Abstract
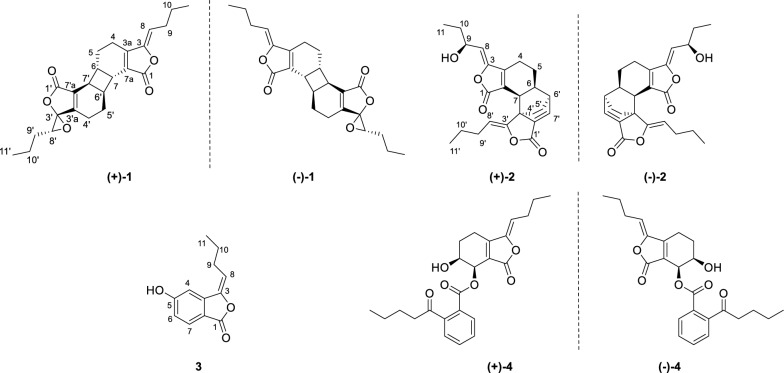
Three pairs of enantiomeric phthalide dimers, including two new ones, angesicolides A (1) and B (2), and a new phthalide monomer (3), were obtained from the rhizomes of Angelica sinensis. Their structures were established through spectroscopic methods, quantum calculations, and chiral HPLC analysis. Compounds 1 and 2 were [2 + 2] and [4 + 2] cycloadducts of phthalide monomers, and their hypothetical biogenetic origin was proposed. Compounds 2, (+)-2, (−)-2, 4, (+)-4, and (−)-4 exhibited significant inhibitory activity against NO production with IC50 values range from 1.23 to 5.55 μM.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13659-025-00512-z.
Keywords: Angelica sinensis, Phthalide dimers, Angesicolides A and B, Anti-inflammatory activity
Introduction
The roots of Angelica sinensis (Oliv.) Diels (Umbelliferae family) is a well-known food and medicine homology herb, which has been widely used as therapeutic treatment for menstrual disorders and chronic constipation for thousands of years [1, 2]. Till now, there are more than ten types of secondary metabolisms have been identified from A. sinensis [3]. Among them, ligustilide and other structure intriguing dimeric phthalides were the representative components [4]. Their diverse structures and biological activities including anti-inflammatory, anticonvulsion, and platelet aggregation inhibition are long-standing attractive targets among natural product chemists and pharmacologists [5–8].
NO is synthesized and released into endothelial cells by nitric oxide synthase and plays vital role in the pathogenesis of inflammatory diseases [9]. Moreover, excessive production of NO is closely related to a variety of inflammatory disorders [10, 11]. Some phthalide dimers have been found to have significant anti-inflammation activity by inhibiting LPS-induced NO production [12]. Recently, an increasing number of studies have been reported on natural products with remarkable biological activities and novel structures from medicinal and dietary plants [13, 14]. To further explore anti-inflammatory natural products from medicinal plants, four phthalides including three pairs of enantiomeric dimers and a monomer (Fig. 1) were isolated and identified from the rhizomes of A. sinensis. Herein, we describe their structural elucidation and anti-inflammatory activities.
Fig. 1.
