed by pathways associated with DSB repair via HR and the cellular response to DNA damage. We observed significant down-regulation of crucial HRR genes, including BRCA1, BRCA2, PALB2, and FANCF (Fig. 2C). Reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis of 22Rv1, LNCaP, and DU145 cells revealed a progressive inhibition of BRCA1/2 mRNA expression with increasing doses and exposure times of BSJ-5-63 (Fig. 2D). Western blot analysis of LNCaP and 22Rv1 cells validated a marked reduction in BRCA1/2 protein levels (Fig. 2E). These effects were also observed after treatment with BSJ-5-63-NC but required a much higher dose to achieve comparable results. RAD51 protein levels were also reduced, likely due to posttranscriptional regulation. An increase in γ-H2AX (H2AX phosphorylated at Ser139), indicative of DSBs, was detected following BSJ-5-63 treatment. Further analyses revealed a time-dependent decrease in BRCA1, BRCA2, and RAD51 protein levels, accompanied by an elevation in γ-H2AX levels across LNCaP, C4-2B, 22Rv1, and DU145 cells (Fig. 2F and fig. S3).
Fig. 2. CDK12 degradation by BSJ-5-63 induces acute BRACness and impairs HRR.
(A) Differentially expressed genes 8 hours after treatment with BSJ-5-63 or BSJ-5-63-NC in 22Rv1 cells. Left: Venn diagram showing the intersection of down-regulated genes between BSJ-5-63 and its negative control analog BSJ-5-63-NC treatment in 22Rv1 cells. Right: Volcano plot showing differentially expressed genes after BSJ-6-63 or BSJ-5-63-NC treatment. Blue dots represent down-regulated genes; red dots represent up-regulated genes. NS, not significant. (B) Top enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in down-regulated genes after BSJ-5-63 treatment in 22Rv1 cells. (C) Scatter blot showing down-regulated BRACness genes after BSJ-5-63 and BSJ-5-63-NC treatment in 22Rv1 cells. (D) mRNA levels of BRCA1/2 were measured after treatment with BSJ-5-63 and BSJ-5-63-NC as indicated in 22Rv1, LNCaP, and DU145 cells by real-time reverse transcription polymerase chain reaction (RT-PCR). Data represent means ± SEM (n = 3). *P < 0.05; ***P < 0.001, one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. (E) Western blots of the indicated proteins in LNCaP and 22Rv1 cells after 48 hours of treatment with BSJ-5-63 or BSJ-5-63-NC in a dose-dependent manner. Representative blots from three independent experiments are shown. (F) Western blots of the indicated proteins in LNCaP and 22Rv1 cells after BSJ-5-63 (100 nM) treatment in a time-course manner. Representative blots from three independent experiments are shown. (G) Representative images of immunofluorescence staining and quantification of the number of RAD51 and γ-H2AX foci in 22Rv1 cells 24 hours after BSJ-5-63 (100 nM) treatment followed by 8-Gy irradiation. Representative images from three independent experiments. For RAD51 foci, data represent means ± SEM (n = 3) with more than 50 cells analyzed each replicate. For γ-H2AX foci, more than 50 cells were analyzed per condition. Dotted lines inside the violin indicate the median. ***P < 0.001; n.s., not significant; the P values were determined using two-sided t test.
To determine whether BSJ-5-63 attenuates HRR function, we examined RAD51 and γ-H2AX foci formation following ionizing radiation (IR) treatment in 22Rv1 cell, with or without BSJ-5-63 (Fig. 2G). We observed an increase in RAD51 foci after IR [8 gray (Gy)], an effect that diminished in the presence of BSJ-5-63, indicating impaired HRR. Subsequently, γ-H2AX foci increased following IR, and this effect was further amplified by BSJ-5-63, suggesting an augmentation of DSBs. Similar results were observed in C4-2B cells (fig. S4). These results indicate that BSJ-5-63 impairs HRR function by diminishing CDK12-mediated BRCA1/2 expression. Treatment with BSJ-4-116 also completely suppressed BRCA1/2 expression in 22Rv1 cells (fig. S5A), disrupting IR-induced RAD51 foci formation and impairing HRR (fig. S5B). This disruption led to a marked increase in γ-H2AX foci, reflecting a higher incidence of DNA DSBs in these cells. Furthermore, we performed direct repeats (DR)–green fluorescent protein (GFP) and end-joining 5 (EJ5)-GFP reporter assays in LNCaP cells for HRR and nonhomologous end joint (NHEJ) function, respectively, demonstrating that BSJ-5-63 specifically inhibits HRR without affecting NHEJ (fig. S6A). These findings were also validated in U2OS stable cell lines harboring DR-GFP and EJ5-GFP reporters (fig. S6B).
Next, we investigated the duration of BRCAness induced by BSJ-5-63 treatment. The 22Rv1 cells were exposed to BSJ-5-63 (100 nM) for 24 hours, followed by a medium change to eliminate the drug. We then measured BRCA1/2 mRNA levels over time and observed that the down-regulation of BRCA1/2 expression persisted for ~48 and 24 hours, respectively (Fig. 3A). To explore potential synergistic effects with PARPis, we designed a sequential combination treatment for LNCaP and 22Rv1 cells, starting with a 48-hour BSJ-5-63 treatment followed by treatment with PARPis—olaparib, rucaparib, niraparib, or talazoparib (Fig. 3B). Short-term, low-dose BSJ-5-63 treatment alone had minimal effects on cell growth, suggesting lower toxicity. However, the combination therapy significantly inhibited cell growth compared to single-agent treatments, as demonstrated by cell viability assays and further validated using colony formation assays in AR-positive LNCaP and 22Rv1 cells, as well as AR-negative DU145 cells (Fig. 3C). BSJ-4-116 also induced sustained BRCA1/2 down-regulation, with effects lasting for ~48 and 24 hours, respectively (Fig. 3D). Sequential administration of BSJ-4-116 with PARPis resulted in a significant enhancement of cell growth inhibition compared to treatment with these agents individually (Fig. 3, E and F). Both BSJ-5-63 and BSJ-4-116 have a minimal effect on the cell cycle when used at low concentrations, indicating the combination effects are cell cycle independent (fig. S7).
Fig. 3. BSJ-5-63 sensitizes PCa cells to PARPis through CDK12 degradation.
(A) 22Rv1 cells were treated with BSJ-5-63 for 2 days, followed by its removal. mRNA levels of BRCA1/2 were measured at the indicated time points using real-time RT-PCR. Data represent means ± SEM (n = 3). (B) Cell viability assays in LNCaP and 22Rv1 cells treated with BSJ-5-63 for 2 days, PARPis [olaparib (Ola), rucaparib (Ruca), niraparib (Nira), or talazoparib (Tala)] for 7 days, or sequential combination where BSJ-5-63 was removed after 2 days and followed by PARPi treatment for additional 5 days. Data represent means ± SEM (n = 3). (C) Colony formation assays in LNCaP, 22Rv1, and DU145 cells treated with BSJ-5-63 for 2 days, PARPis for 14 days, or sequential combination where BSJ-5-63 was removed after 2 days and followed by 12 days of PARPi treatment. Data represent means ± SEM (n = 3). (D) 22Rv1 cells were treated with BSJ-4-116 for 2 days, followed by its removal. mRNA levels of BRCA1/2 were measured at the indicated time points using real-time RT-PCR. Data represent means ± SEM (n = 3). (E) Cell viability assays in LNCaP and 22Rv1 cells treated with BSJ-4-116, PARPis, or a sequential combination as indicated. Data represent means ± SEM (n = 3). (F) Colony formation assays in LNCaP and 22Rv1 cells treated with BSJ-4-116, PARPis, or a sequential combination as indicated. Data represent means ± SEM (n = 3). Experimental procedures are depicted schematically in each panel. *P < 0.05; **P < 0.01; ***P < 0.001, one-way ANOVA with Tukey’s multiple comparisons test. d, days.
Deletion of CDK12 induces transient BRACness and sensitizes PCa cells to PARP inhibition
Many studies have demonstrated the pivotal role of CDK12 in the regulation of the expression of genes involved in HRR (22, 45). CDK12 deletion sensitizes cancer cells to PARP inhibition by inducing HRR defects. CRPC tumors with biallelic CDK12 mutations do not show a favorable response to PARP inhibition (24), despite the fact that most CDK12 mutations lead to truncated proteins with a loss of the kinase domain.
