Abstract
Toxicogenomics study reveals information of gene activity and proteins within the particular cells or tissue of an organism in response to toxic substances. 4-Nonylphenol is a potent environmental contaminant and endocrine disruptor. This study elucidates the toxic and xeno-estrogenic effect of 4-Nonylphenol from the cellular level to the gene level by in vivo and in silico approach. In vivo, studies show that exposure of 4-Nonylphenol at a low dose 64µgL− 1 and a high dose of 160µgL− 1 for 30 days to 60 days of duration during pre-spawning to the spawning period in testes of Heteropneustes fossilis causes cellular level toxicity i.e., dose and duration dependent clumping of spermatocytes. Dose and duration-dependent decrease in superoxide dismutase(SOD), Catalase, glutathione peroxidase(GPx) and increase in lipid peroxidase (LPO) level in testes. There was a dose and duration-dependent decrease in total antioxidant status and increased level of total oxidant status in the testicular tissue of H. fossilis along with an increase in cortisol level 0.4-NP caused alteration in antioxidant enzyme levels impedes the first line of defense mechanism in the body of an organism. There was a dose-dependent increase in necrosis percentage in testicular cells, cell death, and an increase in total ROS (reactive oxygen species) in a dose-dependent manner in testicular cells of H. fossilis. 4-NP causes gene level toxicity i.e., increased DNA migration or DNA fragmentation. Upregulation of gene expression of gonadal aromatase (CYP19a1a) and downregulation of the 3-beta-hydroxysteroid dehydrogenase (3-β HSD) gene in testes were observed. In silico studies also confirmed the interacting potency of 4-NP with steroid enzyme 3- β HSD and CYP19a1a. Present investigations shows that exposure to water bodies contaminated with xenoestrogens like 4-NP has significantly reduced reproductive parameters like fertilization, fecundity, hatching, and larval survival in numerous fish species.4-NP causes alteration in gene expression of the proteins which are very crucial for reproduction and maintenance of maleness. Due to chronic exposure to 4-NP, it becomes a toxicant causing tissue cell death. So, the harmful impact of 4-NP on reproduction in teleost fish is concerning, not just for the fish themselves but for the entire ecosystem. Therefore, efforts should be made to reduce the contamination of water bodies with xenoestrogens.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-92226-y.
Keywords: 4-NP, H. fossilis, SEM, COMET assay, Gene expression
Subject terms: Proteome informatics, Endocrinology
Introduction
The aquatic ecosystem serves as a repository for toxic pollutants introduced from industrial, domestic, and agricultural sources1. 4-Nonylphenol (NP) is byproduct and recalcitrant product of its parent compound alkylphenol ethoxylate compounds (APEs) group within the non-ionic surfactants family, first produced in 19402. It is a xenoestrogen that is produced during the degradation of nonylphenol ethoxylates (NPEO)3. They are widely used in domestic liquid cleaners, industrial application, household application, industrial cleaners, and personal products. However, they pose a challenge for environmental management as they are incompletely biodegraded, leading to the formation of various byproducts in the environment and wastewater treatment plants4. Since its first synthesis in 1940, NP has been produced and used at an exponential rate. With China and India serving as the primary production locations, the NP market is expected to expand significantly on a global scale by 2025 (https://www.persistencemarketresearch.com/market-research/nonylphenol-market.asp).
Therefore, according to the USA Environmental Protection Agency (2010), it is categorized as a high production volume (HPV) chemical2. From the Rural Federal University of Rio de Janeiro conducted a study to look at 4-Nonylphenol (4-NP) levels in the Guandu River surface waters in Rio de Janeiro, Brazil5. The researchers found 4-NP in 12 out of 19 samples, with concentrations ranging from 1.73 to 2.32 µg/L. This study raised concerns about the high levels of nonylphenol in the river and recommended periodic monitoring of its concentration in river water samples.
In India, the presence of 4-NP in the Kaveri, Vellar, and Tamiraparini rivers, Tamil Nadu6, . The presence of 4-NP concentration at various ghats of the Ganga river ranges between 12.40 ± 1.11 µg/L to 16.29 ± 1.18 µg/L in eastern Uttar Pradesh7, . In Chennai marine environment and soil, the concentration of 4-NP varied from 1.22 to 7.24 µg/L and 3.31 to 30.96 µg/kg respectively8.
Nonylphenol (4-NP) accumulates in lipids, cell membranes, and tissues of living beings due to its strong lipophilicity (octanol-water partition coefficient log Kow of 4-Nonylphenol is 4.48)7,9. This accumulation may intensify processes that are susceptible to estrogen7, which may affect the male fish reproductive systems10,110.4-Nonylphenol (4-NP) poses a significant risk to fish because of its high bio-concentration factor, non-biodegradable nature, prolonged environmental persistence, and bio-magnification via the food chain9,12–15.This compound structurally resembles natural estrogens, especially 17-β estradiol, and can mimic or inhibit hormonal responses16.The evident effects of xenoestrogens on sex differentiation and alterations in the structure and function of the gonads were the main focus of the study to show the effects of endocrine disruptors on fish reproduction for a long time. Recognized as an estrogenic compound, 4-NP exposure has been linked to numerous alterations in fish7,17. The threat posed by 4-NP is significant not only to aquatic life but also to human health, as it can accumulate in the lipids of organisms through food intake18. When evaluating the toxicological risks of contaminants in aquatic environments, fish are great biological indicators. Fish can serve as good model organisms for genotoxicological research and can serve as early indicators of environmental deterioration and alterations brought on by toxicants19. The catfish species Heteropneustes fossilis commonaly known as singhi are particularly popular in the Asian subcontinent due to their high protein and low-fat content. It has high economic importance and high demand due to medicinal value20. Aquaculture in rural areas benefits greatly from these fish as well21,220.4-NP ability to bind to estrogen receptors, often referred to as pseudo receptors, recent research has shown that exposure to 4-NP also triggers the production of reactive oxygen species (ROS)23,24. Reactive oxygen species (ROS) generated by the metabolism of several xenobiotics in fish can be reduced by natural defense mechanisms, such as certain antioxidant defense enzymes that assist in scavenging free radicals25. It is believed that oxidative stress caused by pollutants can be indicated by changes in antioxidant enzymes26.These reactive oxygen species (ROS), which include superoxide (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (HO), cortisol is correlated to the total antioxidant capacity which is also an oxidative stress-related parameter can harm unsaturated lipids, proteins, and nucleic acids, among other biological constituents27. Oxidative stress occurs when ROS levels become elevated due to either the activity of scavenger proteins or mitochondrial respiratory chain dysfunction, specifically the reduction of complex I activity. It is speculated that the oxidation of proteins, lipids, and nucleic acids contributes to the onset of various diseases, including cancer, infertility, and neurological conditions like Parkinson’s disease28–31.Due to alteration in antioxidant defense system enzymes and production of ROS, it causes cellular toxicity resulting in cell death i.e., necrosis and apoptosis. The mechanism of cellular harm may change from apoptosis at lower toxicant doses to necrosis at higher concentrations, with apoptosis acting as an early cellular indication of toxicity32. Due to oxidative stress induction from 4-Nonylphenol it causes apoptosis and cell death. The biochemical mechanisms of apoptosis are highly conserved throughout evolution and involve specific DNA fragmentation at internucleosomal sites33. Cell morphological abnormalities, such as alterations in cell shape, nuclear anomalies, and DNA damage, are linked to apoptosis34–37. Alteration in gene expression related to HPG axis, steroidogenesis caused by 4-Nonylphenol. Neuroestrogens play a crucial role in initiating and sustaining reproductive behaviors in both male and female organisms38. 0.4-NP acts as an anti-androgenic and estrogen-like substance, affecting the reproductive system of animals; it is prevalent in aquatic environments and impacts the reproductive system, nervous system, and other organs in both aquatic animals and humans. In vivo approach has been done to elucidate the toxicity, xeno estrogenicity, and underlying molecular mechanism in the context of male reproduction. In silico molecular docking is a novel probing search procedure that simulates the 3D structure of a ligand and receptor binding domains39,40. Understanding the relationship of 4-NP binding with hormones can help explain the concentration-response and time-response of xenoestrogens, it can provide detailed insights into interactions between xenobiotics chemicals and key biomolecules, as a result, serve as powerful tools for evaluating potential endocrine disrupting activities.
Materials and methods
Chemicals
Sodium dihydrogen phosphate monohydrate, a monobasic salt and disodium hydrogen phosphate dehydrate, a dibasic salt (SRL)Bovine serum albumin (for standard) Sodium dodecyl sulphate Merck, Acetic acid Fischer Scientific, Thiobarbituric acid Otto Chemika-Biochemika Reagent, Butylated hydroxyl toluene, Molychem, Absolute alcohol Analytical Reagent. 50 mM Phosphate buffer, 20 mM L-methionine, 1% Triton X-100, 10 mM Hydroxylamine hydrochloride, 50 µM EDTA, 50 µM riboflavin, 1% NED, 1% sulphanilamide, and 5% phosphoric acid, Glutaraldehyde TCI, osmium tetroxide (osmic acid)2% w/v, Ottokemi, Copper sulphate SRL, acetoneSRL, H2O2 and 50 mM Phosphate buffer. 50mM phosphate buffer, 0.4 M of Tris-HCl buffer, 10mM sodium azide, 10% TCA, 0.2 mL of glutathione, 0.1 mL of 0.2 mM of H2O2, DTNB. 150µM xylenol orange, 140mM NaCl, 1.35 M glycerol, 25mM of sulphuric acid, 5mM of ferrous ammonium sulphate and 10mM of o-dianisidine dihydrochloride. 75 mM Clark and Lubs solution and 7.5 mM hydrogen peroxide. p-nitro-N, N-dimethylaniline, sodium hydroxide, potassium ferricyanide. DCFDA and PBS (pH 7.4) all were purchased from SRL.PBS, Ammonium chloride, Sodium bicarbonate, ethylene diamine tetra acetic acid, FBS-Hi Media, Sodium Chloride, Potassium Chloride, Sodium Phosphate dibasic, Monopotassium phosphate, cell staining buffer all were purchased from SRL, Annexin V binding buffer, Annexin V FITC antibody and Propidium iodide ELABSCIENCE APOPTOSIS KIT.
cDNA synthesis kit (verso, Thermofisher, USA), SYBR-Green qPCR thermo fischer. Dimethylsulfoxide (DMSO) - Qualigens (CPW59), Disodium EDTA - HiMedia (RM1370).
