Abstract
Background
The impact of human immunodeficiency virus (HIV) low-level viremia (LLV) on CD4 + T lymphocyte (CD4) cell count recovery during antiretroviral therapy (ART) remains unknown in China. This study aimed to investigate the association between LLV and CD4 count recovery among adults on ART in Southwest China.
Methods
A longitudinal cohort study of persons living with HIV (PLHIV) were conducted in Dehong Prefecture, Southwest China. Incidence of CD4 count recovery (CD4 cell count ≥ 500 cells/µl) was calculated for each follow-up year and characteristics of LLV (VL between 50 and 999 copies/ml) were described. Group-based trajectory model (GBTM) was used to identify and characterize the trajectories of CD4 cell count and VL during follow up. Longitudinal associations between LLV and CD4 count recovery were examined using a generalized estimating equation (GEE) with LLV as a time-updated variable.
Results
The study included a total of 7,485 PLHIV who received ART between 2008 and 2021 in Dehong. The median follow-up duration was 8.5 years. At baseline, the participants had a median age of 36 years, with males accounting for 60.5%. The median CD4 cell count at baseline was 268 cells/µl. Results of GBTM demonstrated that 730 patients (9.8%) experience LLV trajectories and 2,125 patients (28.4%) reached CD4 count recovery during follow-up. Compared to participants with the trajectory of VL < 50 copies/ml, the probability of CD4 count recovery were lower among participants with the trajectories of LLV 50–199 copies/ml (adjusted odds ratio [aOR] 0.69, 95% confidence interval [CI] 0.63–0.76) and LLV 200–999 copies/ml (aOR 0.51, 95% CI 0.45–0.59),
Conclusion
Sustained LLV is associated with poorer CD4 recovery among HIV patients who are receiving ART. Interventions to ensure that PLHIV maintain durably undetectable VL during treatment should be prioritized to achieve immune recovery.
Keywords: HIV, Low-level viremia, CD4 + lymphocyte count, Antiretroviral therapy, Longitudinal study
Introduction
Antiretroviral therapy (ART) can dramatically reduce human immunodeficiency virus (HIV) viral load (VL) for persons living with HIV (PLHIV) [1]. Although the majority of PLHIV achieve viral suppression below a detectable limit (usually < 50 copies/ml) within several months since ART, some proportion of PLHIV experience virological failure [> 200 copies/ml defined by guidelines of European or US HIV associations or > 1,000 copies/ml by the World Health Organization (WHO)] [2–4] during the long-term ART. Meanwhile, there are also a proportion of PLHIV with detectable VL level but < 1,000 copies/ml, an event referred to as low-level viremia (LLV) [5]. Some studies have already suggested adverse impacts of LLV on virological failure [5–7], acquired immunodeficiency syndromes (AIDS) events, serious non-AIDS events and death [8, 9], which attracted attention to optimized management of PLHIV with LLV.
Since ART initiation for PLHIV, CD4 + T lymphocyte (CD4) cell counts can reach normal level and maintain relatively high levels for 7–10 years, especially for most of those with viral suppression [10]. Studies indicated that an CD4 cell count increase to 500 cells/µl or more after ART may be beneficial in improving the survival of PLHIV [11, 12]. However, a small proportion of patients, even with viral suppression, experienced a considerable CD4 cell count decline [13]. Decreased CD4 is detrimental to human health since it may increase the risk of opportunistic infections, cardiovascular disease, and death [12–14]. A CD4 count decline may be HIV-related and concomitant with viral rebound or caused by factors not related to HIV, such as age at ART initiation [15]. Compared with PLHIV complete viral suppression (VL < 50 copies/ml), the proportion of CD4 count recovery was little investigated among those with specific LLV (VL level of 50 − 1,000 copies/ml). Moreover, the association between LLV and CD4 count recovery was investigated in epidemiological settings outside of China [16–19], but rarely in China [20].
Understanding the potential impact of LLV on CD4 count recovery could shed light on adverse health impacts of LLV and provide additional evidence for the PLHIV management. However, further investigation on the association between LLV and CD4 count recovery during ART is warranted. This paper may serve as a way to provide evidence of the magnitude of CD4 count recovery among patients with time-varied LLV and significance of LLV management during long-term ART.
In the present study, we hypothesize that LLV is associated with suboptimal CD4 count recovery. Using longitudinal study data, we characterized trajectories of VL and CD4 count among PLHIV receiving ART treatment. Furthermore, we examined the longitudinal association between LLV and CD4 count recovery, considering time-varying effects of VL and CD4 count during follow-up.