Chemical structures of compounds 1–4
Results and discussion
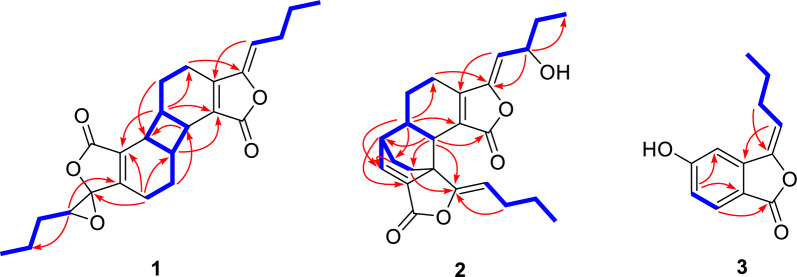
Angesicolide A (1) was isolated as colorless oil. The HR-ESI–MS of 1 (m/z 397.2015 [M + H]+, calcd for 397.2010) indicated its molecular formula of C24H28O5, with 11 degrees of unsaturation. IR band at 1758 cm−1 indicated the presence of an α,β-unsaturated lactone. Compound 1 demonstrated 24 carbon signals in 13C NMR, which was classified into two carbonyl carbons (δC 168.4, 168.2), six olefinic carbons, two oxygenated carbons (δC 89.9, 61.6), four methines, eight methylenes, two methyls (δC 13.8, 13.6) by DEPT experiments. The 1H NMR (Table 1) demonstrated the presence of two butylidene side chains [δH 5.11 (1H), 2.33 (2H), 1.47 (2H), and 0.93 (3H); δH 3.27 (1H), 1.90, 1.78 (each 1H), 1.54 (2H), and 0.93 (3H)], four methine signals (δH 3.04, 3.58, 3.03, 3.57) and four methane signals [δH 2.35 (2H), 1.80 (2H), 2.17, 2.04 (each 1H), 1.86 (2H)]. These signals suggested 1 to be a phthalide dimer with the same basic skeleton as chaxiongnolide A [15]. Striking differences included an extra epoxy group based on same degree of unsaturation and the absence of a double bond. The crucial HMBC correlation between H-8ʹ (δH 3.27) with C-10ʹ (δC 19.2), C-9ʹ (δC 29.8), and C-3ʹa (δC 158.4) indicated that the epoxy group was situated at C-3ʹ and C-8ʹ. Further 1H–1H COSY, HSQC, and HMBC data verified the constructive planar structure (Fig. 2).
Table 1.
NMR (500 MHz) Data of 1–3 (δ in ppm, J in Hz)
| No | 1a | 2a | 3b | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 168.4 | 167.7 | 168.8 | |||
| 3 | 148.9 | 147.5 | 147.1 | |||
| 3a | 152.3 | 155.3 | 142.0 | |||
| 4a | 19.4 | 2.35, mc | 19.7 | 2.20, m | 109.9 | 7.23, d (2.0) |
| 4b | 2.35, mc | 2.08, m | ||||
| 5a | 20.1 | 1.80, mc | 28.8 | 1.95, m | 165.3 | |
| 5b | 1.80, mc | 1.55, m | ||||
| 6 | 34.3 | 3.04, m | 38.2 | 2.56, t (7.5) | 119.3 | 7.00, dd (8.5, 2.0) |
| 7 | 31.9 | 3.58, d (7.0) | 41.6 | 3.25, d (9.0) | 127.9 | 7.70, d (8.5) |
| 7a | 126.4 | 128.0 | 118.1 | |||
| 8 | 110.9 | 5.11, t (8.0) | 113.0 | 5.08, d (8.5) | 114.7 | 5.81, t (8.0) |
| 9a | 27.9 | 2.33, mc | 68.1 | 4.64, m | 28.9 | 2.50, q (7.5)c |
| 9b | 2.33, mc | 2.50, q (7.5)c | ||||
| 10a | 22.5 | 1.47, q (7.0)c | 29.9 | 1.66, mc | 23.9 | 1.62, mc |
| 10b | 1.47, q (7.0)c | 1.66, mc | 1.62, mc | |||
| 11 | 13.8 | 0.93, m | 9.6 | 0.92, t (5.0) | 14.1 | 1.04, t (5.0) |
| 1′ | 168.2 | 164.8 | ||||
| 3′ | 89.9 | 150.3 | ||||
| 3′a | 158.4 | 47.5 | ||||
| 4′a | 19.5 | 2.17, m | 31.0 | 2.04, m | ||
| 4′b | 2.04, m | 1.42, m | ||||
| 5′a | 20.0 | 1.86, mc | 25.8 | 1.89, m | ||
| 5′b | 1.86, mc | 1.30, m | ||||
| 6′ | 34.1 | 3.03, m | 41.5 | 2.99, m | ||
| 7′ | 31.6 | 3.57, d (7.0) | 142.2 | 7.35, d (6.5) | ||
| 7′a | 132.1 | 134.1 | ||||
| 8′ | 61.6 | 3.27, t (6.5) | 108.8 | 4.99, t (7.5) | ||
| 9′a | 29.8 | 1.90, m | 27.5 | 2.16, mc | ||
| 9′b | 1.78, m | 2.16, mc | ||||
| 10′a | 19.2 | 1.54, mc | 22.3 | 1.45, mc | ||
| 10′b | 1.54, mc | 1.45, mc | ||||
| 11′ | 13.6 | 0.93, m | 14.0 | 0.93, t (5.5) | ||
aMeasured in CDCl3
bMeasured in methanol-d4
cOverlapped
Fig. 2.