Ethidium Bromide - Sigma (E-8751), Phosphate Buffered Saline (PBS) (Ca++, Mg + + free) – HiMedia, Sodium Chloride (NaCl) – Thermofisher, Sodium Hydroxide (NaOH) - BDH-Merck (89021), Triton X-100-SRL.
Collection and acclimatization of animals
In order to investigate the impact of 4-nonylphenol on sexually mature male catfish (H. fossilis 40–50 gm; length 15–35 cm), fish were purchased during the preparation phase (March–April; 11.5 L: 12.5D, 22 ± 2˚c; GSI: 1.15 ± 0.04%) from the local fish market in Varanasi, Uttar Pradesh. Fish were kept in a 20-liter flow-through aquarium and treated with 0.1% KMNO4 for disinfection. To recover from the stressful conditions brought on by transit, they were housed for a week in a laboratory setting with normal temperature and photoperiod. They were fed goat liver on demand for the duration of the trial and during the acclimatization period.
All protocols in this study were approved by the Committee on the Animal Ethics of Banaras Hindu University, Varanasi institutional and/or licensing committee and the license number is as follows: B.H.U./IAEC/2019–2020/004 dated 03/03/2020. All methods and procedures were carried out in accordance with ARRIVE guidelines 2.0 for experimentation in animals as well as the rules and regulations of the Animal Ethics Committee of Banaras Hindu University, Varanasi. No extra animal discomfort was caused for sample collection for the purpose of this study. Intensive care was given to prevent cruelty of any kind. All experiments were performed in accordance with relevant guidelines and regulations.
Exposure to 4-NP
A total of 60 male catfish (30–40 g; length = 15 ± 3.5 cm) were purchased from the local fish market to investigate the impact of 4-nonylphenol on male H. fossilis during the pre-spawning phase (March-April,11.5 L: 12.5D, 22 ± 2˚c). 20-liter flow-through aquarium was used to keep the fish, and 0.1% KMNO4 was used to disinfect them. To combat the stressful conditions brought on by transit, they were housed in a lab setting for a week at room temperature and with photoperiod. They were fed on goat liver ad libitum during the experiment and acclimation period.
4-Nonylphenol (4-NP) was diluted with triple distilled water after being dissolved in ethanol to achieve the required concentrations of 64 µg L− 1 and 160 µg L− 1. These concentrations were chosen based on the lethal concentration dosage (LC50) value7. Fish that had been acclimatized from the preliminary phase were kept in three separate 10-L tanks. Control, low dose (64 µg. L− 1 1/25th of LC50) and high dose (160 µg. L− 1 1/10th of LC50) were represented by groups 1, 2, and 3, respectively. Semi-static conditions (renewable of doses were done after 12 h in regular intervals) were maintained for 30 days to 60 days of treatment7.
Dissection
Fish were weighed when the experiment was completed, cold anesthesia was given41 and sacrificed by decapitation22. The testes were removed from the catfish H. fossilis, fixed in Bouin’s fluid, and they were kept in 70% alcohol.
Effect of 4-NP on the morphology of testes through a scanning electron microscope
Testes of control and treated samples from exposure to 4-NP were cut into 1mmx 1 mm size from a sharp blade and fixed into 2.5% glutaraldehyde for 24 h, post-fix for 2 h in 4% of osmium tetraoxide at 4 °C. After fixation, samples were washed three times with 0.1 M phosphate buffer solution (pH 7.4) each of 15 min at 4 °C42. Dehydrated with graded of acetone of 30%, 50%, 70%, 80%, 90%, and 100% dry acetone (mixture of copper sulfate and acetone 1:1 ratio, filtered it with Whatman paper) for 15 min each at 4 °C and testes were then proceed for the critical point of drying. Samples were coated with gold under vacuum with a scanning electron microscope. Finally, the prepared sample was examined under a scanning electron microscope (Carl Zeiss, EVO LS 10) images were taken.
Effects of 4-Nonylphenol on Anti-Oxidative parameters in testes of H. fossilis
Tissues(testes) from each catfish of all the groups were carefully excised, washed in ice-cold physiological saline solution, blotted dry on filter paper, and weighed. 10% homogenate of the tissues was prepared in ice-cold phosphate buffer (0.05 M, pH 7.4). The homogenates were centrifuged at 10,000 X g for 20 min at 4 °C. Different aliquots of the supernatant were prepared and frozen immediately at -80 °C until assayed. The supernatant was used for the estimation of the protein, to assess superoxide dismutase (SOD)43 and the estimation of catalase activity was assayed44, Glutathione peroxidase (GPx) content45, while malondialdehyde (MDA) content was assayed46. Total protein was estimated using the Bradford method. All aforesaid assays were performed by taking OD in 96 non-coated ELISA well plates through multimode reader Agilent BioTek Gen5 version3.12.
Effects of 4-Nonylphenol on total antioxidant status and total oxidant status in testes of H. fossilis
Total antioxidant status and total oxidant status was estimated47,48 respectively. For antioxidant status estimation reagent 1 was prepared also known as Clark and Lubs solution, 75 mM of KCl, 10 mM of o-dianisidine dihydrochloride, 45 µM of Fe(NH4)2(SO4)2 6H2O, and 75 mM of hydrochloric acid were dissolved in 1000 ml of distilled water and pH 1.8. Reagent 2 was prepared by mixing 7.5 mM of hydrogen peroxide in 1000 ml of distilled water. The reaction mixture was initiated by combining 200 µl of reagent 1 with 20 µl of testes homogenate. The initial absorbance was measured at 444 nm, and then 10 µl of reagent 2 was added. After 3–4 min, the final absorbance was measured using a microplate reader from Biotek (USA). The results for each sample were examined as millimolar Trolox equivalents per liter.
The total oxidant state was measured using a well-established protocol as described earlier48. To create reagent 1, 25 mM sulfuric acid was mixed with 150 µM xylenol orange, 140 mM sodium chloride, and 1.35 mM glycerol at a pH of 1.75. Reagent 2 was prepared by mixing 10 mM o-dianisidine dihydrochloride with 5 mM ferrous ammonium sulfate. The process was initiated by combining 35 µl of testes tissue with 225 µl of reagent 1. After measuring the initial absorption at 560 nm, reagent 2 was added and the mixture was allowed to sit for 3–4 min before the final absorbance was measured using a microplate reader from Biotek (USA). The results were reported as hydrogen peroxide equivalents per liter (M H2O2 Equiv./L) for each sample.
Effects of 4-Nonylphenol on cortisol in testes of H. fossilis
A cortisol level was estimated by the well-established protocol49. 10% tissue homogenate of testes of treated and control group of catfish H. fossilis is used for cortisol detection in were, a 100 µl sample mixed with 100 µl of 0.10% solution of p-nitro-N, N-dimethylaniline dissolved in ethanol and then kept in ice water for 5 min and after incubation 25 µl 0.1 N sodium hydroxide in a dark chamber for 5 h49. Followed by the addition of 200 µl Clark and Lubs buffer of pH 9.8 and after that 500 µl 0.10% phenol in ethanol and 50 µl 1% aqueous solution of potassium ferricyanide were added and tubes were kept in the water bath at 24 °C for 10 min and finally the absorbance of the solution was checked at 650 nm using UV-Vis Spectrophotometer.
Effects of 4-Nonylphenol on apoptosis, necrosis, and viable cells in testicular tissue of H. fossilis
Apoptosis and necrosis were estimated by using Annexin V (AV) and propidium iodide (PI) staining. Dissected testes tissues were homogenized and washed with cold FACS buffer. Cells were resuspended in Annexin V binding buffer, stained with AV-FITC and PI, incubated for 15 min at room temperature in the dark, and analyzed using a flow cytometer (BECKMAN COULTER CytoFLEX S).
Effects of 4-Nonylphenol on DNA fragmentation in testicular tissue of H. fossilis
DNA fragmentation by COMET assay was followed42. Tissue homogenate of testes of H. fossilis was centrifuged at 4000 rpm at 4 °C for 5 min, 50 µl homogenate (supernatant) mixed with 50 µl low melting point agarose 1.5% (quick step). 0.8% low melting point agarose was taken and cast on the slide add cover slip and then remove the coverslip after drying the gel (approx.10 min). 100 µl solution (1:1) sample and low melting point agarose were cast on slides. Then slides in the lysing buffer in a coupling jar for 30 min and runs in an electrophoretic buffer solution in a gel electrophoresis compartment at 25 volts for 30 min. After that slides were washed in neutralizing buffer solution 2–3 times. Slides were stained with ethidium bromide for 5 min and scored immediately finally observed under a fluorescence microscope. Analyzation of DNA damage was done by Caps Lab software50.
Effects of 4-Nonylphenol on gene expression of CYPa1a and 3β HSD in testes of H. fossilis
Total RNA from the testes and brain tissue was prepared using Trizol reagent and quantified by ultraviolet absorption at 260/280 nm. Complementary DNA (cDNA) was synthesized from 2 µg total RNA using a cDNA synthesis kit (Thermofisher, USA)51. Further, cDNA gene expression was assayed by qPCR with SYBR-Green qPCR Super Mix-UDG kit on a Mastercycler® ep real lex detection system (Agilent, USA). The relative mRNA levels were calculated with the 2 − ΔΔCt method using β actin as an internal control. The sequences of the forward and reverse primers for the detected gene are mentioned in Table 1.
Table 1.
The sequences of the forward and reverse primers for the detected gene.