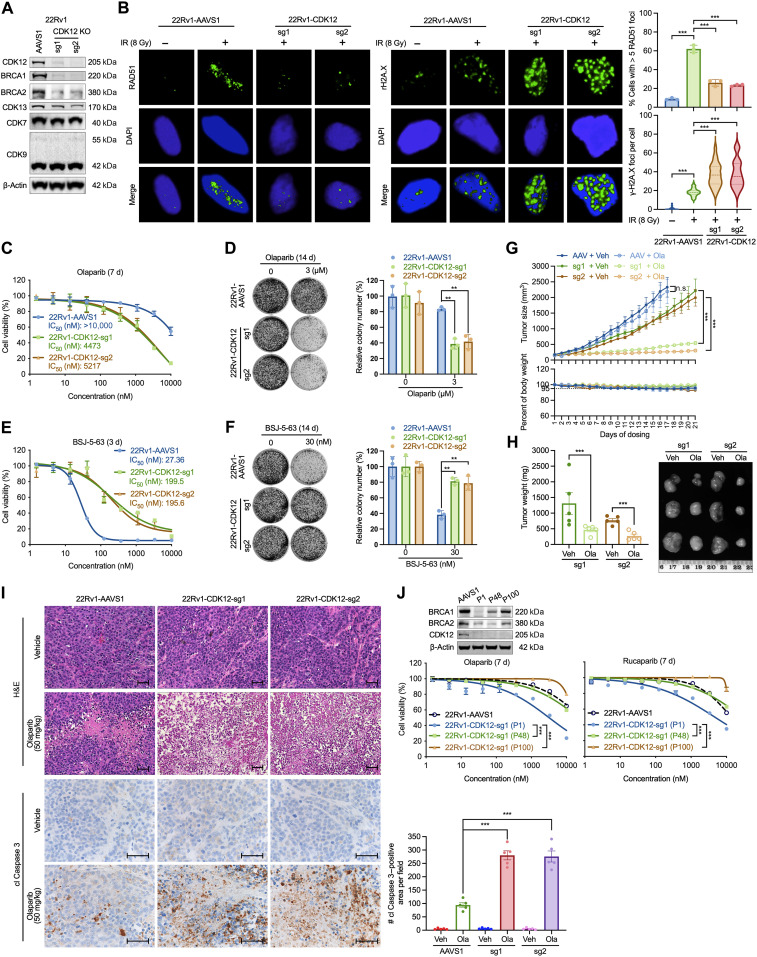
To further investigate this, we used CRISPR-Cas9 gene editing with two single-guide RNAs (sgRNAs) to delete CDK12 in 22Rv1 cells. Western blot analysis confirmed CDK12 knockout (KO), whereas CDK7 and CDK9 protein levels remained unchanged (Fig. 4A). BRCA1/2 protein levels were diminished in CDK12-KO cells. Further examination of RAD51 and γ-H2AX foci formation after IR treatment uncovered an increase in RAD51 foci after IR (8 Gy) in CDK12-intact AAVS1 cells, a phenomenon not observed in CDK12-KO cells, indicating a HRR defect (Fig. 4B). Moreover, we observed a slightly increase in the formation of γ-H2AX foci in CDK12-intact cells after IR. This effect was markedly amplified in CDK12-KO cells, suggesting the augmentation of DNA DSBs. CDK12-KO cells exhibited significantly enhanced sensitivity to olaparib in both the cell viability and colony formation assays (Fig. 4, C and D). CDK12-KO cells displayed reduced sensitivity to BSJ-5-63, implying that CDK12 is a key target of this compound (Fig. 4, E and F). Similar results were observed when CDK12-KO cells were treated with BSJ-4-116 (fig. S8, A and B). To further confirm this, we transiently overexpressed CDK12 in two CDK12-KO single-cell clones, H91 and H92 (fig. S9A), which restored their sensitivity to BSJ-5-63 (fig. S9B). Overexpression of CDK12 in CDK12-KO cells also enhanced HRR function in a reporter assay, while NHEJ function remained unchanged (fig. S9C). This effect is likely attributed to the rescued expression of BRCA1/2 in CDK12-KO cells following CDK12 overexpression, as shown by Western blots (fig. S9D). Moreover, baseline γH2AX levels were elevated in CDK12-KO cells compared to that in CDK12-overexpressing cells, indicating increased DNA DSBs in the absence of CDK12. Notably, both γH2AX and RAD51 levels remained unchanged after BSJ-5-63 treatment in CDK12-KO cells, but γH2AX was increased and RAD51 was decreased in CDK12-overexpressing cells following treatment. Collectively, these results support the conclusion that CDK12 is the primary target of BSJ-5-63. Additionally, CDK12-KO 22Rv1 cells exhibited marked sensitivity to olaparib in xenograft studies, accompanied by a reduction in tumor weight (Fig. 4, G and H). Apoptotic cell death was observed in CDK12-KO tumors using hematoxylin and eosin (H&E) and cleaved Caspase 3 staining (Fig. 4I). However, in prolonged culture under regular medium conditions, the initially observed olaparib sensitivity of CDK12-KO cells was lost. We found that BRCA1/2 expression levels were partially restored after 48 and 100 passages, indicating the potential compensation of CDK12 function through a yet to define mechanism (Fig. 4J). Together, our findings suggest that the acute suppression of CDK12 by genetic and pharmacological means sensitizes PCa cells to PARP inhibition. However, the long-term culture of PCa cells with CDK12 deletion suggests the possibility of compensatory mechanisms may develop over time, consistent with clinical observations in CDK12-mutant CRPC, and warrants further investigation.
Fig. 4. CDK12 KO impairs HRR and sensitizes PCa cells to PARP inhibition.
(A) Western blots showing CDK12 knockout (KO) in 22Rv1 cells using two single-guide RNAs (sgRNAs; sg1 and sg2) compared to the AAVS1 sgRNA control. Representative blots from three independent experiments are shown. (B) Immunofluorescence images and quantification of RAD51 and γ-H2AX foci in CDK12-KO 22Rv1 cells after 8-Gy irradiation, compared to AAVS1-KO control. Data represent means ± SEM (n = 3), with >50 cells analyzed per replicate. Dotted lines in violin plots indicate medians. ***P < 0.001, two-sided t test. (C and D) CDK12 KO significantly sensitized 22Rv1 cells to olaparib in cell viability (C) and colony formation assays (D). Data represent means ± SEM (n = 3). **P < 0.01, one-way ANOVA with Tukey’s test. (E and F) CDK12 KO reduced the antiproliferative effects of BSJ-5-63 in cell viability (E) and colony formation assays (F). Data represent means ± SEM (n = 3). **P < 0.01, one-way ANOVA with Tukey’s test. (G) CDK12 KO enhanced the in vivo antitumor efficacy of olaparib. Tumor volume and mouse body weight curves are shown. Data represent means ± SEM (n = 5). n.s., not significant; ***P < 0.001, two-sided t test. (H) Tumor weights from CDK12-KO 22Rv1 xenografts on day 21 of olaparib treatment with representative images. Data represent means ± SEM (n = 5). ***P < 0.001, two-sided t test. (I) Hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) analysis showing increased cell death in CDK12-KO tumors with olaparib. Right: Quantification of cleaved (cl) Caspase 3 staining. Scale bars, 50 μm. Data represent means ± SEM (n = 5). ***P < 0.001, one-way ANOVA with Tukey’s test. (J) Long-term passaging of CDK12-KO cells led to PARPi resistance. Data represent means ± SEM (n = 3). ***P < 0.001, one-way ANOVA with Tukey’s test. Top: Western blots showing BRCA1/2 expression. Veh, vehicle.
BSJ-5-63 blocks AR signaling through inhibiting MED1 and AR phosphorylation
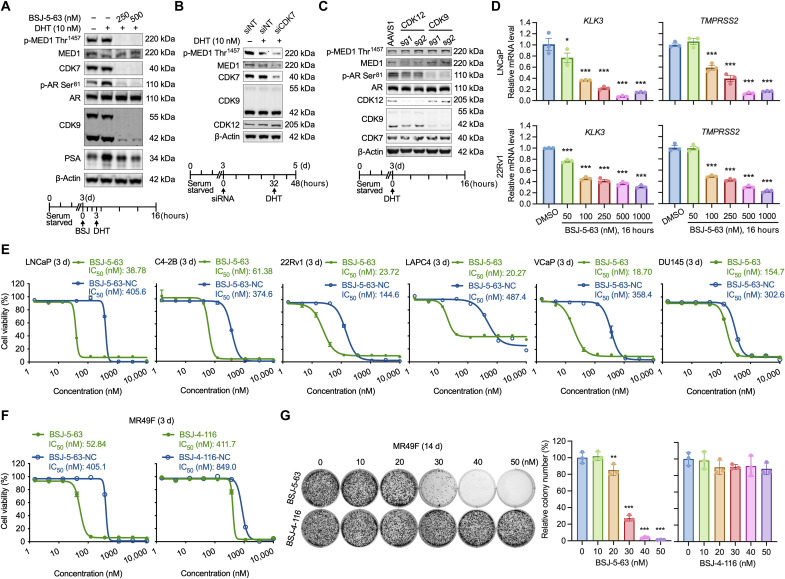
Our proteomic analyses revealed that BSJ-5-63 effectively degrades CDK7 and CDK9, which are crucial kinases in AR-mediated transcription by phosphorylating MED1 and AR (37–41). Upon dihydrotestosterone (DHT) (10 nM) treatment, Western blot analysis of AR-positive LNCaP cells demonstrated increased phosphorylation of MED1 at Thr1457 and AR at Ser81, which was completely abolished by BSJ-5-63 (Fig. 5A). As a result, the levels of PSA, which is encoded by the AR target gene KLK3 and induced by DHT, were reduced. We further confirmed that CDK7 knockdown markedly reduced MED1 phosphorylation at Thr1457 in LNCaP cells (Fig. 5B), an effect that was not observed with CDK9 and CDK12 KO. Conversely, CDK9 KO reduced AR phosphorylation at Ser81 in LNCaP cells (Fig. 5C). RT-qPCR revealed a dose-dependent reduction in the mRNA levels of the AR target genes KLK3 and TMPRSS2 in LNCaP and 22Rv1 cells (Fig. 5D).
Fig. 5. CDK7/9 degradation by BSJ-5-63 blocks MED1/AR-mediated transcription and PCa cell growth.
(A) Western blots showing abolished MED1/AR phosphorylation and reduced PSA expression through CDK7/9 degradation after BSJ-5-63 treatment. LNCaP cells were grown in CSS-containing medium for 3 days. Schematic of drug treatment is shown (bottom). (B) Western blots showing reduced phosphorylation of MED1 at T1457 after CDK7 knockdown. Schematic of drug treatment is shown (bottom). (C) Western blots showing reduced phosphorylation of AR at Ser81 after CDK9 KO. The phosphorylation of MED1 at Thr1457 remains unchanged after CDK12 or CDK9 KO. Schematic of drug treatment is shown (Bottom). (D) The expression of AR target genes significantly down-regulated 16 hours after BSJ-5-63 treatment in LNCaP and 22Rv1 cells. mRNA levels of KLK3 and TMPRSS2 were measured by real-time RT-PCR. Data represent means ± SEM (n = 3). *P < 0.05; ***P < 0.001, one-way ANOVA with Tukey’s multiple comparisons test. (E) Dose-response curves for LNCaP, C4-2B, 22Rv1, LAPC4, VCaP, and DU145 cells treated with BSJ-5-63 or BSJ-5-63-NC at indicated dose range for 72 hours. Data represent means ± SEM (n = 3). (F) Cell viability assay showing the inhibition of MR49F cell growth after treatment with BSJ-5-63, but not BSJ-4-116. Dose-response curves for MR49F cells treated with BSJ-5-63, BSJ-5-63-NC, BSJ-4-116, or BSJ-4-1116-NC at indicated dose range for 72 hours. Data represent means ± SEM (n = 3). (G) Colony formation assay showing inhibition of MR49F colony growth after treatment with BSJ-5-63, but not BSJ-4-116. Representative colonies from three independent experiments are shown. Data represent means ± SEM (n = 3). **P < 0.01; ***P < 0.001, one-way ANOVA with Tukey’s multiple comparisons test.
We evaluated the inhibitory effects of BSJ-5-63 monotherapy on PCa cell growth in vitro. BSJ-5-63 effectively inhibited the growth of PCa cells at nanomolar concentrations, as demonstrated by cell viability assays (Fig. 5E). AR-positive LNCaP, C4-2B, 22Rv1, LAPC4, and VCaP cells were more sensitive to BSJ-5-63 than AR-negative DU145 cells. This increased sensitivity was confirmed through colony formation assays in LNCaP, C4-2B, and 22Rv1 cells compared to that in DU145 cells (fig. S10). These results indicate that BSJ-5-63 is particularly effective against AR-positive PCa cells, likely due to its ability to degrade CDK7 and CDK9. The median inhibitory concentration (IC50) values of BSJ-5-63 in five AR-positive PCa cell lines were 38.78 nM (LNCaP), 61.38 nM (C4-2B), 23.72 nM (22Rv1), 20.27 nM (LAPC4), and 18.7 nM (VCaP), representing a 6.1- to 24-fold increase in potency compared to those of BSJ-5-63-NC, which had IC50 values of 405.6, 374.6, 144.6, 487.4, and 358.4 nM, respectively. These results indicate that the growth inhibitory effect extends beyond CDK7/9 enzymatic inhibition, with protein degradation emerging as a pivotal factor in this context. Similar results were observed with BSJ-4-116 (fig. S11). Furthermore, MR49F, an enzalutamide-resistant AR-positive PCa cell line derived from LNCaP cells (46), exhibited increased sensitivity to BSJ-5-63 compared to BSJ-5-63-NC and BSJ-4-116, as demonstrated by cell viability and colony formation assays (Fig. 5, F and G). The IC50 showed a one-log magnitude improvement, supporting BSJ-5-63 as a viable therapy in cases of direct AR-targeting failure. Notably, the expression levels of BRCA1/2, CDK12/7/9, and AR were similar between LNCaP and MR49F cells (fig. S12), suggesting that the superior efficacy of BSJ-5-63 on MR49F cells is not due to differential expression of its protein targets.
