Abstract
Stent occlusion after stenting for chronic iliofemoral venous disease has an incidence of around 3% to 12% in the literature. The reasons for such occlusion vary, with patient and stent-related factors playing a role. One stent-related issue leading to stent occlusion is the use of undersized stents. Although undersized nitinol stents can be fractured and relined, this is not an option with a woven stent (eg, Wallstent). With an undersized, occluded woven stent, an option would be to bypass the occluded stent. This case report outlines the author’s experience in such a setting where a patient presented with an undersized, occluded iliofemoral venous stent with severe quality-of-life impairing symptoms, and an endovenous bypass was created around the occluded stent column using Wallstent-Z stent combination. Nine months on, the patient remains significantly better with a patent stent.
Keywords: Chronic iliofemoral venous obstruction, Iliofemoral venous stent, Stent occlusion, Venous stenting, Wallstent
Iliofemoral venous stenting for chronic iliofemoral venous obstruction has become the standard of care in symptomatic patients with quality-of-life impairment and who have failed conservative therapy, replacing open surgical procedures like the Palma-Dale procedure.1, 2, 3, 4, 5, 6 However, these patients have a 3% to 12% incidence of stent occlusion, necessitating reintervention for recurrent symptoms.7,8 The treatment for such occlusion is wire recanalization of the stent and utilization of other strategies, including angioplasty, to gain lumen within the stent to as close to the rated diameter of the stent as possible.7,9, 10, 11 However, this works only if the stent that had been placed originally was of an adequate caliber to begin with. If this is not the case, then, although a nitinol stent can be fractured and relined with appropriate caliber stents, such an option is not feasible in woven stents (ie, Wallstents). With the latter, an alternate conduit must be created if the stent cannot be angioplastied open. This involves getting wire access around the occluded stent, thus creating an alternate channel, which is then angioplastied and stented to provide an adequate caliber conduit. This case report describes such an experience where a patient previously stented with suboptimal stents went on to occlude them with severe quality of life impairing symptoms. Permission was obtained from the patient to publish this case report.
Case report
A 66-year-old Caucasian male with multiple medical comorbidities including hypertension, hyperlipidemia, B cell lymphoma (in remission), neuropathy, chronic kidney disease (baseline serum creatinine, 2.3 mg/dL), recurrent lower extremity deep vein thrombosis on anticoagulation (warfarin), in addition to a long-standing history of chronic venous insufficiency for which he underwent left iliofemoral venous stenting before referral to our clinic. His symptoms prior to placement of the original stents (Wallstents) were pain, swelling, heaviness, and tiredness of the leg. However, post stenting, the improvement was mild, and he continued to be symptomatic. Over time these symptoms worsened and imaging done at a local facility revealed an occluded stent. An attempt at recanalization was made at this facility that was unsuccessful. At the time of his presentation to our center, several years after the original stent placement, he had developed significant hyperpigmentation and lipodermatosclerosis as well (Fig 1), alongside a medial malleolar ulcer that had healed (CEAP clinical class 5; Venous Clinical Severity Score of 12). Additionally, he had begun to develop swelling and discomfort of the right leg as well, symptoms that were impairing his quality of life despite compliance with compression stockings. As part of his workup with us, he underwent a duplex ultrasound that demonstrated stent occlusion in addition to post-thrombotic changes in the femoro-popliteal segment. A computed tomography scan (Supplementary Video 1, online only) without contrast (on account of his renal dysfunction) noted sub-optimally sized stents with associated separation of the cranial and caudal stents with large cross-pelvic collaterals below the stent (Fig 2, A-C). Given his quality-of-life impairing symptoms and evidence of end-organ (skin and soft tissue) damage, it was decided to proceed with recanalization of the occluded stent column. The plan was to open up the small caliber stent to an appropriate channel, failing which, an alternate outflow route around the indwelling stent was to be created.
Fig 1.
Image demonstrative of extensive left lower extremity hyperpigmentation and lipodermatosclerosis.
Fig 2.
Computed tomography scan (without contrast) demonstrating coronal image depicting the occluded iliac vein stent (orange arrows) (A); axial image demonstrative of cross pubic collateral (B); and sagittal image noting the end of the occluded stent (orange arrow) and cross-pubic collateral (blue arrow) (C).
