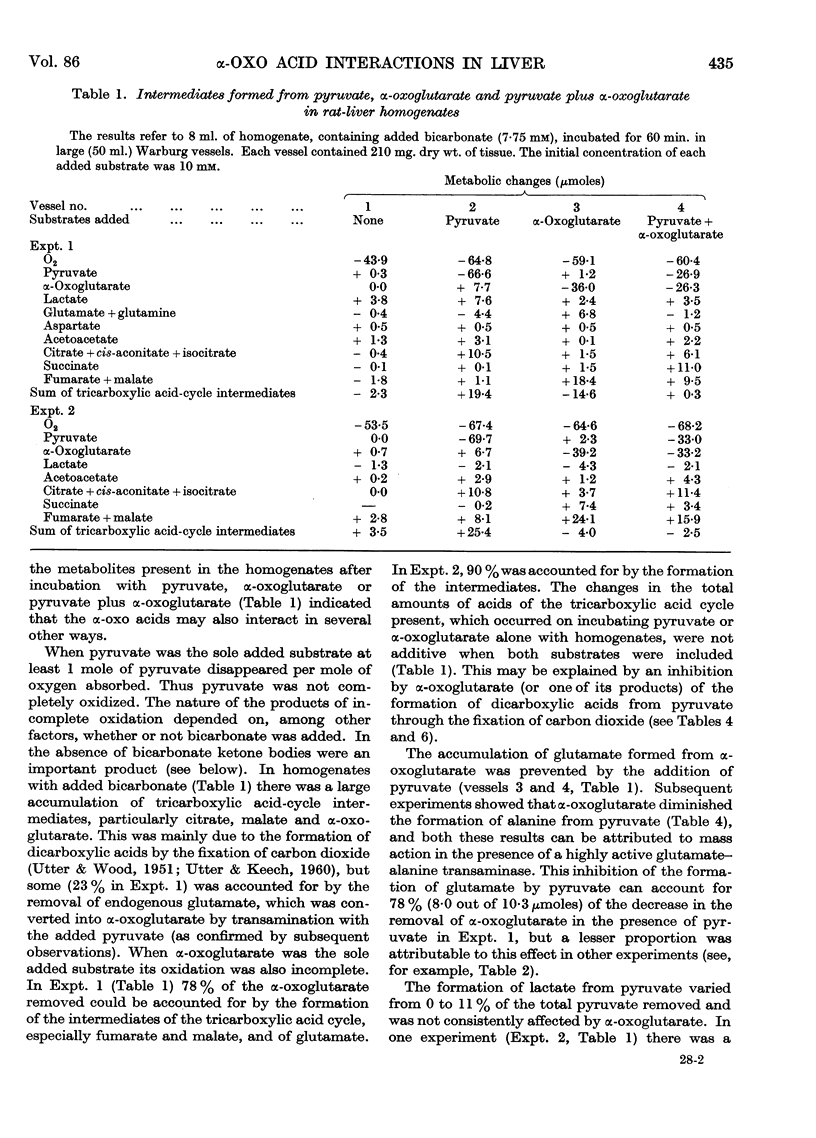
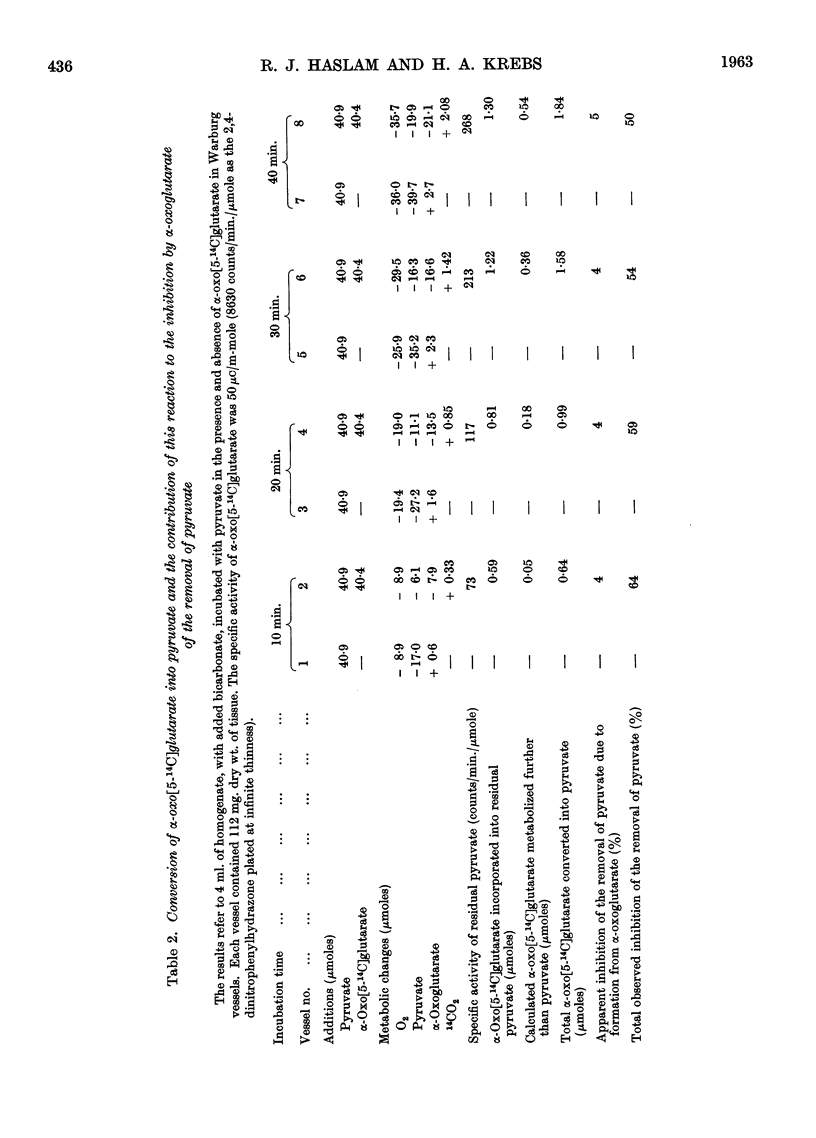
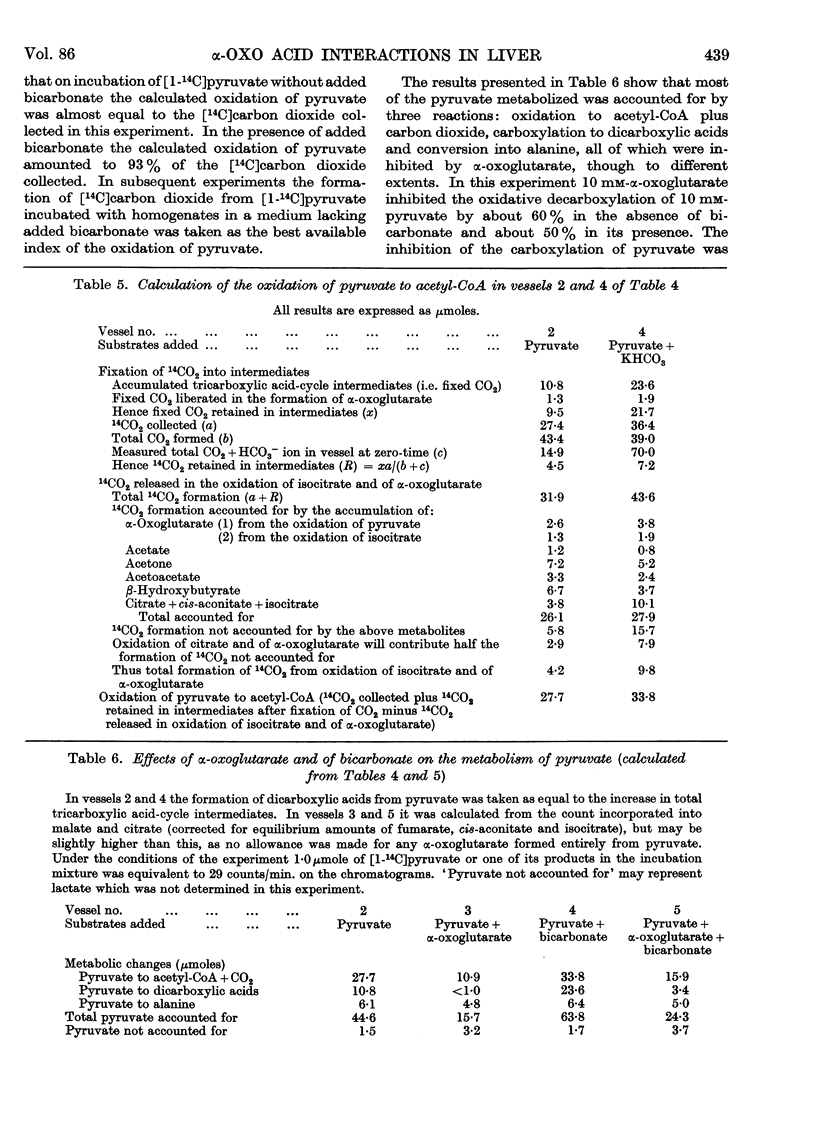
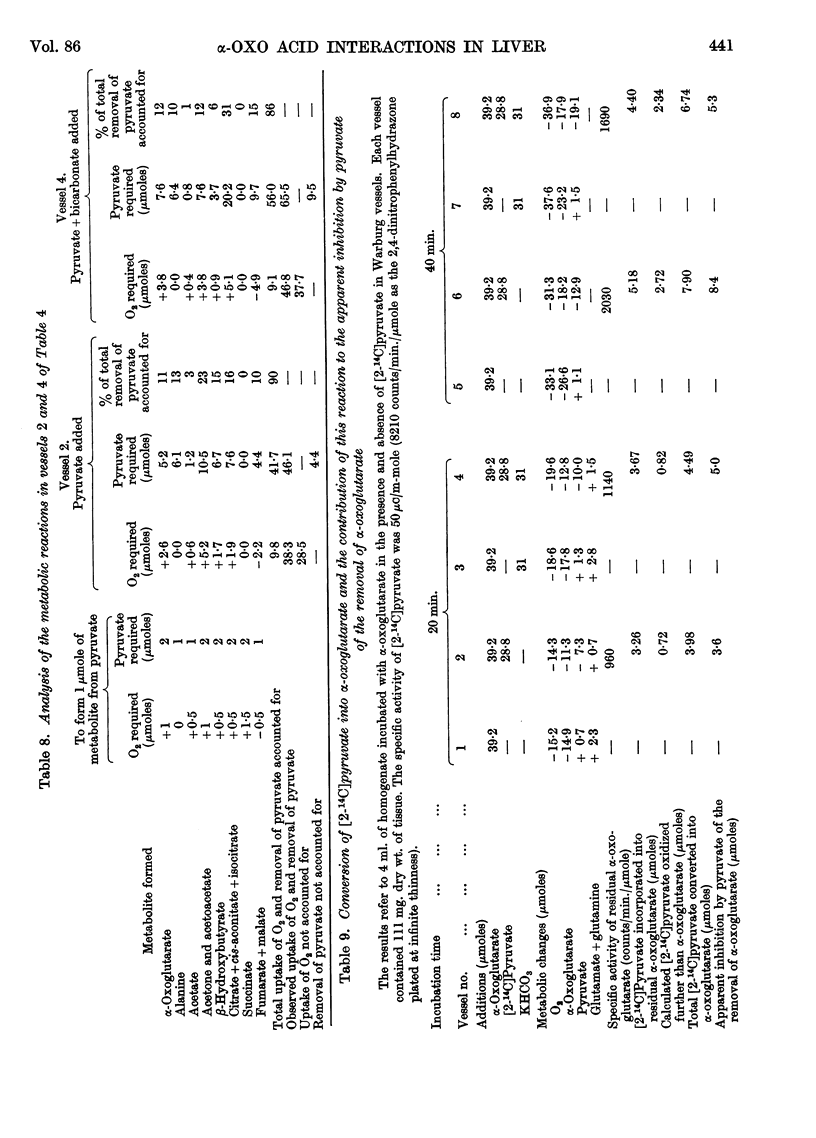
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLEY W. An effect of bicarbonate on the oxidation of pyruvate by kidney homogenates. Biochem J. 1953 Jan;53(2):305–312. doi: 10.1042/bj0530305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLEY W., DAVIES R. E. Active transport of ions by sub-cellular particles. Biochem J. 1954 May;57(1):37–49. doi: 10.1042/bj0570037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLEY W. The formation of phosphopyruvate by washed suspensions of sheep kidney particles. Biochem J. 1954 Mar;56(3):387–390. doi: 10.1042/bj0560387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERKS M., GRISOLIA S. Biosynthesis of a metabolically active citrulline-like material unrelated to carbamyl aspartate and carbamyl phosphate. Biochim Biophys Acta. 1958 Dec;30(3):663–664. doi: 10.1016/0006-3002(58)90131-8. [DOI] [PubMed] [Google Scholar]
- EL HAWARY M. F. S., THOMPSON R. H. S. Separation and estimation of blood keto acids by paper chromatography. Biochem J. 1953 Feb;53(3):340–347. doi: 10.1042/bj0530340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J. Changes in the levels of glycolytic intermediates resulting from the increase in Pasteur effect produced by alpha-ketoglutarate in aerobically-glycolyzing homogenates of the ascites tumor cell. Biochim Biophys Acta. 1961 May 27;49:596–598. doi: 10.1016/0006-3002(61)90263-3. [DOI] [PubMed] [Google Scholar]
