Abstract
Background
Non-alcoholic steatohepatitis-associated hepatocellular carcinoma (NASH-HCC) accounts for an increasing proportion of HCC cases. Currently, effective pharmacological options for treating both NASH and NASH-HCC remain limited, necessitating the identification of novel therapeutic agents. Our previous studies have demonstrated that ginger can ameliorate nonalcoholic fatty liver disease (NAFLD) and prevent the occurrence of NASH. The therapeutic effects and underlying mechanisms of NASH-HCC, however, remain poorly understood.
Methods
Network pharmacology, bioinformatics, single-cell RNA sequencing analysis, and molecular docking were used to identify the main active compounds, targets, and possible mechanisms of ginger in treating NASH-HCC. The anti-tumor efficacy and underlying mechanisms of the selected compound in treating NASH-HCC were validated through in vitro experimentation.
Results
Network pharmacology, bioinformatics, and molecular docking have revealed that 6-gingerol is the main active compound of ginger in treating NASH-HCC. SRC can be an essential target gene for ginger attenuating NASH-HCC progression, while the mitogen-activated protein kinase (MAPK) signaling pathway and reactive oxygen species (ROS) play equally important roles. Single-cell RNA sequencing of the HCC patients shows that the key targets of ginger in treating NASH-HCC are distributed in tumor-associated macrophage (TAMs). It has been reported that NOX2-derived ROS in macrophages can activate Src and then regulate downstream MAPK signaling cascades. 6-Gingerol can inhibit the proliferation, migration and reduce lipid deposition of liver cancer cells in vitro. More importantly, it induces polarization TAMs to M1 and enhances proinflammatory function, which may be achieved via the NOX2/Src/MAPK signaling pathway.
Conclusion
This study proves that 6-gingerol, the primary active compound in ginger, plays a role in attenuating the progression of NASH-HCC by inhibiting the proliferation and migration of tumor cells, or reprogramming TAMs to the M1 phenotype via the NOX2/Src/MAPK signaling pathway and activating the TAM-mediated immune responses.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-025-04890-2.
Keywords: 6-Gingerol, Tumor-associated macrophages, NASH-HCC, Macrophage reprogramming
Introduction
Liver cancer is a common malignant tumor worldwide. According to the latest tumor statistics, the 5-year survival rate of liver cancer is 22% [1]. Hepatocellular carcinoma (HCC) accounts for more than 85% of primary liver cancers [2]. Non-alcoholic fatty liver disease (NAFLD), the most common chronic metabolic disease, occurs in about 30% of adults worldwide and plays an increasingly important role in HCC. NAFLD includes case syndromes ranging from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH). Unfortunately, patients with NAFLD lack obvious clinical symptoms, and some are not diagnosed until the NASH-HCC stage. In addition to surgery and chemoradiotherapy, immune checkpoint inhibitors represented by PD- 1 have achieved promising outcomes in the treatment of HCC in recent years. However, it has been reported that PD- 1 or PD-L1 inhibitors do not improve the survival rate of NASH-HCC patients. When prophylactically administered PD- 1 therapy increases the incidence of NASH-HCC and the number of tumors, which correlated with increased hepatic CD8+PD1+CXCR6+, TOX+, and TNF+ T cells [3]. Spatial proteomics of the immune microenvironment of NASH-HCC revealed that the interaction of myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) with CD8+ T cells in NASH-HCC is the basis of NASH-HCC immunosuppression [4]. Meanwhile, research has been reported that diet-induced NASH is characterized by the induction of TAM-like macrophages and exhaustion of cytotoxic CD8+ T cells in the liver [5].
TAMs are a major component of the tumor microenvironment (TME) and are often associated with poor prognosis and therapy resistance [6]. TAM is usually divided into two functionally contrasting subtypes, namely classically activated M1 macrophages and alternatively activated M2 macrophages [7]. It has been documented that the M2 phenotype exhibits immune suppression and promotes tumor progression [8]. M1 macrophages are historically regarded as anti-tumor macrophage. They can kill tumor cells either by directly mediating cytotoxicity, such as the release of reactive oxygen species (ROS) and nitrogen monoxide (NO), or by antibody dependent cell-mediated cytotoxicity (ADCC) [9]. Accumulating evidence has suggested that immune suppression in TME is the main obstacle to maximizing the clinical potential of immunotherapy. While immunotherapy for NASH-HCC has garnered significant attention in recent years, targeting TAMs in the context of NASH-HCC has been very limited. Therefore, drug research targeting TAMs is a promising study to overcome NASH-HCC immunosuppression.
Traditional Chinese medicine (TCM) plays a significant role in complementary alternative therapy by improving the metabolic reprogramming of cancer cells, enhancing immune function, and increasing the sensitivity to chemotherapy drugs [10–12]. Ginger is one of the most commonly used herbs in TCM. Numerous studies have reported the efficacy of ginger and its active compounds in the treatment of NASH/NAFLD [13, 14]. Our previous studies demonstrated that ginger and its active compounds can mitigate hepatic steatosis, inflammation and oxidative stress, as well as exert a protective effect on liver function [15, 16]. Besides, studies have shown that ginger has immunomodulatory and anti-tumor effects. Ginger polysaccharides can increase the intestinal immunity of immunosuppressed mice by modulating their gut microbiota and promote MDSCs apoptosis by regulating lipid metabolism [17, 18]. In addition, ginger and its extracts can exert anticancer effects against liver cancer, colon cancer and breast cancer by reducing the proliferation, and promoting apoptosis and DNA damage of cancer cells through oxidative stress/inflammatory pathways [19–21].
Although the role of ginger in NASH treatment, immune regulation and anti-tumor has been reported, there is still a large gap in the research of ginger in the treatment of NASH-HCC or targeting TAMs. In the present study we predicted the possible mechanism of ginger in the treatment of NASH-HCC by network pharmacology and bioinformatics and validated it by in vitro co-culture system based on the previous findings and existing literature reports. Interestingly, we found that the mechanism of ginger treating NASH-HCC may be related to TAMs. To our knowledge, current literature on ginger treatment for tumors mainly focuses on directly killing tumor cells, while there are limited reports on ginger regulating TME. Therefore, our study not only fills the gap in the treatment of NASH-HCC with ginger, but also proves that ginger can improve the immunosuppression of TME by targeting TAM, providing a theoretical basis for the subsequent anti-tumor research of ginger.
Materials and methods
Chemicals and reagents
6-Gingerol (Z100111) was purchased from Jingzhu Biological Co., Ltd. (Nanjing, China). Cell Counting Kit- 8 (KTA1020) was purchased from Abbkine Scientific Co., Ltd. (Wuhan, China). The antibodies NADPH oxidase 2 (NOX2) (381,293), Proto-oncogene tyrosine-protein kinase Src (Src) (320,001), Phospho-Src (Tyr418) (680,685), Mitogen-activated protein kinase 14 (p38) (200,782), Phospho-p38 (Thr180/Tyr182) (310,091) were purchased from ZEN-BIOSCIENCE Co., Ltd. (Sichuan, China). Rabbit anti- p44/42 MAPK (Erk1/2) antibody (4695 T), rabbit anti- Phospho-p44/42 MAPK (Erk1/2) antibody (4370 T), rabbit anti- SAPK/JNK antibody (9252S), and rabbit anti- Phospho-SAPK/JNK antibody (4668S) were purchased from Cell Signaling Technology (Danvers, MA, USA). GAPDH Recombinant Rabbit Monoclonal Antibody (ET1601 - 4) was purchased from HUABIO Co., Ltd. (Zhejiang China). ABScript III RT Master Mix for qPCR with gDNA Remover (RK20429), and 2X Universal SYBR Green Fast qPCR Mix (RK21203) were purchased from ABclonal Technology Co.,Ltd. (Wuhan, China). The rat Anti-mouse CD206/FITC (BSM- 30276 A-FITC) was purchased from Bioss Co.,Ltd. (Beijing, China). Triglyceride assay kit (A110 - 1–1) was purchased from Nanjing Jiancheng Bioengineering institute (Nanjing, China).
Common target acquisition
Identification of active compounds and target genes of ginger
The TCMSP database (https://old.tcmsp-e.com/tcmsp.php), TCMSID database (https://tcm.scbdd.com/home/index) were used to screen the active compounds of ginger according to the principle of ADME (absorption, distribution, metabolism, and excretion). The active compounds with OB (Oral bioavailability) ≥ 30% and DL (Drug-likeness) ≥ 0.18 were selected. Then, compounds with anticancer activity and high ginger content reported in the literature were supplemented. The PubChem database (https://pubchem.ncbi.nlm.nih.gov/) was used to find the SMILES (Simplified molecular input line entry system) of the active compounds in ginger. Drug targets of active compounds were obtained from the SwissTargetPrediction database (http://swisstargetprediction.ch/), TCMSP database, and HERB database (http://herb.ac.cn/).
