Abstract
In this study, the impact of light-emitting diodes (LEDs) in different spectrums was investigated on the seed germination and post-germinative performance of Capsicum frutescens seedlings. The seeds were exposed to different LED lights (full spectrum, white, red, blue, and red-blue) for 0, 1, 2, and 4 h (h). The seeds were placed for a week in darkness to investigate germination, and then the growth mechanisms were studied in four-week-old seedlings. Results indicated that germination percentage was promoted markedly under 2 h red and full lights and also in 1 h blue, which was accompanied by the regulation of H2O2 level and NADPH oxidase (NOX) activity. Sprout growth and height were more heightened under 2 h red light, but their contents decreased considerably under blue light with a rising incubation time. Red light induced more biomass yield, chlorophyll (Chl) pigments, Chl a/b ratio and florescence in four-week-old seedlings. Blue light also increased Chl pigments, but decreased biomass yield by enhancing malondialdehyde (MDA) level. Increased growth in seedlings treated to red light was associated with upregulating Rubisco gene expressions (rbcL and rbcS) and its activity. Red and red-blue lights promoted the activity of superoxide dismutase, glutathione reductase, and ascorbate peroxidase enzymes to increase ascorbic acid (ASA) production in the ascorbate–glutathione cycle. Total phenolic (0.22 mg DAG g− 1 DW), ASA (89.58 mg 100 g− 1 FW) and capsaicinoids (2.73 mg g− 1 DW) contents were heightened under red light, while carotenoid (11.78 µg g− 1 FW) content was more accumulated under blue light. The findings of this study suggest red light modulates NOX activity and H2O2 level for inducing seed germination and seedling quality in C. frutescens, which can create important implications for the production of antioxidant metabolites and increase the cultivation area of this plant.
Keywords: Capsicum frutescens, Capsaicinoid, Light emitting diodes (LEDs), NOX activity, Rubisco
Introduction
Capsicum frutescens var. red chili (hot pepper) is an economical, nutritional, and medicinal plant from the family Solanaceae. It is originated from Central America, South of the United States of America and Mexico, and is cultivated in the northern areas of Iran. It is used traditionally in the food and treatment of parasitic infections, toothache, cough, and rheumatism diseases [1]. Pepper is a rich source of colors and antioxidants due to the existence of various compounds such as phenolic, capsaicinoids, terpenes, carotenoids, and vitamin C. Recent studies have shown that capsaicin extract has various biological activities including antioxidant, antimicrobial, cytotoxicity, and anticancer properties [2, 3]. There is a high demand for capsaicin, the main compound of C. frutescens due to its pharmaceutical properties. Many researchers have studied the impact of various factors on capsaicinoid production, including temperature, fertilizer, drought and salinity stresses, chili genotype, mechanical injury, etc [4, 5]. Among all the factors, adequate light can play an important role in plant performance and inducing secondary metabolism. In chili pepper, the full sun showed a 17 and 67% rise in fruit biomass comparing to 50% and 30% sunlight, respectively [6]. In C. chinense (the cultivar of Akanee Pirote), the highest capsaicinoid content (4.820 mg per plant) was obtained in seedlings treated with 50% shading [7].
In addition to sunlight, light-emitting diodes (LEDs) are applied for indoor agriculture, like greenhouse or in vitro culture for plant regenerations and inducing antioxidant metabolites because of their low-energy consumptions, low-cost, and emission of specific wavelength based on plant type being cultivated [8, 9]. For example, Sanoubar et al. [10] mentioned that the red-blue light stimulated seed germination and seedling growth in Artemisia and Chenopodium as compared to fluorescent light. Dong et al. [11] reported that white-red LED light enhanced the harvesting index in Triticum aestivum by decreasing lignin in inedible biomass. The flowering rate, gene expression, and indican content were promoted markedly in Polygonum tinctorium seedlings under blue LED [12]. Moreover, it has been stated that LED lights could influence antioxidative enzyme activities and gene expressions [13]. Until now, few investigations have been done about the impact of various light spectrums on Capsicum species. In C. annuum, blue and red-blue lights stimulated biomass yield, photosynthetic electron transport capacity, and the expression of enzymes related to the Calvin cycle [13]. In Capsicum annuum, applying blue light to seedlings increased considerably capsaicin (43%), dihydrocapsaicin (56%), and homocapsaicin (28%) contents in fruits compared to the control [14]. In C. frutescens, red-blue light induced dry weight, root and shoot lengths as compared to fluorescent lamps [15].
Seed germination is a complex biological process and is affected by genetic, many internal, and environmental factors. Most plants can germinate in the presence of light and use different phytochromes to recognize the specific wavelength to regulate germination and seedling growth [16, 17]. Seed priming is a simple and low-cost method that can stimulate seed germination and subsequent performance of the obtained seedlings by inducing signaling molecules, related gene expressions and enzyme activities [10, 18]. Some recent studies show that seed priming to LED light can act as a metabolite inducer in seedlings. In Stevia rebaudiana, blue LED light promoted seed germination and increased the growth yield, carotenoids and phenolics in 4-week-old plantlets [19]. In C. frutescens, only the germination percentage between different cultivars has been studied which was between 42.15 and 91.95% under darkness [20], and seed priming to LED lights was not investigated.
Light is an important factor in adjusting ROS signaling and H2O2 generation. ROSs can act as decisive mediators of metabolic and molecular events and regulate cell growth. Plasma membrane NADPH oxidases (NOXs) are oxidoreductases that catalyze the one-electron reduction of molecular oxygen (O2) into superoxide anions (O2•-) and oxidize NADPH to NADP+. NOX-generated superoxide is converted to other ROSs [21, 22], which are important signaling molecules in various developmental processes such as root growth, pollen tube tip growth, seed germination, and also in stress tolerance [22–25]. NOX enzymes participate in cell differentiation, cellular defense system, gene expressions, and cell signaling [26]. In diatoms, NOX activity was related to growth phases, and continuous light increased its activity [27]. According to the literature reviews, the impact of light quality on the regulation of NOX activity is not investigated in plants, and it is necessary to study. So, the purpose of this research was to examine the impact of various light qualities in a time-dependent manner on the NOX activity and H2O2 generation at the germination stage; the quality of obtained seedlings was also investigated by Chl pigments and fluorescence, Rubisco activity, enzymatic and non-enzymatic antioxidants in C. frutescens. This study can provide a comprehensive understanding of the mechanisms regulating germination and its impact on seedling quality by investigating the adaptive responses.
Materials and methods
Plant material and growth conditions
C. frutescens var. red chili (a local variety of Isfahan) seeds were bought from Pakan Bazr company and then were disinfected in hypochlorite sodium solution (10% v/v, 10 min), ethanol (70% (v/v), 1 min), sulfuric acid (40% (v/v), 7 min), and finally were washed in sterile distilled water for three times, respectively. The disinfected seeds are placed on sterilized filter paper and put on the shelves (30 cm width × 50 cm depth ×50 cm height) equipped with Linear LED Wall Washer at different LED light spectrums, including full spectrum (100%, 400–840 nm) as a control, cool white (100%, 6000–6500 K), blue (460 nm, 100%), red (660 nm, 100%), and red-blue (3:1 ratio, 75% red, 25% blue) for 0 (dark), 1, 2, and 4 h and then were wrapped with aluminium foil and placed for a week in darkness at 25 ± 2 °C temperature [28] and 55% relative humidity. For each LED light treatment, five Petri dishes were applied. After incubation time, the germination percentage and growth parameters of treatments were compared. After detection of the optimum incubation time (2 h), sprouts were transferred in Hoagland medium until 4 weeks under full spectrum light with 16/8 h (light/dark) to test whether the priming lighting time can be maintained for an extended period of time and the physiological parameters and antioxidant metabolism were compared. The distance between LED light sources and samples was arranged to have a photosynthetic photon flux (PPF) at the intensity of 70 µmol m− 2s− 1. The intensity of LED light was measured by LI-190R device (LI-COR Inc, Lincoln, NE, USA). The relative spectral distribution of LED spectrums is shown in Fig. 1. Four to five samples for each treatment were harvested for physiological, biochemical, and molecular analyses, and the experiments were conducted twice.