BSJ-5-63 inhibits prostate tumor growth in vivo and enhances the efficacy of olaparib
Our in vitro studies revealed that BSJ-5-63 could inhibit the growth of both AR-positive and AR-negative PCa cells when used in conjunction with PARPis. This inhibition is achieved through the degradation of CDK12, leading to a BRCAness state. Alternatively, in AR-positive cells, BSJ-5-63 inhibits growth by targeting CDK7 and CDK9 for degradation, thus attenuating AR-mediated transcription. This finding prompted us to investigate the potential efficacy of this degrader in an in vivo setting. To begin our in vivo exploration, we conducted a comparative analysis of the pharmacokinetics of BSJ-5-63 in mice, in contrast to that of BSJ-4-116. BSJ-5-63 had a longer half-life (2.98 hours) compared to BSJ-4-116 (1.12 hours), indicating a slower clearance from the body (table S2). It also had a higher maximum plasma concentration (Cmax) and a much larger area under the curve, suggesting a prolonged and potentially more potent effect. These data support the in vivo use of BSJ-5-63.
Next, we examined the pharmacodynamics of BSJ-5-63 and its mechanism of action in mice. Western blot analysis of 22Rv1 xenograft tissues revealed the degradation of CDK12, CDK7, and CDK9 following intraperitoneal injection at a dose of 50 mg/kg (Fig. 6A). Furthermore, we examined the efficacy of BSJ-5-63 as a single agent in the 22Rv1 xenograft model. Our results demonstrated that BSJ-5-63 significantly inhibited the growth of 22Rv1 tumors, whether administered daily or every 3 days, at a dosage of 50 mg/kg (Fig. 6B). Tumor weight was significantly reduced (Fig. 6C), and there was an increased in the number of apoptotic cells after BSJ-5-63 treatment (Fig. 6D). However, it is noteworthy that daily administration resulted in ~10% weight loss in mice, suggesting potential toxicity associated with prolonged treatment.
Fig. 6. BSJ-5-63 inhibits the growth of AR-positive 22Rv1 xenografts as a single agent or in combination with olaparib.
(A) Western blot analysis of the expression of CDK12/7/9 in 22Rv1 tumors from mice sacrificed 3 days after BSJ-5-63 treatment as indicated. Representative blots from three independent animals per group are shown. (B) The antitumor efficacy of BSJ-5-63 in 22Rv1 xenograft model. Left: Schematic of the BSJ-5-63 treatment protocol. Right: Tumor volume curve and mouse body weight curve. Data represent means ± SEM (n = 3 or 5 per group). (C) Tumor weights of 22Rv1 xenografts dissected on day 12 of drug treatment with representative images. Data represent means ± SEM (n = 3 or 5 per group). (D) H&E staining and IHC analysis showing significantly increased apoptosis in 22Rv1 tumors after treatment with BSJ-5-63. Scale bars, 50 μm. Right: Quantification of cleaved (cl) Caspase 3 staining. Data represent means ± SEM (n = 3 or 5 per group). (E) Schematic of sequential combination treatment protocol for 22Rv1 xenograft. (F) The in vivo antitumor efficacy of sequential combination of BSJ-5-63 and olaparib in 22Rv1 xenograft model. Top: Tumor volume curve. Bottom: Mouse body weight curve. Data represent means ± SEM (n = 5 per group). (G) Tumor weights of 22Rv1 xenografts dissected on day 21 of drug treatment with representative images. Data represent means ± SEM (n = 5 per group). (H) H&E staining and IHC analysis showing significantly increased apoptosis in 22Rv1 tumors after sequential combination of BSJ-5-63 and olaparib. Scale bars, 50 μm. Right: Quantification of cl Caspase 3 staining. Data represent means ± SEM (n = 5 per group). *P < 0.05; **P < 0.01; ***P < 0.001, one-way ANOVA with Tukey’s multiple comparisons test.
Subsequently, we developed a sequential therapy approach, involving a 1-day treatment with BSJ-5-63 at a dosage of 25 mg/kg followed by a 2-day olaparib (50 mg/kg) treatment, in comparison with treatments involving the vehicle, BSJ-5-63, and olaparib used as single agents (Fig. 6E). When administered as a monotherapy, both olaparib and BSJ-5-63 exhibited partial inhibition of 22Rv1 tumor growth (Fig. 6F). In contrast, the combination therapy had a significantly greater impact on tumor growth without causing a decrease in mouse body weight. This enhanced efficacy was further confirmed by measuring tumor size and weight posttreatment (Fig. 6G). Additionally, the number of apoptotic cells notably increased after treatment with olaparib or BSJ-5-63, and this effect was substantially intensified when the two agents were used in combination (Fig. 6H).
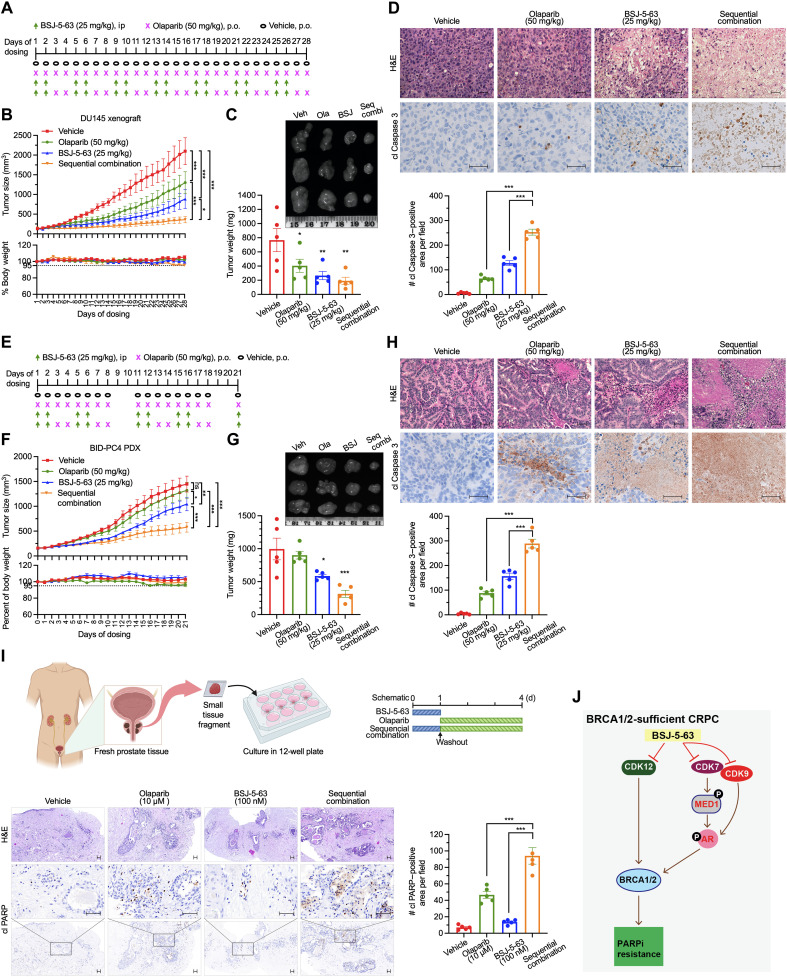
We then turned our attention to the AR-negative DU145 xenograft model and implemented a sequential therapy consisting of 2 days of BSJ-5-63 (25 mg/kg) followed by 2 days of olaparib (50 mg/kg) (Fig. 7A). After treatment with olaparib or BSJ-5-63 alone, we observed only partial inhibition of tumor growth (Fig. 7B). However, the combination therapy effectively halted tumor growth without any adverse effects on the body weight of the mice. Further analysis of tumor size, weight, and detection of apoptotic cells provided strong evidence supporting the synergistic effects of the combination therapy (Fig. 7, C and D).
Fig. 7. BSJ-5-63 sensitizes prostate tumors to PARP inhibition in vivo.
(A) Schematic of sequential treatment protocol for DU145 xenograft. (B) Tumor volume and mouse body weight curves for DU145 xenografts treated with BSJ-5-63, olaparib, or their sequential combination. Data represent means ± SEM (n = 5). (C) Tumor weights from DU145 xenografts on day 28 of treatment with representative images. Data represent means ± SEM (n = 5). (D) H&E staining and IHC for cleaved (cl) Caspase 3 of DU145 tumors reveal increased apoptosis after sequential combination therapy. Scale bars, 50 μm. Quantification of cl Caspase 3 is shown. Data represent means ± SEM (n = 5). (E) Schematic of sequential treatment protocol for BID-PC4 PDX model. (F) Tumor volume and moue body weight curves for BID-PC4 PDX treated with BSJ-5-63, olaparib, or their combination. Data represent means ± SEM (n = 5). (G) Tumor weights from BID-PC4 PDX on day 21 of treatment with representative images. Data represent means ± SEM (n = 5). (H) H&E staining and IHC for cl Caspase 3 of BID-PC4 PDX tumors show enhanced apoptosis following sequential combination therapy. Scale bar, 50 μm. Quantification of cl Caspase 3 is shown. Data represent means ± SEM (n = 5). (I) Short-term ex vivo culture of PCa tissue fragments from patient #1 (Gleason score 7a/ISUP grade group 2) treated with the sequential combination. Top: Experimental schematic. Bottom: H&E staining and IHC for cl PARP reveal increased apoptosis. Scale bars, 50 μm. Quantification of cl PARP is shown. Data represent means ± SEM. (J) Proposed model illustrating how BSJ-5-63 induces acute BRCAness and AR pathway inhibition via CDK12/7/9 degradation, sensitizing PCa cells to PARP inhibitors. n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001, one-way ANOVA with Tukey’s test.
Furthermore, we used a newly developed BID-PC4 patient-derived xenograft (PDX) model, which lacks HRR deficiency. The key genomic alterations of BID-PC4 were determined by targeted exome sequencing (table S3). This model was insensitive to olaparib treatment, as evidenced by tumor growth in severe combined immunodeficient (SCID) mice. BSJ-5-63 demonstrated only a marginal inhibitory effect on tumor growth in this context (Fig. 7, E to G). Nevertheless, when the same sequential treatment approach was applied, combination therapy exerted a substantial inhibitory effect on tumor growth. Histological examination (H&E staining) revealed that the structure of PCa remained largely intact in the control and single-agent–treated groups, whereas the combination therapy led to massive apoptotic cell death, as evidenced by cleaved Caspase 3 staining (Fig. 7H). Last, we extended our findings by using short-term ex vivo cultures of three patient-derived prostate tumors obtained from radical prostatectomy. We observed significantly increased apoptosis in PCa tissues after sequential combination treatment with BSJ-5-63 followed by olaparib as indicated, compared to single-agent treatment, as evidenced by cleaved PARP1 staining (Fig. 7I and fig. S13).