Methods
Study design and data sources
We conducted retrospective analysis of a longitudinal cohort study with the data of treatment-naive PLHIV from China’s National Free Antiretroviral Treatment Program database in Dehong Prefecture, Southwest China. The database consists of ART baseline and follow-up information of PLHIV in over 60 ART clinics in Dehong. ART-eligible individuals were interviewed by well-trained local doctors and nurses for information on demographic characteristics and HIV infection [21, 22]. Patients were requested for blood samples every 12 months to test CD4 cell count and VL during ART follow-ups and the measures were recorded in the ART database. In this study, the follow-up duration of each participant was calculated from the date of ART initiation to the date of the last CD4 or VL measurement by December 31, 2021. The study was approved by the institutional review board of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, with individual-level informed consent waived.
Participants
We included PLHIV in Dehong Prefecture, Southwest China who (1) started ART between 2008 and 2018, (2) were aged 18 years or older at ART initiation, (3) were recorded for at least two VL values, and (4) were recorded for at least two CD4 counts after 6 months of ART initiation.
Definitions of outcomes and exposure
CD4 counts were regarded as the outcome variables, which were regarded as continuous variable or binominal variables (with the cutoff value of 500 cells/µl) based on different statistical models. The CD4 count recovery was defined as CD4 ≥ 500 cells/µl [12, 19]. There were twice or more CD4 tests for each participant, so the outcome is longitudinal.
The exposure of interest was the VL level, which was also retrieved from the ART database in Dehong. The VL level of each testing was categorized into one of the following four groups: <50 copies/ml (defined as viral suppression [VS]), 50–199 copies/ml (defined as LLV 50–199), 200–999 copies/ml (LLV 200–999), and ≥ 1,000 copies/ml (defined as non-suppressed viremia [NSV]) [8]. Participants who had received multiple VL testing during follow-ups could result in changes in the VL level classification of each participant. Therefore, time-depended variable for VL was used in the analysis for the association with the outcome CD4 count recovery.
Covariates
Information on patients regarding age, sex, education, transmission route, duration from HIV acquisition to ART, opportunistic infections (OIs), baseline CD4 cell count, hemoglobin, positive hepatitis B surface Antigen (HBsAg), anti-hepatitis C virus (HCV) antibody, and antiretroviral regimens at baseline. Education was divided into three levels: illiteracy, primary school, and middle school or above [23]. The HIV transmission route was characterized into three groups: injection drug use (IDU), sexual contact, and others [8]. Consistent with published studies, continuous covariates were categorized for analysis: age was grouped into 18–30, 31–50, and > 50 years old, baseline CD4 count was grouped into < 200, 200–349, and ≥ 350 cells/µl [24], and hemoglobin level was grouped into < 90 g/L and ≥ 90 g/L. We described the missing values for variables of OIs, hemoglobin, and HBsAg at baseline as separate categories.
Statistical analysis
The participants’ characteristics were described by proportions of categorical variables and median (interquartile range, IQR) of continuous variables with skewed distribution. The CD4 count recovery rate (CD4 ≥ 500 cells/µl) was calculated according to the follow-up year. The denominator is the length of follow-up (person-years), and the numerator is the number of participants with CD4 ≥ 500 cells/µl. The incidence rate of CD4 count recovery by most recent (time-varied) VL categories (< 50, 50–199, 200–999, and ≥ 1,000 copies/ml) was also calculated, with the total person-years in each corresponding VL categories in the follow-up year interval as the denominator and the total times of CD4 count ≥ 500 cells/µl in next follow-up year interval as the numerator. The incidence and characteristics of LLV occurrence were described.
The participants were divided into several subgroups based on the CD4 count trajectories during the first 5 years of ART with group-based trajectory models (GBTM) [25, 26]. The basis of GBTM is the participants with similar pattern are clustered in the same subgroup to reduce the overall deviance [27]. If there were more than one CD4 testing per follow-up year for the participant, only the last CD4 count measure was used in the GBTM. The same GBTM method was used to group the participants by follow-up VLs. Then the relationship between the CD4 and VL trajectory subgroups were validated by Cochran-Mantel-Haenszel tests.
The Generalized Estimating Equation (GEE) model was used to investigate the association of time-varied LLV with CD4 count recovery. Reclassification occurred if the VL level of a certain participant changed from one category to another. The outcome of follow-up CD4 count was treated as binominal variable (< 500 and ≥ 500 cells/µl) in the GEE model. A combined stepwise method (The significance levels for forward /entering and backward /removing covariates were both at α = 0.05) of variable selection was performed to select the most predictable variables from the following factors: age, sex, education level, infection route, duration from HIV acquisition to ART, ART initiation calendar year, OIs, CD4 count, hemoglobin, HBsAg, Anti-HCV at baseline, and time-varying VL groups, hemoglobin, and antiretroviral regimen during ART follow-ups. Sensitivity analyses were done stratified by follow-up duration or cutoff value of CD4 count (350 cells/µl). The analysis was done after excluding PLHIV with irregular VL detection or with baseline CD4 ≥ 500 cells/µl. A two-sided p-value of less than 0.05 was considered as statistically significant. The analysis was conducted with the Statistical Analysis System (v.9.4; SAS Institute Inc., Cary, NC, US).