Assessment of binding affinity of different protein biomarkers with 4-NP by molecular Docking
i)Software and tools
Biological markers (enzymes) were downloaded from the UniProt database (version) and the ligand which is 4-NonylPhenol is downloaded from PubChem. The Molecular Docking was performed using Auto Dock Vina (version 1.5.7) software. PyMOL (version 4.6.0) was used for the visualization of protein structure, for the addition of missing residue in the structure, and the removal of water molecules. CASTp analysis was used for the prediction of active sites. Chimera (version) used for analysis of docking result. Discovery Studio Visualizer (version 24.1.) is used for analysis of all the interactions between ligand and receptors.
ii)Preparation of PDB files before Docking
Before docking download a protein crystal structure from PDB and if the structure is not predicted download the alpha fold structure PDB file of your proteins from the UniProt database. The proteins used here were obtained among different groups of close species of catfishes which are:3-beta hydroxysteroid dehydrogenase (3-β HSD) of Clarias batrachus and Cytochrome P450 aromatase (CYP19a1a) of Heteropneutes fossilis. Preparation of ligand by downloading the structure from PubChem. For each protein make a folder and keep a copy of ligand_name. pdbqt file in each then use of CASTp for active site prediction. Then use Chimera and PyMol for protein structure and one with the table in which all the active pockets available on that protein are mentioned. Defining the grid box for docking then prepare for configuration file and perform docking by Auto doc Vina.
Statistical analysis
Evaluation of the data using the Bonferroni test (P < 0.05) as a post hoc test after a two-way analysis of variance (ANOVA). The data was presented as mean ± standard error mean (SEM) for n = 15. All statistical data were evaluated using Graph Pad Prism version 3.0.
Results
Effect of 4-NP on the morphology of testes through a scanning electron microscope
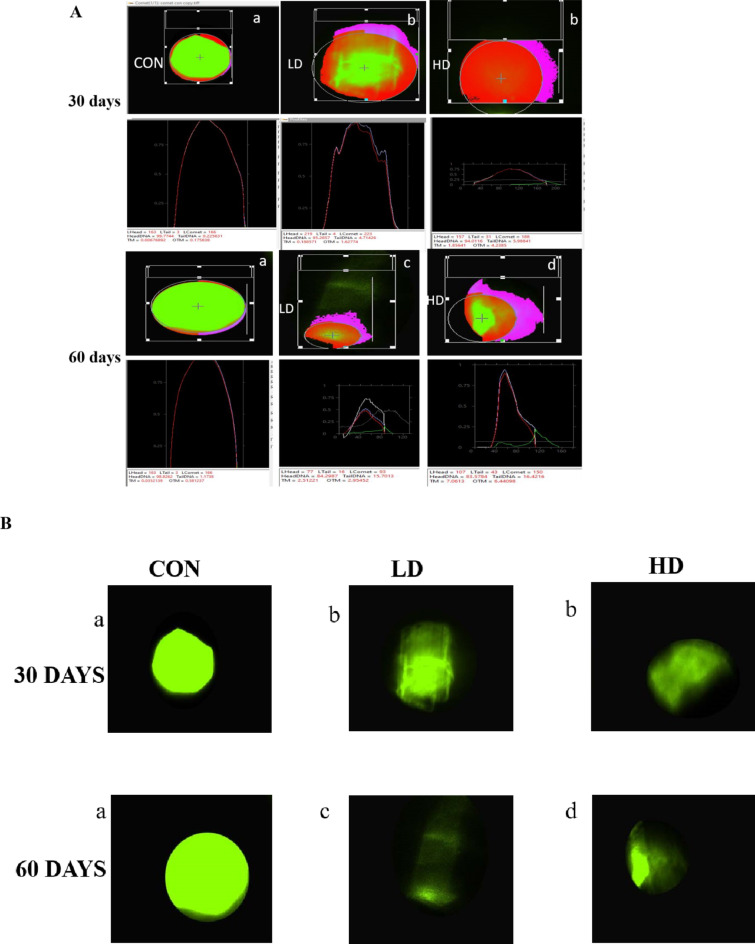
In the present study we have elucidated the effect of 4-Nonylphenol exposure of low dose (64 µg L− 1) and high dose (160 µg L− 1) for 30 days and 60 days respectively on morphology of testes catfish Heteropneustes fossilis by using scanning electron microscope. We have depicted that in 30 days exposure of low dose (64 µg L− 1) and high dose (160 µg L− 1) showed clumping of spermatocytes and this intensity has been increased severely dose and duration manner in 60 days of exposure. Figure 1.
Fig. 1.
Scanning electron microscope images showing the effect of 4-NP on the spermatocytes of testes of H. fossill is at low dose (64 µg/L) and high dose (160 µg/L) for 30 and 60 days of pre-spawning to spawning season. Spermatocytes containing cells in different stages of spermatogenesis; Scale bar 2 μm. Sc-spermatocytes.
Evaluation of antioxidant parameters (SOD, CAT, GPx, LPO, TAS, TOS) in testes tissue of H. fossilis due to exposure of 4-NP
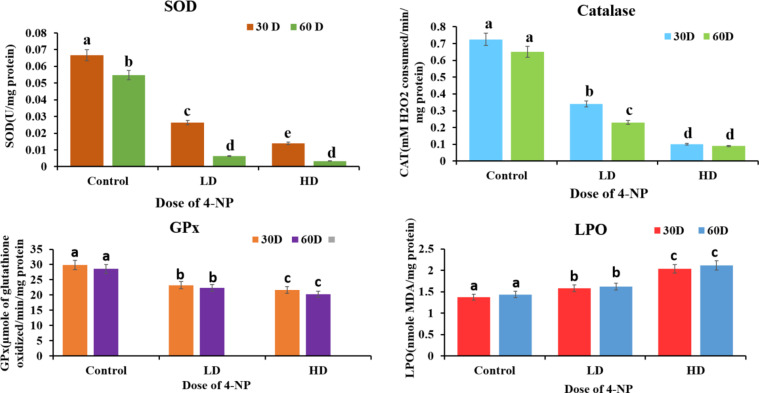
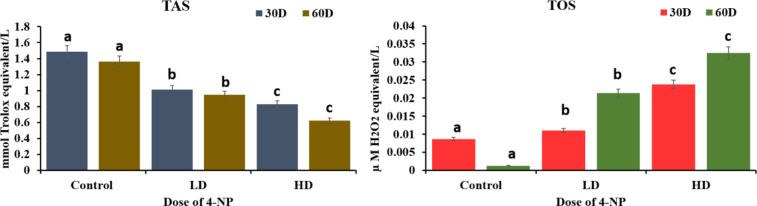
A significant alteration was seen in antioxidant parameters in the testes tissue of H. fossilis due to exposure to 4-NP of a low dose of 64 µg/L and 160 µg/L of 30 and 60 days from the pre-spawning period to the spawning period. Dose and duration-dependent significant decrease in the activity of SOD, CAT, GPx, Total Antioxidant status over control(P < 0.05) and significant increase in the activity of LPO, Total oxidant status was found in testes tissue when compared to control groups of experiments(P < 0.05). Figures 2 and 3 respectively.
Fig. 2.
Effects of 4-NP on antioxidant defense system (SOD, CAT, GPx, LPO) in testes of catfish H. fossilis during pre-spawning and spawning phase of the reproductive cycle of low dose (64 µg/L) and high dose (160 µg/L) of exposure for 30 days and 60 days. Statistical significance is determined using two- way ANOVA and post-hoc test Bonferroni test. Different letters indicate significant differences with respective control (n = 15, P < 0.05).
Fig. 3.
Effects of 4-NP on TAS (total antioxidant status) and TOS (total oxidant status) in testes of catfish H. fossilis during pre-spawning and spawning phase of the reproductive cycle of low dose (64 µg/L) and high dose (160 µg/L) of exposure for 30 days to 60 days. Statistical significance is determined using two-way ANOVA and post hoc test Bonferroni test. Different letters indicate significant differences with respective control (n = 15, P < 0.05).
Effect of 4-NP exposure on cortisol level
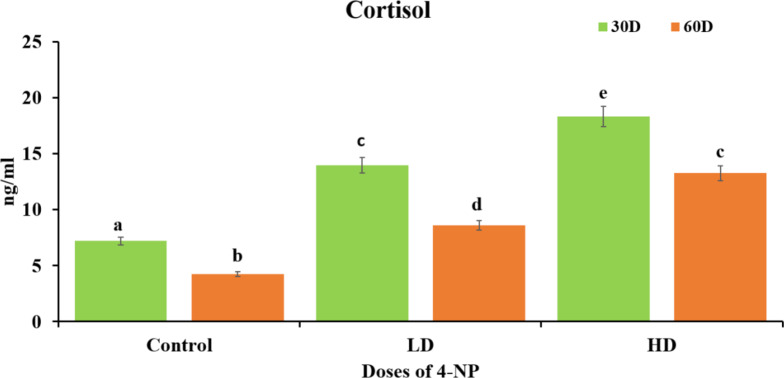
Results depicted that there was dose dependent increase(P < 0.05) in level of cortisol in testes of H. fossilis when expose to 4-NP dose of low dose 64 µg/L and high dose of 160 µg/L for 30 days and 60 days from pre-spawning to spawning period when treated group was compared to control group. It was also observed that cortisol is higher in 30 days of treatment than 60days of treatment in testes tissue of H. fossilis Fig. 4.
Fig. 4.
Effects of 4-NP on stress hormone (cortisol level) in testes of catfish H. fossilis during pre-spawning and spawning phase of the reproductive cycle of low dose (64 µg/L) and high dose (160 µg/L) of exposure for 30 days to 60 days. Statistical significance is determined using two-way ANOVA and post-hoc test Bonferroni test. Different letters indicate significant differences with respective control (n = 15, P < 0.05).
Effect of 4-NP on the apoptosis, necrosis, and cell viability in testes of H. fossilis
Exposure of 4-NP low dose 64 µg/L and high dose 160 µg/L during pre-spawning to the spawning period of 30 days and 60 days to testes of H. fossilis, significantly increase in necrotic cells and dose dependent increase in apoptotic cells percentage but percentage of necrotic cells were more than the percentage of apoptotic cells in testicular cells of 30 and 60 days treatment group when compare to the control group Fig. 5.
Fig. 5.