Disease targets query
The keywords “NASH” and “HCC” were used to collect disease-related targets in the DisGeNET and GeneCards databases, and the combination was used as the target of “NASH-HCC”. In the GEO database using “NASH-HCC” as the keyword, differentially expressed genes (DEGs) analysis was performed on datasets GSE99807 (The dataset included four pairs of paraffin-embedded paired NASH-HCC and peri-carcinoma tissues) and GSE164760 (The dataset included 53 NASH-HCCs, 29 adjacent non-tumor NASH liver specimens, and 74 NASH liver specimens, as well as 6 healthy livers and 8 cirrhotic livers. Transcriptome data of NASH-HCCs and healthy livers were employed in this paper) using “limma” R package (A R software installation package for data analysis, linear models and differential expression for microarray data).
Intersection targets of ginger in the treatment of NASH-HCC
A Venn diagram of common targets in ginger and disease was drawn using the online Venn platform (https://www.bioinformatics.com.cn/).
Network visualization
Cytoscape 3.7.2 was used to draw the networks. The STRING database (https://string-db.org/) was used to determine protein–protein interaction relationships. Network analysis was performed using the plug-in of Cytoscape 3.7.2, and the size of the nodes was adjusted according to the “Degree”. The cytoHubba plug-in was used to search for hub genes in the ginger-disease intersection target networks. Using the NetworkAnalyzer tool in Cytoscape 3.7.2 to analyze the network, the top 20 hub genes were selected as candidate targets according to betweenness centrality (BC), closeness centrality (CC), and degree centrality (DC) rankings. The structures of active compounds were illustrated using ChemDraw 22.0.0.
Single-cell RNA sequencing analysis
R 4.3.2 was used to analyze the single-cell RNA sequencing series GSE242889 (This dataset includs single-cell RNA sequencing of liver cancer tissues and adjacent non-tumor tissues from five HCC patients. In this paper, single-cell RNA sequencing samples from liver cancer tissues were used) obtained from the GEO database. It was also used to calculate the ratio of mitochondria to red blood cells, filter cells, mitochondrial and red blood cell genes, and merge the samples using the “Merge” function. The data were normalized, and cluster visualization was performed using uniform manifold approximation and projection (UMAP). The “Seurat” R package (A toolkit for quality control, analysis, and exploration of single cell RNA sequencing data) was called to search for specific marker genes with high expression in a specific cluster, and the human species were selected on the cellMarker website (http://bio-bigdata.hrbmu.edu.cn/CellMarker/) for subcellular annotation of each cluster.
Molecular docking
The SDF file of the drug was saved in the PubChem database, and the 3D structure of target was downloaded from the PDB database (NOX2:8WEJ, SRC:1FMK, MAPK1:8AOJ, MAPK8:3ELJ, and MAPK14:3FLY). Discovery Studio software was used for the interconnection. All water and heteroatoms were removed from the protein crystal structure to replace the missing atoms in the residue, and for docking ligands, the binding site was determined by the cavity position of the co-crystalline ligand in the initial protein crystal structure based on the CHARMM force field used to optimize the structure and conformation of the side chain residue with the missing atom. The docking module adopts the CDOCKER program, and the scoring system calculates the CDOCKER ENERGY according to CDOCKER to sort the results. Discovery Studio was used to visualize the most stable structures.
Cell culture
Human hepatocellular carcinoma cells (HepG2, and Huh- 7), normal human liver cells (THLE- 3), mouse hepatoma cells (Hepa1 - 6), and mouse macrophages (RAW264.7) were from Cell Bank, Chinese Academy of Sciences (Shanghai, China). HepG2, Huh- 7, Hepa1 - 6, and RAW264.7 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, New York, USA). THLE- 3 was cultured in Roswell Park Memorial Institute (RPMI) 1640 (Gibco, New York, USA). All cell medium supplemented with 10% fetal bovine serum (FBS) (Bio-Channel, Nanjing, China) and 1% penicillin–streptomycin (PS) (Beyotime, Shanghai, China). The cells were maintained under standard cell culture conditions of 5% CO2 and 95% humidity at 37 ℃.
Co-culture system
Control: RAW264.7 cells were cultured in complete medium (10% FBS + 1% PS + 89% DMEM) for 24 h. Mhepa1 - 6: Hepa1 - 6 cells were treated with 100 μM oleic acid (OA) for 24 h, and the medium supernatant was collected. A 0.22 μm filter membrane was used to filter the culture medium supernatant, and the filtered liquid was called conditioned medium (CM). Then CM and normal complete medium (10% FBS + 1% PS + 89% DMEM) were mixed at the ratio of 1:1 to intervene RAW264.7 cells for 24 h. 6 gCM: 100 μM OA and different concentrations of 6-gingerol (10, 20, 40 μM) were employed to induce Hepa1 - 6 cells for 24 h, CM was collected and mixed with normal complete medium at a ratio of 1:1 and then interfered with RAW264.7 cells for 24 h. Mhepa1 - 6 + 6 g: Firstly, intervening Hepa1 - 6 cells with 100 μM OA for 24 h, CM was collected and mixed with normal complete medium in a ratio of 1:1 as the medium of RAW264.7, then 6-gingerol with different concentration (10, 20, 40 μM) was added to the medium to act on RAW264.7 cells for 24 h.
Western blotting
Cells were lysed in RIPA lysis buffer (Beyotime Biotechnology, Shanghai). BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai) was used to detect the total protein concentrations. Equal quantities of boiled protein extracts were separated by 10% SDS-PAGE gels, then transferred to methanol preactivated-polyvinylidene difluoride membranes. Membranes were incubated with 5% skimmed milk for 1.5 h at room temperature and then incubated with primary antibodies overnight at 4℃. Incubated the membrane with secondary antibody at room temperature for 1 h. Protein bands were visually detected using ECL Select (GE Healthcare, Germany) on a ChemiDoc chemiluminescent platform (Bio-Rad, USA).
Determination of gene expression level changes using qRT-PCR
The total RNA was extracted by RNAiso Plus reagent (TaKaRa, Japan) and the RNA concentration was measured by NanoDrop 2000 spectrophotometer. The preparation and reaction conditions of reverse transcription and qRT-PCR system were carried out in strict accordance with the instructions. The reaction was carried out on the CFX Connect System (Bio-Rad, USA). Gapdh was regarded as the reference gene and the 2−ΔΔCT method was carried out to analyze the relative mRNA expressions. The primer sequence of mouse for qRT-PCR analysis is shown in Table 1.
Table 1.
Primer sequences for quantitative real-time PCR
| Target | Primer sequence (5’− 3’) | |
|---|---|---|
| Arg1 | Forward | GGCAACCTGTGTCCTTTCTCCTG |
| Reverse | GGTCTACGTCTCGCAAGCCAATG | |
| Mrc1 | Forward | CTCGTGGATCTCCGTGACAC |
| Reverse | GCAAATGGAGCCGTCTGTGC | |
| Tgfb1 | Forward | GTGGACCGCAACAACGCAATCTATG |
| Reverse | GGCACTGCTTCCCGAATGTCTGA | |
| Pdl- 1 | Forward | ACTTGCTACGGGCGTTTACT |
| Reverse | ACGTCTGTGATCTGAAGGGC | |
| Nos2 | Forward | CCAGCGGAGTGACGGCAAAC |
| Reverse | GCAAGACCAGAGGCAGCACATC | |
| Ptgs2 | Forward | GGTGCCTGGTCTGATGATGTATGC |
| Reverse | GGATGCTCCTGCTTGAGTATGTCG | |
| Tnf | Forward | GTAGCCCACGTCGTAGCAAACC |
| Reverse | CAGCCTTGTCCCTTGAAGAGAACC | |
| Il6 | Forward | TCTATACCACTTCACAAGTCGGA |
| Reverse | GAATTGCCATTGCACAACTCTTT | |
| Gapdh | Forward | TGTTTCCTCGTCCCGTAG |
| Reverse | CAATCTCCACTTTGCCACT |
Flow cytometry
The cells were collected in 1.5 mL EP tubes and centrifuged to remove the supernatant. 100 μL PBS was added to resuspended cells and 1 μL CD86 antibody was added to each tube. Incubated at room temperature for 30 min away from light. PBS was removed and 500 μL fixation buffer (Yeasen, China) was added and fixed for 10 min at room temperature away from light. Centrifuged at 150 g for 5 min to discard the fixating solution, add 500ul of permeabilization wash buffer (Yeasen, China) to each tube, resuspend the cells. centrifuged at 150 g for 5 min, discarded the supernatant, and repeated the steps twice. The cells were resuspended with 100 μL permeabilization wash buffer, and 1 μL CD206 antibody was added, and incubated at room temperature for 30 min away from light. The cells were washed with PBS twice, and 500 μL of PBS was added to resuspended cells for detection on CytoFLEX (Beckman, USA).