Results
Baseline characteristics of study population in Dehong
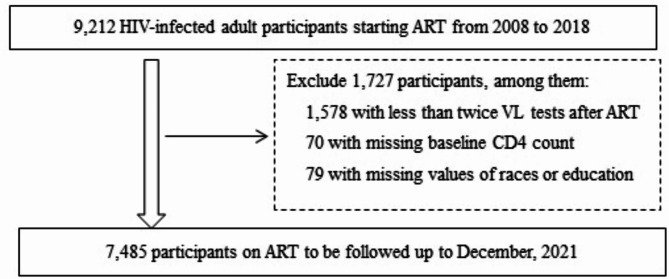
From January 2008 to December 2018, a total of 9,212 PLHIV receiving ART were enrolled in ART database and followed up through December 2021 in Dehong. Among them, 7,485 were eligible for further analysis in the study (Fig. 1) with median follow-up duration of 8.5 years. Table 1 describes the characteristics of the study population. At baseline, the median age of the patients was 36 (IQR: 30–44) years. Most of them were male (60.5%) and with education level of primary school or below (60.3%). The median CD4 count at baseline was 268 cells/µl (IQR: 156–399 cells/µl), with 33.8% of the participants’ baseline CD4 count < 200 cells/µl. The median time duration from HIV diagnosis to ART initiation was 10 months (IQR: 0.5–51). The proportions of patients starting ART in 2008–2011, 2012–2015, 2016–2018 was 38.2, 46.2 and 15.6%, respectively. A proportion of 80.8% started ART with the standard first-line regimen (tenofovir [TDF] or zidovudine [AZT]) + lamivudine (3TC) + (efavirenz [EFV] or nevirapine [NVP]) in China.
Fig. 1.
Participant enrollment flowchart. Abbreviation: ART, antiretroviral therapy; CD4, CD4+ T lymphocytes; HIV, humanimmunodeficiency virus; VL, viral load
Table 2.
Association of CD4 count and VL subgroups by GBTM of 7,485 PLHIV during ART follow-ups in Dehong, Southwest China, 2008–2021
| Follow-up VL | Follow-up CD4 count No. (%), cells/µl | Total | ||
|---|---|---|---|---|
| < 350 | 350 ~ 499 | ≥ 500 | ||
| VL suppression | 1,846 (28.7) | 2,626 (40.8) | 1,959 (30.5) | 6,431 (85.9) |
| LLV | 327 (44.8) | 265 (36.3) | 138 (18.9) | 730 (9.8) |
| NSV | 187 (57.7) | 109 (33.6) | 28 (8.6) | 324 (4.3) |
| Total | 2,360 (31.5) | 3,000 (40.1) | 2,125 (28.4) | 7,485 (100.0) |
Abbreviation: ART, antiretroviral therapy; CD4, CD4 + T lymphocytes; GBTM, group-based trajectory models; HIV, human immunodeficiency virus; LLV, low-level viremia; NSV, non-suppressed viremia; PLHIV, persons living with HIV; VL, viral load
Table 1.