(A) Flow cytometry dot plots of the simultaneous binding of annexin V-fluorescein isothiocyanate (FITC) (FL-1) and propidium iodide uptake (FL-2) in testes of H.fossilis control, Low dose 64 µg/l and high dose 160 µg/l for 30 to 60 days exposed groups to 4-NP. The numbers represent the percentage (%) of cells. The cells from the control H.fossilis were mostly negative for both annexin V binding and uptake of propidium iodide (PI). In contrast, the cells from nonylphenol (NP)treated groups exhibited strong positive staining for annexin V or both annexin V and PI in dose and time-dependent manner, (B) and (C) are graphical representation of necrosis and apoptosis percentage of flow cytometry dot plots. Statistical significance is determined using two-way ANOVA and post hoc test Bonferroni test. Different letters indicate significant differences with respective control (n = 15, P < 0.05).
Effect of 4-NP on intracellular ROS in testes of H. fossilis
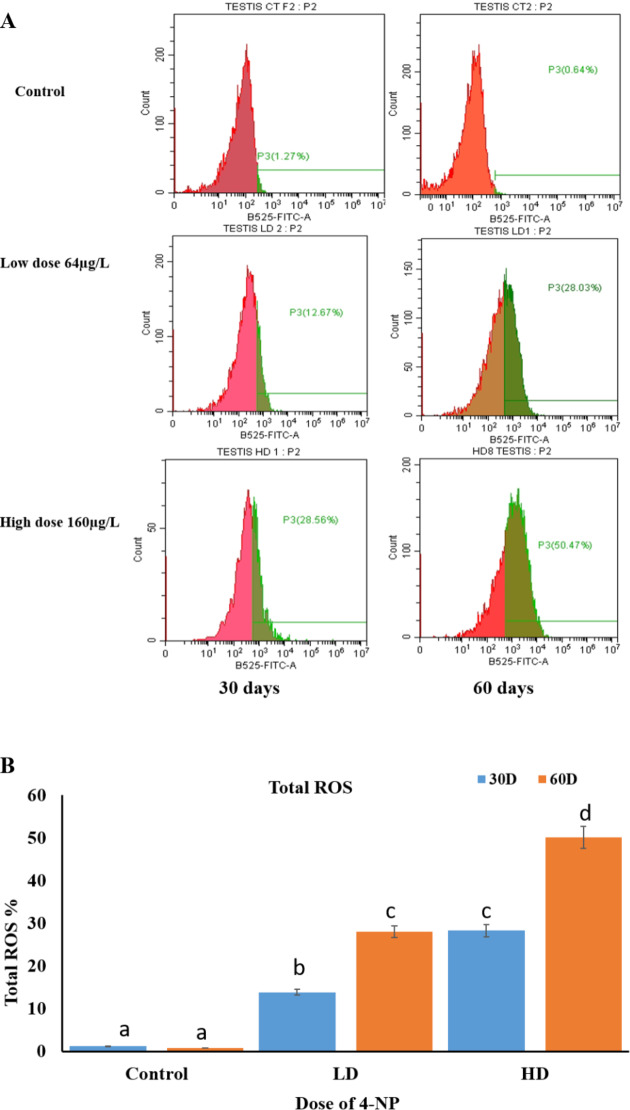
Results depicted that 4-NP exposure to male catfish of low dose 64 µg/L and high dose 160 µg/L for 30 and 60 days during pre-spawning to spawning period cause increased level of intracellular generation of Total ROS (reactive oxygen species) in dose dependent manner in testicular cells of H. fossilis (P < 0.05). Figure 6.
Fig. 6.
(A) Cytofluorometric (ROS-FACS) analysis of the frequency histograms for total ROS (reactive oxygen species) generation percentage by flow cytometer (B) Showing graphical representation of Total ROS generation intensity in testicular cells showing the effect of different concentrations of 4-NP doses low dose 64 µg and high dose of 160 µg on testicular cells of H. fossilis duration of 30 to 60 days pre-spawning to spawning period. Statistical significance is determined using two-way ANOVA and post hoc test Bonferroni test. Different letters indicate significant differences with respective control (n = 15, P < 0.05).
Effect of 4-NP on the DNA fragmentation exposure to testes of H. fossilis
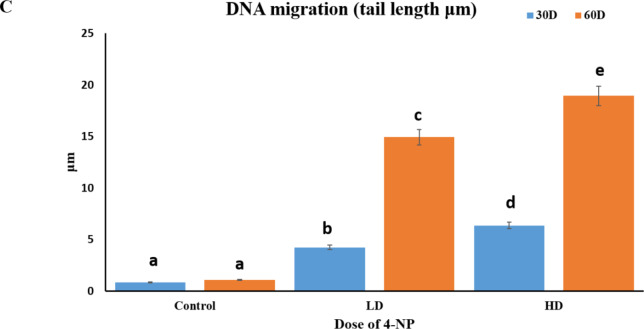
Our results depicted that 4-NP exposure to male catfish of low dose 64 µg/L and high dose 160 µg/L for 30 and 60 days during pre-spawning to spawning period cause increased DNA fragmentation means it makes DNA to migrate forms comet cause tail movement. Increase in DNA fragmentation was dose dependent manner in testicular cells Fig. 7.
Fig. 7.
(A) Measuring DNA damage in cells using CASP lab software showing different degrees of DNA migration (DNA fragmentation) indicated by comet parameters as comet head and tail. (a) Control: No DNA migration in undamaged cell (b) Treated: Mild increase in DNA migration or tail length (c) Treated: Moderate increase in DNA migration; (d) Treated: Severe DNA damage with increased DNA migration or tail length. (B) Unprocessed comet assay image showing control and treated group (C) DNA migration (tail length, µm) in control, low dose 64 µg/l, and high dose 160 µg/l for 30 to 60 days exposed groups to 4-NP in the testes of H. fossilis Statistical significance is determined using Two-way ANOVA and post hoc test Bonefferoni test. Different letters indicate significant differences with respective control (n = 15, P < 0.05).
Effect of 4-NP on the gene expression in testes of catfish H. fossilis
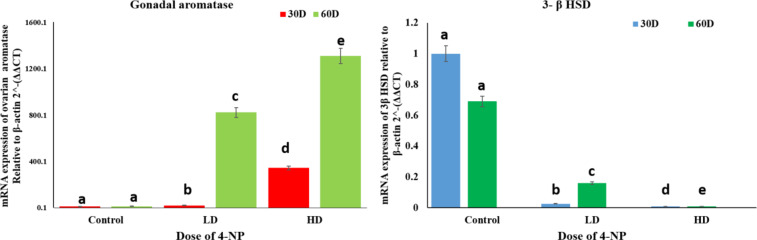
4-NP showed different effects on the gene expression of gonadal aromatase (CYP19a1a) and 3-β Hydroxysteroid dehydrogenase (3-β HSD). It significantly downregulates 3-β HSD expression and significantly upregulates the CYP19a1a expression in the testes of H.fossilis in both low and high doses 64 µg/L and high doses 160 µg/L for 30 and 60 days respectively Fig. 8.
Fig. 8.
Effects of 4-NP on gene expression of Gonadal aromatase and 3-β HSD in the Testes of catfish H. fossilis of 30 to 60 days treatment of low dose (64 µg/L) and high dose (160 µg/L) during pre-spawning and spawning phase of the reproductive cycle. Data were expressed as mean ± SEM (n = 15). Assessment of data by two-way ANOVA followed by post hoc test Bonferroni test. Different letters denote significant changes from the control. (P < 0.05).
Assessment of binding affinity of different protein biomarkers with 4-NP
UniProt id were obtained from the UniProt database for reproductive biomarkers of the same species or if not found in the same species then close species genera were taken, UniProt id mentioned with species and all biomarkers (Table 2).
Table 2.
UniProt id of different species and different biomarkers.
| S.No. | Protein enzyme | Species | UniProt id |
|---|---|---|---|
| 1. | CYP19a1a | Heteropneustes fossilis | A0A068CIW9 |
| 2. | 3-βHSD | Clarias batrachus | A0A075M3S4 |
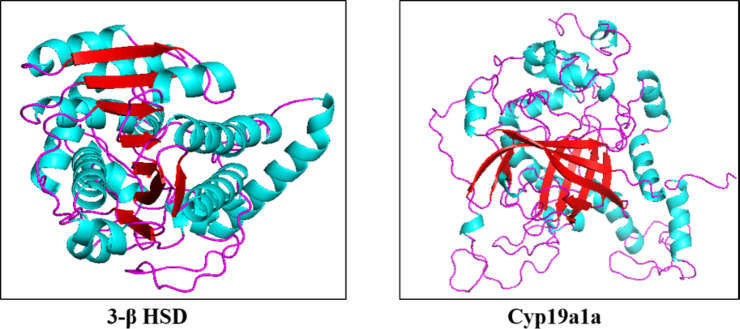
Protein crystal structure from PDB and if the structure is not predicted download the alpha fold structure PDB file of our proteins from uniport. The protein crystal structure from PDB is not available because no work has been done till yet so, we have taken the structure from the UniProt database, Fig. 9, the proteins used here were obtained among different groups of species of catfishes which are: 3-beta hydroxysteroid dehydrogenase (3-β HSD) of Clarias batrachus, Cytochrome P450 aromatase (CYP19a1a) of Heropneustes fosillis. (Table 2)
Fig. 9.
Alpha fold PDB file of proteins of 3-β HSD from UniProt database.