Key 1H–.1H COSY (bold lines) and HMBC (arrows) correlations of compounds 1–3
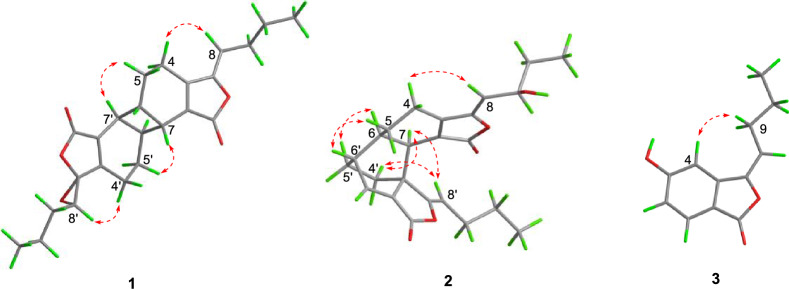
The relative stereochemistry of 1 was elucidated based on interpretation of ROESY interactions. The butylidene side chain took Z-form by the ROESY correlation of H-8 and H-4a. Furthermore, the ROESY correlations of H-7′/H-5a and H-7/H-5′a indicated β-orientations for H-6 and H-7, and α-orientations for H-6′ and H-7′. Consequently, compound 1 was determined to possess the relative configurations of 6R*,7R*,6′R*, and 7′R* (Fig. 3). Although H-4′a showed a strong ROESY correlation with H-8′, it was insufficient to determine the relative configuration of C-3′ and C-8′. Therefore, two possible stereoisomers have been proposed: (6R*,7R*,3′R*,6′R*,7′R*,8′S*)-1A or (6R*,7R*,3′S*,6′R*,7′R*,8′R*)-1B. These configurations were subsequently subjected to NMR calculations by employing the GIAO approach at the wB97XD/6–31+G(d,p) level of theory [16]. The results indicated the whole relative configuration of 1 to be 6R*,7R*,3′R*,6′R*,7′R*,8′S* with a DP4 + MM probability of approximately 75% (Fig. 4B).
Fig. 3.
Key ROESY (dashed lines) correlations of 1–3
Fig. 4.
The.13C NMR calculation results of two plausible stereoisomers and DP4 + MM probability analysis at wB97XD/6–31+G(d,p) level. A,B Linear correlation plots of calculated vs. experimental NMR chemical shift values and DP4 + MM probability analysis for 1A and 1B; C comparison of the experimental and calculated ECD spectra of 1. D,E Linear correlation plots of calculated vs. experimental NMR chemical shift values and DP4 + MM probability analysis for 2A and 2B; F comparison of the experimental and calculated ECD spectra of 2
Angesicolide B (2) was obtained as colorless gum. 2 had the molecular formula of C24H28O5 deduced by the HR-ESI–MS data (m/z 414.2271 [M + NH4]+, calcd for C24H28O5NH4 414.2275), with 11 degrees of unsaturation. The existence of hydroxy and α,β-unsaturated lactone moieties was evidenced by the IR absorption bands at 3437, 1772 and 1710 cm−1. 2 showed 24 carbon signals including eight quaternary carbons, seven methines, seven methylenes and two methyls. The high similarity NMR data with that of levulanolide A indicated both compounds shared the same carbon skeleton [17], except for the existence of an additional hydroxyl group in 2 (δH 4.64; δC 68.1). The HMBC correlations of H-9 (δH 4.64) with C-8 (δC 113.0) and C-10 (δC 29.9) finally established the hydroxyl group was attached to C-9 (Fig. 2).