Characteristics of the 7,485 PLHIV on ART in Dehong, Southwest China, 2008–2021
| Characteristic | No. | % |
|---|---|---|
| Age, y a | 36 (30, 44) | |
| 18 ~ 30 | 2,128 | 28.4 |
| 31 ~ 50 | 4,561 | 60.9 |
| ≥51 | 796 | 10.6 |
| Sex | ||
| Female | 2,957 | 39.5 |
| Male | 4,528 | 60.5 |
| Race | ||
| Han | 3,191 | 42.6 |
| Dai | 2,226 | 29.7 |
| Jingpo | 1,648 | 22.0 |
| Other | 420 | 5.6 |
| Education | ||
| Illiterate | 1,135 | 15.2 |
| Primary School | 3,377 | 45.1 |
| Middle school or above | 2,973 | 39.7 |
| Infection route | ||
| Sexual contact | 5,512 | 73.6 |
| IDU | 1,897 | 25.3 |
| other | 76 | 1.0 |
| HIV infection to ART, y a | 0.8 (0.0, 4.3) | |
| < 1 | 3,861 | 51.6 |
| ≥ 1 | 3,624 | 48.4 |
| Baseline OIs | ||
| No | 1,061 | 14.2 |
| Yes | 5,416 | 72.4 |
| Missing value | 1,008 | 13.5 |
| Baseline WHO clinical stage | ||
| 1 ~ 2 | 4,566 | 61.0 |
| 3 ~ 4 | 2,919 | 39.0 |
| Baseline CD4 count, cells/µl a | 268 (156, 399) | |
| < 200 | 2,533 | 33.8 |
| 200 ~ 349 | 2,569 | 34.3 |
| 350 ~ 499 | 1,256 | 16.8 |
| ≥ 500 | 1,127 | 15.1 |
| Baseline hemoglobin, g/La | 131 (116, 146) | |
| ≥ 90 | 7,050 | 94.2 |
| < 90 | 313 | 4.2 |
| Missing value | 122 | 1.6 |
| Baseline HBsAg | ||
| Negative | 5,139 | 68.7 |
| Positive | 405 | 5.4 |
| Missing value | 1,941 | 25.9 |
| Baseline anti-HCV antibody | ||
| Negative | 3,963 | 52.9 |
| Positive | 1,277 | 17.1 |
| Missing value | 2,245 | 30.0 |
| Baseline regimen | ||
| TDF + 3TC + EFV/NVP | 3,147 | 42.0 |
| AZT + 3TC + EFV/NVP | 2,906 | 38.8 |
| LPV/r + 3TC + AZT/TDF | 651 | 8.7 |
| other | 781 | 10.4 |
| ART initiation calendar year | ||
| 2008–2011 | 2,863 | 38.2 |
| 2012–2015 | 3,455 | 46.2 |
| 2016–2018 | 1,167 | 15.6 |
| Follow-up duration, ya | 8.5 (6.2, 10.2) | |
| ≤5 | 1,246 | 16.6 |
| >5 | 6,239 | 83.4 |
Data are presented as No. (%) unless otherwise specified
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; AZT, zidovudine; CD4: CD4 + T lymphocytes; EFV, efavirenz; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; LPV/r, lopinavir/ritonavir; NVP, nevirapine; OI, opportunistic infection; PLHIV, persons living with HIV; TDF, tenofovir
a Median (interquartile range)
CD4 count recovery in Dehong
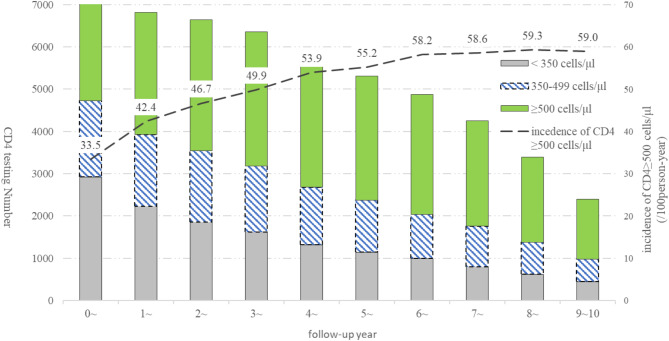
A total of 91,545 CD4 tests were performed in 7,485 PLHIV, and the median number of tests (IQR) was 12 (7–17). The incidence rates of CD4 count recovery (≥ 500 cells/µl) by follow-up year in 7,485 PLHIV increased from 33.5/100 person-years in the first ART year to 59.0/100 person-years in the tenth year (Fig. 2). After the VL levels were categorized into four groups: <50 copies/ml, LLV 50–199, LLV 200–999, and ≥1,000 copies/ml, the incidence rates of CD4 count recovery in the following year were 58.9 (26,333/44,714.6), 52.4 (1,446/2,761.6), 45.5 (707/1,553.5), and 24.7/100 person-years (909/3,680.2), respectively.
Fig. 2.
CD4 count recovery stratified by follow-up year among PLHIV on ART in Dehong, Southwest China, 2008–2021. Abbreviation: ART, antiretroviral therapy; CD4, CD4 + T lymphocytes; HIV, human immunodeficiency virus; PLHIV, persons living with HIV
Incidence of LLV in Dehong
Among the 7,485 PLHIV, 3,058 (40.9%) experienced LLV (50–999 copies/ml) during the follow-up years, and the numbers of participants with 1, 2 and 3 or more LLV occurrences were 1,861 (24.9%), 709 (9.5%), and 488 (6.5%), respectively. The median (IQR) time from ART to the first LLV occurrence was 2.0 (1.0–4.0) years.