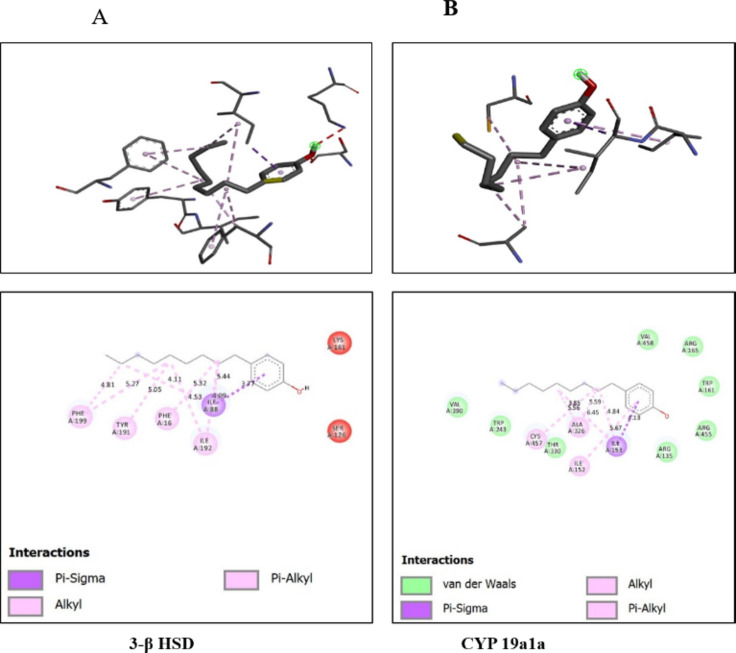
Molecular docking was done by auto doc vina, ligand-receptor interaction and obtained the binding affinity of given biomarkers with the 4-Nonylphenol in 3-D form of representation complex with 4-NP and amino acid residue in the binding pocket of given reproductive, biomarkers. (Fig. 10) Higher binding energy was obtained from the interaction between 4-NP and 3-βHSD − 6.2 kcal/mol with number of hydrogen bonds is zero > CYP 19a1a with 4-NP -5.3 kcal/mol with the number of hydrogen bonds is zero. (Table 3)
Fig. 10.
3-D form representation of docking complex of A-3β HSD, B-CYP19a1a with 4-Nonylphenol Amino-acid residues in the binding pocket of 3-βBHD and CYP19a1a involved in interactions with 4-Nonylphenol.
Table 3.
Binding affinity of different reproductive biomarkers with 4-NP.
| Enzymes | Ligand | Binding affinity (Kcal/mol) | No. of H bonds | Amino acids involved in the interaction |
|---|---|---|---|---|
| 3-β HSD | 4-Nonylphenol | -6.2 | 0 | PheA199, TyrA191,PheA16,IleA88,IleA192 |
| CYP19 a1a | 4-Nonylphenol | -5.3 | 0 | CysA457, AlaA326, IleA152, IleA153 |
Discussion
Effect of 4-NP on morphology of testes of H. fossilis
In the present studies, elucidated the effect of 4-Nonylphenol exposure of low dose (64 µg L− 1) and high dose (160 µg L− 1) for 30 days to 60 days respectively on the morphology of testes catfish Heteropneustes fossilis by using a scanning electron microscope (SEM). It was depicted that in 30 days exposure of low dose (64 µg L− 1) and high dose (160 µg L− 1) showed clumping of spermatocytes54 and this intensity has been increased severely in dose and duration manner in 60 days of exposure. SEM images reveal that spermatocytes in fish testes exposed to 4-NP tend to aggregate or clump together. In control groups normally, spermatocytes are well-organized within the seminiferous tubules, each in distinct developmental stages. However, 4-NP disrupts this organization, leading to clusters of spermatocytes. The disruption of cellular junctions often accompanies the clumping. Tight junctions and desmosomes, which normally hold spermatocytes in place, are compromised, leading to the loose association and subsequent clumping of these cells. SEM studies show that the surface morphology of clumped spermatocytes differs significantly from normal cells. The cell membranes of clumped spermatocytes appear irregular and show signs of membrane fusion or adherence to adjacent cells54. The clumping of spermatocytes leads to disorganized seminiferous tubules. Instead of the orderly arrangement of germ cells, SEM images show disarray, with clumps of spermatocytes disrupting the tubular architecture. The clumping of spermatocytes disrupts the normal process of spermatogenesis. Proper spacing and organization are crucial for the maturation of sperm cells and clumping hinders this process, resulting in fewer mature sperm. Fish exposed to 4-NP and exhibiting clumped spermatocytes generally show reduced fertility. The quality and quantity of sperm are adversely affected, leading to lower reproductive success. The stress from clumping and disrupted cellular environments can lead to increased apoptotic or necrosis (cell death) of spermatocytes55, further reducing the sperm count and impairing testicular function56. This is reported first time the clumping of spermatocytes in male catfish H.fossilis by using scanning electron microscope for morphological view.
Evaluation of antioxidant parameters (SOD, CAT, GPx, and TAS) and LPO, TOS in testes tissue of H. fossilis due to exposure of 4-NP
Oxidative stress occurs when there is an imbalance between reactive oxygen species (ROS) and reactive nitrogen species (RNS) in cells and tissues, overwhelming the body’s antioxidant defenses. These defenses, including antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH), serve as the primary line of protection against free radicals, which contribute to oxidative stress. As such, they are important markers for determining how harmful the environment is to different aquatic species57,58. Antioxidant enzyme activities of aquatic animals are affected by 4-nonylphenol (4-NP) exposure59. Lipid peroxidation (LPO), which is caused by oxidative damage to circulating lipids and phospholipids in cell membranes, yields malondialdehyde (MDA). An increase in MDA levels indicates oxidative stress caused by a variety of substances, including xenobiotics60. The results of this investigation into the effects of 4-NP exposure on lipid peroxidation are consistent with those of previous studies41,61–63. Oxidative stress is indicated by increased lipid peroxidation (LPO) and decreased CAT, SOD and GPX activities. This oxidative stress can further impair steroidogenic activity in the gonads, as can respiratory stress and genotoxicity. Exposure to the pyrethroid esbiothrin in Cyprinus carpio resulted in a decreased plasma total antioxidant status64.
Oxidative stress and cellular abnormalities were recently observed in the testes of Clarias gariepinus (Burchell) from the Yamuna River in the Delhi region65. In our laboratory similar studies has depicted the decreased level of SOD, CAT, GPx and increased level of LPO in testicular tissues due to effect of 4-NP of dose 80 µg/L for 21 days of treatment22.
Effect of 4-NP on cortisol level in testes of H. fossilis
Cortisol has detrimental effects on reproductive function66,67. In Pacific salmon, hypercortisolism may lead to apoptosis after spawning68. Studies on the impact of estradiol (E2) or 4-nonylphenol (4-NP) on stress responses in Atlantic salmon yolk-sac larvae over an extended period of time have revealed a rise in plasma cortisol levels69. Another study reported that juvenile Sparus aurata exhibited a substantial reduction in cortisol concentrations following an acute simultaneous exposure to 4-NP and E270.
Fish exposed to the highest dose of 4-NP exhibited high cortisol levels along with low total antioxidant capacity (TAC). This result could indicate that after being exposed to 4-NP, the plasma antioxidant network reacts to the increased generation of reactive oxygen species (ROS). As a result, TAC may be a useful marker for determining how pollution affects animals in the ecosystem71. Increased cortisol levels can cause a decrease in TAC. This can be because different cortisol pathways activate sequentially, or because ROS directly affect cortisol metabolism72.
Effect of 4-NP on the apoptosis, necrosis, and cell viability in testes of H. fossilis
Results show that exposure of 4-NP with low dose and high dose i.e.,64 µg/L and 160 µg/L of 30 days and 60 days induces necrosis or cell death in testicular tissue it means 4-NP is extremely toxic to the testicular cells causes direct cell damage without entering into programmed cell death apoptosis mechanism. And necrosis percentage increases with dose and duration-dependent induction to 4-NP. Other than xenoestrogenic and as an endocrine disrupting chemical role of 4-Nonylphenol, it also causes cytotoxicity of gonadal tissues leading to reproductive toxicity. For instance, it has been demonstrated that 4-NP causes testicular cell death by preventing endoplasmic reticulum Ca2 + pumps from functioning73. Through the production of reactive oxygen species (ROS), including superoxide anion (O2−) and hydrogen peroxide (H2O2) a variety of environmental pollutants can cause oxidative stress. ROS can start a chain of events that harm cellular constituents and ultimately result in cell death74,65. Alteration in antioxidant parameters and increased levels of ROS induce apoptosis cell death and eventually cause cell death of the tissues. Testicular apoptosis of zebrafish increased under Bisphenol A induction75.
Effect of 4-NP on intracellular ROS in testes of H. fossilis
In our study, it was observed that exposure of 4-NP of 64 µg/L and 160 µg/L causes induction of intracellular ROS in testicular cells in dose dose-dependent manner. Oxidative stress-induced cellular activation typically results from ROS-mediated oxidation of DNA, polyunsaturated fatty acids in lipids76, and amino acids in proteins77. Beyond these direct oxidation effects, cellular oxidative responses also occur. According to recent studies, 4-NP can cause the generation of ROS in yeast, bacterial, and cultured vertebrate cells at doses ranging from 50 to 200 µM23,24,78,79. Additionally, it has been observed that 4-NP responds to oxidative stress in vivo80,81. These findings indicate that 4-NP within this concentration range can cause both estrogenic effects and ROS production. Although these 4-NP levels are higher than those typically found in the environment, significant quantities of 4-NP have been identified in some sewage and in numerous plants and animals in aquatic habitats12.
Effect of 4-NP on the DNA fragmentation exposure to testes of catfish H. fossilis
4-Nonylphenol induces genotoxicity, and cytotoxicity causes DNA damage or DNA fragmentation. Our investigation shows that DNA fragmentation by alkaline COMET assay showed tail DNA movement increases in a dose-dependent manner in the testes of catfish H. fossilis when they are exposed to 4-NP doses. Reactive species such as free radicals are continuously generated in vivo, with DNA being the most significant target of oxidative stress. Oxidative DNA damage serves as a predictive biomarker for monitoring the risk of developing various diseases. In the comet assay, the percentage of tail DNA is used to evaluate DNA damage50. This is a highly important and sensitive parameter that has been extensively utilized in various studies82,83. The excessive production of ROS can damage lipids, proteins, and DNA84. 0.4-NP has been reported to disrupt microtubules, induce DNA adduct formation, and cause point mutations, strand breaks, and genomic rearrangements83.