DISCUSSION
In this study, we demonstrated the efficacy of BSJ-5-63 in degrading CDK12, CDK7, and CDK9, supporting its potential as a versatile therapeutic agent (Fig. 7J). The degradation of CDK12, coupled with the down-regulation of long HRR genes, including BRCA1/2, aligns with previous studies highlighting the importance of CDK12 in controlling HRR function (22, 45). This degradation induces a state of BRCAness, rendering CRPC cells sensitive to PARP inhibition. The temporal persistence of this BRCAness state following BSJ-5-63 treatment paves the way for a rational sequential combination therapy, potentially minimizing toxicity and overcoming resistance issues commonly associated with single-agent treatments. The extended mechanism of BSJ-5-63, targeting CDK7 and CDK9, further enhances its therapeutic potential. The inhibition of these kinases not only disrupts AR signaling, a primary therapeutic target in CRPC, but also interferes with the expression of AR-controlled HRR genes (17, 18), providing additional pathway to enhance BRCAness. RNA-seq analysis confirmed that the impaired HRR pathway is the primary mechanism of action induced by BSJ-5-63. Therefore, the proposed combinatorial approach involving the degradation of CDK12, CDK7, and CDK9 holds great promise for a broad spectrum of patients with CRPC, extending beyond those with BRCA1/2 alterations.
Both genetic deletion of CDK12 and suppression of its activity using BSJ-5-63 demonstrated that acute CDK12 inhibition can sensitize CRPC cells to PARP inhibition. However, prolonged CDK12 deletion in PCa cells led to a gradual and partial restoration of BRCA1/2 expression, resulting in a subsequent loss of sensitivity to PARP inhibition. This observation is in line with clinical data showing the insensitivity of PCa with CDK12 loss-of-function mutations (24, 27–29), indicating the complexity of cellular responses to CDK12 genetic alterations and prompting further exploration of the underlying mechanisms. These mechanisms may include substantial functional redundancy between CDK12 and its paralog kinase, CDK13 (47, 48). Furthermore, our results revealed that BSJ-5-63 can target AR-positive CRPC cells by degrading CDK7 and CDK9, key players in AR-mediated transcription. Targeting AR through CDK7 and CDK9, without involving AR ligand binding, may prove effective in CRPC with AR amplification, mutations, or variants. Inhibition of AR signaling by BSJ-5-63 offers an alternative therapeutic strategy when direct AR-targeted therapies fail, potentially overcoming ARPI resistance, a major challenge in CRPC treatment. In vivo experiments using cell line–derived xenograft and PDX models demonstrated the translational potential of BSJ-5-63. While both olaparib and BSJ-5-63 as single agents showed limited impact, the sequential combination of BSJ-5-63 with olaparib produced synergistic effects, indicating the clinical relevance of this approach. Sequential combination therapy was not only well tolerated but also effective for CRPC, irrespective of genetic alteration status. Ex vivo experiments also demonstrated the efficacy of the sequential combination therapy, reinforcing its potential application in patients.
While both BSJ-5-63 and BSJ-4-116 degrade CDK12 and suppress HRR genes BRCA1/2, BSJ-5-63 distinguishes itself as a CDK12/7/9 triple degrader. This distinction is likely attributed to the use of the E3 ubiquitin ligase VHL ligand, as opposed to CRBN in BSJ-4-116. The choice of VHL versus CRBN is crucial for achieving effective targeted protein degradation. This is likely due to tissue-specific expression of E3 ligase, substrate specificity, and ubiquitination efficiency, which profoundly affect degradation profiles. Consequently, BSJ-5-63 has an additional impact on AR signaling by targeting both CDK7 and CDK9, in contrast to BSJ-4-116, which targets only CDK9 in PCa cells. This differential targeting led to more potent inhibition of the AR pathway with BSJ-5-63, as CDK7 acts as a master regulator in AR-mediated transcription (36). BSJ-5-63 exhibited favorable pharmacokinetic properties, including a longer half-life, reduced clearance rate, and a higher maximum plasma concentration than BSJ-4-116. These characteristics suggest that BSJ-5-63 may offer enhanced efficacy in vivo. However, our study has limitations. The potential off-target effects of BSJ-5-63, a common concern with compounds of this class, remain unexplored, which could affect its clinical utility and safety profile. The primary toxicity may arise from targeting CDK7, CDK9, and CDK12, which are key transcriptional regulators. While these CDKs are well-established cancer therapy targets, with various inhibitors and degraders in development, further research is needed to determine whether simultaneous inhibition of multiple CDKs is essential. The specific contributions of CDK7 and CDK9 degradation in inducing BRCAness require further validation.
In conclusion, clinical investigations over the past several years have shown that PARP inhibition is particularly effective in the context of BRCA1/2 alterations. However, the extent to which patients with non-BRCA1/2 genomic alterations can benefit from PARPis remains unclear, as gene-by-gene analyses have been inconclusive (49, 50). Mutations in non-BRCA HRR genes may not induce an HRR deficiency comparable to that caused by BRCA1/2 loss. Even in patients with BRCA mutations, acquired resistance can develop because of BRCA reversion mutations (51), which restore BRCA1/2 expression and HRR function. Therefore, it is critical to develop approaches that effectively suppress BRCA1/2 expression and synergize PARP inhibition. One such approach involves ARPIs, which have been shown to down-regulate HRR gene expression to some extent in preclinical models (43). However, the clinical benefit of combining AR and PARP inhibition may not be attributable to AR-mediated transcription of the HRR genes (21). Further evidence is needed to determine whether ARPIs can induce a true BRCAness state. Our findings present a promising avenue for CRPC treatment through the distinctive profile of BSJ-5-63, which targets CDK12, CDK7, and CDK9 to transiently but thoroughly abolish BRCA1/2 expression and HRR function. Combination therapies with PARPis exhibit remarkable efficacy in preclinical models, laying the groundwork for future clinical studies.
MATERIALS AND METHODS
Cells
Parental PCa cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). LNCaP (catalog no. CRL-1740; RRID: CVCL_0395), C4-2B (catalog no. CRL-3315; RRID: CVCL_4784), 22Rv1 (catalog no. CRL-2505; RRID: CVCL_1045), DU145 (catalog no. HTB-81; RRID: CVCL_0105), and LAPC4 (catalog no. CRL-13009; RRID: CVCL_4744) cells were cultured in RPMI 1640 medium (Gibco, catalog no. 11875093). VCaP (catalog no. CRL-2876; RRID: CVCL_2235) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) with GlutaMAX (Gibco, catalog no. 10566016). Human embryonic kidney (HEK) 293T (catalog no. CRL-3216; RRID: CVCL_0063) cells were grown in DMEM (Gibco, catalog no. 21013024). The enzalutamide-resistant cell line MR49F (RRID: CVCL_RW53) was obtained from A. Zoubeidi (University of British Columbia, Canada) and maintained in RPMI 1640 medium supplemented with 10 μM enzalutamide (46). U2OS-DR-GFP (RRID: CVCL_B0A7) and U2OS-EJ5-GFP were obtained from L. Lan (Duke University) and maintained in DMEM (52). The medium was supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, catalog no. F0926) and 1% penicillin-streptomycin solution (Gibco, catalog no. 15140122). The cell lines were maintained in an incubator at 37°C and 5% CO2. All cell lines were tested negative for mycoplasma contamination using MycoAlert (Lonza, catalog no. LT07-318).
Animals
Male SCID mice aged 4 to 5 weeks were obtained from Taconic Laboratories and acclimated for 1 week in a pathogen-free enclosure before the start of the study. Mice were maintained in a 12-hour light/12-hour dark cycles with free access to food and water. All procedures were conducted in compliance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the Center for Comparative Medicine in Brigham and Women’s Hospital.
Human prostate carcinoma tissue
Fresh prostate carcinoma tissue samples were collected at Goethe University Hospital Frankfurt, Germany, from patients undergoing radical prostatectomy under license UCT-38-2023 approved by the University Cancer Center (UCT) Scientific Board and Ethics Committee and compliant with all relevant ethical regulations regarding research involving human participants. Signed consent was obtained from all patients. Samples were de-identified before transport to the laboratory.
Compounds
BSJ-5-63, BSJ-5-63-NC, BSJ-4-116, and BSJ-4-116-NC were synthesized in-house. The medicinal chemistry and pharmacological profiles of the compounds are described in Supplementary Text. Olaparib (catalog no. HY-10162), rucaparib (catalog no. HY-10617A), niraparib (catalog no. HY-10619), talazoparib (catalog no. HY-16106), sunitinib (catalog no. HY-10255A), enzalutamide (catalog no. HY-70002), and MG-132 (catalog no. HY-13259) were purchased from MedChemExpress (Monmouth Junction, NJ, USA). For cell cultures, all compounds were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog no. D8418) to prepare a stock solution (10 mM) and stored at −20°C. The final dosing solution was prepared on the day of use by diluting the stock solution. For in vivo assays, the compounds were dissolved in a vehicle composed of 5% DMSO, 15% (w/v) Kolliphor HS 15 (Sigma-Aldrich, catalog no. 42966), and 80% normal saline.
Generation of gene KO cell lines using CRISPR-Cas9
LentiGuide-Puro (Addgene, catalog no. 52963; RRID: Addgene_52963) and lentiCas9-Blast (Addgene, catalog no. 52962; RRID: Addgene_52962) plasmids were used to generate CDK12 and CDK9 KO cell lines. CRISPR sgRNAs targeting CDK12 and CDK9 were cloned into a lentiGuide-Puro vector. AAVS1 sgRNAs were cloned into the same vector and served as controls. The sgRNAs used in this study are listed in table S4. Lentiviruses were generated by transfecting sub-confluent HEK293T cells along with the lentiviral vector and packaging vectors pCMV-VSV-G (Addgene, catalog no. 8454; RRID: Addgene_8454) and psPAX2 (Addgene, catalog no. 12260; RRID: Addgene_12260) at a 2:1:1 ratio using Lipofectamine 3000 transfection reagents (Invitrogen, catalog no. L3000015) according to the manufacturer’s protocol. Viral supernatants were collected 48 hours after transfection and filtered through a 0.45-μm membrane (Corning, catalog no. 431220). For cell infection, 1.5 ml of viral supernatant, 0.5 ml of fresh medium, and polybrene (8 μg ml−1) were added to 5 million cells per well in six-well plates. Plates were centrifuged at 600g for 90 min at 37°C during the spin infection. The virus-containing medium was removed after 24 hours. Initially, cells overexpressing Cas9 were generated under blasticidin selection (20 μg ml−1). Subsequently, CDK12 and CDK9 KO cells were further generated under puromycin selection (2 μg ml−1). The KO efficiency was validated using Western blotting.