- Edson N. L. Ketogenesis-antiketogenesis: Substrate competition in liver. Biochem J. 1936 Oct;30(10):1862–1869. doi: 10.1042/bj0301862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson N. L. Ketogenesis-antiketogenesis: The influence of ammonium chloride on ketone-body formation in liver. Biochem J. 1935 Sep;29(9):2082–2094. doi: 10.1042/bj0292082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER H., HOLLDORF A. Isolierung von D-Glycerat-dehydrogenase, einige Eigenschaften des Enzyms und seine Verwendung zur enzymatisch-optischen Bestimmung von Hydroxypyruvat neben Pyruvat. Biochem Z. 1957;329(4):292–312. [PubMed] [Google Scholar]
- JENKINS W. T., SIZER I. W. Glutamic aspartic transaminase. II. The influence of pH on absorption spectrum and enzymatic activity. J Biol Chem. 1959 May;234(5):1179–1181. [PubMed] [Google Scholar]
- KORNBERG H. L. The metabolism of C2 compounds in micro-organisms. I. The incorporation of [2-14C] acetate by Pseudomonas fluorescens, and by a Corynebacterium, grown on ammonium acetate. Biochem J. 1958 Mar;68(3):535–542. doi: 10.1042/bj0680535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BELLAMY D. The interconversion of glutamic acid and aspartic acid in respiring tissues. Biochem J. 1960 Jun;75:523–529. doi: 10.1042/bj0750523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., EGGLESTON L. V., D'ALESSANDRO A. The effect of succinate and amytal on the reduction of acetoacetate in animal tissues. Biochem J. 1961 Jun;79:537–549. doi: 10.1042/bj0790537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. Equilibria in transamination systems. Biochem J. 1953 Apr;54(1):82–86. doi: 10.1042/bj0540082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. The equilibrium