The α-orientations of H-6 and H-7, and the β-orientation of H-6′ were confirmed by the ROESY correlations of H-4′a/H-7, H-4′a/H-8′, H-7/H-8′, H-5/H-6′, and H-6/H-5′. Consequently, the relative configurations of C-6, C-7, C-3′a, and C-6′ were assigned as 6R*,7R*,3′aR*, and 6′R*, respectively (Fig. 3). The stereochemistry of the hydroxyl group at C-9 could not be determined through ROESY correlations. Consequently, two possible relative configurations were proposed for compound 2: (6R*,7R*,9R*,3′aR*,6′R*)-2A and (6R*,7R*,9S*,3′aR*,6′R*)-2B. The subsequent experimental and the calculated NMR data established the relative configuration of 2 as 6R*,7R*,9R*,3′aR*,6′R*, with a probability of approximately 85% for DP4 + MM (Fig. 4E).
Angesicolide C (3) was purified as yellow oil. It had the same molecular formula of C12H12O3 with that of senkyunolide C [18], based on the HR-ESI–MS data at m/z 205.0861 [M + H]+ (calcd for 205.0859). Upon analysis of the 1D and 2D NMR data, 3 possessed the same planar structure as that of senkyunolide C. The most pronounced difference was the enolicdouble bond in 3 took E-configuration, as verified by the absence of ROESY correlation of H-8 and H-4 and the corresponding upfield chemical shifts at C-4 (ΔδC − 8.2 ppm) and C-8 (ΔδC − 3.7 ppm) in 3.
Based on the specific optical rotation and chiral HPLC data, compounds 1 and 2 were a mixture of enantiomers, which were further treated by chiral separation to give two pairs of enantiomers [(+)-1/(−)-1, (+)-2/(−)-2]. As shown in Fig. 4, the calculated ECD results finally established the absolute configurations of (+)-1 and (−)-1 as (6S,7R,3ʹS,6′S,7′R,8ʹR)-1 and (6R,7S,3ʹR,6′R,7′S,8ʹS)-1 (Fig. 4C), respectively. Similarly, the absolute configurations of (+)-2 and (−)-2 were assigned as (6R,7R,9R,3′aR,6′R)-2 and (6S,7S,9S,3′aS,6′S)-2 (Fig. 4F).
Compound 4 was firstly isolated from A. sinensis. Comparing its spectral data with the reported data, compound 4 was identified as (±)-lyocasuarolide A [19]. Compounds (+)-4 and (−)-4 were obtained by chiral HPLC analysis, see Supporting Information for details.