Western blotting
Cells were washed with phosphate-buffered saline (PBS) buffer (Gibco, catalog no. 10010023) and lysed in 1× radioimmunoprecipitation assay lysis buffer (Thermo Scientific, catalog no. 89901) supplemented with protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, catalog no. 78442). The total protein concentration was measured using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, catalog no. 23227). Western blotting was performed as previously described (53). Briefly, equal amounts of proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (MilliporeSigma, catalog no. IPVH00010). Membranes were incubated with primary antibodies in 5% bovine serum albumin (BSA) in 1× TBST buffer overnight at 4°C. After primary antibody incubation, membranes were probed with a secondary antibody at room temperature for 1 hour. The antibodies used in this study are listed in table S4.
Immunofluorescence staining
Cells were seeded into four-well Millicell EZ slide (MilliporeSigma, catalog no. PEZGS0416) pre-coated with poly-l-lysine (Sigma-Aldrich, catalog no. P4707-50ml) overnight. For treatment, 22Rv1 and C4-2B cells were treated with compound as indicated for 24 hours before irradiation (8 Gy). For CDK12-KO 22Rv1 and AAVS1 control cells, they were incubated for 24 hours after irradiation (8 Gy). Subsequently, cells were washed three times with PBS and then fixed in 4% formaldehyde (Electron Microscopy Sciences, catalog no. 15714) for 10 min at room temperature. Fixed cells were further incubated with 0.1% Triton X-100 in PBS for 10 min to permeabilize the cells and then washed with PBST (0.1% Tween 20 in PBS) three times for 5 min. Fixed cells were blocked in PBS containing 5% BSA for H2AX or in PBS containing 1% BSA and 20% FBS for RAD51 at room temperature for 1 hour. After washing with PBST three times for 5 min each, cells were incubated with phospho-histone H2AX Ser139 or RAD51 antibodies diluted in the same blocking buffer overnight at 4°C. After washing with PBST, the cells were further incubated with the appropriate secondary antibodies for 1 hour at room temperature. Cells were washed with PBST, mounted, and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Mounting Medium With DAPI-Aqueous, Fluoroshield; Abcam, catalog no. ab104139) overnight at 4°C. The antibodies used are listed in table S4. Each experiment was performed independently in triplicate. To calculate the γ-H2AX foci, the number of foci was counted from at least 50 cells for each condition. To calculate RAD51 foci, the number of cells containing at least five foci was counted in at least 50 cells for each replicate under each condition.
Colony formation assay
Cells were seeded at a density of 15,000 cells per well for LNCaP, C4-2B, and DU145 cells and 30,000 cells per well for 22Rv1 cells in six-well plates and incubated overnight before treatment as indicated. For the colony formation assay, cells were incubated for 14 days at 37°C with 5% CO2, with the medium changed once per week. For sequential combination treatment, cells were treated with BSJ-5-63 or BSJ-4-116 at the indicated dose for 2 days. Cells were rinsed with 1× PBS and then treated with PARPis olaparib, rucaparib, or niraparib at the indicated doses for an additional 12 days. The colonies were subsequently washed with PBS and stained with 0.1% crystal violet as previously described (54). Colony images were quantified using the ImageJ software (National Institutes of Health). Three independent experiments were performed.
Cell viability assay
Cells were seeded at a density of 7200 cells per well (for 3 days of treatment) or 2700 cells per well (for 7 days of treatment) in 96-well plates and incubated overnight before treatment. Subsequently, the cells were treated with the indicated compounds. Cell viability was determined using alamarBlue Cell Viability Reagent (Thermo Fisher Scientific, catalog no. DAL1025) according to the manufacturer’s instructions. IC50 values were estimated using GraphPad Prism 9 (GraphPad software). Three independent experiments were performed in triplicates.
RNA interference
For knockdown experiments, cells were transfected with 100 nM ON-TARGETplus SMARTpool small interfering RNA (siRNA) targeting CDK7 (Dharmacon, L-003241-00-0005) or a non-targeting pool (Dharmacon, D-001810-10-05) as a negative control, using Lipofectamine RNAiMAX (Invitrogen, catalog no. 13778150) according to the manufacturer’s instructions. Cells were harvested at 48 hours post-transfection.
DHT stimulation
LNCaP, CDK12-KO 22Rv1, and CDK9-KO 22Rv1cells were grown in medium containing charcoal-stripped FBS (CSS) for 3 days. Subsequently, the cells were treated with 10 nM 5-α-DHT (Sigma-Aldrich, catalog no. D-073-1ML) in ethanol or with ethanol alone as a control. Cells were harvested at 16 hours poststimulation. For DHT stimulation with BSJ-5-63, LNCaP cells grown in CSS-containing medium for 3 days were treated with BSJ-5-63 as indicated. Three hours posttreatment, the cells were stimulated with 10 nM DHT for an additional 13 hours. For DHT stimulation with CDK7 RNA interference, LNCaP cells grown in CSS-containing medium for 3 days were transfected with 100 nM ON-TARGETplus SMARTpool siRNA targeting CDK7 or a non-targeting pool as indicated. Thirty-two hours post-transfection, the cells were stimulated with 10 nM DHT for an additional 16 hours.
RNA isolation and RT-qPCR
Total RNA was extracted using the RNeasy Plus Mini Kit (QIAGEN, catalog no. 74134) according to the manufacturer’s protocol. One microgram of RNA was reverse transcribed to complementary DNA in 20 μl of reaction volumes using iScript Reverse Transcription Supermix (Bio-Rad, catalog no. 1708841) according to the manufacturer’s protocol. RT-qPCR was performed using the SYBR Select Master Mix for CFX (Applied Biosystems, catalog no. 4472942) using the Bio-Rad CFX Connect Real-Time System. Briefly, 100 ng of cDNA was used as a template in 20 μl of reaction volumes with 250 nM primers. Amplification reactions were run for 40 thermocycles of 15 s at 95°C, 1 min at 59°C. The primers used are listed in table S4.
Cell cycle analysis
Cells were seeded at a density of 1 × 106 cells per well in six-well plates and incubated overnight before the indicated treatments. Cell cycle analysis was then performed as previously described (55). Briefly, cells were harvested, washed with cold 1× PBS, and fixed in cold 70% ethanol, followed by overnight incubation at 4°C. On the day of analysis, cells were centrifuged, washed with 1× PBS, and stained with PI staining buffer [propidium iodide (50 μg/ml; Sigma-Aldrich, catalog no. 81845) and ribonuclease (10 mg/ml; Sigma-Aldrich, catalog no. 11119915001) in 1× PBS] for 30 min at room temperature. Flow cytometry was performed, and the results were analyzed using FlowJo 10 software (RRID: SCR_008520). Experiments were performed independently three times.
DR-GFP and EJ5-GFP reporter assay
The plasmids pDEST-3xHA-I-SceI-T2A-mCherry, pDRGFP, and pimEJ5GFP were provided by L. Lan (Duke University) (52, 56). Cells were seeded at 8 × 105 cells per well in six-well plates and incubated overnight before treatment. For HRR and NHEJ efficiency in LNCaP cells, 1.25 μg of I-SceI plasmid was co-transfected with 1.25 μg of DR-GFP plasmid (HRR) or 1.25 μg of EJ5-GFP plasmid (NHEJ) using Lipofectamine 3000, following the manufacturer’s protocol. Three hours post-transfection, cells were treated with BSJ-5-63 (100 nM) or DMSO for 72 hours.
For stable U2OS-DR-GFP and U2OS-EJ5-GFP cells, 2.5 μg of I-SceI plasmid was transfected. Twenty-four hours later, cells were treated with BSJ-5-63 (100 nM) or DMSO for 48 hours. Cells were subsequently harvested, washed with 1× PBS, and filtered through a 100-μm membrane. GFP and mCherry expression levels were analyzed by flow cytometry, and the GFP:mCherry ratio was calculated using FlowJo 10 software. Three independent experiments were performed.
CDK12 rescue assay
The pHAGE-CMV-Flag-CDK12_IDG-K plasmid was obtained from Addgene (catalog no. 135281; RRID: Addgene_135281). Full-length CDK12 was amplified with a 3× Flag tag at the 5′ end and subcloned into the pcDNA3.1+ vector using primers listed in table S4. CDK12-KO single-cell clone lines H91 and H92 were seeded at a density of 1 × 107 cells in 100-mm dishes and incubated overnight. Cells were transfected with 10 μg of 3× Flag-CDK12 plasmid using Lipofectamine 3000, as per the manufacturer’s protocol. After 24 hours, cells were harvested for downstream applications.
For Western blot analysis, cells were reseeded at 1 × 106 cells per well in six-well plates. After 48 hours, cells were harvested for CDK12 rescue verification or treated with BSJ-5-63 for 48 hours before protein extraction for immunoblotting. For DR-GFP and EJ5-GFP reporter assays, cells were reseeded at a density of 1 × 106 cells per well in six-well plates. I-SceI (1.25 μg) and either DRGFP (HRR) or EJ5GFP (NHEJ) plasmids (1.25 μg) were co-transfected. After 48 hours, cells were harvested, washed with 1× PBS, filtered through a 100-μm membrane, and analyzed for GFP and mCherry expression using flow cytometry. The GFP:mCherry ratio was calculated with FlowJo 10 software. Three independent experiments were performed. For cell viability assays, cells were reseeded at 1 × 104 cells per well in 96-well plates and incubated overnight. Cells were then treated with BSJ-5-63 for 72 hours, and viability was measured using the alamarBlue Cell Viability Reagent according to the manufacturer’s instructions. Dose-response curves were generated with GraphPad Prism 9. Three independent experiments were performed in triplicate.
RNA-seq assay
22Rv1 cells were treated with DMSO, BSJ-5-63 (100 nM), or BSJ-5-63-NC (100 nM) for 8 hours. Treatments were performed in duplicated. Cells were then rinsed with ice-cold 1× PBS. Total RNA was extracted using the RNeasy Plus Mini Kit according to the manufacturer’s protocol. The samples were sent to Genewiz for RNA-seq. RNA-seq data analysis was performed as previously described (54). Gene set enrichment analysis (GSEA) was performed using GSEA software (57, 58). RNA-seq data in this study have been deposited in the GEO database under the accession number GSE263218 and are publicly available.
Proteomics
22Rv1 cells were seeded in 10-mm dishes overnight before treatment with DMSO, BSJ-5-63 (100 nM), BSJ-5-63-NC (250 nM), or BSJ-4-116 (100 nM) for 8 hours. Cells were rinsed with cold PBS and stored at −80°C. Treatments were performed in biological triplicate. Proteomics and data analysis were performed as previously described (34). The mass spectrometry (MS) proteomics data in this study are provided within data S1 and S2.