constants of the fumarase and aconitase systems. Biochem J. 1953 Apr;54(1):78–82. doi: 10.1042/bj0540078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULKA R. G., KREBS H. A., EGGLESTON L. V. The reduction of acetoacetate to beta-hydroxybutyrate in animal tissues. Biochem J. 1961 Jan;78:95–106. doi: 10.1042/bj0780095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Johnson W. A. Metabolism of ketonic acids in animal tissues. Biochem J. 1937 Apr;31(4):645–660. doi: 10.1042/bj0310645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Metabolism of amino-acids: Deamination of amino-acids. Biochem J. 1935 Jul;29(7):1620–1644. doi: 10.1042/bj0291620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Quantitative determination of glutamine and glutamic acid. Biochem J. 1948;43(1):51–57. doi: 10.1042/bj0430051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Smyth D. H., Evans E. A. Determination of fumarate and malate in animal tissues. Biochem J. 1940 Jul;34(7):1041–1045. doi: 10.1042/bj0341041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER H. The ninhydrin reaction and its analytical applications. Biochem J. 1957 Oct;67(2):333–340. doi: 10.1042/bj0670333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., BARNES F. W., Jr, ENNS T., VON SCHUCHING S. Mechanisms in enzymatic transamination reaction between carbon chains of same length. Bull Johns Hopkins Hosp. 1954 Mar;94(3):117–127. [PubMed] [Google Scholar]