Statistical analysis
The data are presented as mean ± standard error of mean (SEM). Means between multiple groups were compared using one-way ANOVA with Tukey’s post hoc test, while those between two groups were compared using Student’s t-test (GraphPad Prism 9.0, USA). Differences were considered significant at p < 0.05 (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Additional detailed methods are included in Supplementary-methods.
Results
Active compounds and hub genes acquisition
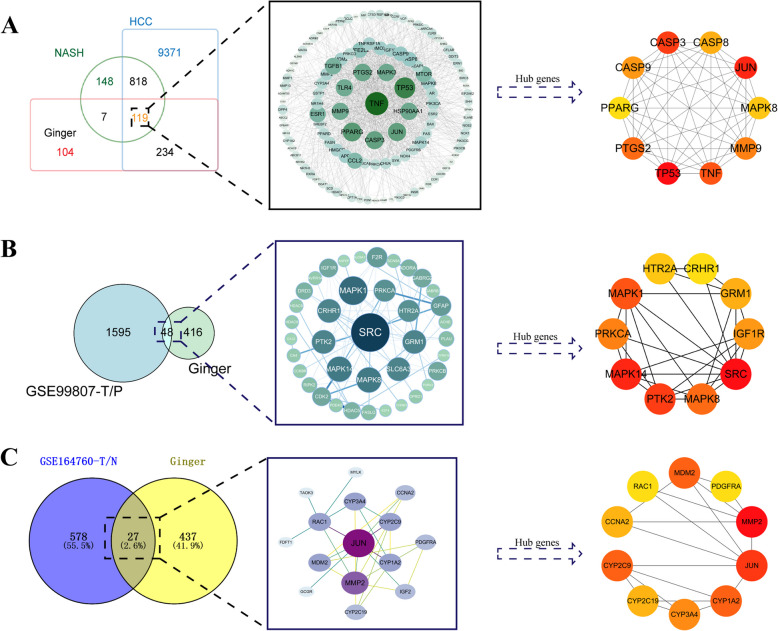
A total of 464 ginger targets were found in fifteen ginger compounds gathered from SwissTargetPrediction, TCMSP, TCMSID and HERB database. Nine hundred thirty-seven genes of NASH-HCC were obtained from DisGeNET and GeneCards database, and 119 intersection genes were found with ginger targets (Fig. 1A). The 1645 NASH-HCC-related DEGs were obtained from the NASH-HCC transcriptome dataset GSE99807, including 48 genes in common with ginger targets (Fig. 1B). Meanwhile, 612 DEGs of NASH-HCC were found from GSE164760 transcriptome dataset with 27 DEGs crossed with ginger targets (Fig. 1C). Since hub genes are usually characterized as possessing the strongest regulatory effects on DEGs, in order to better understand the biological processes of DEGs, we used Cytoscape 3.7.2 to extract the hub genes of each component. After merging these hub genes, duplicate genes were removed, and finally 28 hub genes of ginger treating NASH-HCC were obtained.
Fig. 1.
Targets acquisition of ginger treating NASH-HCC. A Nine hundred and thirty-seven NASH-HCC related genes were obtained from DisGeNET and GeneCards database. Among them, 119 genes overlapped with ginger related genes and were visualized by Cytoscape 3.7.2. Hub genes of the 119 genes were searched by Cytoscape 3.7.2. B 1595 differentially expressed genes (DEGs) between paired NASH-HCC and peri-carcinoma tissues in GSE99807 dataset (GSE99807-T/P) were obtained. Forty-eight intersection genes of ginger targets with DEGs were visualized by Cytoscape 3.7.2 and the hub genes were identified. C 578 DEGs between NASH-HCCs and healthy livers in GSE164760 dataset (GSE164760-T/N) were obtained. Twenty-seven intersection genes of ginger targets with DEGs were visualized by Cytoscape 3.7.2 and the hub genes were identified
MAPK signaling pathway and ROS play a major role in ginger treating NASH-HCC
The ginger active compound-hub gene network was constructed, where gingerdione was not connected to any hub gene was excluded. Finally, 14 active compounds and 28 hub genes were identified (Fig. 2A). Based on the analysis function of Cytoscape 3.7.2, the active compounds were sorted according to the “node degree” (Table 2), and the top 5 compounds (6-shogaol, beta-sitosterol, 6-gingerol, 8-shogaol and 10-shogaol) were selected for subsequent research. The GO enrichment analyses of hub genes showed that among the top 10 enrichment results of the biological process (GO-BP) term, four were related to Reactive Oxygen Species (ROS), such as cellular response to oxidative stress, cellular response to reactive oxygen species, response to oxidative stress, and response to reactive oxygen species (Fig. 2B). The molecular function (GO-MF) term was mainly related to the activity of multiple enzymes, including cysteine-type endopeptidase, MAP kinase and oxidoreductase (Fig. 2C). The top 25 KEGG enrichment pathways of hub genes are shown in the bubble map, which include the MAPK signaling pathway, TNF signaling pathway and IL- 17 signaling pathway. (Fig. 2D). Then, Cytoscape 3.7.2 was used to evaluate the key hub genes in the network, and the top 20 genes with the highest scores of BC, CC and DC were selected as candidate targets (Fig. 2E). The X2K platform was used to enrich transcription factors (TFs) and kinases that may regulate candidate targets, and the top 10 TFs and kinases were visualized based on the p-value results. The top three TFs were PPARG, CTCF, and EGR1 (Fig. 2F), and interestingly two of the top three kinases are located in the MAPK signaling pathway (MAPK1 and MAPK14) (Fig. 2G). The GO and KEGG results of hub genes, as well as the kinase prediction of candidate targets showed that ginger treating NASH-HCC were strongly associated with MAPK signaling pathway. We hypothesized that main active compounds of ginger may regulate the progression of NASH-HCC through the MAPK signaling pathway.
Fig. 2.
Hub genes analysis. A. All hub genes obtained in Fig. 1 were collected and the ginger active compound-hub gene network was constructed. B The GO-Biological Process (BP) of hub genes, and the top 10 was shown. C The GO-Molecular Function of hub genes. D The top 25 KEGG signaling pathways of hub genes. E Hub genes were sorted by BC, CC, and DC, and the top 20 candidate genes were selected by red dotted boxes. F The top 10 transcription factors that may have regulatory effects on candidate genes. G The top 10 kinases that may have regulatory effects on candidate genes
Table 2.