Trajectories of CD4 count and LLV
Based on the GBTM model, the 7,485 PLHIV in Dehong were categorized into three subgroups by CD4 count trajectories: CD4 count < 350, 350–499, ≥ 500 cells/µl, and the proportions of the three subgroups were 31.5, 40.1, and 28.4%, respectively (Fig. 3). With similar method, the participants were also grouped into three subgroups by follow-up VL trajectories: VL < 50 copies/ml, 50–999 (LLV), and ≥ 1,000 copies/ml with the proportions of 85.9, 9.8 and 4.3%, respectively. The proportions of CD4 count recovery to ≥ 500 cells/µl among the three VL groups were 30.5, 18.9 and 8.6%, respectively, which was significantly different (P < 0.001) (Table 2).
Fig. 3.
CD4 count subgroup by group-based trajectory model among PLHIV on ART in Dehong, Southwest China, 2008–2021. Abbreviation: ART, antiretroviral therapy; CD4: CD4 + T lymphocytes; HIV, human immunodeficiency virus; PLHIV, persons living with HIV
CD4 count recovery of LLV groups lower than that of VL < 50 copies/ml with GEE
By treating the follow-up CD4 count as a dichotomous variable (≥ 500 cells/µl as the event outcome), when compared with participants with VL < 50 copies/ml, GEE model suggested that the adjusted odds ratios (ORs) of follow-up CD4 count recovery to ≥ 500 cells/µl in the groups of LLV 50–199 and 200–999 copies/ml were 0.69 (95% CI: 0.63–0.76) and 0.51 (95% CI: 0.45–0.59), respectively. The OR of VL ≥ 1,000 copies/ml group was 0.22 (95% CI: 0.19–0.24) compared with VL < 50 copies/ml group (Table 3).
Table 3.
Association of time-dependent LLV with CD4 count recovery by GEE model among 7,485 PLHIV during ART follow-ups in Dehong, Southwest China, 2008–2021
| Variable | Adjusted OR | 95% CI | P value |
|---|---|---|---|
| Recent VL level, copies/ml | |||
| < 50 | 1.0 (Ref) | - | - |
| 50 ~ 199 a | 0.69 | 0.63–0.76 | < 0.0001 |
| 200 ~ 999 a | 0.51 | 0.45–0.59 | < 0.0001 |
| ≥ 1,000 | 0.22 | 0.19–0.24 | < 0.0001 |
a two categories of LLV. The GEE model adjusted for the covariates of age at ART initiation, sex, education, infection route, duration from HIV acquisition to ART, ART initiation calendar year, baseline CD4 count, hemoglobin, HbsAg, anti-HCV, OIs, recent hemoglobin and recent ART regimen
Abbreviation: ART, antiretroviral therapy; CD4, CD4 + T lymphocytes; CI, confidence interval; GEE, generalized estimating equation; HbsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; LLV, low-level viremia; OI, opportunistic infection; OR, odds ratio; PLHIV, persons living with HIV; Ref, reference group; VL, viral load
When stratified by the follow-up duration (< 5 and ≥ 5 years) or excluding the PLHIV with baseline CD4 count ≥ 500 cells/µl, there was no change in the directions of the OR estimates for groups of LLV 50–199, LLV 200–999 copies/ml and VL ≥ 1,000 copies/ml. When analysed with the cutoff value of 350 cells/µl for the CD4 count recovery, the results were similar to the cutoff of 500 cells/µl.
Discussion
This longitudinal cohort study of 7,485 participants on ART from 2008 to 2021 in Dehong Prefecture, Southwest China, finds that the follow-up CD4 count of more than half of the participants recovered to ≥ 500 cells/µl level with 5-year ART. However, there were only about 30% participants with CD4 count recovery to ≥ 500 cells/µl when considering the CD4 count longitudinal trajectories during the whole ART follow-ups. Moreover, this study finds an inverse relationship between follow-up VL and CD4 cell recovery, offering clues on the importance of routine monitoring on VL during ART.
This study showed that PLHIV in Dehong could be partitioned to three main subgroups by different longitudinal CD4 count trajectories during ART follow-ups. The average CD4 count increased over time in all the different subgroups, which indicates short-term and long-term benefit of ART. CD4 count recovery was affected by a variety of factors, including baseline CD4 count [28, 29], gender [30], infection route [31] and baseline VL [18]. Generally, the duration of CD4 increase during ART in this study is consistent with the results of other studies [18, 32]. When evaluating CD4 count recovery throughout the whole ART process, only 30% participants reached the CD4 ≥ 500 cells/µl level, lower than those in other studies [19, 30]. This finding suggests that longitudinal CD4 trajectory may provide additional information than previous endpoint.