Fig. 1.
Relative spectral distribution of different LED spectrums (full spectrum), white, red, blue, and red-blue (3:1)
Germination percentage and seedling growth
After 7 days of seed culture, germination percentage was detected in Petri dishes containing 20 seeds at four replications under different LED spectrums.
The dry weight of seedlings was obtained in an oven at 65 °C until containing the constant mass (dry weight).
NOX activity and H2O2 content
The plasma membrane fractions were isolated from fresh sprouts (0.5 g) based on the procedure of Jiang and Zhang [29]. NOX activity was evaluated using the reaction mixture of 50 mM TrisHCl buffer (pH 7.5), 0.5 mM 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2-tetrazolium 5-carboxanilide (XTT), and 100 µM NADPH. XTT reduction was measured at 470 nm in the presence and absence of 50 units of SOD [30].
H2O2 level was assayed by the procedure of Velikova et al. [31]. Sprout fresh weight (0.4 g) was homogenized in 5 ml cold trichloroacetic acid (TCA, 0.1% (w/v) in an ice bath and certificated at 12,000×g for 10 min at 4 C˚. The reaction solution was 0.5 mL potassium phosphate buffer (pH 7.0, 10 mM), 1 mL potassium iodide (KI, 1 M), and 0.2 ml extract. The absorbance was read by a UV–vis spectrophotometer (UNICO, 4802, USA) at 390 nm and calculated by the standard curve of H2O2.
Chl pigments and fluorescence
Chl pigments and carotenoids were quantified using the procedure explained by Arnon [32]. Fresh leaves (0.25 g) were homogenized in 80% (v/v) acetone, and after centrifugation (5000×g for 5 min), the absorbance was read spectrophotometrically at 470, 646, and 663 nm, and their contents were calculated using the formula explained by Wellburn [33].
Chl a = 12.21 A663–2.81 A646.
Chl b = 20.13 A646–5.03 A663.
Total Car = 1(000 A470–3.27 Chl a – 104 Chl b)/198.
The maximum quantum yield of PSII photochemistry (Fv/Fm) value was determined in leaves, which had been previously located in darkness for 30 min with a photosynthesis system (Handy- PEA (Plant Efficiency Analyser) Instrument, Hansatech) according to the procedure of Ben Saad et al. [34]. The transients were induced by 1− s illumination with an array of light-emitting diodes and homogeneous irradiation over a 4 mm diameter leaf area. The fast fluorescence kinetics (F0 to Fm) was recorded from 10µs to 1s. The fluorescence intensity at 50µs was considered as F0.
Malondialdehyde (MDA) and antioxidant enzyme activities
To determine MDA, Fresh leaves (0.5 g) were added to 10% TCA and extracted by centrifugation at 4000 rpm for 10 min. Afterwards, the supernatant (2 mL) was added to the reaction mixture of 0.6% TBA in 10% TCA, incubated in a water bath (100 ◦C temperature) for 20 min, cooled, and centrifuged. The absorption was recorded at 450, 532, and 600 nm [35].
The fresh leaves (0.25 g) were extracted with 2 mL of cold extraction buffer (1 M Tris-HCl, pH 6.8). The supernatant was used for antioxidant enzyme analyses. Superoxide dismutase (SOD) activity was evaluated by quantifying the inhibition of nitroblue tetrazolium reduction, as described by Giannopolitis and Ries [36]. The reaction mixture was nitroblue tetrazolium (NBT, 75 µM), 0.1 mM ethylenediaminetetraacetic acid (EDTA, 0.1 mM), potassium phosphate buffer (50 mM), 13 mM methionine, 75 µM riboflavin, and 150 µl of enzyme extract. The reaction mixture was irradiated for 10 min, and then the absorbance of NBT reduction was recorded at 560 nm. One unit of SOD activity was defined as the amount of enzyme which caused 50% inhibition in NBT reduction. Ascorbate peroxidase (APX) activity was assayed by a decline in the absorbance of oxidized ascorbate at 290 nm, according to Jebara et al. [37]. The reaction mixture (1 ml) was 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM H2O2, 0.5 mM ascorbate, and 10 µl enzyme extract. The addition of H2O2 started the reaction, and ascorbate oxidation was measured at 290 nm for 1 min. Enzyme activity was quantified using the molar extinction coefficient for ascorbate (2.8 mM− 1 cm− 1), and the results were expressed as 1 µM of ascorbate oxidized per min per mg protein at 25 ± 2_C. The activity of glutathione reductase (GR) was measured at 340 nm by the rate of NADPH oxidation. The reaction solution was oxidized glutathione (1mM) in potassium phosphate buffer (50mM, pH 8.2), EDTA (1mM), and NADPH (0.2mM) in the final volume of 1mL [38].
Rubisco activity, gene expression, and total carbohydrate content
For quantification of Rubisco activity, fresh leaves were extracted with 2 mL cold buffer (0.1 M Tris-HCl, pH 7.8), EDTA (0.0025 M), MgCl2 (0.05 M), and 37.5 mg of dithiothreitol (DTT), and its activity was detected by the procedure of Usuda et al. [39]. The reaction mixture was enzyme extract (25 µL) and assay mixture (925 µL) containing 40 mM NaHCO3,100 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 0.2 mM NADH, 5 mM DTT, 4 mM ATP, 0.2 mM EDTA, 0.2 mM ribulose1,5-bisphosphate, 3-phosphoglyceratekinase (1 Unite), and glyceraldehyde- 3-phosphodehydrogenase (1 Unite). After 10 min, the absorption was obtained at 340 nm, and the enzyme activity was displayed as µmol CO2 mg− 1 protein min− 1.
Semi-quantitative RT-PCR was applied for the determination of the expressions of Rubisco subunits (rbcL and rbcS). The Oligo 5 software was used to design specific primers (Table 1). Total RNA was extracted from leaves through RNX-P reagent based on the instructions recommended by the manufacturer. The cDNA was prepared through a cDNA synthesis kit (Pishgam, Iran) according to the protocol. The conditions of polymerase chain reaction (PCR) were improved for each primer, as displayed in Table 1. Products of PCR were monitored on agarose gel electrophoresis and the nucleotide sequences were confirmed by the biotechnological company of Macrogen in Seoul, Korea. Relative gene expression was evaluated by Total Lab software, and normalization was conducted based on the positive control actin.
Table 1.
Sequences of primer pairs used in the sqRT-PCR analysis
| Gene and accession no. | Size (bp) | Primer sequences | PCR conditions |
|---|---|---|---|
| rbcL (HF572816.1) | 561 |
Forward primer 5’- TCCTGAGTACCAAACCAA-3’ |
94 °C/2 min; 94 °C/50 s, 54 °C/50 s, 72 °C/1 min, 35cycles; 72 °C/7 min |
|
Reverse primer 5’- CGCATAAATGGTTGTGAG − 3’ |
|||
| rbcS (KC176707.1) | 664 |
Forward primer 5’- AGGAAAGCATAGCAGTAG − 3’ |
94 °C/2 min; 94 °C/50 s, 52 °C/50 s, 72 °C/1 min, 35cycles; 72 °C/7 min |
|
Reverse primer 5’- GAAAAAACTTGAGGAGGA − 3’ |
|||
| Actin (XM026571753.1) | 600 |
Forward primer 5’-GGAGAAGATTTGGCATCACAC TTTCTACAATGAG-3’ |
94 °C/2 min; 94 °C/50 s, 54 °C/50 s, 72 °C/1 min, 35cycles; 72 °C/7 min |
|
Reverse primer 5’-CTTCCTGATATCCACAATCAC ACTTCATGATGG-3’ |
For determination of total carbohydrate content, the dried leaves (0.5 g) were homogenized in 3 mL distilled water. After centrifugation (5000×g for 7 min), 0.5 mL of extract was added to 0.5 mL of 5% phenol. Then, 2.5 mL of 97% sulfuric acid was added, and the reaction solution was mixed quickly. The solution absorption was measured at 485 nm after 30 min [40]. Total carbohydrate content was calculated by a glucose standard curve.