Effect of 4-NP on the gene expression in testes of catfish H. fossilis
The present investigation depicts the profound effect of 4-Nonylphenol on gene expression of CYP19a1a, and 3β HSD in testes of H. fossilis. CYP19a1a drastically increase in testes in a dose-dependent manner and 3-β HSD decrease in dose-dependent manner due to endocrine disrupting and xenoestrogen effect of 4-NP exposure of 64 µg/L as a low dose and 160 µg/L as a high dose given for period of 30 days to 60 days of exposure.
Nonylphenol (4-NP) has been shown to have demasculinizing and estrogenic effects in teleost fish such as impaired testicular development and increased hepatic vitellogenin production76,85–87,]. Xenoestrogens influence sex differentiation by altering the expression levels of steroid receptors and steroidogenic enzymes, which are crucial for this process. In the South American teleost pejerrey, Odontesthes bonariensis, exposure to EE2 was associated with a skewed sex ratio and a downregulation of 11-β hydroxysteroid dehydrogenase type 2 (11-β 2-HSD), which is necessary for the production of both androgen receptors (Arɑ and Arβ) and the principal male androgen, 11-ketotestosterone (11-KT). Simultaneously, there was a notable rise in the expression of cytochrome P450 aromatase cyp19a1a, an essential enzyme involved in the synthesis of estrogen88. Similarly, an augmentation of ovarian aromatase expression was observed in zebrafish exposed to 10 µg/L BPF89.
Similar results were found that the impact of xenoestrogens on the expression of steroidogenic genes has been investigated, with most studies indicating a downregulation of these genes in the gonads. Specifically, gonadal expression of steroidogenic acute regulatory protein (star), cytochrome P450-mediated side-chain cleavage enzyme (cyp11a1), cytochrome P450 17 or 17-β hydroxysteroid dehydrogenase (cyp17a1 or 17-β HSD) and 3-β hydroxysteroid dehydrogenase (3-β HSD) significantly decreased in male and female adult rare minnows exposed to 25 µg/L of EE290,91.
Assessment of binding affinity of different protein biomarkers with 4-NP
Our docking study revealed that 4-NP elicits higher binding affinity with 3-β HSD followed by CYP19a1a,4-NP interacted with 3-βHSD PheA199, TyrA191, PheA16, IleA88, IleA192 amino residue followed by Cyp19a1a interacted 4-NP with its amino acid residue CysA457, AlaA326, IleA152, IleA153. It has been described that amino acid residue plays crucial role in the connection between the ligand and the receptor. Organophosphate compounds are known to alter steroid hormones in aquatic organisms92–94.Molecular docking results elucidated that 4-NP has higher interaction with 3-β HSD followed by CYP19a1a which is proved in vivo studies elicits that gene expression of 3-βHSD drastically decreases due to the effect of 4-NP showing its anti-androgenic effect 0.4-NP also act as xenoestrogen which was depicted in vivo result having gene expression of ovarian aromatase increases abruptly in male catfish H. fossilis showing the xenoestrogen attributes of 4-NP.
Conclusion
In vivo studies elucidated the various changes induced by 4-NP in fish gametogenesis and steroidogenesis ultimately leading to a decline in their reproductive success. Research has demonstrated that exposure to water bodies contaminated with xenoestrogens like 4-NP causes oxidative stress at the cellular level and genotoxicity at the gene level. In silico studies elicit the interaction or binding affinity of 4-NP with steroid enzyme 3-β HSD and CYP19a1a which were validated the 4-NP detrimental effects. Due to chronic exposure to 4-NP, it becomes toxic and causes tissue cell death. So, the harmful impact of 4-NP on reproduction in teleost fish is concerning, not just for the fish themselves but for the entire ecosystem. Therefore, efforts should be made to reduce the contamination of water bodies with xenoestrogens.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Suman would like to express her gratitude to the Indian Council of Medical Research (ICMR), New Delhi, India, for awarding a Senior Research Fellowship (File No.45/05/2022/TRM/BMS). IoE SRICC Banaras Hindu University credit passbook for monetary assistance. The author acknowledges the Interdisciplinary School of Life Sciences (ISLS), SATHI, Central Discovery Centre (CDC), CIL (M.M.V), Banaras Hindu University for assisting the instrumental support.
Author contributions
Suman, GGJ and RC were involved in the conception, design, and execution of the experiments. In silico part was done by MJ. All experimental data was analyzed by Suman, GGJ, contributed to the article’s drafting. PG and Suman assisted with reagent preparation and experimentation. It was critically revised for significant knowledgeable content by Suman and GGJ. The final version to be published was approved by all of the authors.
Data availability
All data supporting the findings of this study are available within the paper.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Okai, Y., Sato, E. F., Higashi-Okai, K. & Inoue, M. Enhancing effect of the endocrine disruptor para-nonylphenol on the generation of reactive oxygen species in human blood neutrophils. EnvironmentalHealthPerspectives112 (5), 553–556. 10.1289/ehp.6584 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Bruin, W., Kritzinger, Q., Bornman, R. & Korsten, L. Occurrence, fate and toxic effects of the industrial endocrine disrupter, nonylphenol, on plants-a review. Ecotoxicol. Environ. Saf.181, 419–427. 10.1016/j.ecoenv.2019.06.009 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Chokwe, T. B., Okonkwo, J. O. & Sibali, L. L. Distribution, exposure pathways, sources and toxicity of nonylphenol and nonylphenol ethoxylates in the environment. Water Sa. 43 (4), 529–542. 10.4314/wsa.v43i3.01 (2017). [Google Scholar]
- 4.Sharma, M., Chadha, P. & Sharma, P. DNA damage in spleen as a indicator of genetoxicity in Channa punctatus exposed to 4-nonylphenol. J. Environ. Biol.41 (1), 53–58. 10.22438/jeb/41/1/MRN-1099 (2020). [Google Scholar]
- 5.Araujo, F. G. D., Bauerfeldt, G. F. & Cid, Y. P. Determination of 4-nonylphenol in surface waters of the Guandu river basin by high performance liquid chromatography with ultraviolet detection. Journal of the Brazilian Chemical Society, 29(10), 2046–2053. (2018). 10.21577/0103-5053.20180079
- 6.Sayed, A. E. D. H., Eid, Z., Mahmoud, U. M., Lee, J. S. & Mekkawy, I. A. Reproductive toxicity and recovery associated with 4-Non-ylphenol exposure in juvenile African catfish (Clarias garepinus). Front. Physiol.13, 851031. 10.3389/fphys.2022.851031 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erel O.A new automated colorimetric method for measuring total oxidant status. Clin. Biochem.38 (12), 1103–1111. 10.1016/j.clinbiochem.2005.08.008 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Rajizadeh, M. A., Hosseini, M. H., Bahrami, M., Hosseini, N. S., Rostamabadi, F.,Bagheri, F., … Bejeshk, M. A. Comparison of preventive and therapeutic effects of continuous exercise on acute lung injury induced with methotrexate. Experimental Physiology,108(9), 1215–1227. https://doi.org/10.1113/EP091162 (2023). [DOI] [PMC free article] [PubMed] [Retracted]
- 9.Lushchak, V. I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol.101 (1), 13–30. 10.1016/j.aquatox.2010.10.006 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Gautam, G. J., Chaube, R. & Joy, K. Toxicity and tissue accumulation of 4-nonylphenol in the catfish Heteropneustes fossilis with a note on prevalence of 4-NP in water samples. Endocr. Disruptors. 3 (1), e981442. 10.4161/23273747.2014.981442 (2015). [Google Scholar]
- 11.Yin, H., Xu, L. & Porter, N. A. Free radical lipid peroxidation: mechanisms and analysis. Chemicalreviews111 (10), 5944–5972. 10.1021/cr200084z (2011). [DOI] [PubMed] [Google Scholar]
- 12.Ahel, M., McEvoy, J. & Giger, W. Bioaccumulation of the lipophilic metabolites of nonionic surfactants in fresh water organisms. Environ. Pollut.79 (3), 243248. 10.1016/0269-7491(93)90096-7 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Limberaki, E. et al. Cortisol levels and serum antioxidant status following chemotherapy. Health3 (08), 512. 10.4236/health.2011.38085 (2011). [Google Scholar]
- 14.Martinez-Alvarez, R. M., Morales, A. E. & Sanz, A. Antioxidant defenses in fish: biotic and abiotic factors.reviews. Fish. Biology Fisheries. 15, 75–88. 10.1007/s11160-005-7846-4 (2005). [Google Scholar]
- 15.Rego, A. C. & Oliveira, C. R. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochemicalresearch28, 1563–1574. 10.1023/A:1025682611389 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal, A., Dash, D. & Singh, R. Intranasal Curcumin and dexamethasone combination ameliorates inflammasome (NLRP3) activation in lipopolysaccharide exposed asthma exacerbations. Toxicol. Appl. Pharmcol.436, 115861. 10.1016/j.taap.2021.115861 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Sayed, A. E. D. H., Mahmoud, U. M. & Mekkawy, I. A. Erythrocytes alterations of monosex tilapia (Oreochromis niloticus, Linnaeus, 1758) produced using Methyltestosterone. Egypt J. Aquat. Res.42 (1), 83–90. 10.1016/j.ejar.2015.10.004 (2016). [Google Scholar]
- 18.De Falco, M. et al. V.Nonylphenol effects on the HPA axis of the bioindicator vertebrate, Podarcis sicula Lizard. Chemosphere104, 190–196. 10.1016/j.chemosphere.2013.11.014 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Ololade, I. A. & Oginni O.Toxic stress and hematological effects of nickel on African catfish, Clarias Gariepinus, fingerlings. J. Environ. Chem. Ecotoxicol.2 (2), 014–019 (2010). [Google Scholar]