The structures of ginger active compounds and the degree assesed by Cytoscape 3.7.2
| Number | Compound name | Degree | Formula | Structure |
|---|---|---|---|---|
| 1 | 6-shogaol | 11 | C17H24O3 |
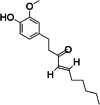
|
| 2 | beta-sitosterol | 11 | C29H50O |

|
| 3 | 6-gingerol | 9 | C17H26O4 |
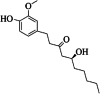
|
| 4 | 8-shogaol | 7 | C19H28O3 |

|
| 5 | 10-shogaol | 7 | C21H32O3 |
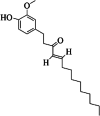
|
| 6 | 6-methylgingediacetate2 | 6 | C22H34O6 |

|
| 7 | dihydrocapsaicin | 5 | C18H29NO3 |
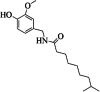
|
| 8 | caffeic acid | 5 | C9H8O4 |

|
| 9 | 8-gingerol | 5 | C19H30O4 |
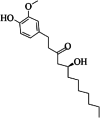
|
| 10 | stigmasterol | 4 | C29H48O |
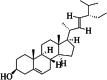
|
| 11 | clionasterol | 1 | C29H50O |

|
| 12 | zingerone | 1 | C11H14O3 |
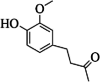
|
| 13 | alpha-curcumene | 1 | C15H22 |

|
| 14 | 10-gingerol | 1 | C21H34O4 |

|
| 15 | gingerdione | 0 | C17H24O4 |
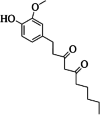
|
SRC, CCNA2, and MMP9 as the target genes in ginger treating NASH-HCC
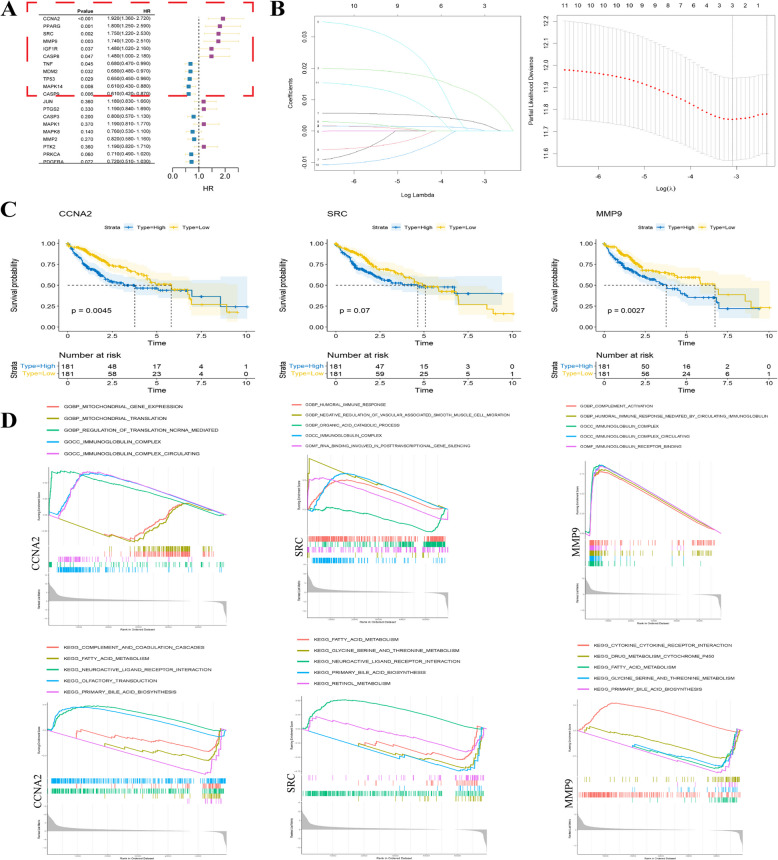
Univariate Cox regression analysis of candidate targets showed that CCNA2, PPARG, SRC, MMP9, IGF1R, CASP8, TNF, MDM2, TP53, MAPK14, and CASP9 have great value in the clinical diagnosis of HCC (Fig. 3A). Lasso-Cox regression was used to further screen the risk factors for these 11 genes. When the lambda was equal to lambda.min, CCNA2, SRC, and MMP9 were considered as risk factors to be included in the subsequent study (Fig. 3B). After that, the transcriptome and clinical information of the TCGA-LIHC dataset from the TCGA liver cancer database were used to analyze the prognosis of target genes (CCNA2, SRC, and MMP9). Initially, target genes were divided into low- and high-expression groups based on the median. Survival curves showed that the high CCNA2 and MMP9 expression groups were associated with a reduced survival probability in patients with HCC (p < 0.05), but there was no statistically significant difference in survival probability between the low and high SRC expression groups (p = 0.07) (Fig. 3C). Single-gene GSEA analysis of target genes showed that among the top five GO enrichment results, CCNA2, SRC, and MMP9 were all related to the immunoglobulin complex, whereas among the top five KEGG results, they were all related to fatty acid metabolism and primary bile acid biosynthesis (Fig. 3D). Dysregulation of bile acid and fatty acid metabolism can lead to the malignant transformation of NASH into HCC [22, 23]. The GSEA results suggest that the target genes may play an important role in the progression of NASH-HCC. Interestingly, we used regression analysis to screen target genes of ginger treating NASH-HCC from candidate targets, one of which is SRC, a member of the non-receptor tyrosine kinase family. It has been reported that phosphorylated Src can activate the MAPK signaling pathway in various cancers.
Fig. 3.
Finding and analyzing target genes. A Forest map was drawn based on univariate COX regression analysis of candidate genes. B Lasso regression analysis coefficient graph and parameter graph based on univariate COX regression analysis and three target genes (CCNA2, SRC, MMP9) with the ability to regulate HCC were identified. C Survival curves of target genes based on clinical information of TCGA-LIHC data. D GSEA analysis of target genes
Ginger may exert anti-NASH-HCC effects via the NOX2/Src/MAPK signaling pathway in macrophages
To further investigate the detail functions of these target genes on specific individual cells, we analyzed a single-cell RNA sequencing dataset to understand the profiles of the target genes in HCC. We first used R 4.3.2 to analyze the five HCC tissue samples from the single-cell RNA sequencing set GSE242889, and 14 clusters were obtained (Fig. 4A). Subsequently, 11 cell types were identified using marker genes (Fig. 4B), and the distribution and expression of CCNA2, SRC, and MMP9 in HCC were calculated. As shown in the violin and feature plots, CCNA2 was highly expressed mainly in cancer cells, cancer stem cells, and T cells, while SRC and MMP9 were highly expressed in macrophages (Fig. 4C, D).
Fig. 4.
Single-cell RNA sequencing dataset and transcriptome dataset revealed that ginger treatment for NASH-HCC is mainly associated with TAMs. A Five HCC tissue samples from single-cell RNA sequencing dataset GSE242889 were analyzed using R 4.3.2, and 14 clusters were obtained. B 11 cell types were identified by marker genes. C Violin diagram shows the expression level of CCNA2, SRC and MMP9 in different cell types. D Feature plot shows the distribution of target genes on umap. E Box diagram shows transcription levels of the NADPH oxidase family and SRC in healthy and NASH-HCC livers of GES164760 dataset. F Feature plot shows the distribution of NOX2 and key genes on MAPK signailing pathway on umap. G Feature plot shows that both NOX2 and SRC are expressed at a high level in TAMs
It has been reported that SRC can be activated by ROS, NADPH oxidase (NOX) is the main source of ROS [24–27]. We obtained a box diagram of differential expression in the NOXs family between healthy liver and NASH-HCC from the GSE164760 dataset, and only the differential expression of NOX2 was statistically significant (p < 0.05) (Fig. 4E). It has been reported that NOX2 was also mainly expressed in liver macrophages [28]. We analyzed the distribution of NOX2 (CYBB) and key genes on the MAPK signaling pathway (MAPK1, MAPK8, MAPK14) in HCC using a single-cell RNA sequencing dataset, and found that the above genes were expressed in tumor-associated macrophages, and that NOX2 (CYBB) was highly expressed in macrophages (Fig. 4F, G).
By analyzing the single-cell RNA sequencing dataset and the transcriptome data, we found that the target gene SRC and its upstream gene NOX2 were highly expressed in tumor-associated macrophage (TAMs), and the key genes on the downstream MAPK signaling pathway of SRC were also expressed in TAMs. Therefore, we hypothesized that the main active compounds of ginger act effects mainly by regulating macrophages in the NASH-HCC tumor microenvironment, and this effect may be achieved via the NOX2/Src/MAPK signaling pathway.
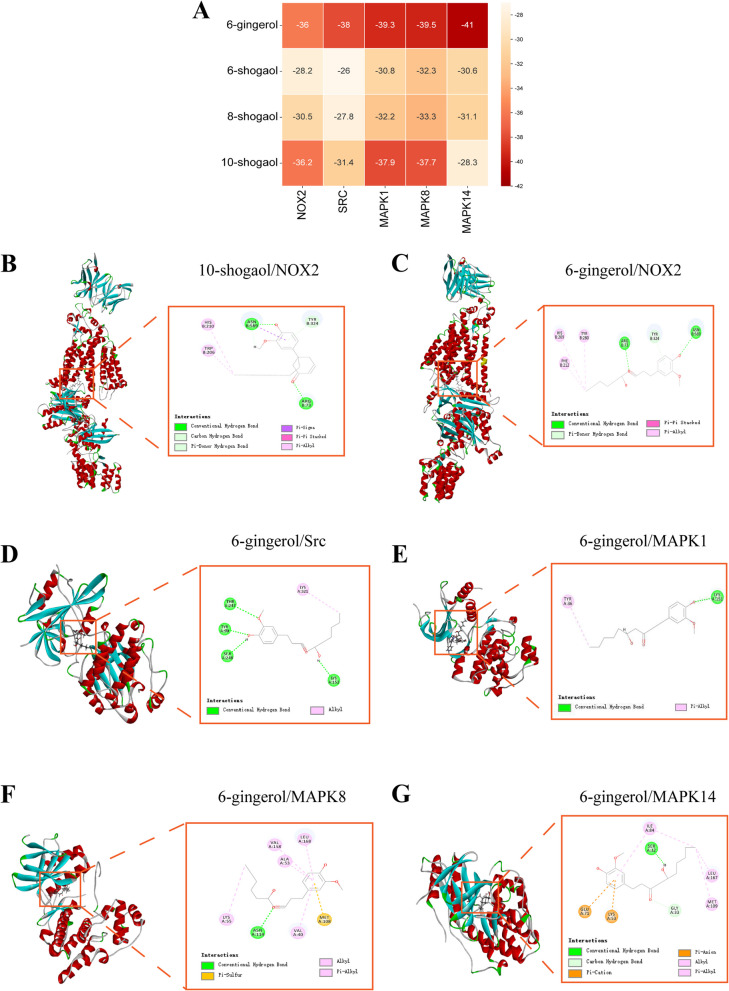
6-Gingerol exhibit the best binding ability to core proteins in the NOX2/Src/MAPK signaling pathway among the compounds of ginger
We took the top five compounds of ginger (including 6-shogaol, 6-gingerol, 8-shogaol, and 10-shogaol) in the ginger active compound-hub gene network, performed molecular docking with NOX2, SRC, and the key targets in the MAPK signaling pathway (MAPK1, MAPK8, MAPK14). Although beta-sitosterol was among the top five compounds, it has also been reported to be present in various plants and is not specific to ginger; therefore, it was not included in this study. The docking score results are displayed as a heat map (Fig. 5A), and the compounds with the best binding ability to each protein were selected for docking visualization. The docking score of molecular docking provides a reference for the binding ability of small molecules to proteins. It is generally believed that the lower the binding energy, the better the binding effect between small molecules and proteins. The docking results of 6-gingerol (− 36) (Fig. 5C) and 10-shogaol (− 36.2) (Fig. 5B) with NOX2 were lower than − 30, indicating that 6-gingerol and 10-shogaol May have a good combination with NOX2. But when considered with the docking results of other targets (Fig. 5D-G), 6-gingerol is more prominent. Therefore, we hypothesized that 6-gingerol, the main active compound of ginger, plays an anti-NASH-HCC role through the NOX2/Src/MAPK signaling pathway.