Total capsaicinoid, phenolic, and ascorbic acid (ASA) contents
Capsaicinoid extraction was conducted by the procedure of González-Zamora et al. [41]. Dried leaf (0.5 g) was extracted with 10 mL acetonitrile at 65˚C for 15 min under sonication (40 kHz). After centrifugation (10000 × g, 10 min), the supernatant evaporated to dryness at 60 °C, re-suspended in acetonitrile (2 mL), and centrifuged again. The absorption was read at 650 nm, and total capsaicinoids were quantified with capsaicin as standard, and the coefficient connected with the Scoville heat value (1.6 × 107) for capsaicin.
Total phenolics were evaluated by the procedure explained by Singleton and Rossi [42] with some modifications. Dried leaves (0.1 g) were homogenized in 3 ml methanol (80%, v/v) and then centrifugated at 5000× g for 5 mine. The 0.5 ml methanolic extract was added to 0.5 ml Folin–Ciocalteu reagent (10%), followed by mixing with 500 µl sodium carbonate (10%) and placed in the dark condition for 30 min. The absorption was measured at 725 nm, and its content was calculated by a gallic acid standard curve.
Fresh leaf tissue (1 g) was homogenized in 5 ml TCA 10% (w/v), and the supernatant was used for quantification of ASA spectrophotometrically at 525 nm according to the method explained by Law et al. [43]. The reaction mixture was 50 µL supernatant, EDTA (5 mM), FeCl3 (16 mM) provided in potassium phosphate buffer (100 Mm, pH 7.5), 1.7% TCA, 7.6% O-phosphoric acid, and 44 mM bipyridyl. After 45 min at 40 °C, the absorption was read at 525 nm. Ascorbate content was acquired using a standard curve of ASA.
Statistical analysis
Data analyses were conducted through the One-way ANOVA test with SPSS, version 21.0. The experiments were performed with four or five replications for growth and other analyses. Duncan’s multiple range test acquired the statistical difference between means. The principal component analysis (PCA) test for sprout growth and germination was conducted by the XLSTAT 2021.2.2 software. Hierarchical cluster analysis (HCA) and partial least squares discriminate analysis (PLS-DA) on the data obtained from seedlings were obtained by MetaboAnalyst software on the website (https:\\www.metaboanalyst.ca), and the Pearson correlation coefficient compared all data.
Results
Seed germination and growth parameters
Different LED lights showed various germination responses in C. frutescens (Fig. 2a). Seeds incubated in 2 h full and red lights showed a 2-fold rise for germination percentage comparing to control. Blue light after 1 h also promoted the germination percentage with a 2.13-fold raise comparing to control, but its percentage decreased gradually with raising the incubation time. White and red-blue light showed a similar trend for germination percentage, and the maximum percentage was obtained after 2 h white and red-blue lights with 1.39 and 1.47-fold raise comparing to control, and then its content decreased slightly after 4 h incubation time (Fig. 3a).
Fig. 2.
Depiction of C. frutescens sprouts under 2 h various LED lights (a), the effect of various LED lights at different incubation times (0, 1, 2, and 4 h) on the sprout growth (a) and length (b) in C. frutescens. The scale bar was 4 cm. Values are means ± SE of five replicates. Different letters indicated significant (p ≤ 0.05) differences
Fig. 3.
Effect of various LED lights at different incubation times (0, 1, 2, and 4 h) on the seed germination (a), H2O2 (b), and NOX activity (c) in C. frutescens sprouts. Values are means ± SE of five replicates for germination and four replicates for others. Different letters indicated significant (p ≤ 0.05) differences
Seeds incubated in 2 h full and red lights showed a potential response for sprout growth and showed a 2.17 and 2.65-fold rise in biomass yield comparing to their controls. Increasing incubation time (4 h) caused a 16.27 and 24.48% decline of fresh weight in full and red treatments comparing to 2 h, respectively. However, blue light declined sprout growth with rising incubation time and the lowest growth was observed after 4 h of applying light treatment (Fig. 2b).
Sprout length changed significantly under various LED lights and incubation time as similar to sprout biomass (Fig. 1c). As priming seeds to 2 h full and red lights maximized sprout length with a 2.8-fold raise comparing to control. Blue light showed a logarithmic decline in sprout length with increasing incubation time, and a 4.2-fold decline was observed after 2 h (Fig. 2c).
H2O2 level and NOX activity
H2O2 content increased in sprouts with increasing incubation time under various LED lights (Fig. 3b). Blue light more promoted H2O2 generation comparing to the other lights. The highest H2O2 accumulation was obtained after 4 h of blue light with a 4.7-fold raise comparing to the control. Moreover, white and red-blue lights induced H2O2 content with increasing incubation time, but its accumulation was significantly lower than blue light after 4 h.
NOX activity increased significantly under various LED lights. Blue light (2 and 4 h incubation time) and red-blue light (4 h) heightened markedly NOX activity in sprouts that were in agreement with the results of H2O2 accumulation in these treatments (Fig. 3c). No light condition (0 h) showed the minimum NOX and H2O2 level comparing to the light treatment.
Biomass, photosynthetic pigments, and Fv/Fm
The dry weight changed under LED lights in four-week-old seedlings. The red (53.4%) and red-blue (25.8%) lights showed a potential impact on the biomass yield comparing to the full light. However, seedlings treated to blue light showed the lowest biomass with a 93.5% decline comparing to full light.
Chl a and b contents changed under various LED lights in seedlings. As red and blue lights more promoted Chl a and b synthesis in seedling leaves, although the stimulatory impact of red light on Chl a pigment (41.9%) was slightly higher than blue light (40.3%). However, seedlings treated to white light showed the lowest Chl a (23.3%) and b (25.6%) pigments comparing to control. The ratio of Chl a/b was heightened in seedlings primed with red and red-blue lights (Fig. 4 and 5. There was no significant alteration between blue and white treatments with the control sample for Chl ratio.
Fig. 4.

PCA plot of various light factors at different times on the seed germination, sprout growth parameters, and redox responses of C. frutescens. NOX–NADPH oxidase activity
Fig. 5.
Depiction of four-week-old seedlings treated to various LED lights at 2 h incubation time (a), Effect of LED lights on the dry weight (b), chlorophyll pigments (c), Chlorophyll a/b ratio (d), and Fv/Fm (e) contents in four-week-old seedlings of C. frutescens. The scale bar was 6 cm. Values are means ± SE of four replicates. Different letters indicated significant (p ≤ 0.05) differences
Red (27.4%) and red-blue (14.5%) lights increased significantly Fv/Fm compared to control; however, its content decreased considerably under blue light (26.5%). The results of Fv/Fm were in accordance with the dry weight responses under different LED lights (Fig. 5d).