In vitro mouse liver microsomal stability assay
The stability of BSJ-5-63 and BSJ-4-116 was determined in mouse liver microsomes using the service of Scripps Florida (FL, USA). Mouse liver microsomes (0.5 mg/ml) were incubated with BSJ-5-63 (1 μM) or BSJ-4-116 (1 μM) at 37°C in the presence of the cofactor reduced form of nicotinamide adenine dinucleotide phosphate (1 mM). Sunitinib, a multi-targeted receptor kinase inhibitor, was used as a positive control in parallel. Samples were collected at time points 0, 5, 10, 20, 40, and 60 min for the measurement of remaining compounds by liquid chromatography–tandem MS (LC-MS/MS).
In vivo xenograft model
All procedures were performed in compliance with the guidelines from the IACUC at the Brigham and Women’s Hospital under protocol no. 2019 N000125. Cells were filtered through 70-μm cell strainers (BD, NJ, USA) and suspended in serum-free medium. Xenografts were initiated by subcutaneous injection of cells (5 × 106 cells/50 μl per mouse with an additional 50 μl of Matrigel, n = 5 per group) into the right flank near the axillary fossa of the mice. Tumor growth was monitored daily using the standard formula: V = length × width2 × 0.5. When the tumor volume approached ~150 mm3, the mice were randomly assigned into treatment groups.
For BSJ-5-63 single-reagent treatment, mice bearing 22Rv1 xenografts were randomly assigned to three groups (n = 5 per group) and treated with vehicle [5% DMSO/15% Solutol HS-15/80% saline, intraperitoneally (ip), daily], BSJ-5-63 (50 mg/kg, ip, daily), or BSJ-5-63 (50 mg/kg, ip, 1 day on/2 days off). Mice were euthanized after 12 days of treatment. For sequential combination treatment, mice bearing 22Rv1 or DU145 xenografts were randomly assigned to four groups (n = 5 per group) and treated with vehicle (orally, p.o.), olaparib (50 mg/kg, p.o.), BSJ-5-63 (25 mg/kg, ip), or a sequential combination of BSJ-5-63 with olaparib as indicated. Mice were euthanized after 3 or 4 weeks of treatment or when the tumor length reached 2 cm. For olaparib single-reagent treatment, mice bearing CDK12-KO 22Rv1 xenografts (using gRNA sg1 and sg2) and AAVS1-KO control xenografts were randomly assigned to two groups separately and treated with vehicle (p.o.) or olaparib (50 mg/kg, p.o.) daily. Mice were euthanized after 3 weeks of treatment or when the tumor length reached 2 cm. All compounds were formulated in the vehicle. The injection volume was 0.1 ml/10 g of mice body weight. Daily body weight was monitored. Mice were euthanized 3 hours post–final dose, and tumors were excised, weighted, and subjected to routine histopathological examination.
In vivo PDX model
The human PCa BID-PC4 PDX was generated through the Dana-Farber/Harvard Cancer Center Genitourinary Oncology Rapid Autopsy Program, as previously described (59). The PDX was subjected to targeted exome sequencing, and genetic alterations in key genes are listed in table S3. No deleterious biallelic mutations were identified in the HRR genes. BID-PC4 was maintained by constant passaging in male SCID mice. Intact male SCID mice were implanted subcutaneously with BID-PC4 tumor bits in 50 μl of 1× PBS per mouse with an additional 50 μl of Matrigel into the right flank near the axillary fossa of mice. When tumors reached ~150 mm3, mice were randomly assigned to four groups (n = 5 per group) and treated with vehicle (5% DMSO/15% Solutol HS-15/80% saline, p.o.), olaparib (50 mg/kg, p.o.), BSJ-5-63 (25 mg/kg, ip), and sequential combination of BSJ-5-63 with olaparib as indicated. All compounds were formulated in the vehicle. The injection volume was 0.1 ml/10 g of mouse body weight. Daily body weight was monitored. Mice were euthanized after 3 weeks of treatment or when tumor length reached 2 cm. The tumors were excised, weighted, and subjected to routine histopathological examination.
H&E staining
Tumor tissues were fixed in 4% formalin, embedded in paraffin, cut into 4-μm-thick sections, and mounted onto slides. Standard staining with H&E was performed.
Immunohistochemistry
Immunohistochemistry staining of 4-μm-thick tumor tissue sections was conducted as previously described (55). Briefly, after dewaxing, rehydration, and antigen retrieval, the sections were blocked with 5% serum for 1 hour. Subsequently, the sections were incubated with primary antibodies overnight at 4°C, followed by incubation with Polymer Helper for 20 min and secondary antibody for another 20 min. After the color reaction, the slides were counterstained with hematoxylin.
In vivo pharmacokinetic analysis
The in vivo pharmacokinetic analysis was conducted using the service of Scripps Florida (FL, USA). The pharmacokinetic properties of BSJ-5-63 and BSJ-4-116 were determined in male C57BL/6 mice following intraperitoneal administration of 10 mg/kg. The compounds were formulated in 5% DMSO/15% Solutol HS-15/80% saline. Blood samples were collected up to 8 hours post-dosing for the measurement of compound concentrations in the blood using LC-MS/MS.
In vivo pharmacodynamic analysis
The in vivo pharmacodynamic properties and efficacy of BSJ-5-63 were determined using 22Rv1 xenografts in male SCID mice. Xenografts were initiated by subcutaneous injection of 22Rv1 cells (5 × 106 cells/50 μl per mouse with an additional 50 μl of Matrigel, n = 3 per group) into the right flank near the axillary fossa of the mice. Tumor growth was monitored daily using the standard formula: V = length × width2 × 0.5. Mice bearing tumors of ~200 mm3 were randomly assigned to three groups and treated with vehicle (5% DMSO/15% Solutol HS-15/80% Saline, ip, twice a day, bid), BSJ-5-63 (25 mg/kg, ip, bid), or BSJ-5-63 (50 mg/kg, ip, bid) for 3 days. Three hours after the final dose, the mice were euthanized, and the tumors were excised and subjected to Western blotting.
Ex vivo human PCa patient model
Fresh prostate carcinoma tissue samples were collected at Goethe University Hospital Frankfurt, Germany, from patients undergoing radical prostatectomy under license UCT-38-2023 approved by the UCT Scientific Board and Ethics Committee and compliant with all relevant ethical regulations regarding research involving human participants. Signed consent was obtained from all patients. Samples were de-identified before transport to the laboratory. Tissue specimens from patients #1 (Gleason score 7a/ISUP grade group 2), #2 (Gleason Score 7a/ISUP grade group 2), and #3 (Gleason score 9/ISUP grade group 5) were obtained from radical prostatectomy without prior therapy. Tissues were cut into small fragments and transferred into 12-well plates with 2 ml of culture medium per well (DMEM/F-12 containing 10% FBS, 1% GlutaMAX, and 1% penicillin-streptomycin solution). The tissue slices were treated with 100 nM BSJ-5-63 for 24 hours followed by washout of BSJ-5-63 and treatment with 10 μM olaparib for 3 to 4 days. As controls, treatments with DMSO, BSJ-5-63 (100 nM), or olaparib (10 μM) were performed for 4 to 5 days as indicated. Multiple tumor pieces were used for each condition. The plates were incubated at 37°C and 5% CO2. Subsequently, tissue fragments were fixed in 4% paraformaldehyde for 24 hours and embedded in paraffin. Immunohistochemistry staining for cleaved PARP (Asp214) (Cell Signaling Technology, catalog no. 5625; dilution ratio, 1:100) was performed, followed by counterstaining with hematoxylin.
Statistical analysis
All data are presented as means ± SEM. Comparisons between two groups were analyzed using two-tailed unpaired Student’s t test. For multiple group comparisons, one-way or two-way analysis of variance (ANOVA) followed by the Tukey-Kramer test for post hoc analysis was applied. Statistical analyses were performed using GraphPad Prism 9 (GraphPad software). The significance levels are denoted as *P < 0.05, **P < 0.01, and ***P < 0.001.
Acknowledgments
We thank N. Gray and T. Zhang for providing the compounds BSJ-4-116 and BSJ-5-63, reviewing the manuscript, and engaging in insightful discussions. The development of BSJ-5-63 was supported, in part, by NIH R01CA218278 to N. Gray.
Funding: This work was supported by the National Institutes of Health, grants R01CA262524, R01CA279410, and R21CA267496 (L.J.); Department of Defense, grant W81XWH-22-1-0477 (L.J.); Dana Farber/Harvard Cancer Center Incubator Award (S.P.B. and L.J.); Cancer Prevention and Research Institute of Texas, CPRIT Scholar in Cancer Research, grant RR220032 (M.K.); and Mildred Scheel Career Center Frankfurt, Deutsche Krebshilfe (A.F.).