Fig. 5.
Molecular docking demonstrates the binding of major compounds and targets of ginger therapy for NASH-HCC. A Heat map of docking result scores. B Demonstration of NOX2 and 10-shogaol docking results. C Demonstration of NOX2 and 6-gingerol docking results. D Displayed the results of Src docking with 6-gingerol. E Demonstration of the docking results between MAPK1 and 6-gingerol. F Demonstration of MAPK8 and 6-gingerol docking results. G Demonstration of MAPK14 and 6-gingerol docking results
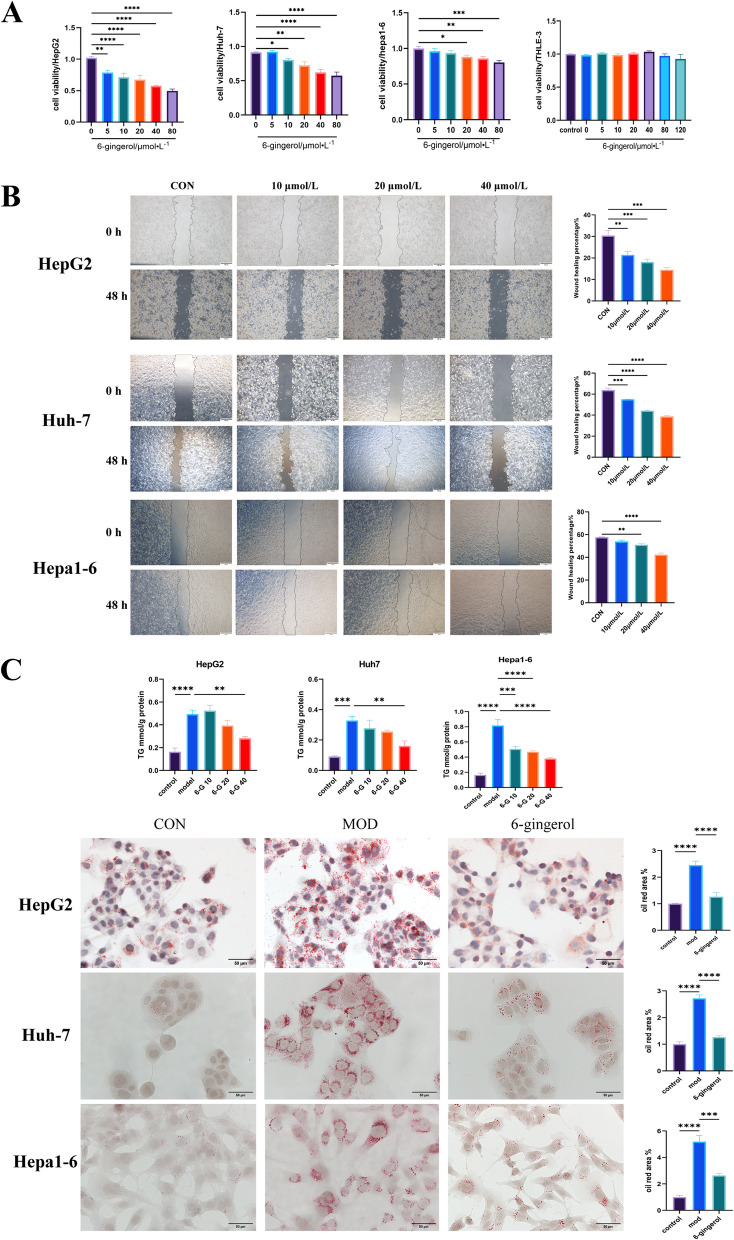
6-Gingerol exhibits anticancer and lipid-lowering effects in vitro
The results of molecular docking showed that 6-gingerol interacted well with core proteins on the NOX2/Src/MAPK signaling pathway. We studied the anticancer activity of 6-gingerol in vitro. The results showed that 6-gingerol could inhibit the proliferation and decrease the cell activity of human (HepG2, Huh- 7) and murine-derived (Hepa1 - 6) liver cancer cells in vitro. And it is not cytotoxic to normal human liver cells (THLE- 3) in the effective dose range of the drug (Fig. 6A). According to the results of CCK- 8 and the previous experimental experience of our team, 6-gingerol with the concentration of 10, 20, 40 μM was selected for the follow-up experimental studies. The results showed that 6-gingerol has an inhibitory effect on the migration of liver cancer cells in vitro (Fig. 6B). Severe steatosis in tumor cells is commonly seen in obesity-driven and non-alcoholic steatohepatitis (NASH) -driven HCC mouse models, and this has also been confirmed in human NASH-HCC, which may reflect a characteristic metabolic alteration of NASH-HCC [29]. We treated HepG2, Huh- 7 and Hepa1 - 6 cells with 100 μM oleic acid (OA) for 24 h to establish in vitro models of lipid accumulation. The results showed that 6-gingerol could reduce lipid deposition in HCC cells (Fig. 6C). It is suggested that 6-gingerol has the effects of anti-cancer and lipid-lowering.
Fig. 6.
The anticancer effects of 6-gingerol. A The anti-cancer effect of 6-gingerol on three kinds of liver cancer cells (HepG2, Huh- 7, and Hepa1 - 6) and the cytotoxicity detection of 6-gingerol on human normal hepatocytes (THLE- 3) were detected by CCK- 8 assay HepG2 (n = 5), Huh- 7 (n = 5), Hepa1 - 6 (n = 6), THLE- 3 (n = 6). B The migration effect of 6-gingerol on hepatocellular carcinoma cells was detected by wound healing assay, HepG2 (n = 3), Huh- 7 (n = 3), Hepa1 - 6 (n = 3). C Three kinds of hepatocellular carcinoma cells (HepG2, Huh- 7, and Hepa1 - 6) were treated with oleic acid (OA) for 24 h to induce intracellular lipid deposition. The drug group was treated with OA and 6-gingerol together for 24 h. The effects on lipid deposition of 6-gingerol were observed by intracellular TG levels and oil red O staining, HepG2 (n = 5), Huh- 7 (n = 5), Hepa1 - 6 (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
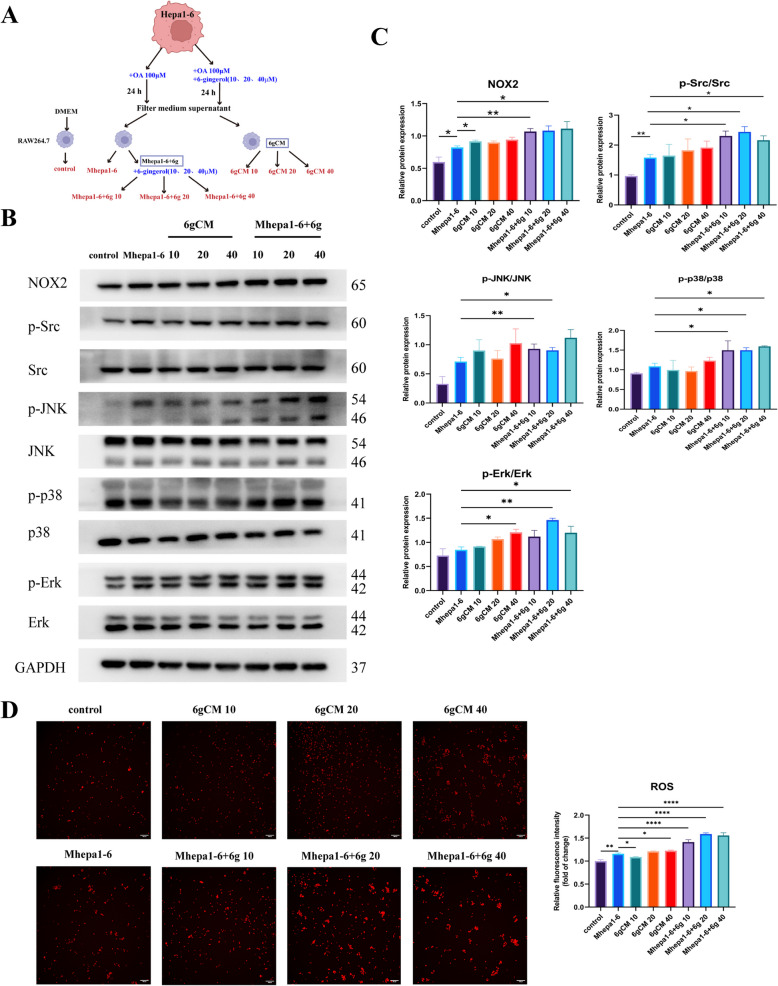
6-Gingerol regulates TAMs in the TME through NOX2/Src/MAPK signaling pathway
Combining network pharmacology, bioinformatics, and single-cell RNA sequencing analysis results, we hypothesized that 6-gingerol may play an anti-NASH-HCC role through the NOX2/Src/MAPK signaling pathway, and that this role may be related to macrophages in TME. Since 6-gingerol has anti-tumor and lipid-lowering effects in vitro, we designed the 6 gCM groups and the Mhepa1 - 6 + 6 g groups to determine whether 6-gingerol can directly regulate macrophages through the NOX2/Src/MAPK signaling pathway or indirectly affected by changes of soluble factors in the microenvironment after anti-tumor (Fig. 7A). The results of Western blot showed that the key targets NOX2 and Src in Mhepa1 - 6 were elevated compared with those in the control group, suggesting that NOX2 and Src may be involved in macrophages regulation in the tumor microenvironment, which was consistent with the previous hypothesis. Compared with Mhepa1 - 6, the expressions of NOX2, p-Src, p-p38 and p-JNK in 6 gCM groups were not significantly increased (Fig. 7B, C). However, compared with Mhepa1 - 6 group, NOX2, p-Src, p-p38, p-JNK and p-Erk in Mhepa1 - 6 + 6 g group were increased (Fig. 7B, C). These findings suggested that 6-gingerol can directly regulate macrophages through the NOX2/Src/MAPK signaling pathway, rather than the influence of soluble factors changed in the microenvironment after anti-tumor treatment. The results of intracellular ROS levels were similar to those of Western blot (Fig. 7D), indicating that 6-gingerol can directly regulate macrophages through the NOX2/ROS/Src/MAPK signaling pathway, and its effect is superior to the microenvironment changes after 6-gingerol intervention in tumor cells.