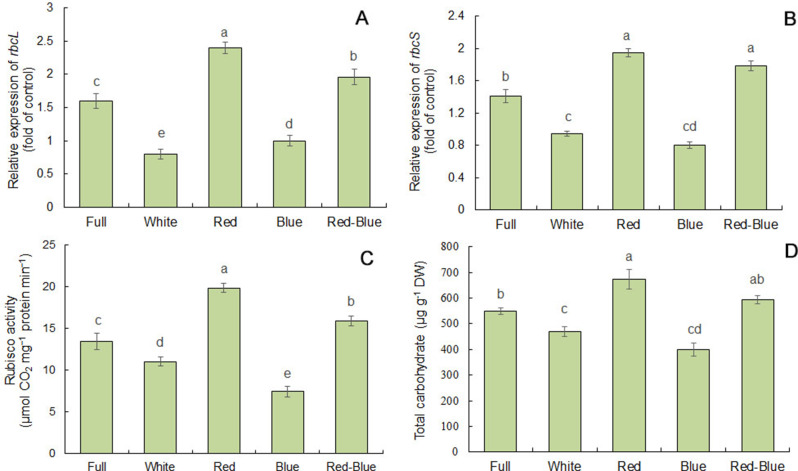
Rubisco gene expressions, Rubisco activity, and carbohydrate content
Alterations in gene expressions of large and small subunits of Rubisco (rbcL and rbcS, respectively) were studied using sqRT-PCR under LED lights. The rbcL gene was upregulated in four-week-old seedlings under red light (50%) and red-blue light (22.5%) comparing to control (Fig. 6a). However, seedlings treated to blue and white lights showed a marked decline (1.6 and 2-folds) in rbcL expression comparing to control, respectively.
Fig. 6.
Effect of LED lights on the gene expressions of rbcL (a), rbcS (b), Rubisco activity (c), and total carbohydrate (d) content in four-week-old seedlings of C. frutescens. Values are means ± SE of four replicates. Different letters indicated significant (p ≤ 0.05) differences
The maximum rbcS gene expression observed in seedlings treated with red (35.7%) and followed by red-blue (27.14%) lights in C. frutescens comparing to control (Fig. 6b). However, white treatment decreased markedly the rbcS transcript accumulation (a 2.1-fold decline) comparing to the control.
Rubisco activity was increased considerably in seedlings treated to red light (23.8%) comparing to control, but its activity showed a 21.8 and 81.2% decline under white and blue lights, respectively (Fig. 6c). The result of Rubisco activity was in accordance with dry weight in C. frutescens seedlings.
Total carbohydrates showed a significant alteration in seedlings, with a 17.1 and 37.6% decline under white and blue lights, respectively. Red light significantly induced total carbohydrates (22.9%) in C. frutescens comparing to the control (Fig. 6d).
MDA and antioxidant enzymes
MDA level changed variously under light quality (Fig. 7). Red light diminished the MDA level with a 21.05% decline comparing to full light. However, seedlings treated to blue light showed a 39.1% rise comparing to full light.
Fig. 7.
Effect of LED lights on the MDA (a) content, SOD (b), APX (c), and GR (d) activities in four-week-old seedlings of C. frutescens. Values are means ± SE of four replicates. Different letters indicated significant (p ≤ 0.05) differences
The activity of some antioxidant enzymes related to the ascorbate–glutathione (ASA-GSH) pathway, including SOD, APX, and GR activities, were investigated under various LED lights. Red and red-blue lights showed the maximum SOD activity with a 29.8 and 25.7% raise comparing to full light. However, seedlings treated to white lights displayed the lowest SOD activity, with a 33.2% decline comparing to control.
APX activity showed a significant rise in seedlings treated to red light, with a 43.6% rise comparing to control. Seedlings treated to blue light showed the lowest APX activity with a 2.5-fold decline comparing to the control.
Seedlings treated to red and red-blue light showed the maximum GR activity. However, the activity of GR decreased in white and blue light with a 20.1 and 33.1% decline comparing to the control, respectively.
ASA, carotenoids, phenolics, and capsaicinoid contents
ASA content reached to maximum level in seedlings treated with red (89.58 mg 100 g− 1 FW) and red-blue (82.45 mg 100 g− 1 FW) lights, while its content did not change under white light comparing to control (58.12 mg 100 g FW) (Table 2). Seedlings treated to blue light also promoted ASA content (75.34 mg 100 g− 1 FW).
Table 2.
Effect of various LED lights on some antioxidant compounds of four-week-old C. frutescens seedlings
| Parameters | Full | White | Red | Blue | Red-Blue |
|---|---|---|---|---|---|
| Ascorbic acid (mg 100 g− 1 FW) | 58.12 ± 4.23 c | 61.43 ± 2.21 c | 89.58 ± 3.45 a | 75.34 ± 5.98 ab | 82.45 ± 3.11 a |
| Carotenoids (µg g− 1 FW) | 7.81 ± 0.67 b | 9.42 ± 0.77 ab | 10.34 ± 0.91 c | 11.78 ± 0.55 a | 10.03 ± 0.66 b |
| Phenolics (mg GAE g− 1 DW) | 0.13 ± 0.005 c | 0.15 ± 0.006 bc | 0.22 ± 0.007 a | 0.17 ± 0.008 b | 0.20 ± 0.009 ab |
| Capsaicinoids (mg g− 1 FW) | 1.72 ± 0.09 c | 2.23 ± 0.17 b | 2.73 ± 0.11 a | 2.49 ± 0.18 ab | 2.68 ± 0.12 a |
Values are means ± SE of four replicates. Different letters indicated significant (p ≤ 0.05) differences
Carotenoid content increased under various LED lights comparing to full light. Treatment of red, blue, and red-blue lights induced more carotenoid content, as maximum content (11.78 µg g− 1 FW) was observed in seedlings treated with blue light comparing to control (7.81 µg g− 1 FW).
Total phenolic content was more enhanced in seedlings treated to red and red-blue lights, and the highest content was obtained in seedlings treated to red light (0.22 mg DAG g− 1 DW) comparing to control (0.13 mg DAG g− 1 DW) (Table 2).
Capsaicinoid content increased significantly under various LED lights. The maximum content obtained in seedlings treated with red (2.73 mg g− 1 DW) and red-blue (2.68 mg g− 1 DW) lights comparing to control (1.72 mg g− 1 DW) (Table 2). Also, blue light significantly enhanced capsaicinoid content (2.49 mg g− 1 DW; 44.7% raise) comparing to the control.
Correlation analysis of sprouts and metabolic profiles of C. frutescens seedlings
The PCA test was used to determine the correlation between each variable and light treatments in sprouts with principal component 1 (PC1) at 63.94%) and PC2 at 25.59% (Fig. 4). The positive correlation was observed between sprout growth and length, and their negative relations with H2O2 level and NOX activity. The red and full lights showed a potential impact on the sprout growth parameters. Moreover, the positive effect of H2O2 induced by blue light was identified in the inducing seed germination (Fig. 4).
PLS-DA analysis was performed to determine which light quality promoted more variability in the data sets of seedlings (Fig. 7). The PLS-DA plot indicated an acceptable accuracy of sample separation with the first and second components of 26.5 and 53.3%, respectively. As shown in Fig. 8, seedlings treated with red-blue and red lights are closely related together in terms of data variability and separated from blue light treatment.
Fig. 8.