- NOSSAL P. M. Estimation of L-malate and fumarate by malic decarboxylase of Lactobacillus arabinosus. Biochem J. 1952 Jan;50(3):349–355. doi: 10.1042/bj0500349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODGERS K. Estimation of succinic acid in biological materials. Biochem J. 1961 Aug;80:240–244. doi: 10.1042/bj0800240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERLIN I., COTZIAS G. C. Microdiffusion of acetic acid as an assay for acetylcholinesterase. J Biol Chem. 1955 Jul;215(1):263–268. [PubMed] [Google Scholar]
- STICKLAND R. G. Some properties of the malic enzyme of pigeon liver. 1. Conversion of malate into pyruvate. Biochem J. 1959 Dec;73:646–654. doi: 10.1042/bj0730646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR T. G. A modified procedure for the microdetermination of citric acid. Biochem J. 1953 Apr;54(1):48–49. doi: 10.1042/bj0540048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIN C., ROBERTSON A. The estimation of acetone bodies. Biochem J. 1952 May;51(2):218–223. doi: 10.1042/bj0510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYLER D. B. Effect of citric acid-cycle intermediates on oxaloacetate utilization and succinate oxidation. Biochem J. 1960 Aug;76:293–297. doi: 10.1042/bj0760293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER P. G. A colorimetric method for the estimation of acetoacetate. Biochem J. 1954 Dec;58(4):699–704. doi: 10.1042/bj0580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER P. G. A colorimetric method for the estimation of acetoacetate. Biochem J. 1954 Dec;58(4):699–704. doi: 10.1042/bj0580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIELAND O. Eine optisch-enzymatische Bestimmung der L-(+)-Milchsäure mit DPN-unabhängiger Milchsäure-dehydrogenase aus Hefe. Biochem Z. 1958;329(7):568–576. [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]