Fig. 7.
6-gingerol regulates macrophages in the TME through NOX2/Src/MAPK signaling pathway. A Experimental grouping diagram. Detailed procedures are described in the Methods section. B According to the experimental grouping, WB was used to detect protein expression in each group. Corresponding uncropped full-length gels and blot have been included in the supplementary files. C Histogram of protein statistics of each group, NOX2 (n = 3), p-Src/Src (n = 4), p-JNK/JNK (n = 3), p-p38/p38 (n = 3), p-Erk/Erk (n = 3). D Intracellular ROS expression level of each group (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
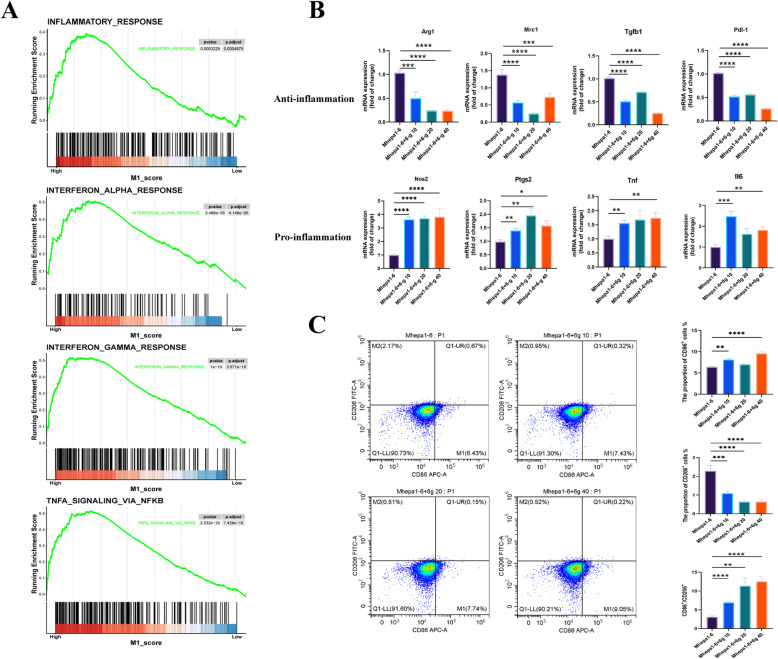
6-Gingerol remodel tumor-associated macrophages toward the M1 phenotype
The MAPK signaling pathway is widely recognized for its role in mediating inflammation. Given the close associations among inflammation, NOX2, and ROS with M1-like macrophages, we postulate that 6-gingerol may facilitate the remodeling of tumor-associated macrophages towards the M1 phenotype. Initially, the analysis of the transcriptome data GSE164760 of NASH-HCC showed that the high tumors of “M1” enriched the gene set related to immune activity such as INFLAMATORY_RESPONSE, INTERFERON_ALPHA_RESPONSE, INTERFERON_GAMMMA_RESPONSE and TNFA_SIGNALING_VIA_NFKB. Suggesting that a M1-like TAM in NASH-HCC is associated with higher immune activity (Fig. 8A). Subsequent qRT-PCR was used to detect the expression of M1-like and M2-like macrophage related factors. The results showed that 6-gingerol increased the expression of M1-related proinflammatory factors INOS, Ptgs2, Tnf, and Il6 in tumor-associated macrophages, while decreased the expression of M2-related anti-inflammatory factors Arg1, Mrc1, Tgfb, and Pdl- 1 (Fig. 8B). Flow cytometry results showed that compared with Mhepa1 - 6, the proportion of M1 was increased in Mhepa1 - 6 + 6 g groups, while the proportion of M2 was decreased. Besides, the proportion of M1/M2 was increased, and Mhepa1 - 6 + 6 g 40 had the best effect (Fig. 8C). In GSEA analysis of NASH-HCC patients, M1-like TAM was indeed associated with a stronger immune response, while PCR and flow cytometry demonstrated the potential of 6-gingerol to reprogram M2-like TAM into the M1 phenotype.
Fig. 8.
6-gingerol reshaped tumor-associated macrophages to M1 phenotype. A Analysis of TAM in tumor samples from the NASH-HCC transcriptome dataset GSE164760 showed that high expression of M1 enriched the gene set associated with immune activity. B The marker genes of M1-like macrophages and M2-like macrophages were detected by qRT-PCR (n = 5). C Flow cytometry was used to detecte the ratio of M1 and M2 in TAM after 6-gingerol intervention (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Discussion
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality globally. Non-alcoholic steatohepatitis (NASH) and alcohol-associated liver disease (ALD), contribute to the majority of HCC in North America, Europe, Latin America, and Australasia [30, 31]. Simultaneously, NASH has emerged as the fastest growing cause of liver disease among liver transplant recipients from 2002 to 2022, surpassing chronic hepatitis C (CHC) as the primary cause of HCC in this population [32]. Although the prevalence of NASH-HCC is increasing annually and may soon become the leading cause of HCC, the treatment of which remains under extensive investigation.
NASH-HCC demonstrates a reduced responsiveness to current immunotherapies compared to virus-induced HCC attributed primarily to the unique TME and the presence of NASH-related comorbidities, including obesity and diabetes [33]. Thus, there is an urgent need for the identification of novel therapeutic agents and targets. Traditional Chinese medicine (TCM) constitutes a vital component of complementary and alternative medicine, and its combined anti-tumor efficacy has been demonstrated by experimental and clinical evidence [34]. Additionally, TCM modulates immune responses in cancer patients and enhances chemotherapy efficacy. Even within the immunosuppressive tumor microenvironment, TCM can also play an anti-tumor role by up-regulating the immune response [11]. Ginger is one of the most commonly used herbs in TCM. It has been reported to possess immunomodulatory properties, but but studies on its effects on tumor-associated macrophages (TAMs) remain limited [35, 36]. While research has shown that a combination of curcumin, resveratrol, and epicatechin gallate could polarize M2-TAMs into M1 macrophages in HPV+ tumors, there is a notable lack of studies focused solely on ginger components. Moreover, the application of ginger in the treatment of NASH-HCC is underexplored. In this study, we demonstrate that 6-gingerol, the main active compound in ginger, reprograms M2-like macrophages in the TME to the M1 phenotype through the NOX2/Src/MAPK signaling pathway, thereby enhancing immune responses against NASH-HCC.