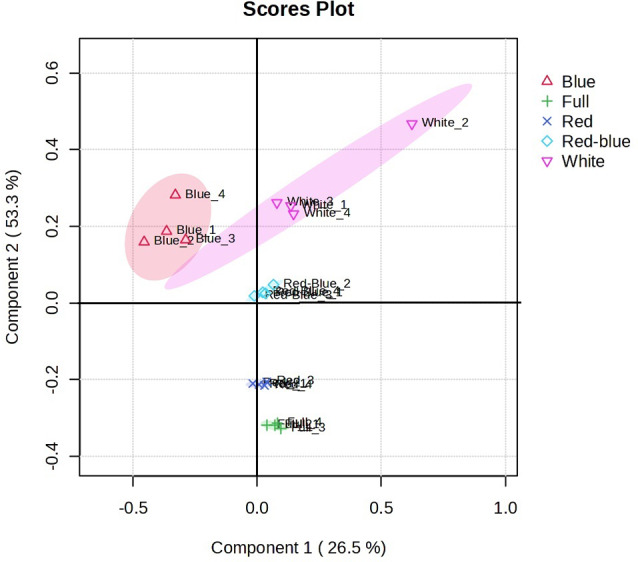
PLS-DA plot was performed on the metabolomics data with components 1 (26.5%) and 2 (53.3%) for samples. A 95% confidence ellipse draws each group of samples. The numbers 1, 2, 3, and 4 display four replicates mediated for each sample
HCA cluster analysis of seedling metabolic responses is displayed in Fig. 9. Two main clusters have been shown in which Rubisco activity and gene expressions, Chl fluorescence, antioxidant enzymes, and growth are set in cluster 1, and antioxidant metabolites (carotenoids, ASA, capsaicinoids, and total phenolics), MDA, and Chl pigments are placed in cluster 2. The HCA graph shows the potential impact of red light on the induction of antioxidant enzymes associated with the ASA-GSH cycle and antioxidant metabolites in response to ROS accumulations, which can promote seedling growth. However, blue light only showed an inducing impact on the antioxidant metabolite accumulations (carotenoids, capsaicinoids, and ASA) (Fig. 9).
Fig. 9.

HCA graph displays the clustering of the physiological parameters, enzyme activities, and antioxidant metabolites under various LED lights based on the Pearson correlations coefficient. Colors in matrix boxes show the direction of correlation: intense red indicates strong positive correlations, and blue indicates strong negative correlations. Chl- chlorophyll, Car-carotenoid, MDA-malondialdehyde, and ASA-ascorbic acid
Discussion
This research was conducted to elucidate the priming impact of light spectrums on the seed germination and post-germinative performance of C. frutescens seedling by investigating some physiological parameters and antioxidant defense systems. Germination is a complex process which can be affected by various optical factors such as light type, intensity, and photoperiod and its rate varies between genotypes [44]. Solano et al. [28] reported that red light did not change the germination percentage in pea seeds, but improved considerably the sprout growth and adventitious roots. Zaghdoud et al. [45] stated that red light induced the high germination index in C. annuum through more water uptake and up-regulation of aquaporin isoforms. In this study, C. frutescens seeds showed a low germination percentage (46%) under darkness. Seed priming to LED lights displayed a time-dependent impact on seed germination. In addition, germination percentage reached to maximum level under 1 h blue and 2 h full and red lights (Fig. 2). Nishii et al. [46] reported that seeds can comprehend and collect light signals and stimulate photomorphogenesis processes through inducing the related genes. ROSs act as signals to mobilize resources to initiate germination and contribute to the oxidative modification of stored proteins [47]. NOXs are known to be involved in ROS production during seed germination [48]. The PCA graph showed a positive relation between H2O2 level and NOX activity and the potential impact of blue light on the induction of these parameters (Fig. 8). However, Full and red lights modulate the NOX activity and H2O2 accumulation after 2 h incubation time (Fig. 3). It has been reported that NOX-dependent H2O2 is needed in improving seed germination in tobacco [49] and barley [50] due to increasing a-amylase activity and sugar content in seeds [51, 52]. In Salvia officinalis, seed priming with H2O2 showed a dose-dependent effect on seed germination and seedling vigor, and H2O2 at 5 mM concentration increased the mentioned parameters under saline conditions [18]. Results of this research suggest that light quality in a time-dependent manner regulates NOXs activity to induce the H2O2-signaling pathway and enhance seed germination.
Seed priming to LEDs lights also affected sprout growth parameters in a time-dependent manner, and 2 h red light showed an optimum lighting condition for inducing sprout growth (Fig. 2). Similarly, red light promoted sprout biomass, hypocotyl length, and cotyledon expansion in tomato [53]. In tobacco, red light stimulated root elongation, formation of lateral roots, and auxin polar transport from shoot to root to produce high-quality transplants [54]. Ca2+ influx is required for cell elongation in roots. NOXs can control development by generating ROS that regulates plant cell expansion and growth through the activation of Ca2+ channels [55].
Four-week-old seedlings also showed the maximum biomass yield under red light, which was followed by red-blue light (Fig. 5a). Li et al. [13] reported that increasing biomass under red light can be associated with the impact of light on the leaf anatomy and chloroplast ultrastructure. In Brosimum gaudichaudii, red light enhanced leaf area, but decreased leaf thickness for high capture rate of radiant energy [56]. The PCA graph showed the positive impact of red light on the pigments, Chl a/b ratio, and Chl fluorescence (Fig. 9). The photosynthetic pigments act as a biological indicator for several physiological processes related to photosynthetic capacity and changing in the Chl a/b ratio expresses an alteration in the organization and performance of thylakoids [57, 58]. Moreover, Chl fluorescence (Fv/Fm) can act as another sensitive indicator to show the impact of stress on photosynthetic electron transport systems [59]. Similarly, Red light improved the maximum quantum yield (Fv/Fm), effective quantum yield, and light energy efficiency in Hypnum plumaeforme due to its regulatory impact on ROS production [60]. On the other hand, blue light increased Chl pigments, but decreased Fv/Fm ration and biomass yield in four-week-old seedlings (Fig. 5). In accordance with these results, Wang et al. [61] reported that the Chl a/b ratio decreased in grapes under blue light. In rapeseed, enhancing the blue-light ratio effectively enhanced leaf mass, leaf thickness, and Chl content, but induced the palisade mesophyll cells in leaves, which may be due to oxidative damage induced by H2O2 on the grana structure and proteins related to thylakoids [62, 63]. In addition, lipid peroxidation-related products are recognized as a potent cause of oxidative modification of the PSII proteins and decrease the photosynthesis performance [63].
Antioxidant enzymes act as the defense mechanisms against ROS-induced oxidative damage and interfere with stress resistance and developmental processes [64]. Investigation of antioxidant enzymes (SOD, APX, and GR) showed that these enzymes were activated in four-week-old seedlings. The upregulation of enzyme activities (SOD, APX, and GR) and antioxidant metabolites were observed in seedlings treated to red and red-blue lights. On the other hand, the HCA graph displayed the positive impact of red light on the activity of antioxidant enzymes related to the ASA-GSH cycle and antioxidant metabolites. SOD activity increased considerably in seedlings treated with red and red-blue lights (Fig. 7). Enhanced SOD activity rapidly converted the dismutation of the superoxide radical to H2O2 [9]. APX activity was heightened significantly in seedlings treated to red and red-blue lights. Increased APX activity converts H2O2 to water by helping ASA, and then monodehydroascorbate and dehydroascorbate (DHA) are produced by the activity of the monodehydroascorbate reductase enzyme. Afterwards, DHA is converted to ASA through the activity of GR and ASA is produced again [65]. HCA graph shows the positive impact of red and red-blue lights on the GR activity and ASA production in four-week-old seedlings. The findings of this research display that red and red-blue lights promote the enzyme activities related to the ASA-GSH pathway to accumulate ASA.
Rubisco activity also showed the maximum activity under red light, and its activity was associated with the upregulation of rbcL and rbcS gene expressions and carbohydrate accumulation (Fig. 6). Ke et al. [66] stated that Rubisco carboxylase activity and the expression of genes related to Calvin cycle altered considerably under light quality. Carbohydrates, as a product of photosynthesis, participate in the adjustment of growth and development and photosynthesis in a feedback manner [67]. This research displayed that red light with stimulating antioxidant enzymes related to the ASA-GSH cycle could manage the H2O2 level to up-regulate Rubisco gene expressions, Rubisco activity, and following growth seedlings. However, blue light down-regulates Rubisco activity and promotes MDA levels, which can be related to inducing oxidative stress and more lipid peroxidation [11].