Author contributions: Conceptualization: F.G., B.J., T.Ts., S.P.B., A.S.K., and L.J. Methodology: F.G., B.J., T.Ts., S.A., C.P., G.A.B., A.F., A.S.K., and L.J. Investigation: F.G., B.J., J.J., T.Ta., C.P., G.A.B., R.E., M.K., A.F., A.S.K., and L.J. Visualization: F.G., B.J., G.A.B., A.F., and L.J. Data curation: F.G., M.K., and A.S.K. Software: G.A.B. Resources: F.G., B.J., Z.H., S.A., J.K., A.F., S.P.B., A.S.K., and L.J. Validation: F.G., B.J., T.Ta., C.P., A.F., and L.J. Formal analysis: F.G., B.J., T.Ts., T.Ta., R.E., M.K., and L.J. Funding acquisition: A.F., S.P.B., A.S.K., and L.J. Project administration: F.G., A.F., A.S.K., and L.J. Supervision: M.K., A.F., A.S.K., and L.J. Writing—original draft: F.G., Z.H., and L.J. Writing—review and editing: F.G., B.J., Z.H., S.A., R.E., M.K., A.F., S.P.B., A.S.K., and L.J.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
The PDF file includes:
Supplementary Text
Figs. S1 to S13
Tables S1 to S4
Legends for data S1 to S3
Other Supplementary Material for this manuscript includes the following:
Data S1 to S3
REFERENCES AND NOTES
- 1.Siegel R. L., Giaquinto A. N., Jemal A., Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Einstein D. J., Arai S., Balk S. P., Targeting the androgen receptor and overcoming resistance in prostate cancer. Curr. Opin. Oncol. 31, 175–182 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.E. Lee, Z. Zhang, C. C. Chen, D. Choi, A. C. A. Rivera, E. Linton, Y. J. Ho, J. Love, J. LaClair, J. Wongvipat, C. L. Sawyers, Timing of treatment shapes the path to androgen receptor signaling inhibitor resistance in prostate cancer. bioRxiv 585532 [Preprint] (2024). 10.1101/2024.03.18.585532. [DOI]
- 4.Abida W., Cyrta J., Heller G., Prandi D., Armenia J., Coleman I., Cieslik M., Benelli M., Robinson D., Van Allen E. M., Sboner A., Fedrizzi T., Mosquera J. M., Robinson B. D., De Sarkar N., Kunju L. P., Tomlins S., Wu Y. M., Nava Rodrigues D., Loda M., Gopalan A., Reuter V. E., Pritchard C. C., Mateo J., Bianchini D., Miranda S., Carreira S., Rescigno P., Filipenko J., Vinson J., Montgomery R. B., Beltran H., Heath E. I., Scher H. I., Kantoff P. W., Taplin M. E., Schultz N., deBono J. S., Demichelis F., Nelson P. S., Rubin M. A., Chinnaiyan A. M., Sawyers C. L., Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 116, 11428–11436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord C. J., Ashworth A., PARP inhibitors: Synthetic lethality in the clinic. Science 355, 1152–1158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krastev D. B., Wicks A. J., Lord C. J., PARP inhibitors - trapped in a toxic love affair. Cancer Res. 81, 5605–5607 (2021). [DOI] [PubMed] [Google Scholar]
- 7.de Bono J., Mateo J., Fizazi K., Saad F., Shore N., Sandhu S., Chi K. N., Sartor O., Agarwal N., Olmos D., Thiery-Vuillemin A., Twardowski P., Mehra N., Goessl C., Kang J., Burgents J., Wu W., Kohlmann A., Adelman C. A., Hussain M., Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Hussain M., Mateo J., Fizazi K., Saad F., Shore N., Sandhu S., Chi K. N., Sartor O., Agarwal N., Olmos D., Thiery-Vuillemin A., Twardowski P., Roubaud G., Ozguroglu M., Kang J., Burgents J., Gresty C., Corcoran C., Adelman C. A., de Bono J., Investigators P. R. T., Survival with olaparib in metastatic castration-resistant prostate cancer. N. Engl. J. Med. 383, 2345–2357 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Fizazi K., Piulats J. M., Reaume M. N., Ostler P., McDermott R., Gingerich J. R., Pintus E., Sridhar S. S., Bambury R. M., Emmenegger U., Lindberg H., Morris D., Nole F., Staffurth J., Redfern C., Saez M. I., Abida W., Daugaard G., Heidenreich A., Krieger L., Sautois B., Loehr A., Despain D., Heyes C. A., Watkins S. P., Chowdhury S., Ryan C. J., Bryce A. H., Investigators T., Rucaparib or physician’s choice in metastatic prostate cancer. N. Engl. J. Med. 388, 719–732 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abida W., Patnaik A., Campbell D., Shapiro J., Bryce A. H., McDermott R., Sautois B., Vogelzang N. J., Bambury R. M., Voog E., Zhang J., Piulats J. M., Ryan C. J., Merseburger A. S., Daugaard G., Heidenreich A., Fizazi K., Higano C. S., Krieger L. E., Sternberg C. N., Watkins S. P., Despain D., Simmons A. D., Loehr A., Dowson M., Golsorkhi T., Chowdhury S., TRITON2 investigators , Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 38, 3763–3772 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lord C. J., Ashworth A., BRCAness revisited. Nat. Rev. Cancer 16, 110–120 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Fizazi K., Azad A. A., Matsubara N., Carles J., Fay A. P., De Giorgi U., Joung J. Y., Fong P. C. C., Voog E., Jones R. J., Shore N. D., Dunshee C., Zschabitz S., Oldenburg J., Ye D., Lin X., Healy C. G., Di Santo N., Laird A. D., Zohren F., Agarwal N., First-line talazoparib with enzalutamide in HRR-deficient metastatic castration-resistant prostate cancer: The phase 3 TALAPRO-2 trial. Nat. Med. 30, 257–264 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad F., Clarke N. W., Oya M., Shore N., Procopio G., Guedes J. D., Arslan C., Mehra N., Parnis F., Brown E., Schlurmann F., Joung J. Y., Sugimoto M., Sartor O., Liu Y. Z., Poehlein C., Barker L., Del Rosario P. M., Armstrong A. J., Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): Final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 24, 1094–1108 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Chi K. N., Rathkopf D., Smith M. R., Efstathiou E., Attard G., Olmos D., Lee J. Y., Small E. J., de Santana Gomes A. J. P., Roubaud G., Saad M., Zurawski B., Sakalo V., Mason G. E., Francis P., Wang G., Wu D., Diorio B., Lopez-Gitlitz A., Sandhu S., MAGNITUDE Principal Investigators , Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 41, 3339–3351 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi K. N., Sandhu S., Smith M. R., Attard G., Saad M., Olmos D., Castro E., Roubaud G., de Santana Gomes A. J. P., Small E. J., Rathkopf D. E., Gurney H., Jung W., Mason G. E., Dibaj S., Wu D., Diorio B., Urtishak K., Del Corral A., Francis P., Kim W., Efstathiou E., Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: Second interim analysis of the randomized phase III MAGNITUDE trial. Ann. Oncol. 34, 772–782 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal N., Azad A. A., Carles J., Fay A. P., Matsubara N., Heinrich D., Szczylik C., De Giorgi U., Young Joung J., Fong P. C. C., Voog E., Jones R. J., Shore N. D., Dunshee C., Zschabitz S., Oldenburg J., Lin X., Healy C. G., Di Santo N., Zohren F., Fizazi K., Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomised, placebo-controlled, phase 3 trial. Lancet 402, 291–303 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Polkinghorn W. R., Parker J. S., Lee M. X., Kass E. M., Spratt D. E., Iaquinta P. J., Arora V. K., Yen W. F., Cai L., Zheng D., Carver B. S., Chen Y., Watson P. A., Shah N. P., Fujisawa S., Goglia A. G., Gopalan A., Hieronymus H., Wongvipat J., Scardino P. T., Zelefsky M. J., Jasin M., Chaudhuri J., Powell S. N., Sawyers C. L., Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 3, 1245–1253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asim M., Tarish F., Zecchini H. I., Sanjiv K., Gelali E., Massie C. E., Baridi A., Warren A. Y., Zhao W., Ogris C., McDuffus L. A., Mascalchi P., Shaw G., Dev H., Wadhwa K., Wijnhoven P., Forment J. V., Lyons S. R., Lynch A. G., O'Neill C., Zecchini V. R., Rennie P. S., Baniahmad A., Tavare S., Mills I. G., Galanty Y., Crosetto N., Schultz N., Neal D., Helleday T., Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 8, 374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiewer M. J., Goodwin J. F., Han S., Brenner J. C., Augello M. A., Dean J. L., Liu F., Planck J. L., Ravindranathan P., Chinnaiyan A. M., McCue P., Gomella L. G., Raj G. V., Dicker A. P., Brody J. R., Pascal J. M., Centenera M. M., Butler L. M., Tilley W. D., Feng F. Y., Knudsen K. E., Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2, 1134–1149 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madan R. A., Karzai F., VanderWeele D. J., Cheng H. H., de Bono J. S., Poly(ADP-ribose) polymerase inhibitor combinations in first-line metastatic castration-resistant prostate cancer: Increasing toxicity with unclear benefits. J. Clin. Oncol. 41, 5501–5504 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasterok S., Scott T. G., Roller D. G., Spencer A., Dutta A. B., Sathyan K. M., Frigo D. E., Guertin M. J., Gioeli D., The androgen receptor does not directly regulate the transcription of DNA damage response genes. Mol. Cancer Res. 21, 1329–1341 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubbury S. J., Boutz P. L., Sharp P. A., CDK12 regulates DNA repair genes by suppressing intronic polyadenylation. Nature 564, 141–145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsin J. P., Manley J. L., The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 26, 2119–2137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y. M., Cieslik M., Lonigro R. J., Vats P., Reimers M. A., Cao X., Ning Y., Wang L., Kunju L. P., de Sarkar N., Heath E. I., Chou J., Feng F. Y., Nelson P. S., de Bono J. S., Zou W., Montgomery B., Alva A., PCF/SU2C International Prostate Cancer Dream Team, Robinson D. R., Chinnaiyan A. M., Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 173, 1770–1782.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armenia J., Wankowicz S. A. M., Liu D., Gao J., Kundra R., Reznik E., Chatila W. K., Chakravarty D., Han G. C., Coleman I., Montgomery B., Pritchard C., Morrissey C., Barbieri C. E., Beltran H., Sboner A., Zafeiriou Z., Miranda S., Bielski C. M., Penson A. V., Tolonen C., Huang F. W., Robinson D., Wu Y. M., Lonigro R., Garraway L. A., Demichelis F., Kantoff P. W., Taplin M. E., Abida W., Taylor B. S., Scher H. I., Nelson P. S., de Bono J. S., Rubin M. A., Sawyers C. L., Chinnaiyan A. M., PCF/SU2C International Prostate Cancer Dream Team, Schultz N., Van Allen E. M., The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 50, 645–651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajrami I., Frankum J. R., Konde A., Miller R. E., Rehman F. L., Brough R., Campbell J., Sims D., Rafiq R., Hooper S., Chen L., Kozarewa I., Assiotis I., Fenwick K., Natrajan R., Lord C. J., Ashworth A., Genome-wide profiling of genetic synthetic lethality identifies CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity. Cancer Res. 74, 287–297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonarakis E. S., Isaacsson Velho P., Fu W., Wang H., Agarwal N., Sacristan Santos V., Maughan B. L., Pili R., Adra N., Sternberg C. N., Vlachostergios P. J., Tagawa S. T., Bryce A. H., McNatty A. L., Reichert Z. R., Dreicer R., Sartor O., Lotan T. L., Hussain M., CDK12-altered prostate cancer: Clinical features and therapeutic outcomes to standard systemic therapies, poly (ADP-ribose) polymerase inhibitors, and PD-1 inhibitors. JCO Precis. Oncologia 4, 370–381 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reimers M. A., Yip S. M., Zhang L., Cieslik M., Dhawan M., Montgomery B., Wyatt A. W., Chi K. N., Small E. J., Chinnaiyan A. M., Alva A. S., Feng F. Y., Chou J., Clinical outcomes in cyclin-dependent kinase 12 mutant advanced prostate cancer. Eur. Urol. 77, 333–341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo J., Antonarakis E. S., PARP inhibition - not all gene mutations are created equal. Nat. Rev. Urol. 16, 4–6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson S. F., Cruz C., Greifenberg A. K., Dust S., Stover D. G., Chi D., Primack B., Cao S., Bernhardy A. J., Coulson R., Lazaro J.