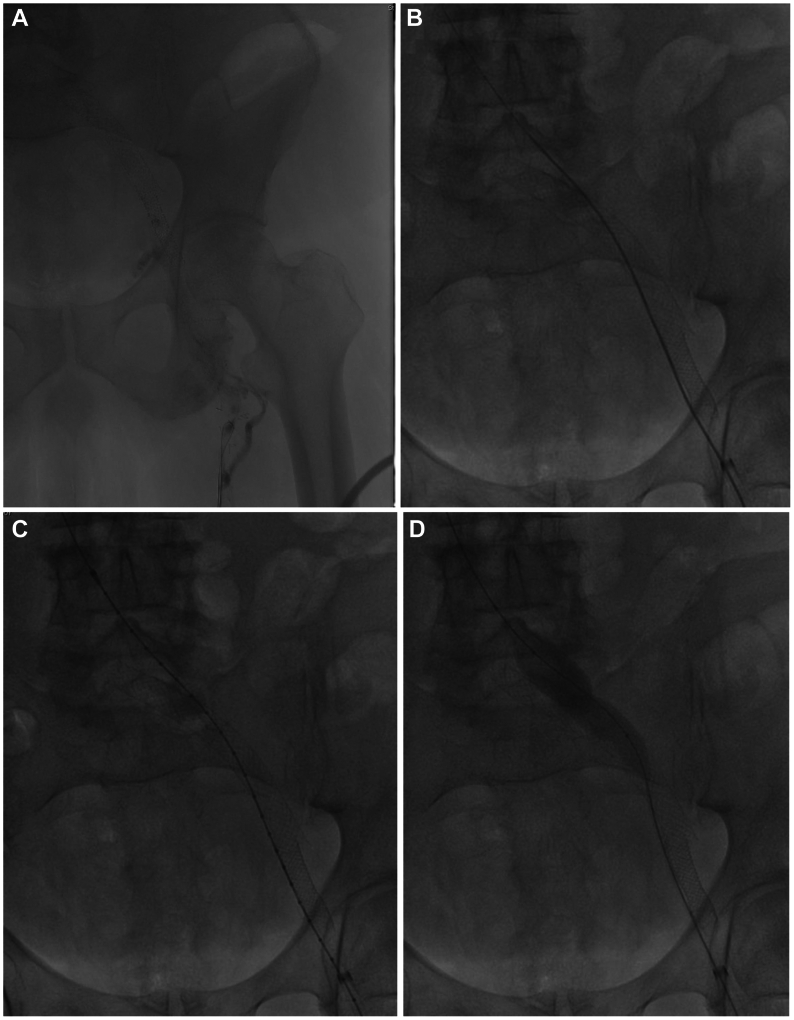
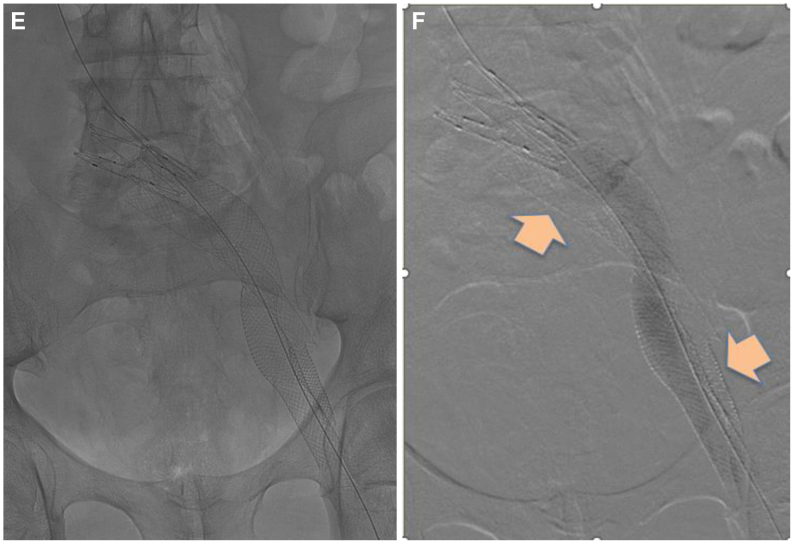
The procedure was performed under general anesthesia with preprocedural thromboprophylaxis in the form of enoxaparin (40 mg) subq and bivalirudin (75 mg) intravenously. Under ultrasound guidance, access was obtained in the midthigh femoral vein, and an 11-French access sheath was placed. A diagnostic ascending venogram of the left lower extremity and inferior vena cava was performed (Fig 3, A). This confirmed the diagnosis of an occluded iliofemoral stent column with occlusion of the caudad native common femoral vein, as well as a large cross-pubic collateral that provided outflow. An attempt was made to recanalize the stent using a 0.035 Glidewire in isolation and then with the support of an 8.5 Fr 63-cm Swartz Braided Transseptal Guiding Introducer (Abbott Labs). This enabled recanalization of the native common femoral vein and caudal-most aspect of the stent. Intravascular ultrasound (IVUS) (Visions PV .035 digital IVUS catheter, Philips) was used to confirm our location within the stent. However, no further progress up the stent could be made. Given that we were within the stent, a 2.3-mm Spectranetics laser catheter (Spectranetics Corp) was used to recanalize the cranial portion of the common femoral stent as well as the caudal external iliac portion of the stent. Before proceeding further, we attempted to open up the caudal stent initially using an 8 mm × 40 mm angioplasty balloon, and subsequently a 14 mm × 60 mm angioplasty balloon. However, despite the angioplasty, the Wallstent could not be opened up to the appropriate caliber. This was indicative of the fact that it was highly unlikely that we would have good inflow into the stent even if we were able to recanalize the remainder of the stent column. Considering this, we decided to proceed with the creation of an alternate outflow channel. Through the same access and using the angled Swartz sheath, we angled the 0.035 Glidewire around the caudal aspect of the stent, instead of into the stent, and guided the wire across the entire iliac segment and into the inferior vena cava (IVC) cranially (Fig 3, B). This required redirecting the Swartz sheath on multiple occasions so the trajectory of the Glidewire was always towards the IVC. The cranial aspect of the conduit creation required the use of sharp recanalization with the stiff end of the Glidewire to enter the IVC. IVUS interrogation was then carried out to ensure we were within the vein lumen cranially (Fig 3, C). Following such confirmation, angioplasty was then carried out using initially the 8 mm × 40 mm angioplasty balloon and subsequently an 18 mm × 60 mm angioplasty balloon across the femoro-ilio-caval segments (Fig 3, D). Stenting was then carried out to create an alternate channel using two 20 mm × 80 mm Wallstents with about 25 mm overlap. The entry point into the IVC was handled with an additional a 25 mm × 50 mm Z-stent that was deployed mostly within the cranial Wallstent to buttress this area. Stenting was thus carried out from a point of relatively good inflow to one of good outflow, from the common femoral vein to the distal IVC just cranial to the iliac confluence (Fig 3, E). Post-dilation of the entire stent column was carried out using the previously used 18 mm × 60 mm angioplasty balloon. A completion IVUS interrogation was then performed, followed by a completion venogram (Fig 3, F). Total contrast usage for the entire procedure was 6 mL of iodixanol. Once we were satisfied with the results, the 11-French sheath was withdrawn to outside the vein, and a fibrillar patch was introduced to enable hemostasis. A compressive dressing was then applied over the site after a period of manual pressure. The patient was woken up and transferred to the post-anesthesia care unit in a stable condition. Post-intervention, he was switched from warfarin to apixaban 5 mg twice daily and aspirin 81 mg daily, both to be continued long term.