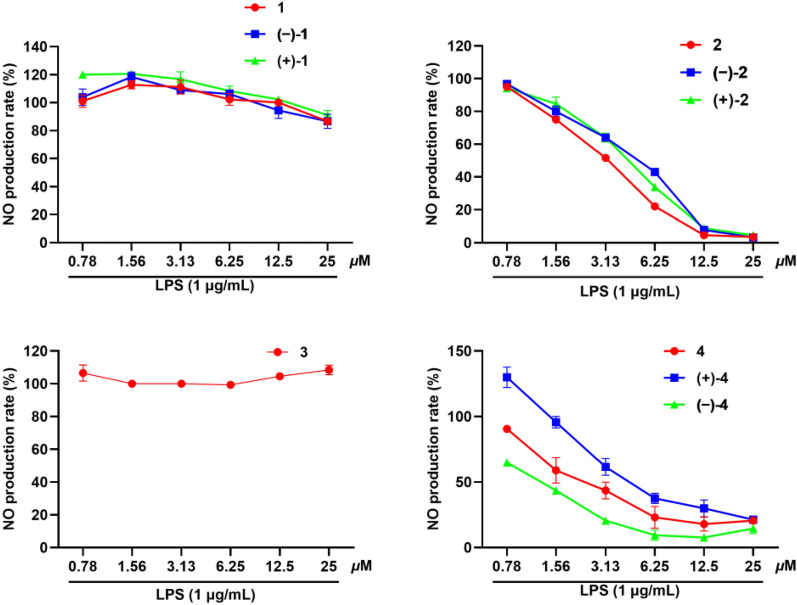
All the isolates (1–4) were evaluated in vitro for their inhibitory effects against NO production in LPS-induced RAW 246.7 mouse macrophages. As shown in Fig. 5 and Figure S1, compounds 2 and 4 and their enantiomers exhibit inhibitory activity against LPS-induced NO production without cytotoxicity. As shown in Table 2, compounds 2, (+)-2, (−)-2, 4, (+)-4, and (−)-4 exhibited significant NO inhibitory effects with IC50 values between 1.23 and 5.55 μM, which were more potential than the positive control l-NMMA.
Fig. 5.
The inhibition rates of compounds 1–4 against LPS-induced NO production in RAW 264.7 cells
Table 2.
IC50 values of compounds 1–4 and l-NMMA against LPS-induced NO production in RAW 264.7 cells
| Compounds | Inhibition of NO production | |
|---|---|---|
| IC50 (μM) | NO inhibition at 25 μM (%) | |
| 1 | NDa | 13.33 ± 2.54 |
| (+)-1 | NDa | 8.89 ± 3.33 |
| (−)-1 | NDa | 13.33 ± 5.09 |
| 2 | 3.12 | 96.44 ± 2.22 |
| (+)-2 | 4.20 | 95.37 ± 1.63 |
| (−)-2 | 4.44 | 96.80 ± 1.63 |
| 3 | NDa | – |
| 4 | 2.70 | 79.49 ± 2.96 |
| (+)-4 | 5.55 | 78.63 ± 2.56 |
| (−)-4 | 1.23 | 85.47 ± 3.92 |
| l-NMMAb | 33.0 | 38.32 ± 4.89 |
aND, not detected
bl-NMMA, positive control
Materials and methods
General experimental procedures
NMR, HR-ESI–MS, UV, and IR spectra were measured as described previously [20].
Plant materials
Details regarding the plant material, including collection and identification information, have been reported previously [20].
Extraction and isolation
Detailed information for compounds 1–4 isolation procedures please see the Supporting Information.
Chiral separation and characterization
Compounds 1 and 2 were analyzed and isolated with a CHIRALPAK AD-H chiral column to give (+)-1/(−)-1, (+)-2/(−)-2. Chiral separation details are shown in the Supporting Information.
Compound 1: Colorless oil; UV (MeOH) λmax (log ε) 278 (3.56) nm; HR-ESI–MS m/z 397.2015 [M + H]+ (calcd for C24H28O5 + H, 397.2010). IR (KBr) νmax 2960, 1758, 1717, 1635, 679 cm−1; 1H (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) data see Table 1. (+)-(6R,7R,3′R,6′R,7′R,8′S)-1: [α]25D + 17 (c 0.08, MeOH); ECD (MeOH) λmax (Δε) 195 (−11.6), 226 (−0.6), 291 (+11.3) nm. (−)-(6S,7S,3′S,6′S,7′S,8′R)-1: [α]25D − 21 (c 0.07, MeOH); ECD (MeOH) λmax (Δε) 195 (+15.3), 219 (+2.0), 256 (−8.3) nm.