-B., Kochupurakkal B., Sun H., Unitt C., Moreau L. A., Sarosiek K. A., Scaltriti M., Juric D., Baselga J., Richardson A. L., Rodig S. J., D’Andrea A. D., Balmaña J., Johnson N., Geyer M., Serra V., Lim E., Shapiro G. I., CDK12 inhibition reverses de novo and acquired PARP inhibitor resistance in BRCA wild-type and mutated models of triple-negative breast cancer. Cell Rep. 17, 2367–2381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shyamsunder P., Sridharan S. P., Madan V., Dakle P., Zeya C., Kanojia D., Chng W.-J., Ong S. T., Koeffler H. P., THZ531 induces a state of BRCAness in multiple myeloma cells: Synthetic lethality with combination treatment of THZ 531 with DNA repair inhibitors. Int. J. Mol. Sci. 23, 1207 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iniguez A. B., Stolte B., Wang E. J., Conway A. S., Alexe G., Dharia N. V., Kwiatkowski N., Zhang T., Abraham B. J., Mora J., Kalev P., Leggett A., Chowdhury D., Benes C. H., Young R. A., Gray N. S., Stegmaier K., EWS/FLI confers tumor cell synthetic lethality to CDK12 inhibition in Ewing sarcoma. Cancer Cell 33, 202–216.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Hao M., Leggett A. L., Gao Y., Ficarro S. B., Che J., He Z., Olson C. M., Marto J. A., Kwiatkowski N. P., Zhang T., Gray N. S., Discovery of MFH290: A potent and highly selective covalent inhibitor for cyclin-dependent kinase 12/13. J. Med. Chem. 63, 6708–6726 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Jiang B., Gao Y., Che J., Lu W., Kaltheuner I. H., Dries R., Kalocsay M., Berberich M. J., Jiang J., You I., Kwiatkowski N., Riching K. M., Daniels D. L., Sorger P. K., Geyer M., Zhang T., Gray N. S., Discovery and resistance mechanism of a selective CDK12 degrader. Nat. Chem. Biol. 17, 675–683 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dias M. P., Moser S. C., Ganesan S., Jonkers J., Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 18, 773–791 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Constantin T. A., Greenland K. K., Varela-Carver A., Bevan C. L., Transcription associated cyclin-dependent kinases as therapeutic targets for prostate cancer. Oncogene 41, 3303–3315 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasool R. U., Natesan R., Deng Q., Aras S., Lal P., Sander Effron S., Mitchell-Velasquez E., Posimo J. M., Carskadon S., Baca S. C., Pomerantz M. M., Siddiqui J., Schwartz L. E., Lee D. J., Palanisamy N., Narla G., Den R. B., Freedman M. L., Brady D. C., Asangani I. A., CDK7 inhibition suppresses castration-resistant prostate cancer through MED1 inactivation. Cancer Discov. 9, 1538–1555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo J. W., Nouri M., Balk S. P., Androgen receptor interaction with mediator complex is enhanced in castration-resistant prostate cancer by CDK7 phosphorylation of MED1. Cancer Discov. 9, 1490–1492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richters A., Doyle S. K., Freeman D. B., Lee C., Leifer B. S., Jagannathan S., Kabinger F., Koren J. V., Struntz N. B., Urgiles J., Stagg R. A., Curtin B. H., Chatterjee D., Mathea S., Mikochik P. J., Hopkins T. D., Gao H., Branch J. R., Xin H., Westover L., Bignan G. C., Rupnow B. A., Karlin K. L., Olson C. M., Westbrook T. F., Vacca J., Wilfong C. M., Trotter B. W., Saffran D. C., Bischofberger N., Knapp S., Russo J. W., Hickson I., Bischoff J. R., Gottardis M. M., Balk S. P., Lin C. Y., Pop M. S., Koehler A. N., Modulating androgen receptor-driven transcription in prostate cancer with selective CDK9 inhibitors. Cell Chem. Biol. 28, 134–147.e14 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Gao X., Liang J., Wang L., Zhang Z., Yuan P., Wang J., Gao Y., Ma F., Calagua C., Ye H., Voznesensky O., Wang S., Wang T., Liu J., Chen S., Liu X., Phosphorylation of the androgen receptor at Ser81 is co-sustained by CDK1 and CDK9 and leads to AR-mediated transactivation in prostate cancer. Mol. Oncol. 15, 1901–1920 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z., Ye Z., Soccio R. E., Nakadai T., Hankey W., Zhao Y., Huang F., Yuan F., Wang H., Cui Z., Sunkel B., Wu D., Dzeng R. K., Thomas-Ahner J. M., Huang T. H. M., Clinton S. K., Huang J., Lazar M. A., Jin V. X., Roeder R. G., Wang Q., Phosphorylated MED1 links transcription recycling and cancer growth. Nucleic Acids Res. 50, 4450–4463 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mbonye U., Wang B., Gokulrangan G., Shi W., Yang S., Karn J., Cyclin-dependent kinase 7 (CDK7)-mediated phosphorylation of the CDK9 activation loop promotes P-TEFb assembly with Tat and proviral HIV reactivation. J. Biol. Chem. 293, 10009–10025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L., Karanika S., Yang G., Wang J., Park S., Broom B. M., Manyam G. C., Wu W., Luo Y., Basourakos S., Song J. H., Gallick G. E., Karantanos T., Korentzelos D., Azad A. K., Kim J., Corn P. G., Aparicio A. M., Logothetis C. J., Troncoso P., Heffernan T., Toniatti C., Lee H. S., Lee J. S., Zuo X., Chang W., Yin J., Thompson T. C., Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci. Signal. 10, eaam7479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaborowska J., Egloff S., Murphy S., The pol II CTD: New twists in the tail. Nat. Struct. Mol. Biol. 23, 771–777 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Krajewska M., Dries R., Grassetti A. V., Dust S., Gao Y., Huang H., Sharma B., Day D. S., Kwiatkowski N., Pomaville M., Dodd O., Chipumuro E., Zhang T., Greenleaf A. L., Yuan G. C., Gray N. S., Young R. A., Geyer M., Gerber S. A., George R. E., CDK12 loss in cancer cells affects DNA damage response genes through premature cleavage and polyadenylation. Nat. Commun. 10, 1757 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop J. L., Sio A., Angeles A., Roberts M. E., Azad A. A., Chi K. N., Zoubeidi A., PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 6, 234–242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan Z., Devlin J. R., Hogg S. J., Doyle M. A., Harrison P. F., Todorovski I., Cluse L. A., Knight D. A., Sandow J. J., Gregory G., Fox A., Beilharz T. H., Kwiatkowski N., Scott N. E., Vidakovic A. T., Kelly G. P., Svejstrup J. Q., Geyer M., Gray N. S., Vervoort S. J., Johnstone R. W., CDK13 cooperates with CDK12 to control global RNA polymerase II processivity. Sci. Adv. 6, eaaz5041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tien J. C., Luo J., Chang Y., Zhang Y., Cheng Y., Wang X., Yang J., Mannan R., Mahapatra S., Shah P., Wang X. M., Todd A. J., Eyunni S., Cheng C., Rebernick R. J., Xiao L., Bao Y., Neiswender J., Brough R., Pettitt S. J., Cao X., Miner S. J., Zhou L., Wu Y. M., Labanca E., Wang Y., Parolia A., Cieslik M., Robinson D. R., Wang Z., Feng F. Y., Chou J., Lord C. J., Ding K., Chinnaiyan A. M., CDK12 loss drives prostate cancer progression, transcription-replication conflicts, and synthetic lethality with paralog CDK13. Cell Rep. Med. 5, 101758 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abida W., Campbell D., Patnaik A., Shapiro J. D., Sautois B., Vogelzang N. J., Voog E. G., Bryce A. H., McDermott R., Ricci F., Rowe J., Zhang J., Piulats J. M., Fizazi K., Merseburger A. S., Higano C. S., Krieger L. E., Ryan C. J., Feng F. Y., Simmons A. D., Loehr A., Despain D., Dowson M., Green F., Watkins S. P., Golsorkhi T., Chowdhury S., Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: Analysis from the phase II TRITON2 study. Clin. Cancer Res. 26, 2487–2496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fallah J., Xu J., Weinstock C., Gao X., Heiss B. L., Maguire W. F., Chang E., Agrawal S., Tang S., Amiri-Kordestani L., Pazdur R., Kluetz P. G., Suzman D. L., Efficacy of poly(ADP-ribose) polymerase inhibitors by individual genes in homologous recombination repair gene-mutated metastatic castration-resistant prostate cancer: A US Food and Drug Administration pooled analysis. J. Clin. Oncol. 42, 1687–1698 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loehr A., Hussain A., Patnaik A., Bryce A. H., Castellano D., Font A., Shapiro J., Zhang J., Sautois B., Vogelzang N. J., Chatta G., Courtney K., Harzstark A., Ricci F., Despain D., Watkins S., King C., Nguyen M., Simmons A. D., Chowdhury S., Abida W., Emergence of BRCA reversion mutations in patients with metastatic castration-resistant prostate cancer after treatment with rucaparib. Eur. Urol. 83, 200–209 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H., Yang H., Zhu X., Yadav T., Ouyang J., Truesdell S. S., Tan J., Wang Y., Duan M., Wei L., Zou L., Levine A. S., Vasudevan S., Lan L., m5C modification of mRNA serves a DNA damage code to promote homologous recombination. Nat. Commun. 11, 2834 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gui B., Gui F., Takai T., Feng C., Bai X., Fazli L., Dong X., Liu S., Zhang X., Zhang W., Kibel A. S., Jia L., Selective targeting of PARP-2 inhibits androgen receptor signaling and prostate cancer growth through disruption of FOXA1 function. Proc. Natl. Acad. Sci. U.S.A. 116, 14573–14582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsujino T., Takai T., Hinohara K., Gui F., Tsutsumi T., Bai X., Miao C., Feng C., Gui B., Sztupinszki Z., Simoneau A., Xie N., Fazli L., Dong X., Azuma H., Choudhury A. D., Mouw K. W., Szallasi Z., Zou L., Kibel A. S., Jia L., CRISPR screens reveal genetic determinants of PARP inhibitor sensitivity and resistance in prostate cancer. Nat. Commun. 14, 252 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gui F., Jiang J., He Z., Li L., Li Y., Deng Z., Lu Y., Wu X., Chen G., Su J., Song S., Zhang Y.-M., Yun C.-H., Huang X., Weisberg E., Zhang J., Deng X., A non-covalent inhibitor XMU-MP-3 overrides ibrutinib-resistant BtkC481S mutation in B-cell malignancies. Br. J. Pharmacol. 176, 4491–4509 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ouyang J., Yadav T., Zhang J. M., Yang H., Rheinbay E., Guo H., Haber D. A., Lan L., Zou L., RNA transcripts stimulate homologous recombination by forming DR-loops. Nature 594, 283–288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P., Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mootha V. K., Lindgren C. M., Eriksson K.-F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E., Houstis N., Daly M. J., Patterson N., Mesirov J. P., Golub T. R., Tamayo P., Spiegelman B., Lander E. S., Hirschhorn J. N., Altshuler D., Groop L. C., PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Einstein D. J., Arai S., Calagua C., Xie F., Voznesensky O., Capaldo B. J., Luffman C., Hecht J. L., Balk S. P., Sowalsky A. G., Russo J. W., Metastatic castration-resistant prostate cancer remains dependent on oncogenic drivers found in primary tumors. JCO Precis. Oncol. 5, 1514–1522 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S13
Tables S1 to S4
Legends for data S1 to S3
Data S1 to S3