The proportion of LLV in Dehong from 2008 to 2021 was about 10% when defined as two or more LLV measures, or by group-based trajectory model. This was similar to the results of Elvstam [8], Ding [33] and Bai [34], and lower than the study result by Hermans [5] in South Africa and by Zhang [35] in Northeast China. The difference of LLV incidence may be due to the different study design and population characteristics. It is suggested that LLV is common in the ART population and should be taken into consideration of PLHIV management.
The associations of follow-up LLV and CD4 count recovery, resulted from the two markers’ trajectories contingency table and GEE model with follow-up VL as time-dependent variable, indicated that the LLV of 50–999 copies/ml impact the CD4 count recovery during ART. The similar results were shown in other studies [18, 36]. LLV may be one of the factors affecting CD4 count recovery. It is also possible that a certain factor (older age) contributes to the occurrence of LLV and CD4 count decline [36].
Monitoring the two markers may be important for ART management, but VL during ART follow-ups predicts adverse outcomes faster than CD4 count due to the relatively slow change of CD4 count. Better ART management with LLV is warranted to reduce the adverse impact of LLV on CD4 count recovery.
The limitations of this study include: (1) The data are only from one city in China. Caution is required when extrapolating data to other sites. (2) The data were collected from routine working data, and there may be certain errors in data entry and detection. (3) we could not infer a causal relationship between LLV and CD4 count recovery based on current study design. In addition, it is possible that there are other omitted factors that may also contribute to the LLV and suboptimal CD4 count recovery.
In conclusion, our analysis of a longitudinal study involving PLHIV receiving ART revealed that despite VL being controlled below the threshold for virological failure (i.e. VL < 200 or 1,000 copies/ml), persistent LLV (VL between 50 and 999 copies/ml) was associated with suboptimal CD4 count recovery during ART treatment. Efforts should be made to develop strategies that ensure sustained undetectable VL in PLHIV receiving ART, thereby promoting immune recovery.
Acknowledgements
We thank Dr. Zunyou Wu who deceased on 27 October 2023 for his contribution to this work. We thank the staff in Dehong for the continuous support.
Abbreviations
- 3TC
lamivudine
- AIDS
Acquired immunodeficiency Syndrome
- ART
Antiretroviral therapy
- AZT
zidovudine
- CD4
CD4 + T lymphocyte
- EFV
Efavirenz
- GBTM
Group-based trajectory model
- GEE
Generalized estimating equation
- HBsAg
Hepatitis B surface antigen
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- IDU
Injection drug use
- LLV
Low-level viremia
- LPV/r
Lopinavir/ritonavir
- NVP
Nevirapine
- NSV
Non-suppressed viremia
- OIs
Opportunistic infections
- PLHIV
Persons living with HIV
- TDF
Tenofovir
- VL
Viral load
- VS
Viral suppression
- WHO
World Health Organization
Author contributions
HY, CJ, and RY contributed to the conception and design of the study. YY, RY, DC, YC, YS, GX, PL, YF, HW, JS, and SD contributed to data collection and checking. HY, RY, and CJ contributed to analyses, interpretation of data and making figures. The manuscript was drafted by HY and was critically reviewed and subsequently approved by all authors.
Funding
This work was funded by the Beijing Municipal Science and Technology Program [No. D171100006717001] and the project from the National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.
Data availability
The data that support the findings of this study are available from the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, but restrictions apply to the availability of these data and so are not publicly available. The data are, however, available from the corresponding authors upon reasonable request and with the permission of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.
Declarations
Ethics approval and consent to participate
The study was approved by the institutional review board (IRB) of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention (No. KX210330646), with individual-level informed consent waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hailiang Yu and Yuecheng Yang contributed equally to this work.
Cong Jin was declared as the primary corresponding author.
Contributor Information
Runhua Ye, Email: 122022116@qq.com.
Cong Jin, Email: jinc@chinaaids.cn.