It is well-known that antioxidant compounds protect cell components from oxidation and oxidant-mediated alterations.
oxidant-mediated alterations. These compounds react with oxidants through redox reactions and decrease or cancel the oxidant activity [68], and the balance between oxidant production and antioxidant defense causes to maintain redox homeostasis [57]. Light plays an important role in the biosynthesis of secondary metabolites through the stimulation of gene expressions and enzyme activities, and its impact is species-specific in plants. Liu et al. [69] reported that blue light markedly enhanced flavonoid biosynthesis in Cyclocarya paliurus through the stimulation of phenylalanine ammonia-lyase (PAL) and chalcone synthase activities. Gene expressions related to flavonoid biosynthesis (GmCHSs) in soybeans were upregulated under red light against blue light [70]. Seed priming to H2O2 intensified PAL1 gene expression and PAL enzyme activity in Salvia officinalis; also the transcription levels of RBOH (encoding NADPH oxidase) and rosmarinic acid synthase enhanced in leaves [71, 72]. In Catharanthus roseus, red light increased vindoline accumulation by increasing the expression of transcription factors and linked genes [73]. In this study, the maximum ascorbic acid (89.58 mg g− 1 FW), total phenolic (0.22 mg GAE g− 1 DW), and capsaicinoid (mg 100 g− 1 DW) content were accumulated in seedlings treated to red light, followed by red-blue and blue lights (Table 2). Red light induced kaempferol and quercetin biosynthesis through stimulation of phytochromes in Pisum sativum [74]. Phenolic compounds contribute to reducing oxidative stress in plants by ROS scavenging and limiting Chl excitation in the photosynthetic apparatus under stress conditions [68]. Capsaicinoids are shown to have antioxidant and anti-inflammatory activities, DNA protection against strand breaks, scavenging of free radical-mediated injuries, etc [75]. Besides, carotenoids play main roles in photosynthesis and transfer energy to Chl [76] and have antioxidant characteristics, including singlet oxygen quenching and radical suppressing capacities [77]. In this study, the maximum carotenoid content (11.78 µg g− 1 FW) was obtained under blue light and followed by red and red-blue lights (Table 2). On the other hand, antioxidant compounds can regulate NOX expression to generate O2− or H2O2. When antioxidants are presented in excess, they can decrease the level of cellular oxidative damage [76]. In this study, red light showed the maximum potential for the accumulation of total phenolics, capsaicinoids, and ASA in four-week-old C. frutescens seedlings. These metabolites with several bioactive capacities have a high interest in pharmaceutical industries.
Conclusion
This study shows that light quality in a time-dependent manner affects seed germination and sprout growth by regulating NOX activity and H2O2 level. Red and full lights were the suitable light conditions to acquire the optimum germination percentage and sprout growth in C. frutescens. Blue light decreased sprout growth through high H2O2 accumulation and oxidative damage during the time. Therefore, LED light-induced responses for germination are likely mediated by NOX-mediated H2O2. Red light showed the optimum light condition to promote seedling growth through promoting Chl pigment synthesis, Chl fluorescence, antioxidant enzymes related to ASA-GSH cycle, Rubisco activity and gene expression, carbohydrate synthesis, and antioxidant metabolites. These findings highlight the role of light quality on the ROS signaling in the germination stage, which can act as a signal memory to affect seedlings’ growth and secondary metabolism. More detail should be addressed in future studies using patterns of expression of genes related to NOXs and phytochromes in LED light-primed seeds and seedlings.
Acknowledgements
The Author gives thanks to the Aerospace Research Institute for providing the research laboratory.
Author contributions
Halimeh Hassanpour performed the experiments and statistical analyses and also wrote the manuscript.
Funding
This work was supported by the Project from Bagh Rezvan Malair Company (PHG-Indust 2).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The author declares that any human participants, human tissue, or clinical trial are not involved in this study. So, it is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muthuswamy R, Asish S, Nison M, Review. on Capsicum frutescens, A Tribal herbal food used as Medicine. Research Journal of Pharmacognosy and Phytochemistry. 2021;13(4):191-4. 10.52711/0975-4385.2021.00033
- 2.Periferakis AT, Periferakis A, Periferakis K, Caruntu A, Badarau IA, Savulescu-Fiedler I, Scheau C, Caruntu C. Antimicrobial properties of capsaicin: available data and future research perspectives. Nutrients. 2023;15(19):4097. 10.3390/nu15194097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos VA, Bressiani PA, Zanotto AW, Almeida IV, Berti AP, Lunkes AM, Vicentini VE, Düsman E. Cytotoxicity of capsaicin and its analogs in vitro. Brazilian J Biology. 2023;83:e268941. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Gao C, Ye Q, Liu C, Wan H, Ruan M, Zhou G, Wang R, Li Z, Diao M, Cheng Y. The influence of different factors on the metabolism of capsaicinoids in pepper (Capsicum annuum L). Plants. 2024;13(20):2887. 10.3390/plants13202887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed IHM, Ali EF, Gad AA, Bardisi A, El-Tahan AM, Abd Esadek OA, El-Saadony MT, Gendy AS. Impact of plant growth regulators spray on fruit quantity and quality of pepper (Capsicum annuum L.) cultivars grown under plastic tunnels. Saudi J Biol Sci. 2022;29:2291–8. 10.1016/j.sjbs.2021.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun F, Xiong S, Zhu Z. Dietary capsaicin protects cardiometabolic organs from dysfunction. Nutrients. 2016;8(5):174. 10.3390/nu8050174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeeatid N, Techawongstien S, Suriharn B, Bosland PW, Techawongstien S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum Chinense Jacq.) cultivars. Environ Biotechnol. 2017;58:103–10. 10.1007/s13580-017-0165-6. Horticulture. [Google Scholar]
- 8.Aasim M, Ali SA, Bekiş P, Nadeem MA. Light-emitting diodes induced in vitro regeneration of Alternanthera reineckii mini and validation via machine learning algorithms. Vitro Cell Dev Biology-Plant. 2022;58(5):816–25. 10.1007/s11627-022-10312-6. [Google Scholar]
- 9.Hassanpour H. Potential impact of red-blue LED light on callus growth, cell viability, and secondary metabolism of Hyoscyamus reticulatus. Vitro Cell Dev Biology-Plant. 2022;58(2):256–65. 10.1007/s11627-021-10232-x. [Google Scholar]
- 10.Sanoubar R, Calone R, Noli E, Barbanti L. Data on seed germination using LED versus fluorescent light under growth chamber conditions. Data Brief. 2018;19:594–600. 10.1016/j.dib.2018.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong C, Fu Y, Liu G, Liu H. Growth, photosynthetic characteristics, antioxidant capacity and biomass yield and quality of wheat (Triticum aestivum L.) exposed to LED light sources with different spectra combinations. J Agron Crop Sci. 2014;200(3):219–30. 10.1111/jac.12059. [Google Scholar]
- 12.Nakai A, Tanaka A, Yoshihara H, Murai K, Watanabe T, Miyawaki K. Blue LED light promotes indican accumulation and flowering in Indigo plant, Polygonum tinctorium. Ind Crops Prod. 2020;155:112774. 10.1016/j.indcrop.2020.112774. [Google Scholar]
- 13.Li Y, Xin G, Liu C, Shi Q, Yang F, Wei M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020;20:1–6. 10.1186/s12870-020-02523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap ES, Uthairatanakij A, Laohakunjit N, Jitareerat P, Vaswani A, Magana AA, Morre J, Maier CS. Plant growth and metabolic changes in ‘super hot’chili fruit (Capsicum annuum) exposed to supplemental LED lights. Plant Sci. 2021;305110826. 10.1016/j.plantsci.2021.110826. [DOI] [PubMed]
- 15.Gris T, Pinheiro MV, Thiesen LA, Webler AR, Junges DL, Holz E, Naibo I, Batista DS, Otoni WC, Schmidt D. Light quality and sealing type affect in vitro growth and development of Capsicum frutescens cultivars. An Acad Bras Cienc. 2021;93(e20190061). 10.1590/0001-3765202120190061.