Compound 2: colorless gum; UV (MeOH) λmax (log ε) 274 (4.40) nm; HR-ESI–MS m/z 414.2271 [M + NH4]+ (calcd for C24H28O5 + NH4, 414.2275). IR (KBr) νmax 3437, 2958, 2934, 2872, 1772, 1710, 1674, 1461 cm−1; 1H (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) data see Table 1. (+)-(6R,7R,9R,3ʹaR,6ʹR)-2: [α]25D + 246 (c 0.18, MeOH); ECD (MeOH) λmax (Δε) 202 (+24.8), 238 (−4.5), 261 (+2.7), 292 (−10.2) nm. (−)-(6S,7S,9S,3ʹaS,6ʹS)-2: [α]25D − 346 (c 0.13, MeOH); ECD (MeOH) λmax (Δε) 202 (−27.3), 238 (+6.7), 261 (−1.6), 292 (+12.7) nm.
Compound 3: yellow oil; HR-ESI–MS m/z 205.0861 [M + H]+ (calcd for C24H28O5 + H, 205.0859). IR (KBr) νmax 3182, 1721, 1670, 1620, 1589 cm−1; 1H (500 MHz, methanol-d4) and 13C NMR (125 MHz, methanol-d4) data see Table 1.
ECD calculation
The theoretical calculations of compounds 1 and 2 were calculated employing the Gaussian program package. Conformational analysis was performed using CONFLEX software. Conformations with a Boltzmann population exceeding 2% were chosen for optimization and spectral calculation. The theoretical calculation of ECD was performed using Time Dependent Density Functional Theory (TDDFT) at B3LYP/6-31G (d,p) (compound 1) and CAM-B3LYP/DGDZVP (compound 2) level. The ECD spectra of the compounds 1 and 2 were obtained by considering the Boltzmann distribution of each geometric conformation [21].
NO inhibitory assay
The NO inhibitory assay was carried out as described previously [20]. For details, see the Supporting Information.
Statistical analysis
The statistical analysis was carried out as described previously [20].
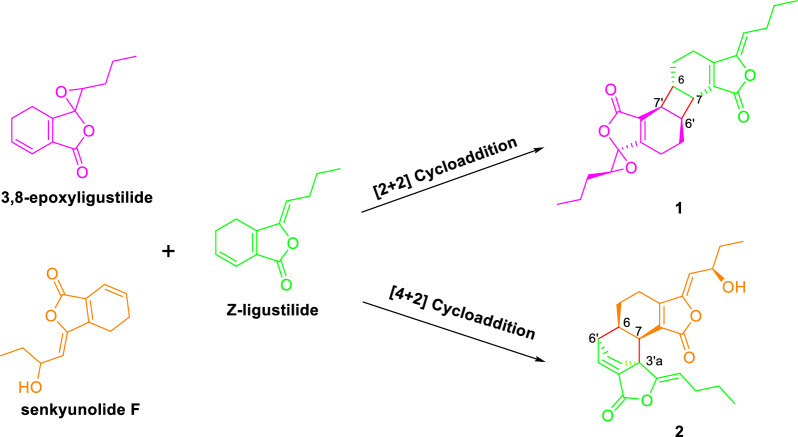
Plausible biosynthetic pathway for 1 and 2
Phthalide dimers with different linkages are generated by [4 + 2] or [2 + 2] cycloaddition of two monomeric phthalate units. As for 1 and 2, they might be formed by cycloaddition reaction between Z-ligustilide and the molecule of phthalide monomer (3,8-epoxyligustilide [22] and senkyunolide F [23]) (Scheme 1).
Scheme 1.