References
- 1.Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryom L, Cotter A, De Miguel R, Béguelin C, Podlekareva D, Arribas JR, Marzolini C, Mallon P, Rauch A, Kirk O, et al. 2019 Update of the European AIDS clinical society guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020;21(10):617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidelines for the Use of Antiretroviral Agents in. Adults and Adolescents with HIV [https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/guidelines-adult-adolescent-arv.pdf]
- 4.Consolidated guidelines on the use. of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach - Second edition [https://www.who.int/hiv/pub/arv/arv-2016/en/]
- 5.Hermans LE, Moorhouse M, Carmona S, Grobbee DE, Hofstra LM, Richman DD, Tempelman HA, Venter WDF, Wensing AMJ. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis. 2018;18(2):188–97. [DOI] [PubMed] [Google Scholar]
- 6.Inzaule SC, Bertagnolio S, Kityo CM, Siwale M, Akanmu S, Wellington M, de Jager M, Ive P, Mandaliya K, Stevens W, et al. The relative contributions of HIV drug resistance, nonadherence and low-level viremia to viremic episodes on antiretroviral therapy in sub-Saharan Africa. AIDS. 2020;34(10):1559–66. [DOI] [PubMed] [Google Scholar]
- 7.Joya C, Won SH, Schofield C, Lalani T, Maves RC, Kronmann K, Deiss R, Okulicz J, Agan BK, Ganesan A. Persistent Low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis. 2019;69(12):2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elvstam O, Marrone G, Medstrand P, Treutiger CJ, Sonnerborg A, Gisslen M, Bjorkman P. All-Cause mortality and serious Non-AIDS events in adults with Low-level human immunodeficiency virus viremia during combination antiretroviral therapy: results from a Swedish nationwide observational study. Clin Infect Dis. 2021;72(12):2079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernal E, Gomez JM, Jarrin I, Cano A, Munoz A, Alcaraz A, Imaz A, Iribarren JA, Rivero M, Arazo P, et al. Low-Level viremia is associated with clinical progression in HIV-Infected patients receiving antiretroviral treatment. J Acquir Immune Defic Syndr. 2018;78(3):329–37. [DOI] [PubMed] [Google Scholar]
- 10.Guihot A, Tubiana R, Breton G, Marcelin AG, Samri A, Assoumou L, Goncalves E, Bricaire F, Costagliola D, Calvez V, et al. Immune and virological benefits of 10 years of permanent viral control with antiretroviral therapy. AIDS. 2010;24(4):614–7. [DOI] [PubMed] [Google Scholar]
- 11.Aksak-Wąs BJ, Kowalska JD, Ząbek P, Serwin K, Rafalska-Kosior M, Gołąb J, Chober D, Skonieczna-Żydecka K, Hackiewicz M, Parczewski M. Immune restoration affects 10-year survival in people living with HIV/AIDS. HIV. 2023;24(3):325–34. [DOI] [PubMed]
- 12.Liu J, Wang L, Hou Y, Zhao Y, Dou Z, Ma Y, Zhang D, Wu Y, Zhao D, Liu Z, et al. Immune restoration in HIV-1-infected patients after 12 years of antiretroviral therapy: a real-world observational study. Emerg Microbes Infect. 2020;9(1):2550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helleberg M, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Obel N, Gerstoft J. CD4 decline is associated with increased risk of cardiovascular disease, cancer, and death in virally suppressed patients with HIV. Clin Infect Dis. 2013;57(2):314–21. [DOI] [PubMed] [Google Scholar]
- 14.Young J, Psichogiou M, Meyer L, Ayayi S, Grabar S, Raffi F, Reiss P, Gazzard B, Sharland M, Gutierrez F, et al. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med. 2012;9(3):e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, Rupert A, Baseler M, Tagaya Y, Roby G, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A. 2008;105(50):19851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Margolick JB, Jamieson BD, Rinaldo CR, Phair JP, Jacobson LP. CD4 + T-cell counts and plasma HIV-1 RNA levels beyond 5 years of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;57(5):421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford N, Stinson K, Gale H, Mills EJ, Stevens W, Pérez González M, Markby J, Hill A. CD4 changes among virologically suppressed patients on antiretroviral therapy: a systematic review and meta-analysis. J Int AIDS Soc. 2015;18(1):20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantazis N, Papastamopoulos V, Paparizos V, Metallidis S, Adamis G, Antoniadou A, Psichogiou M, Chini M, Sambatakou H, Sipsas NV, et al. Long-term evolution of CD4 + cell count in patients under combined antiretroviral therapy. AIDS. 2019;33(10):1645–55. [DOI] [PubMed] [Google Scholar]
- 19.Inzaule SC, Kroeze S, Kityo CM, Siwale M, Akanmu S, Wellington M, de Jager M, Ive P, Mandaliya K, Stevens W, et al. Long-term HIV treatment outcomes and associated factors in sub-Saharan Africa: multicountry longitudinal cohort analysis. AIDS. 2022;36(10):1437–47. [DOI] [PubMed] [Google Scholar]