- 16.Wei Y, Wang S, Yu D. The role of light quality in regulating early seedling development. Plants. 2023;12(14):2746. 10.3390/plants12142746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng MC, Kathare PK, Paik I, Huq E. Phytochrome signaling networks. Annu Rev Plant Biol. 2021;72(1):217–44. 10.1146/annurev-arplant-080620-024221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mardani-Korrani F, Amooaghaie R, Ahadi A. He–Ne laser enhances seed germination and salt acclimation in Salvia officinalis seedlings in a manner dependent on phytochrome and H2O2. Protoplasma. 2023;260(1):103–16. 10.1007/s00709-022-01762-1. [DOI] [PubMed] [Google Scholar]
- 19.Simlat M, Ślęzak P, Moś M, Warchoł M, Skrzypek E, Ptak A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci Hort. 2016;211:295–304. 10.1016/j.scienta.2016.09.009. [Google Scholar]
- 20.Hasegawa H, Tate Y, Ogino M, Maki T, Begum ZA, Ichijo T, Rahman IM. Laboratory culture experiments to study the effect of lignite humic acid fractions on iron solubility and iron uptake rates in phytoplankton. J Appl Phycol. 2017;29:903–15. 10.1007/s10811-016-0982-5. [Google Scholar]
- 21.Bailly C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem J. 2019;476(20):3019–32. 10.1042/BCJ20190159. [DOI] [PubMed] [Google Scholar]
- 22.Taylor JP, Hubert MT. The role of NADPH oxidases in infectious and inflammatory diseases. Redox Biol. 2021;48:102159. 10.1016/j.redox.2021.102159. [DOI] [PMC free article] [PubMed]
- 23.Chang YL, Li WY, Miao H, Yang SQ, Li R, Wang X, Li WQ, Chen KM. Comprehensive genomic analysis and expression profiling of the NOX gene families under abiotic stresses and hormones in plants. Genome Biol Evol. 2016;8:791–810. 10.1093/gbe/evw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaya H, Iwano M, Takeda S, Kanaoka MM, Kimura S, Abe M, Kuchitsu K. Apoplastic ROS production upon pollination by RbohH and RbohJ in Arabidopsis. Plant Signal Behav. 2015;10:e989050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li WY, Chen BX, Chen ZJ, Gao YT, Chen Z, Liu J. Reactive oxygen species generated by NADPH oxidases promote radicle protrusion and root elongation during rice seed germination. Int J Mol Sci. 2017;18(1):110. 10.3390/ijms18010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermot A, Petit-Härtlein I, Smith SM, Fieschi F. NADPH oxidases (NOX): an overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants. 2021;10(6):890. 10.3390/antiox10060890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laohavisit A, Anderson A, Bombelli P, Jacobs M, Howe CJ, Davies JM, Smith AG. Enhancing plasma membrane NADPH oxidase activity increases current output by diatoms in biophotovoltaic devices. Algal Res. 2015;12:91–8. 10.1016/j.algal.2015.08.009. [Google Scholar]
- 28.Solano CJ, Hernández JA, Suardíaz J, Barba-Espín G. Impacts of leds in the red spectrum on the germination, early seedling growth and antioxidant metabolism of pea (Pisum sativum L.) and Melon (Cucumis Melo L). Agriculture. 2020;10(6):204. 10.3390/agriculture10060204. [Google Scholar]
- 29.Jiang M, Zhang J. Involvement of plasma-membrane NADPH oxidase in abscisic acid-and water stress-induced antioxidant defense in leaves of maize seedlings. Planta. 2002;215:1022–30. 10.1007/s00425-002-0829-y. [DOI] [PubMed] [Google Scholar]
- 30.Duan ZQ, Bai L, Zhao ZG, Zhang GP, Cheng FM, Jiang LX, Chen KM. Drought-stimulated activity of plasma membrane nicotinamide adenine dinucleotide phosphate oxidase and its catalytic properties in rice. J Integr Plant Biol. 2009;51(12):1104–15. 10.1111/j.1744-7909.2009.00879.x. [DOI] [PubMed]
- 31.Velikova V, Yordanov I, Edreva AJ. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151(1):59–66. 10.1016/S0168-9452(99)00197-1. [Google Scholar]
- 32.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellburn AR. The spectral determination of chlorophylls a and B, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144(3):307–13. 10.1016/S0176-1617(11)81192-2. [Google Scholar]
- 34.Ben Saad R, Fabre D, Mieulet D, Meynard D, Dingkuhn M, AL-DOSS AB, Guiderdoni E, Hassairi A. Expression of the Aeluropus littoralis AlSAP gene in rice confers broad tolerance to abiotic stresses through maintenance of photosynthesis. Plant Cell Environ. 2012;35(3):626–43. 10.1111/j.1365-3040.2011.02441.x. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zhang S, Jiang W, Liu D. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ Sci Pollut Res. 2013;20:1117–23. 10.1007/s11356-012-1054-2. [DOI] [PubMed] [Google Scholar]
- 36.Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59(2):309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jebara S, Jebara M, Limam F, Aouani ME. Changes in ascorbate peroxidase, catalase, Guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol. 2005;162(8):929–36. [DOI] [PubMed] [Google Scholar]
- 38.Hassanpour H. ROS regulation in Dunaliella salina by fulvic acid: induction of enzymes related to the ascorbate–glutathione pathway and antioxidant metabolites. J Appl Phycol. 2024;16:1–1. 10.1007/s10811-024-03346-3. [Google Scholar]
- 39.Usuda H. The activation state of ribulose 1, 5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985;26(8):1455–63. 10.1093/oxfordjournals.pcp.a077047. [Google Scholar]
- 40.Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–6. 10.1021/ac60111a017. [Google Scholar]
- 41.González-Zamora A, Sierra-Campos E, Pérez-Morales R, Vázquez-Vázquez C, Gallegos-Robles MA, López-Martínez JD, García-Hernández JL. Measurement of capsaicinoids in Chiltepin hot pepper: a comparison study between spectrophotometric method and high performance liquid chromatography analysis. J Chem. 2015;2015(1):709150. 10.1155/2015/709150. [Google Scholar]
- 42.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticulture. 1965;16:144–58. 10.5344/ajev.1965.16.3.144. [Google Scholar]
- 43.Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. Biochem J. 1983;210:899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al Murad M, Razi K, Jeong BR, Samy PM, Muneer S. Light emitting diodes (LEDs) as agricultural lighting: impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability. 2021;13(4):1985. 10.3390/su13041985. [Google Scholar]
- 45.Zaghdoud C, Ollio I, Solano CJ, Ochoa J, Suardiaz J, Fernández JA, Martínez Ballesta MD. Red LED light improves pepper (Capsicum annuum L.) seed radicle emergence and growth through the modulation of Aquaporins, hormone homeostasis, and metabolite remobilization. Int J Mol Sci. 2023;24(5):4779. 10.3390/ijms24054779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishii K, Nagata T, Wang CN, Möller M. Light as environmental regulator for germination and macrocotyledon development in Streptocarpus rexii (Gesneriaceae). South Afr J Bot. 2012;81:50–60. 10.3390/molecules19045434. [Google Scholar]