Fig 3.
(A) Initial venogram demonstrating an occluded iliofemoral venous stent; (B) Successful wire recanalization around the pre-existing stent; (C) Intravascular ultrasound (IVUS) confirmation of entry into caudal inferior vena cava; (D) Sequential angioplasty of endobypass tract; (E) Fluoroscopic image depicting the configuration of the original stent and the new conduit around it. (F) Completion venogram depicting a patent endobypass conduit of Wallstent-Z stent combination with orange arrow depicting the occluded original stent column.
Post-stenting, he had significant improvement of his symptoms with the Venous Clinical Severity Score improving from a preoperative score of 12 to 6 post-stenting and the grade of swelling going from 4 (swelling involving the leg up above the knee) to 0 (no swelling). He noted improvement in the right leg swelling and pain as well. At 9 months post-procedure, he continues to do well without any limiting symptoms/signs and a widely patent stent. He will have lifelong follow-up duplex ultrasound exams alongside clinical visits.
Discussion
Femoroiliocaval stenting has become the first line of treatment for patients presenting with quality-of-life impairing symptoms of chronic iliofemoral venous obstruction that have failed conservative therapy. This has led to the increased utilization of stenting over the past decade, with a consequent overall increase in stent-related complications. Although techniques currently exist for the treatment of in-stent restenosis, stent compression, or stent occlusion, they are not uniformly successful.12 Additionally, when undersized stents are used, the options available to treat the indwelling stent are limited. Although this is clearly an avoidable problem, it continues to persist. Moreover, leaving behind residual stenosis when treating in-stent restenosis, stent compression, or stent occlusion leads to residual venous hypertension and persistent symptoms or early recurrence of symptoms. So, in patients in whom it is not possible to restore an adequate luminal caliber, consideration should be given to the creation of an alternate outflow tract. This is best created endovascularly, given the problems associated with open bypass, especially the short patency associated with longer bypasses (eg, femoro-caval).13 The use of an endovenous conduit helps mitigate these issues. Given that veins can withstand much dilation without rupture, an endovenous bypass offers promise. In our patient, we were able to create a femoro-caval bypass from an area of relatively good inflow to an area of good outflow using bare metal woven stents (Wallstents). Given the nature of these stents, the risk of leak/rupture is minimal. We proceeded down this path only after attempting to salvage his indwelling stent. Thorough knowledge of the patient’s anatomy, familiarity with the use of angulated sheaths, frequent use of IVUS to provide guidance, a healthy comfort level with sharp recanalization techniques, and knowing when to quit are all important when deciding to pursue this technique. It is conceivable that such endovenous bypasses could be used to replicate Palma-Dale and May-Husni open bypasses as well. However, only when such bypasses have been successfully attempted with long-term follow-up, will we be truly able to assess the role of their role. For now, it represents an option in patients with quality-of-life impairing symptoms where no other choice exists.