Cancer cells can actively modulate their microenvironment through the secretion of various cytokines, chemokines, which lead to reprogramming of surrounding cells [37]. The tumor immune microenvironment consists of both innate immune cells (such as macrophages, neutrophils, dendritic cells, natural killer cells, and myeloid-derived suppressor cells) and adaptive immune cells (including T and B cells) [38]. Owing to the relative insensitivity of NASH-HCC to conventional immune checkpoint inhibition (ICI), numerous studies are currently focused on identifying novel therapeutic targets and developing agents that could enhance sensitivity to treatment. Analysis of spatially resolved single-cell atlas from patients with NASH-HCC, virus-HCC (HBV-HCC and HCV-HCC), and healthy donors has revealed that interactions between MDSCs and TAMs with effector T cells underlie immunosuppression in NASH-HCC, presenting targetable opportunities [4]. In diet-induced mouse models of NASH over a five-month period, two significant characteristics were observed within the liver immune microenvironment during NASH pathogenesis: the expansion of macrophages exhibiting TAM molecular signatures and depletion of CD8+ T cells [5].
As a crucial element within the tumor microenvironment (TME), tumor-associated macrophages (TAMs) exhibit substantial plasticity. Consequently, several therapeutic strategies aimed at targeting macrophages have been proposed in both animal models and clinical trials. These strategies include reduction or depletion of TAMs, repolarization of TAMs towards an M1-like phenotype, and blocking the inhibitory receptors (immune checkpoints) on TAMs [39]. IL4R Exo (si/mi) has been found to enhance anti-tumor immunity and inhibit tumor growth by reprogramming TAMs into M1-like macrophages [40]. The TLR9 agonist CpG ODN downregulates glycolysis levels. It also inhibits the M2 polarization of HCC- TAMs, and reverses the tumor-promoting effect of TAMs in vitro and in vivo [41]. Therefore, reprogramming M2-like macrophages to the M1 phenotype may represent an effective strategy in the immunotherapy of NASH-HCC.
MAPK is a recognized pro-inflammatory pathway that can be involved in the regulation of macrophage polarization in TME. For instance, chenodeoxycholic acid suppresses acute myeloid leukemia (AML) progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization [42]. USP7 inhibitors delayed tumor growth in mice with Lewis lung carcinoma, promoted tumor infiltration of M1 MΦs and IFN-γCD8 T cells and mediate MΦs reprogramming by activating the p38 MAPK pathway [43]. This study predicts that the NOX2/Src/MAPK signaling pathway and TAMs may play a key role in attenuating NASH-HCC by considering of GO and KEGG enrichment, as well as transcriptome results. It has been reported that the activation of NOX2 can promote inflammasome activation, and plays an antitumor role in the tumor microenvironment [44]. Moreover, in M1-like macrophages, macrophages augment NOX2, inducible nitric oxide synthase (iNOS), and tumor necrosis factor receptor-associated factor 6 contribute to increased ROS production, triggering inflammatory responses, phagocytosis, and cytotoxicity [45].
In present study, we observed that compared with Mhepa1 - 6, 6-gingerol activated the NOX2/Src/MAPK signaling pathway after direct intervention in macrophages (Mhepa1 - 6 + 6 g), elevating intracellular ROS levels. It has been reported that Nox-derived ROS production regulates M1/M2 macrophage polarization, affecting inflammation, infection, and tumor progression [46]. IL4 receptor targeting enables nab-paclitaxel to enhance reprogramming of M2-type macrophages into M1-like phenotype via ROS-HMGB1-TLR4 axis and inhibition of tumor growth and metastasis [47]. In line with these findings, we observed that 6-gingerol treatment increased pro-inflammatory markers associated with M1-like macrophages while simultaneously reducing M2 macrophage markers and enhancing the M1/M2 ratio.
Taken together, our study suggests that ginger and its main active compound 6-gingerol can reprogram M2-like macrophages toward M1 phenotype through NOX2/Src/MAPK signaling pathway and increase NOX2-derived ROS to play an anti-tumor role. Nevertheless, the present study has several limitations. First, the predicted results of this experiment are mainly based on computer simulation. Although the in vitro experiment can demonstrate that 6-gingerol can improve the immunosuppression of TME by reprogramming TAMs, further studies involving animal models are required to elucidate the effects of TME complexity and individual drug tolerance. Second, represent only a simplified aspect of the disease and lack comprehensive simulation. Therefore, more complex experiments are needed to prove the interaction between macrophages and other cells, such as T cells and MDSCs. Finally, although we did conclude that 6-gingerol has no cytotoxicity to normal liver cells (THLE- 3) at effective doses range, additional in vivo studies are essential to assess potential drug toxicity and side effects.
Conclusion
The principal conclusion to be drawn is that 6-gingerol, the primary active compound in ginger, effectively reprograms tumor-associated macrophages via the NOX2/Src/MAPK signaling pathway, transforming them from the M2 to the M1 phenotype. This reprogramming process helps mitigate immunosuppression in the TME while enhancing ROS production to promote cytotoxicity against tumor cells. Our study provides evidence that ginger and its active compounds may offer potential therapeutic avenues for tumor immunotherapy. Further in vivo validation is required to confirm these findings and better understand the interactions between macrophages and tumor cells in vivo.
Supplementary Information
Acknowledgements
Not applicable.
Clinical trial number
Not applicable.
Abbreviations
- ADME
Absorption, distribution, metabolism and excretion
- Arg1
Arginase-1
- BC
Betweenness centrality
- BP
Biological process
- CC
Closeness centrality
- CC
Cell composition
- CCNA2
Cyclin-A2
- CTCF
CCCTC-binding factor
- CSNK2 A1
Casein kinase 2 alpha 1
- DC
Degree centrality
- Erk
Extracellular signal-regulated kinase
- EGR1
Early growth response factor 1
- GO
The Gene Ontology
- GEO
Gene Expression Omnibus
- ICI
Immune checkpoint inhibition
- Il6
Interleukin-6
- JNK
C-Jun NH2- terminal kinase
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MDSC
Myeloid-derived suppressor cells
- MAPK
Mitogen-activated protein kinase
- MMP9
Matrix metalloproteinase-9
- Mrc1
Macrophage mannose receptor 1
- MF
Molecular function
- NOX2(CYBB)
NADPH (nicotinamide adenine dinucleotide phosphate) oxidases 2
- Nos2
Nitric oxide synthase, inducible
- PPARG
Peroxisome proliferator-activated receptor gamma
- PD1
Programmed cell death protein 1
- PD-L1
Programmed cell death 1 ligand 1
- Ptgs2
Prostaglandin G/H synthase 2
- SRC
Proto-oncogene tyrosine-protein kinase Src
- TAM
Tumor-associated macrophages
- TME
Tumor microenvironment
- TCM
Traditional Chinese medicine
- TCMSP
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform
- TCMSID
Traditional Chinese Medicine Simplified Integrated Database
- Tgfb1
Transforming growth factor beta-1 proprotein
- Tnf
Tumor necrosis factor
- TCGA
The Cancer Genome Atlas Program
- VEGF-R2
Vascular endothelial growth factor receptor 2
Authors’ contributions
Q L Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. M W Data curation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. X H Conceptualization, Project administration, Supervision, Writing – review & editing. S W Methodology, Supervision, Validation, Writing – review & editing. C L Funding acquisition, Writing – review & editing. P L Funding acquisition, Writing – review & editing. W X Methodology, Writing – review & editing. L Y Supervision, Writing – review & editing. C D Conceptualization, Supervision, Writing – review & editing. M Z Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. J W Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (NO. 82274164, NO. 81973653, NO. 81673659, NO.82104469), Chongqing Natural Science Foundation (cstc2021jcyj-msxmX0320) and Chongqing traditional Chinese Medicine inheritance and Innovation team project (2023090006KJZX2022WJW008).
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mingming Zhang, Email: zhangmm@cqmu.edu.cn.