- 20.Lee CT, Chen HP, Lin HH, Ke MY, Wu PF. The influence of low-level viremia on CD4 + cell count in human immunodeficiency virus-infected patients. J Chin Med Assoc. 2022;85(12):1126–30. [DOI] [PubMed] [Google Scholar]
- 21.Hou Y, Liu J, Zhao Y, Wu Y, Ma Y, Zhao D, Dou Z, Liu Z, Shi M, Jiao Y, et al. Epidemiological trends of severely immunosuppressed people living with HIV at time of starting antiretroviral treatment in China during 2005–2018. J Infect. 2022;84(3):400–9. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Yang Y, Cao D, Zhao Y, Jin C, Sun H, Cao Y, Ye R, Yao S, Duan S, et al. Association of low-level viremia with mortality among people living with HIV on antiretroviral therapy in Dehong, Southwest China: A retrospective cohort study. HIV Med. 2023;24(1):37–45. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Tang Z, Mao Y, Van Veldhuisen P, Ling W, Liu D, Shen Z, Detels R, Lan G, Erinoff L, et al. Testing and linkage to HIV care in China: a cluster-randomised trial. Lancet HIV. 2017;4(12):e555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Wu Z, McGoogan JM, Sha Y, Zhao D, Ma Y, Brookmeyer R, Detels R, Montaner JSG. Nationwide cohort study of antiretroviral therapy timing: treatment dropout and virological failure in China, 2011–2015. Clin Infect Dis. 2019;68(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27(7):2015–23. [DOI] [PubMed] [Google Scholar]
- 26.Yuen T, Brouillette MJ, Fellows LK, Ellis RJ, Letendre S, Heaton R, Mayo N. Personalized risk index for neurocognitive decline among people with Well-Controlled HIV infection. J Acquir Immune Defic Syndr. 2017;76(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Ann Rev Clin Psychol. 2010;6:109–38. [DOI] [PubMed] [Google Scholar]
- 28.Kanters S, Nachega J, Funk A, Mukasa B, Montaner JS, Ford N, Bucher HC, Mills EJ. CD4(+) T-cell recovery after initiation of antiretroviral therapy in a resource-limited setting: a prospective cohort analysis. Antivir Ther. 2014;19(1):31–9. [DOI] [PubMed] [Google Scholar]
- 29.Handoko R, Colby DJ, Kroon E, Sacdalan C, de Souza M, Pinyakorn S, Prueksakaew P, Munkong C, Ubolyam S, Akapirat S, et al. Determinants of suboptimal CD4(+) T cell recovery after antiretroviral therapy initiation in a prospective cohort of acute HIV-1 infection. J Int AIDS Soc. 2020;23(9):e25585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiseha T, Ebrahim H, Ebrahim E, Gebreweld A. CD4 + cell count recovery after initiation of antiretroviral therapy in HIV-infected Ethiopian adults. PLoS ONE. 2022;17(3):e0265740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montarroyos UR, Miranda-Filho DB, César CC, Souza WV, Lacerda HR, Albuquerque Mde F, Aguiar MF, Ximenes RA. Factors related to changes in CD4 + T-cell counts over time in patients living with HIV/AIDS: a multilevel analysis. PLoS ONE. 2014;9(2):e84276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gras L, May M, Ryder LP, Trickey A, Helleberg M, Obel N, Thiebaut R, Guest J, Gill J, Crane H, et al. Determinants of restoration of CD4 and CD8 cell counts and their ratio in HIV-1-Positive individuals with sustained virological suppression on antiretroviral therapy. J Acquir Immune Defic Syndr. 2019;80(3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding H, Xu J, Liu J, Wang Q, Kang J, Li X, Zhang Z, Han X, Jiang Y, Geng W, et al. Outcomes of persistent low-level viremia among HIV patients on antiretroviral therapy: A prospective cohort study. HIV Med. 2022;23(Suppl 1):64–71. [DOI] [PubMed] [Google Scholar]
- 34.Bai R, Lv S, Hua W, Su B, Wang S, Shao Y, Li Z, Liu A, Sun L, Dai L. Factors associated with human immunodeficiency virus-1 low-level viremia and its impact on virological and immunological outcomes: A retrospective cohort study in Beijing, China. HIV Med. 2022;23(Suppl 1):72–83. [DOI] [PubMed] [Google Scholar]
- 35.Zhang T, Ding H, An M, Wang X, Tian W, Zhao B, Han X. Factors associated with high-risk low-level viremia leading to virologic failure: 16-year retrospective study of a Chinese antiretroviral therapy cohort. BMC Infect Dis. 2020;20(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natural History Project Working Group for the Collaboration of Observational HIV Epidemiological Research Europe in EuroCoord. Factors associated with short-term changes in HIV viral load and CD4(+) cell count in antiretroviral-naive individuals. AIDS. 2014;28(9):1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, but restrictions apply to the availability of these data and so are not publicly available. The data are, however, available from the corresponding authors upon reasonable request and with the permission of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.