Conclusion
Endovenous bypass is an option in symptomatic patients with unsalvageable occluded iliofemoral venous stents. Although the technique appears to be safe and effective, further study is required to corroborate these findings.
Funding
None.
Disclosures
None.
Footnotes
Additional material for this article may be found online at www.jvscit.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Appendix
Additional material for this article may be found online at www.jvscit.org.
Appendix (online only)
Computed tomography scan (abdomen-pelvis) of the patient before intervention to create the endovenous bypass.
References
- 1.Neglen P., Hollis K.C., Olivier J., Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 2.Hartung O., Loundou A.D., Barthelemy P., Arnoux D., Boufi M., Alimi Y.S. Endovascular management of chronic disabling ilio-caval obstructive lesions: long-term results. Eur J Vasc Endovasc Surg. 2009;38:118–124. doi: 10.1016/j.ejvs.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Gutzeit A., Zollikofer Ch L., Dettling-Pizzolato M., Graf N., Largiader J., Binkert C.A. Endovascular stent treatment for symptomatic benign iliofemoral venous occlusive disease: long-term results 1987-2009. Cardiovasc Intervent Radiol. 2011;34:542–549. doi: 10.1007/s00270-010-9927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seager M.J., Busuttil A., Dharmarajah B., Davies A.H. Editor's choice - a systematic review of endovenous stenting in chronic venous disease secondary to iliac vein obstruction. Eur J Vasc Endovasc Surg. 2016;51:100–120. doi: 10.1016/j.ejvs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Ye K., Lu X., Li W., et al. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol. 2012;23:497–502. doi: 10.1016/j.jvir.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Jayaraj A., Noel C., Kuykendall R., Raju S. Long-term outcomes following use of a composite Wallstent-Z stent approach to iliofemoral venous stenting. J Vasc Surg Venous Lymphat Disord. 2021;9:393–400.e2. doi: 10.1016/j.jvsv.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Jayaraj A., Crim W., Knight A., Raju S. Characteristics and outcomes of stent occlusion after iliocaval stenting. J Vasc Surg Venous Lymphat Disord. 2019;7:56–64. doi: 10.1016/j.jvsv.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Badesha A.S., Black S.A., Khan G., et al. A meta-analysis of the medium- to long-term outcomes in patients with chronic deep venous disease treated with dedicated venous stents. J Vasc Surg Venous Lymphat Disord. 2024;12 doi: 10.1016/j.jvsv.2023.101722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raju S., Knight A., Buck W., May C., Jayaraj A. Caliber-targeted reinterventional overdilation of iliac vein Wallstents. J Vasc Surg Venous Lymphat Disord. 2019;7:184–194. doi: 10.1016/j.jvsv.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Jayaraj A., Fuller R., Raju S. Role of laser ablation in recalcitrant instent restenosis post iliofemoral venous stenting. J Vasc Surg Cases Innov Tech. 2021;7:298–301. doi: 10.1016/j.jvscit.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montoya C., Polania-Sandoval C., Almeida J.I. Endovascular mechanical thrombectomy of iliofemoral venous stent occlusion with the novel RevCore thrombectomy system: case reports and literature review. J Vasc Surg Cases Innov Tech. 2024;10 doi: 10.1016/j.jvscit.2024.101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayaraj A., Fuller R., Raju S., Stafford J. In-stent restenosis and stent compression following stenting for chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2022;10:42–51. doi: 10.1016/j.jvsv.2021.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Jayaraj A., Gloviczki P. In: Handbook of venous Disorders - Guidelines of the American venous Forum. 5th ed. Gloviczki P., editor. Taylor and Francis Group - CRC Press; 2024. Open surgical reconstructions for non-malignant occlusion of the inferior vena cava and iliofemoral veins; pp. 553–562. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Computed tomography scan (abdomen-pelvis) of the patient before intervention to create the endovenous bypass.