- 47.Verma G, Mishra S, Sangwan N, Sharma S. Reactive oxygen species mediate axis-cotyledon signaling to induce reserve mobilization during germination and seedling establishment in Vigna radiata. J Plant Physiol. 2015;184:79–88. 10.1016/j.jplph.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 49.Li W, Li X, Chao J, Zhang Z, Wang W, Guo Y. NAC family transcription factors in tobacco and their potential role in regulating leaf senescence. Front Plant Sci. 2018;9:1900. 10.3389/fpls.2018.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishibashi Y, Kasa S, Sakamoto M, Aoki N, Kai K, Yuasa T, Hanada A, Yamaguchi S, Iwaya-Inoue M. A role for reactive oxygen species produced by NADPH oxidases in the embryo and aleurone cells in barley seed germination. PLoS ONE. 2015;10(11):e0143173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemahunguni NK, Gupta S, Kulkarni MG, Finnie JF, Van Staden J. The effect of biostimulants and light wavelengths on the physiology of Cleome gynandra seeds. Plant Growth Regul. 2020;90:467–74. 10.1007/s10725-019-00546-7. [Google Scholar]
- 52.Zhang T, Shi Y, Piao F, Sun Z. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult (PCTOC). 2018;134:231–40. [Google Scholar]
- 53.Izzo LG, Mele BH, Vitale L, Vitale E, Arena C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ Exp Bot. 2020;179:104195. 10.1016/j.envexpbot.2020.104195. [Google Scholar]
- 54.Jinxiu S, Qingwu M, Weifen D, Dongxian H. Effects of light quality on growth and development of cucumber seedlings in controlled environment. Int J Agricultural Biol Eng. 2017;10(3):312–8. [Google Scholar]
- 55.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422(6930):442–6. 10.1681/ASN.2012111112. [DOI] [PubMed] [Google Scholar]
- 56.Costa ÉL, Farnese FD, de Oliveira TC, Rosa M, Rodrigues AA, Resende EC, Januario AH, Silva FG. Combinations of blue and red leds increase the morphophysiological performance and furanocoumarin production of Brosimum gaudichaudii trécul in vitro. Front Plant Sci. 2021;12:680545. 10.3389/fpls.2021.680545/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen LL, Wang HY, Gong XC, Zeng ZH, Xue XZ, Hu YG. Transcriptome analysis reveals effects of red and blue light-emitting diodes (LEDs) on the growth, chlorophyll fluorescence, and endogenous plant hormones of potato (Solanum tuberosum L.) plantlets cultured in vitro. J Integr Agric. 2021;20(11):2914–31. 10.1016/S2095-3119(20)63393-7. [Google Scholar]
- 58.Krupinska K, Mulisch M, Hollmann J, Tokarz K, Zschiesche W, Kage H, Humbeck K, Bilger W. An alternative strategy of dismantling of the chloroplasts during leaf senescence observed in a high-yield variety of barley. Physiol Plant. 2012;144(2):189–200. 10.1111/j.1399-3054.2011.01545.x. [DOI] [PubMed] [Google Scholar]
- 59.Faseela P, Sinisha AK, Brestič M, Puthur JT. Chlorophyll a fluorescence parameter as indicators of a particular abiotic stress in rice. Photosynthetica. 2020;2:58. 10.32615/ps.2019.147. [Google Scholar]
- 60.Xie M, Wang X, Zeng Q, Shen J, Huang B. Growth physiology and chlorophyll fluorescence analysis of two moss species under different LED light qualities. Plant Physiol Biochem. 2024;108777. 10.1016/j.plaphy.2024.108777. [DOI] [PubMed]
- 61.Wang S, Wang X, Shi X, Wang B, Zheng X, Wang H, Liu F. Red and blue lights significantly affect photosynthetic properties and ultrastructure of mesophyll cells in senescing grape leaves. Hortic Plant J. 2016;2(2):82–90. 10.1016/j.hpj.2016.03.001. [Google Scholar]
- 62.Yao XY, Liu XY, Xu ZG, Jiao XL. Effects of light intensity on leaf microstructure and growth of rape seedlings cultivated under a combination of red and blue leds. J Integr Agric. 2017;16(1):97–105. 10.1016/S2095-3119(16)61393-X. [Google Scholar]
- 63.Pospíšil P, Yamamoto Y. Damage to photosystem II by lipid peroxidation products. Biochim Et Biophys Acta (BBA)-General Subj. 2017;1861(2):457–66. 10.1016/j.bbagen.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Khorshidi N, Hassanpour H, Ziyadi H. Static magnetic field improved growth and Astaxanthin production in Haematococcus lacustris via the regulation of carbohydrate accumulation, H2O2 level, and antioxidant defense system. J Appl Phycol. 2022;34(5):2283–95. 10.1007/s10811-022-02758-3. [Google Scholar]
- 65.Hasanuzzaman M, Bhuyan MB, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8(9):384. 10.3390/antiox8090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ke X, Li JK, Xu CH, Gong M. Effects of different light quality on anatomical structure, carboxylase activity of ribulose 1,5-bisphosphate carboxylase Oxygenase and expression of Rbc and Rca genes in tobacco (Nicotiana tabacum L.) leaves. Plant Physiol J. 2012;48:251–9. [Google Scholar]
- 67.Paul MJ, Pellny TK. Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot. 2003;54(382):539–47. 10.1093/jxb/erg052. [DOI] [PubMed] [Google Scholar]
- 68.Hassanpour H, Hassanpour S. Promoting impact of electromagnetic field on antioxidant system and performance of vascular tissues in Physalis alkekengi. Russ J Plant Physiol. 2021;68(3):545–51. [Google Scholar]
- 69.Liu Y, Fang S, Yang W, Shang X, Fu X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J Photochem Photobiol B. 2018;179:66–73. 10.1016/j.jphotobiol.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Floris M, Bassi R, Robaglia C, Alboresi A, Lanet E. Post-transcriptional control of light-harvesting genes expression under light stress. Plant Mol Biol. 2013. 10.1007/s11103-013-0046-z. 82:147– 54. [DOI] [PubMed] [Google Scholar]
- 71.Mardani-Korrani F, Amooaghaie R, Ahadi A, Ghanadian M. RBOH-dependent signaling is involved in He-Ne laser-induced salt tolerance and production of Rosmarinic acid and carnosol in Salvia officinalis. BMC Plant Biol. 2024;24(1):798. 10.1186/s12870-024-05502-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amooaghaie R, Mardani Korrani F, Ghanadian M, Ahadi A, Pak A, Mardani G. Hybrid priming with He–Ne laser and hydrogen peroxide advances phenolic composition and antioxidant quality of Salvia officinalis under saline and non-saline condition. J Plant Growth Regul. 2024;43(4):1012–25. 10.1007/s00344-023-11156-z. [Google Scholar]
- 73.Liu Y, Patra B, Pattanaik S, Wang Y, Yuan L. GATA and phytochrome interacting factor transcription factors regulate light-induced Vindoline biosynthesis in Catharanthus roseus. Plant Physiol. 2019;180(3):1336–50. 10.1104/pp.19.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bottomley W, Smith H, Galston AW. Flavonoid complexes in Pisum sativum—III.: the effect of light on the synthesis of Kaempferol and Quercetin complexes. Phytochemistry. 1966;5(1):117–23. 10.1016/S0031-9422(00)85089-X. [Google Scholar]
- 75.Hashimoto H, Sugai Y, Uragami C, Gardiner AT, Cogdell RJ. Natural and artificial light-harvesting systems utilizing the functions of carotenoids. J Photochem Photobiol C. 2015;25:46–70. 10.1016/j.jphotochemrev.2015.07.004. [Google Scholar]
- 76.El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430(1):37–48. 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 77.Cremonini E, Daveri E, Mastaloudis A, Adamo AM, Mills D, Kalanetra K, Hester SN, Wood SM, Fraga CG, Oteiza PI. Anthocyanins protect the Gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019;26:101269. 10.1016/j.redox.2019.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.