Plausible biosynthetic pathway of compounds 1 and 2
Conclusion
In conclusion, three pairs of enantiomeric phthalides characterized as [2 + 2] and [4 + 2] cycloadducts and a phthalide monomer were obtained from the rhizomes of A. sinensis. The racemates were further separated by chiral column and all of them were evaluated for their inhibitory effects on LPS-induced NO production. Interestingly, the current results demonstrated that the phthalide dimers have more potential anti-inflammatory activity than that of monomeric phthalides. The discovery of 1–3 are enriching supplement for the diversity of the phthalides category and provides a new insight for their biosynthetic, synthetic, and pharmacological investigation.
Supplementary Information
Additional file 1. 1D and 2D NMR, HR-ESI-MS, and UV data of compounds 1–3, the chiral separation of 1 and 2.
Author contributions
Hongyan Wen, finished the isolation and identification studies; Sheng Li implemented the anti-inflammatory studies. Formal analysis and data compilation by Hongyan Wen and Sheng Li. Hongyan Wen wrote the first draft; Yu Zhang conceived the projects, revised manuscript, and provided financial support; All authors approved the final version of the manuscript.
Funding
This work was financially supported by National Key R&D Program of China (2022YFF1100301), Major Science and Technology Project of Henan Province (231100310200), Yunnan Province Science and Technology Department (202305AH340005).
Data availability
All data generated or analyzed during this study are included in this published article and its Additional file 1.
Declarations
Competing interests
The authors declare no competing financial interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang HY, Bi WG, Yu Y, Liao WB. Angelica sinensis (Oliv.) Diels in China: distribution, cultivation, utilization and variation. Genet Resour Crop Evol. 2012;59(4):607–13. [Google Scholar]
- 2.Fang L, Xiao X, Liu C, He X. Recent advance in studies on Angelica sinensis. Chin Herb Med. 2012;4(1):12–25. [Google Scholar]
- 3.Ma JP, Guo ZB, Jin L, Li YD. Phytochemical progress made in investigations of Angelica sinensis (Oliv.) Diels. Chin J Nat Med. 2015;13(4):241–9. [DOI] [PubMed] [Google Scholar]
- 4.Li XN, Chen YY, Cheng DP, Tong SQ, Qu HB, Yan JZ. Two phthalide dimers from the radix of Angelica sinensis. Nat Prod Res. 2011;26:1782–6. [DOI] [PubMed] [Google Scholar]
- 5.Su YW, Chiou WF, Chao SH, Lee MH, Chen CC, Tsai YC. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol. 2011;11(9):1166–72. [DOI] [PubMed] [Google Scholar]
- 6.Zou J, Chen GD, Zhao H, Huang Y, Luo X, Xu W, et al. Triligustilides A and B: two pairs of phthalide trimers from Angelica sinensis with a complex polycyclic skeleton and their activities. Org Lett. 2018;20(3):884–7. [DOI] [PubMed] [Google Scholar]
- 7.Tang F, Yan YM, Yan HL, Wang LX, Hu CJ, Wang HL, et al. Chuanxiongdiolides R4 and R5, phthalide dimers with a complex polycyclic skeleton from the aerial parts of Ligusticum chuanxiong and their vasodilator activity. Bioorg Chem. 2021;107: 104523. [DOI] [PubMed] [Google Scholar]
- 8.Zou J, Chen GD, Zhao H, Wang XX, Zhang ZJ, Qu YB, et al. Triangeliphthalides A–D: bioactive phthalide trimers with new skeletons from Angelica sinensis and their production mechanism. Chem Commun. 2019;55(44):6221–4. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Lee D, Lee SO, Ryu JY, Choi SZ, Kang KS, et al. Anti-inflammatory activity of the sclerotia of edible fungus, Poria cocos Wolf and their active lanostane triterpenoids. J Funct Foods. 2017;32:27–36. [Google Scholar]