Jianwei Wang, Email: wjwcq68@163.com.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: a cancer journal for clinicians. 2024;74(1):12–49. 10.3322/caac.21820. [DOI] [PubMed]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. [DOI] [PubMed]
- 3.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–6. 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Wang L, Cong L, Wong CC, Zhang X, Chen H, et al. Spatial proteomics of immune microenvironment in nonalcoholic steatohepatitis-associated hepatocellular carcinoma. Hepatology (Baltimore, MD). 2024;79(3):560–74. 10.1097/hep.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang P, Chen Z, Kuang H, Liu T, Zhu J, Zhou L, et al. Neuregulin 4 suppresses NASH-HCC development by restraining tumor-prone liver microenvironment. Cell Metab. 2022;34(9):1359-76.e7. 10.1016/j.cmet.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6(1):75. 10.1038/s41392-021-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084. 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Sun M, Yao W, Wang F, Li X, Wang W, et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer. 2020;8(1). 10.1136/jitc-2019-000317. [DOI] [PMC free article] [PubMed]
- 9.Boutilier AJ, Elsawa SF. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021;22(13). 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed]
- 10.Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front Immunol. 2020;11:609705. 10.3389/fimmu.2020.609705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother Biomedecine & pharmacotherapie. 2020;121:109570. 10.1016/j.biopha.2019.109570. [DOI] [PubMed]
- 12.Wang S, Fu JL, Hao HF, Jiao YN, Li PP, Han SY. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res. 2021;170:105728. 10.1016/j.phrs.2021.105728. [DOI] [PubMed] [Google Scholar]
- 13.Samadi M, Moradinazar M, Khosravy T, Soleimani D, Jahangiri P, Kamari N. A systematic review and meta-analysis of preclinical and clinical studies on the efficacy of ginger for the treatment of fatty liver disease. Phytotherapy research : PTR. 2022;36(3):1182–93. 10.1002/ptr.7390. [DOI] [PubMed] [Google Scholar]
- 14.Ahn J, Lee H, Jung CH, Ha SY, Seo HD, Kim YI, et al. 6-Gingerol Ameliorates Hepatic Steatosis via HNF4α/miR-467b-3p/GPAT1 Cascade. Cell Mol Gastroenterol Hepatol. 2021;12(4):1201–13. 10.1016/j.jcmgh.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Li D, Wang S, Peng Z, Tan Q, He Q, et al. 6-Gingerol Ameliorates Hepatic Steatosis, Inflammation and Oxidative Stress in High-Fat Diet-Fed Mice through Activating LKB1/AMPK Signaling. Int J Mol Sci. 2023;24(7). 10.3390/ijms24076285. [DOI] [PMC free article] [PubMed]
- 16.Li J, Wang S, Yao L, Ma P, Chen Z, Han TL, et al. 6-gingerol ameliorates age-related hepatic steatosis: Association with regulating lipogenesis, fatty acid oxidation, oxidative stress and mitochondrial dysfunction. Toxicol Appl Pharmacol. 2019;362:125–35. 10.1016/j.taap.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Liu JP, Wang J, Zhou SX, Huang DC, Qi GH, Chen GT. Ginger polysaccharides enhance intestinal immunity by modulating gut microbiota in cyclophosphamide-induced immunosuppressed mice. Int J Biol Macromol. 2022;223(Pt A):1308–19. 10.1016/j.ijbiomac.2022.11.104. [DOI] [PubMed] [Google Scholar]
- 18.Song C, Ji Y, Wang W, Tao N. Ginger polysaccharide promotes myeloid-derived suppressor cell apoptosis by regulating lipid metabolism. Phytotherapy research : PTR. 2023;37(7):2894–901. 10.1002/ptr.7784. [DOI] [PubMed] [Google Scholar]
- 19.Hamza AA, Heeba GH, Hamza S, Abdalla A, Amin A. Standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/ inflammation pathway. Biomed Pharmacother. 2021;134:111102. 10.1016/j.biopha.2020.111102. [DOI] [PubMed]
- 20.Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z, et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–40. 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CY, Chen YN, Shiau JP, Tang JY, Hou MF, Chang HW. Ginger-Derived 3HDT Exerts Antiproliferative Effects on Breast Cancer Cells by Apoptosis and DNA Damage. Int J Mol Sci. 2023;24(6). 10.3390/ijms24065741. [DOI] [PMC free article] [PubMed]
- 22.Pinyol R, Torrecilla S, Wang H, Montironi C, Piqué-Gili M, Torres-Martin M, et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2021;75(4):865–78. 10.1016/j.jhep.2021.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie G, Wang X, Huang F, Zhao A, Chen W, Yan J, et al. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int J Cancer. 2016;139(8):1764–75. 10.1002/ijc.30219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi S, Feng Z, Li Q, Qi Z, Zhang Y. Inhibition of ROS-mediated activation Src-MAPK/AKT signaling by orientin alleviates H(2)O(2)-induced apoptosis in PC12 cells. Drug Des Dev Ther. 2018;12:3973–84. 10.2147/dddt.S178217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Wang Y, Zhang W, Zhao D, Zhang L, Fan J, et al. Membranous NOX5-derived ROS oxidizes and activates local Src to promote malignancy of tumor cells. Signal Transduct Target Ther. 2020;5(1):139. 10.1038/s41392-020-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong Q, Ma M, Zhang L, Liu S, He S, Wu J, et al. Icariside II potentiates the anti-PD-1 antitumor effect by reducing chemotactic infiltration of myeloid-derived suppressor cells into the tumor microenvironment via ROS-mediated inactivation of the SRC/ERK/STAT3 signaling pathways. Phytomedicine : Int J Phytother Phytopharmacol. 2023;110:154638. 10.1016/j.phymed.2022.154638. [DOI] [PubMed] [Google Scholar]
- 27.Luo HM, Wu X, Xian X, Wang LY, Zhu LY, Sun HY, et al. Calcitonin gene-related peptide inhibits angiotensin II-induced NADPH oxidase-dependent ROS via the Src/STAT3 signalling pathway. J Cell Mol Med. 2020;24(11):6426–37. 10.1111/jcmm.15288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crosas-Molist E, Fabregat I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 2015;6:106–11. 10.1016/j.redox.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujiwara N, Nakagawa H, Enooku K, Kudo Y, Hayata Y, Nakatsuka T, et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67(8):1493–504. 10.1136/gutjnl-2017-315193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younossi ZM, Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP reports : Innov Hepatol. 2021;3(4):100305. 10.1016/j.jhepr.2021.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PK, et al. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology. 2023;164(5):766–82. 10.1053/j.gastro.2023.01.033. [DOI] [PubMed] [Google Scholar]
- 32.Koh JH, Ng CH, Nah B, Tan DJH, Loomba R, Huang DQ. NASH is the Leading Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2024;22(1):197-9.e3. 10.1016/j.cgh.2023.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwälder M, El-Serag HB, et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. 2023;20(8):487–503. 10.1038/s41575-023-00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15(5):283–98. 10.5582/bst.2021.01318. [DOI] [PubMed] [Google Scholar]
- 35.Al-Ataby IA, Talib WH. Daily Consumption of Lemon and Ginger Herbal Infusion Caused Tumor Regression and Activation of the Immune System in a Mouse Model of Breast Cancer. Front Nutr. 2022;9:829101. 10.3389/fnut.2022.829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Wei S, Lu X, Qiao X, Simal-Gandara J, Capanoglu E, et al. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2021;350:129261. 10.1016/j.foodchem.2021.129261. [DOI] [PubMed] [Google Scholar]
- 37.Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Can Res. 2019;79(18):4557–66. 10.1158/0008-5472.Can-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu T, Dai LJ, Wu SY, Xiao Y, Ma D, Jiang YZ, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. 2021;14(1):98. 10.1186/s13045-021-01103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Xu X, Wei S, Jiang P, Xue L, Wang J. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. 2021;9(1). 10.1136/jitc-2020-001341. [DOI] [PMC free article] [PubMed]
- 40.Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137. 10.1016/j.biomaterials.2021.121137. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Han Q, Zhao H, Zhang J. Promotion of epithelial-mesenchymal transformation by hepatocellular carcinoma-educated macrophages through Wnt2b/β-catenin/c-Myc signaling and reprogramming glycolysis. J Experiment Clin Cancer Res. 2021;40(1):13. 10.1186/s13046-020-01808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Wei Y, Jia W, Can C, Wang R, Yang X, et al. Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. 2022;56:102452. 10.1016/j.redox.2022.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai X, Lu L, Deng S, Meng J, Wan C, Huang J, et al. USP7 targeting modulates anti-tumor immune response by reprogramming Tumor-associated Macrophages in Lung Cancer. Theranostics. 2020;10(20):9332–47. 10.7150/thno.47137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu X, Ding S, Lu G, Lin Z, Liao L, Xiao W, et al. Apolipoprotein C-III itself stimulates the Syk/cPLA2-induced inflammasome activation of macrophage to boost anti-tumor activity of CD8(+) T cell. Cancer Immunol Immunother. 2023;72(12):4123–44. 10.1007/s00262-023-03547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pérez S, Rius-Pérez S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants (Basel, Switzerland). 2022;11(7). 10.3390/antiox11071394. [DOI] [PMC free article] [PubMed]
- 46.Shiau DJ, Kuo WT, Davuluri GVN, Shieh CC, Tsai PJ, Chen CC, et al. Hepatocellular carcinoma-derived high mobility group box 1 triggers M2 macrophage polarization via a TLR2/NOX2/autophagy axis. Sci Rep. 2020;10(1):13582. 10.1038/s41598-020-70137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vadevoo SMP, Kang Y, Gunassekaran GR, Lee SM, Park MS, Jo DG, et al. IL4 receptor targeting enables nab-paclitaxel to enhance reprogramming of M2-type macrophages into M1-like phenotype via ROS-HMGB1-TLR4 axis and inhibition of tumor growth and metastasis. Theranostics. 2024;14(6):2605–21. 10.7150/thno.92672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.