- 20.Robertson, J. D. & Orrenius, S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit. Rev. Toxicol.30 (5), 609–627. 10.1080/10408440008951122 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Chaube, R. et al. Molecular cloning and characterization of a gonadotropin-releasing hormone 2 precursor cDNA in the catfish Heteropneustes fossilis: expression profile and regulation by ovarian steroids. Fish Physiol. Biochem.280, 134–146. 10.1016/j.ygcen.2019.04.021 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Suman, R. C. & Jiwatram, G. G. 4-Nonylphenol affects the structure and function of testis in catfish H.fossilis and C.batrachus. Journal of Scientific Research,65(5). (2021). 10.37398/JSR.2021.650517
- 23.Okai, Y. et al. Protective effect of antioxidants against para-nonylphenol-induced Inhibition of cell growth in Saccharomyces cerevisiae. FEMS Microbiol. Lett.185 (1), 65–70. 10.1111/j.1574-6968.2000.tb09041.x (2000). [DOI] [PubMed] [Google Scholar]
- 24.Okai, Y., Sato, E. F., Higashi-Okai, K. & Inoue, M. Effect of endocrine disruptor para-nonylphenol on the cell growth and oxygen radical generation in Escherichia coli mutant cells deficient in catalase and superoxide dismutase. Free Radic. Biol. Med.37 (9), 1412–1418. 10.1016/j.freeradbiomed.2004.07.001 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Suman, R. C. & Gautam, G. J. Modulation of testicular activity in Indian stinging catfish Heteropneustes fossilis under acute exposure to 4-Nonylphenol.Journal of Environmental Biology,43 (2022). 10.22438/jeb/43/2/MRN-1989
- 26.Mao, Z. et al. Occurrence and biodegradation of nonylphenol in the environment. Int. J. Mol. Sci.13 (1), 491–505. 10.3390/ijms13010491 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, H., Yang, X., Zhang, Z. & Xu, H. Both calcium and ROS as common signals mediate Na2SeO3-induced apoptosis in SW480 human colonic carcinoma cells. J. Inorg. Biochem.97 (2), 221–230. 10.1016/S0162-0134(03)00284-8 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Agarwal, A., Saleh, R. A. & Bedaiwy, M. A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril.79 (4), 829–843. 10.1016/s0015-0282(02)04948-8 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Benhar, M., Engelberg, D. & Levitzki, A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. Doi. 10.1093/embo-reports/kvf094 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong, Y. & Han, X. D. Nonylphenol-induced oxidative stress and cytotoxicity in testicular Sertoli cells. Reprodive Toxicol.22 (4), 623–630. 10.1016/j.reprotox.2006.04.019 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Raju, S., Sivamurugan, M., Gunasagaran, K., Subramani, T. & Natesan, M. Preliminary studies on the occurrence of nonylphenol in the marine environments, Chennai—a case study. J. Basic. Appl. Zool.79, 1–7. 10.1186/s41936-018-0063-1 (2018). [Google Scholar]
- 32.Rivero, C. L., Barbosa, A. C., Ferreira, M. F. N., Dorea, J. G. & Grisolia, C. K. Evaluation of genotoxicity and effects on reproduction of nonylphenol in Oreochromis niloticus (Pisces: Cichlidae). Ecotoxicology17, 732–737. 10.1007/s10646-008-0222-0 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Gautam, G. J., Chaube, R. & December Toxic impact of alkylphenols on the fish reproduction and endocrine disruption. In Proceedings of the Zoological Society (Vol. 74, No. 4, pp. 648–659). New Delhi: Springer India. (2021). 10.1007/s12595-021-00416-z
- 34.Cavas, T., Garanko, N. N. & Arkhipchuk, V. V. Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food andChemicalToxicology. 43 (4), 569–574. 10.1016/j.fct.2004.12.014 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Monnet-Tschudi, F., Zurich, M. G., Boschat, C., Corbaz, A. & Honegger, P. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev. Environ. Health, 21(2),105–118. 10.1515/REVEH.2006.21.2.105(2006). [DOI] [PubMed]
- 36.Saha, S. K., Ali, M. H. & Rashid, H. Gametogenesis in Captive Stinging Catfish (Heteropneustes fossilis) During Spawning Season. In Proceedings of 5th International Conference on Environmental Aspects of Bangladesh (pp. 126–128) (2014).
- 37.Tabassum, H., Afjal, M. A., Khan, J., Raisuddin, S. & Parvez, S. Neurotoxicological assessment of pendimethalin in freshwater fish Channa punctata Bloch. Ecol. Ind.58, 411–417. 10.1016/j.ecolind.2015.06.008 (2015). [Google Scholar]
- 38.Bakker, J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J. Neuroendocrinol.15 (6), 615–621. 10.1046/j.1365-2826.2003.01036.x (2003). [DOI] [PubMed] [Google Scholar]
- 39.Chen, X. et al. Bioconcentration and developmental neurotoxicity of novel brominated flame retardants, hexabromobenzene and pentabromobenzene in zebrafish. Environ. Pollut.268, 115895. 10.1016/j.envpol.2020.115895 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Teles, M., Pacheco, M. & Santos, M. A. Sparus aurata L. liver EROD and GST activities, plasma cortisol, lactate, glucose and erythrocytic nuclear anomalies following short-term exposure either to 17β-estradiol (E2) or E2 combined with 4-nonylphenol. Sci. Total Environ.336 (1–3), 57–69. 10.1016/j.scitotenv.2004.05.004 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Mittal, A. K. & Whitear, M. A note on cold anesthesia of poikilotherms [hypothermia]. (1978). 10.1111/j.1095-8649.1978.tb03462.x
- 42.de Siqueira-Silva, D. H., dos Santos Silva, A. P., Ninhaus-Silveira, A. & Veríssimo-Silveira, R. The effects of temperature and Busulfan (Myleran) on the Yellowtail tetra Astyanax altiparanae (Pisces, Characiformes) spermatogenesis. Theriogenology84 (6), 1033–1042. 10.1016/j.theriogenology.2015.06.004 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Das, K. C. & Das, C. K. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: redox independent functions. Biochem. Biophys. Res. Commun.277 (2), 443–447. 10.1006/bbrc.2000.3689 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Claiborne, A. J. F. C. P. Handbook of Methods for Oxygen Radical ResearchBoca Raton, 283–284 (CRC, 1985).
- 45.Perez, M. R., Fernandino, J. I., Carriquiriborde, P. & Somoza, G. M. Feminization and altered gonadal gene expression profile by ethinylestradiol exposure to Pejerrey, Odontesthes bonariensis, a South American teleost fish. Environ. Toxicol. Chem.31 (5), 941–946. 10.1002/etc.1789 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Murugananthkumar, R., Rajesh, D. & Senthilkumaran, B. Copper nanoparticles differentially target testis of the catfish, Clarias Batrachus: in vivo and in vitro study. Front. Environ. Sci.4, 67. 10.3389/fenvs.2016.00067 (2016). [Google Scholar]
- 47.Dhawan, A., Bajpayee, M. M., Pandey, A. K. & Parmar Protocol for the single cell gel electrophoresis/comet assay for rapid genotoxicity assessment. Sigma1077 (1), 1–10 (2003). [Google Scholar]
- 48.Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem.37 (2), 112–119. 10.1016/j.clinbiochem.2003.10.014 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Bartos, J. & Pesez, M. Colorimetric and fluorimetric determination of aldehydes and ketones. Pure Appl. Chem.51 (8), 1803–1814. 10.1351/pac197951081803 (1979). [Google Scholar]
- 50.Yang, F. W., Li, Y. X., Ren, F. Z., Luo, J. & Pang, G. F. Assessment of the endocrine-disrupting effects of organophosphorus pesticide Triazophos and its metabolites on endocrine hormones biosynthesis, transport and receptor binding in Silico. Food Chem. Toxicol.13310.1016/j.fct.2019.110759 (2019). [DOI] [PubMed]
- 51.Hughes, P. J. et al. Estrogenic alkylphenols induce cell death by inhibiting testis Endoplasmic reticulum Ca2 + pumps. Biochem. Biophys. Res. Communication. 277 (3), 568–574. 10.1006/bbrc.2000.3710 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Chaube, R., Rawat, A. & Joy, K. P. Molecular cloning and characterization of brain and ovarian cytochrome P450 aromatase genes in the catfish Heteropneustes fossilis: sex, tissue and seasonal variation in, and effects of gonadotropin on gene expression. Gen. Comp. Endocrinol.221, 120–133. 10.1016/j.ygcen.2015.06.004 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Mekkawy, I. A., Mahmoud, U. M. & Sayed, A. E. D. H. Effects of 4-nonylphenol on blood cells of the African catfish Clarias gariepinus (Burchell, 1822). Tissue Cell.43 (4), 223–229. 10.1016/j.tice.2011.03.006 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Wang, L. et al. T.Psychological stress-induced oxidative stress as a model of sub–healthy condition and the effect of TCM. Evidence–Based Complement. Altern. Med.4 (2), 195–202. 10.1093/ecam/nel080 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murakawa, M., Jung, S. K., Iijima, K. & Yonehara, S. Apoptosis-inducing protein, AIP, from parasite-infected fish induces apoptosis in mammalian cells by two different molecular mechanisms. Cell. Death Differ.8 (3), 298–307. 10.1038/sj.cdd.4400811 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Stein-Behrens, B. A. & Sapolsky, R. M. Stress, glucocorticoids, and aging. Aging (Milan Italy). 4 (3), 197–210. 10.1007/BF03324092 (1992). [DOI] [PubMed] [Google Scholar]
- 57.Mommsen, T. P., Vijayan, M. M. & Moon, T. W. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish.9, 211–268. 10.1023/A:1008924418720 (1999). [Google Scholar]
- 58.Lovasova, E. & Sesztakova, E. Total antioxidant status-a possible marker of environmental influences on animal organism. Slovak J. Anim. Sci.42 (Supplement), 42–45 (2009). [Google Scholar]