- 10.Hu YS, Han X, Yu PJ, Jiao MM, Liu XH, Shi JB. Novel paeonol derivatives: design, synthesis and anti-inflammatory activity in vitro and in vivo. Bioorg Chem. 2020;98: 103735. [DOI] [PubMed] [Google Scholar]
- 11.Xu ZP, Liu Y, Wang SY, Li ZW, Lu DX, Jiang P, et al. Phenolic compounds of Solanum xanthocarpum play an important role in anti-inflammatory effects. Arab J Chem. 2022;15(7): 103877. [Google Scholar]
- 12.Fu RH, Hran HJ, Chu CL, Huang CM, Liu SP, Wang YC, et al. Lipopolysaccharide-stimulated activation of murine DC2.4 cells is attenuated by n-butylidenephthalide through suppression of the NF-κB pathway. Biotechnol Lett. 2011;33(5):903–10. [DOI] [PubMed] [Google Scholar]
- 13.Chen JB, Li G, Chen X, Liao LH, He YQ, Ye F, et al. Nutritional value and antioxidant activity of Artemisia princeps, an edible plant frequently used in folk food in the Xiangxi region. Food Med Homol. 2025;2(2):9420043. [Google Scholar]
- 14.Xie YK, Pan XY, Liang XR, Zhai KF, Yu Q. Research progress on structural characterization and bioactivities of Poria cocos and Ganoderma polysaccharides. Food Med Homol. 2025;2(1):9420040. [Google Scholar]
- 15.Yang JB, Wang AG, Wei Q, Ren J, Ma SC, Su YL. New dimeric phthalides from Ligusticum sinense Oliv cv. Chaxiong. J Asian Nat Prod Res. 2014;16(7):747–52. [DOI] [PubMed] [Google Scholar]
- 16.Franco BA, Luciano ER, Sarotti AM, Zanardi MM. DP4+App: finding the best balance between computational cost and predictive capacity in the structure elucidation process by DP4+. factors analysis and automation. J Nat Prod. 2023;86(10):2360–7. [DOI] [PubMed] [Google Scholar]
- 17.Naito T, Katsuhara T, Niitsu K, Ikeya Y, Okada M, Mitsuhashi H. Phthalide dimers from Ligusticum chuanxiong Hort. Heterocycles. 1991;32(12):2433–42. [Google Scholar]
- 18.Kobayashi M, Fujita M, Mitsuhashi H. Studies on the constituents of Umbelliferae plants. XV. Constituents of Cnidium officinale: occurrence of pregnenolone, coniferylferulate and hydroxyphthalides. Chem Pharm Bull. 1987;35(4):1427–33. [Google Scholar]
- 19.Liu Y, Chen X, Liu XJ, Liu R, Hu HL, Min SX, et al. Five racemic phthalides from the aerial parts of Lycopodiastrum casuarinoides and their neuroprotective activities. Phytochemistry. 2025;233: 114384. [DOI] [PubMed] [Google Scholar]
- 20.Wen HY, Li S, Wei YL, Sun Y, et al. Bioassay and NMR-HSQC guided isolation and identification of phthalide dimers with anti-inflammatory activity from the rhizomes of Angelica sinensis. J Agric Food Chem. 2025;73(8):4630–41. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Luo RC, Liang ZZ, Zhang BD, Wei YL, Wen HY, et al. Corydecusines A–H, new phthalideisoquinoline hemicetal alkaloids from the bulbs of Corydalis decumbens inhibit Tau pathology by activating autophagy mediated by AMPK-ULK1 pathway. Bioorg Chem. 2024;144: 107166. [DOI] [PubMed] [Google Scholar]
- 22.Schinkovitz A, Pro SM, Main M, Chen SN, Jaki BU, Lankin DC, et al. Dynamic nature of the ligustilide complex. J Nat Prod. 2008;71(9):1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchida T, Kobayashi M, Kaneko K, Mitsuhashi H. Studies on the constituents of Umbelliferae plants. XVI. Isolation and structures of three new ligustilide derivatives from Angelica acutiloba. Chem Pharm Bull. 1987;35(11):4460. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. 1D and 2D NMR, HR-ESI-MS, and UV data of compounds 1–3, the chiral separation of 1 and 2.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional file 1.