- 59.Chitra, K. C. & Mohan, M. Response of the freshwater fish, Oreochromis mossambicus to the environmental pollutant, nonylphenol. Int. J. Adv. Res.2 (12), 85–91 (2014). [Google Scholar]
- 60.Banerjee, B. D., Seth, V., Bhattacharya, A., Pasha, S. T. & Chakraborty, A. K. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol. Lett.107 (1–3), 33–47. 10.1016/S0378-4274(99)00029-6 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Aydogan, M., Korkmaz, A., Barlas, N., & Kolankaya, D. The effect of vitamin C on bisphenol A, nonylphenol and octylphenol induced brain damages of male rats. Toxicology, 249(1), 35–39. doi: 10.1016/j.tox.2008.04.002 (2008). [DOI] [PubMed]
- 62.Weber, A. A. et al. Environmental exposure to oestrogenic endocrine disruptors mixtures reflecting on gonadal sex steroids and gametogenesis of the neotropical fish Astyanax rivularis. General and comparative endocrinology,279,pp.99–108. (2019). 10.1016/j.ygcen.2018.12.016 [DOI] [PubMed]
- 63.Abd-Elkareem, M., Abou Khalil, N. S. & Sayed, A. H. Hepatotoxic responses of 4-nonylphenol on African catfish (Clarias gariepinus): antixoidant and histochemical biomarkers. Fish Physiol. Biochem.44, 969–981. 10.1007/s10695-018-0485-1 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Selvaraj, K. K., Shanmugam, G., Sampath, S., Larsson, D. J. & Ramaswamy, B. R. GC–MS determination of bisphenol A and alkylphenol ethoxylates in river water from India and their ecotoxicological risk assessment. Ecotoxicol. Environ. Saf.99, 13–20. 10.1016/j.ecoenv.2013.09.006 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Tyor, A. K. & Pahwa, K. Testicular oxidative stress and cellular deformities in Clarias gariepinus (Burchell) from river Yamuna in Delhi region, India. Bull. Environ Contam. Toxicol.100, 659–664. 10.1007/s00128-018-2286-8 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Campbell, P. M., Pottinger, T. G. & Sumpter, J. P. Preliminary evidence that chronic confinement stress reduces the quality of gametes produced by brown and rainbow trout. Aquaculture120 (1–2), 151–169. 10.1016/0044-8486(94)90230-5 (1994). [Google Scholar]
- 67.Carragher, J. F., Sumpter, J. P., Pottinger, T. G. & Pickering, A. D. The deleterious effects of cortisol implantation on reproductive function in two species of trout, Salmo trutta L. and Salmo gairdneri Richardson. Gen. Comp. Endocrinol.76 (2), 310–321. 10.1016/0016-6480(89)90163-9 (1989). [DOI] [PubMed] [Google Scholar]
- 68.Stadtman, E. R. & Levine, R. L.Free radical-mediated oxidation of free amino acids and amino acid residues in proteins.Aminoacids,25,207–218. (2003). 10.1007/s00726-003-0011-2 [DOI] [PubMed]
- 69.Jobling, S., Sumpter, J. P., Sheahan, D., Osborne, J. A. & Matthiessen, P. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ. Toxicol. Chem. : Int. J.15 (2), 194–202. 10.1002/etc.5620150218 (1996). [Google Scholar]
- 70.Talapatra, S. N. & Banerjee, S. K. Detection of micronucleus and abnormal nucleus in erythrocytes from the gill and kidney of Labeo bata cultivated in sewage-fed fish farms. Food Chemicaltoxicology. 45 (2), 210–215. 10.1016/j.fct.2006.07.022 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Liu, Y. et al. DNA methylation in the 5′ flanking region of cytochrome P450 17 in adult rare minnow Gobiocypris rarus-tissue difference and effects of 17α-ethinylestradiol and 17α-methyltestosterone exposures. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 16–22. 10.1016/j.cbpc.2014.03.001 (2014). ,162,. [DOI] [PubMed]
- 72.Lerner, D. T., Björnsson, B. T. & McCormick, S. D. Larval exposure to 4-nonylphenol and 17β-estradiol affects physiological and behavioral development of seawater adaptation in Atlantic salmon Smolts. Environ. Sci. Technol.41 (12), 4479–4485. 10.1021/es070202w (2007). [DOI] [PubMed] [Google Scholar]
- 73.Golden, T. R., Hinerfeld, D. A. & Melov, S. Oxidative stress and aging: beyond correlation. Aging Cell.1 (2), 117–123. 10.1046/j.1474-9728.2002.00015.x (2002). [DOI] [PubMed] [Google Scholar]
- 74.Chitra, K. C., Latchoumycandane, C. & Mathur, P. P.Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology185 (1–2), 119–127. 10.1016/S0300-483X(02)00597-8 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Zade, S., Nagwanshi, A., Shinkhede, M. & Agase, D. A study on the toxicity of 4-nonylphenol on the histopathology of testes of African catfish Clarias gariepinus (Burchell, 1822). J. Appl. Nat. Sci.10 (2), 676–680. 10.31018/jans.v10i2.1765 (2018). [Google Scholar]
- 76.Yang, X., Liu, Y., Li, J., Chen, M., Peng, D., Liang, Y., … Jiang, G. Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish.Environmental toxicology, 31(3),285–294.https://doi.org/10.1002/tox.22043 (2016). [DOI] [PubMed]
- 77.Soares, A., Guieysse, B., Jefferson, B., Cartmell, E. & Lester, J. N. Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int.34 (7), 1033–1049. 10.1016/j.envint.2008.01.004 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Giesy, J. P., Pierens, S. L., Snyder, E. M., Miles-Richardson, S., Kramer, V. J.,Snyder, S. A., … Villeneuve, D. A. Effects of 4‐nonylphenol on fecundity and biomarkers of estrogenicity in fathead minnows (Pimephales promelas). Environmental Toxicology and Chemistry.: An International Journal,19(5),1368–1377.https://doi.org/10.1002/etc.5620190520 (2000).
- 79.Ohkawa, H., Ohishi, N. & Yagi K.Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.95 (2), 351–358. 10.1016/0003-2697(79)90738-3 (1979). [DOI] [PubMed] [Google Scholar]
- 80.Chitra, K. C. & Mathur, P. P. Vitamin E prevents nonylphenol-induced oxidative stress in testis of rats. Indian J. Exp. Biol.42 (2), 220–223 (2004). [PubMed] [Google Scholar]
- 81.Chitra, K. C., Latchoumycandane, C. & Mathur, P. Effect of nonylphenol on the antioxidant system in epididymal sperm of rats. Arch. Toxicol.76, 545–551. 10.1007/s00204-002-0372-4 (2002). [DOI] [PubMed] [Google Scholar]
- 82.Ali, T. et al. Genotoxicity and repair capability of Mus musculus DNA following the oral exposure to Tramadol. Saudi J. Biol. Sci.27 (1), 12–17. 10.1016/j.sjbs.2019.03.008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Selvi, M., Çavaş, T., Cağlan Karasu Benli, A., Koçak Memmi, B., Çinkılıç, N., Dincel,A. S., … Erkoc, F.Sublethal toxicity of esbiothrin relationship with total antioxidant status and in vivo genotoxicity assessment in fish (Cyprinus carpio L., 1758) using the micronucleus test and comet assay. Environmental toxicology,28(11),644–651.https://doi.org/10.1002/tox.20760 (2013). [DOI] [PubMed]
- 84.Baudou, F. G. et al. Use of integrated biomarker indexes for assessing the impact of receiving waters on a native Neotropical teleost fish. Sci. Total Environ. 1779–1786. 10.1016/j.scitotenv.2018.09.342 (2019). ,650,. [DOI] [PubMed]
- 85.Gavrieli, Y., Sherman, Y. & Ben-Sasson, S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol.119 (3), 493–501. 10.1083/jcb.119.3.493( (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jie, X. et al. Y.Neurotoxic effects of nonylphenol: a review.Wiener Klinische Wochenschrift,125. (2013). 10.1007/s00508-012-0221-2 [DOI] [PubMed]
- 87.Wu, M., Xu, H., Shen, Y., Qiu, W. & Yang, M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ. Toxicol. Chem.30 (10), 2335–2341. 10.1002/etc.634 (2011). [DOI] [PubMed] [Google Scholar]
- 88.Pawar, D. River water pollution, an environmental crisis a case study of Panchaganga river of Kolhapur City. Int. J. Ecol. Develop Sum. 9 (1), 131–133 (2012). [Google Scholar]
- 89.Yang, F. W., Zhao, G. P., Ren, F. Z., Pang, G. F. & Li, Y. X. Assessment of the endocrine-disrupting effects of diethyl phosphate, a nonspecific metabolite of organophosphorus pesticides, by in vivo and in Silico approaches. Environ. Int. 135105383. 10.1016/j.envint.2019.105383 (2020). [DOI] [PubMed]
- 90.Linhua, H. A. O., Zhenyu, W. A. N. G. & Baoshan, X. I. N. G. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in juvenile carp (Cyprinus carpio). J. Environ. Sci.21 (10), 1459–1466. 10.1016/S1001-0742(08)62440-7 (2009). [DOI] [PubMed] [Google Scholar]
- 91.Liu, S. et al. Effects of 17α-ethinylestradiol and bisphenol A on steroidogenic messenger ribonucleic acid levels in the rare minnow gonads. Aquatictoxicology122, 19–27. 10.1016/j.aquatox.2012.05.010 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Xiao, Q., Zhang, S., Guo, H., Su, F. & Xu, Y. Nonylphenol causes decrease in antioxidant enzyme activities, increase in O2 – content, and alteration in ultrastructures of FG cells, a flounder (Paralichthys olivaceus) gill cell line. Toxicol. Mech. Methods. 17 (3), 127–134. 10.1080/15376510600860227 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Yahia, D. & Ali, M. F. .Assessment of neurohepatic DNA damage in male Sprague–Dawley rats exposed to organophosphates and pyrethroid insecticides. Environ. Sci. Pollut. Res.25, 15616–15629 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Zhao, Y. et al. Syringin alleviates bisphenol A-induced spermatogenic defects and testicular injury by suppressing oxidative stress and inflammation in male zebrafish. Int. Immunopharmacol.131, 111830. 10.1016/j.intimp.2024.111830 (2024). [DOI] [PubMed] [Google Scholar]
- 98.Zhu LiFei, Z. L. et al. ZiJian, W. Z. Chronic Thiamethoxam exposure impairs the HPG and HPT axes in adult Chinese rare minnow (Gobiocypris rarus): docking study, hormone levels, histology, and transcriptional responses. (2019). 10.1016/j.ecoenv.2019.109683 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper.