Abstract
Observationally, higher basal metabolic rate (BMR) is associated with metabolism-related disorders, cancer, aging, and mortality. In this Mendelian randomization (MR) phenome-wide association study, using two-sample MR methods, we systematically and comprehensively investigated the health effects of genetically predicted BMR across the phenome sex-specifically. We obtained sex-specific genetic variants strongly (p < 5 × 10− 8) and independently (r2 < 0.001) predicting BMR from the UK Biobank and applied them to over 1,000 phenotypes within the same study. We combined genetic variant-specific Wald estimates using inverse-variance weighting, supplemented by sensitivity analysis. We used a false-discovery rate correction to allow for multiple comparisons as well as multivariable MR adjusted for body mass index and testosterone to investigate the independent effects of BMR on phenotypes with significant univariable associations. We obtained 217/219 genetic variants predicting BMR and applied them to 1,150/1,242 phenotypes in men/women, respectively. BMR was associated with 190/270 phenotypes in univariable analysis and 122/123 phenotypes in multivariable analysis in men/women. Examples of robust associations in multivariable analysis included those with neoplasms, diseases of the circulatory system, and growth and reproductive investment. In conclusion, BMR might affect a wide range of health-related outcomes. The underlying mechanisms and interactions between phenotypes warrant further study, as BMR is modifiable.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-98017-9.
Keywords: Body mass index, Basal metabolism, Mendelian randomization analysis, Metabolism, Phenome-wide association study, Testosterone.
Subject terms: Evolutionary biology, Genetic association study, Endocrine system and metabolic diseases, Metabolic disorders, Genetic variation, Genetic variation, Endocrinology, Epidemiology
Introduction
Basal metabolic rate (BMR) refers to the energy requirement needed for the body to perform basic functions at rest, including the activities of the brain, heart, and skeletal muscle. Contributed to predominately by muscle mass1, BMR has been extensively investigated in relation to metabolism-related disorders, such as obesity2–4 and type 2 diabetes5. BMR is also a concern in cancer because BMR is often higher in people with cancer6,7, which may have nutritional implications during and after cancer management8,9. Notably, cancer is also increasingly perceived as a metabolic disease10. So, the age-related changes in BMR11–14 and the association of BMR with health status15,16, mortality17–20, and longevity21,22 could be highly relevant to aging.
Observational studies of BMR are open to confounding, especially by physical activity because some forms of physical exercise increase BMR23. BMR also declines with age11–14, making comprehensive adjustment difficult. Randomized controlled trials of BMR are largely limited to those studying short-term effects of thermogenic agents, which act to raise BMR, on hemodynamic parameters24–30 and some biomarkers24,28.
Mendelian randomization (MR) is a form of instrumental variable analysis that uses genetic variants (GVs) as instruments31. An MR study can circumvent confounding, for example by lifestyle and socioeconomic position, thus giving a less biased estimate32,33. MR studies have suggested that higher BMR is a potential cause of multiple sclerosis34, cancer35–40, COVID-1941,42, erectile dysfunction43, shorter lifespan44 and potentially protects against osteoporosis45. Nevertheless, sex-specific effects on these outcomes have rarely been investigated36, although men have higher BMR than women46.
Benefitting from large-scale biobanks, a hypothesis-free approach, known as a phenome-wide association study (PheWAS), which allows investigation of the effect of a GV on many health outcomes (or many exposures on an outcome), is now possible47. A PheWAS also using MR may generate new knowledge by identifying unexpected associations of an exposure with outcomes that have not been considered from a theoretical approach.
To date, no MR-PheWAS of BMR has been conducted. Given the importance of BMR in the development of non-communicable diseases and in aging, as well as it being a readily modifiable target, such as through diet (e.g., caloric and protein intake48) and exercise (e.g., resistance training23,49), here we sought to clarify its causal roles and their underlying mechanisms. In this study, we systematically investigated sex-specific effects of genetically predicted BMR on a wide range of health-related outcomes in the extensively phenotyped UK Biobank. Given that BMR and body mass index (BMI) are correlated50,51 and BMI is generally considered harmful, along with evidence from a trial showing that exogenous testosterone increases BMR52, we also used multivariable MR to evaluate the effects of BMR independent of BMI and testosterone.
Results
Genetic predictors of basal metabolic rate, body mass index, and testosterone
An overview of the study is shown in Fig. 1. We identified 217 and 219 GVs which strongly and independently predicted BMR in men and women, respectively; all were available for the outcomes because they originate from the same study (Supplementary Table S1). These GVs explained 7.7% and 6.6% of the variance in BMR, with a mean F-statistic of 60 (range, 30 to 363) and 59 (range, 30 to 400), in men and women, respectively.
Fig. 1.
Overview of the study. The numbers denote the number of phenotypes in men/women. BMI: body mass index; FDR: false discovery rate; IVW: inverse-variance weighting; MR: Mendelian randomization; MR-RAPS: Mendelian Randomization Robust Adjusted Profile Score.
Similarly, we identified 124 and 141 GVs strongly and independently predicting BMI in men and women, respectively; all were available for the outcomes because they originate from the same study (Supplementary Table S2). These GVs explained 3.9% and 3.7% of the variance in BMI, with a mean F-statistic of 52 (range, 30 to 521) and 50 (range, 30 to 427), in men and women, respectively.
We identified 93 and 216 GVs strongly and independently predicting bioavailable testosterone in men and total testosterone in women, respectively; 91 and 213 were available for the outcomes (Supplementary Table S3). These available GVs explained 3.9% and 7.3% of the variance in bioavailable testosterone in men and total testosterone in women, with a mean F-statistic of 92 (range, 27 to 1363) and 86 (range, 24 to 1656), in men and women, respectively.
Phenotype selection
After excluding irrelevant phenotypes, 1,150 and 1,242 were retained for analysis in men and women, respectively (Supplementary Fig. S1).
Univariable Mendelian randomization
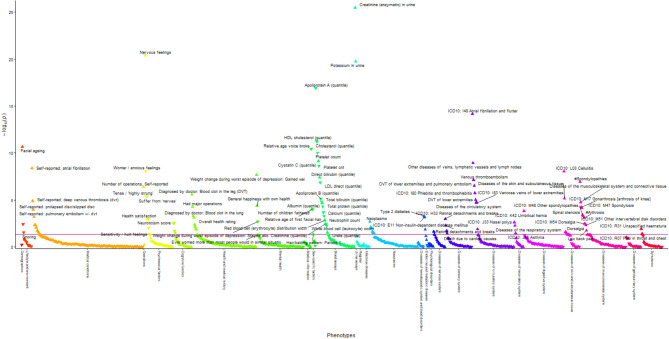
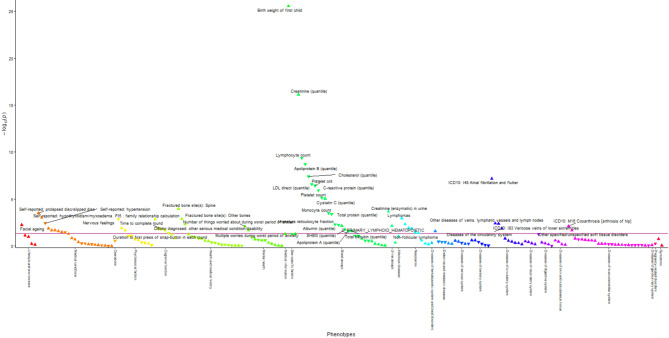
BMR was associated with 190 phenotypes in men (Fig. 2 and Supplementary Table S4) and 270 phenotypes in women (Fig. 3 and Supplementary Table S5) using inverse-variance weighting (IVW) at a false discovery rate (FDR)-corrected significance. BMR was positively associated with several body composition attributes, such as muscle mass and fat mass, anthropometrics, such as waist and hip circumference and heel bone density in both sexes. BMR was generally positively associated with International Classification of Diseases-10 (ICD-10) coded diseases in both sexes, except for diseases of the blood and blood-forming organs, some disorders involving immune mechanisms (ICD-10 III), some infectious and parasitic diseases (ICD-10 I), and in women with diseases of the genitourinary system (ICD-10 XIV). BMR was inversely associated with disorders related to pregnancy, childbirth, and the puerperium (ICD-10 XV). The associations of BMR with diseases of the eye and adnexa (ICD-10 VII) were mixed, with positive associations in men and both positive and inverse associations in women. BMR was not associated with mental and behavioral disorders (ICD-10 V). BMR was also associated with medical conditions in both sexes except for mental health, which showed mixed associations. BMR was positively associated with cognitive function, exercise electrocardiogram parameters, hand grip strength, and spirometry in both sexes. BMR was associated with growth-related phenotypes including facial aging in both sexes, no balding, younger age at voice breaking, younger age of first facial hair in men, and younger age when menarche started in women. BMR was associated with reproduction-related phenotypes including more children fathered in men and more lifetime sexual partners, a shorter length menstrual cycle, and higher birth weight of first child in women. BMR was associated with some biomarkers for renal disorders, liver disorders, and inflammation, lipids, a sex hormone and its related proteins, nutrients and blood cell-related parameters in men and/or women. Comparison of estimates showed no directionally inconsistent results between men and women (Supplementary Table S6).
Fig. 2.
Manhattan plot showing phenotypes with false discovery rate-significant univariable Mendelian randomization associations using inverse-variance weighting in men. Phenotypes in the group of physical measures are omitted for better visualization. The red line corresponds to a p-value of 0.05. The triangles facing upwards represent positive associations, whereas the triangles facing downwards represent inverse associations. Some phenotypes are not labelled due to overlapping data points.
Fig. 3.
Manhattan plot showing phenotypes with false discovery rate-significant univariable Mendelian randomization associations using inverse-variance weighting in women. Phenotypes in the group of physical measures are omitted for better visualization. The red line corresponds to a p-value of 0.05. The triangles facing upwards represent positive associations, whereas the triangles facing downwards represent inverse associations. Some phenotypes are not labelled due to overlapping data points.
Different MR methods gave directionally consistent results except those for spondylopathies, spondylosis, and self-reported apnea in men (Supplementary Table S4) and unspecified acute lower respiratory infection, hearing difficulty/problems with background noise, and self-reported disc degeneration in women (Supplementary Table S5), where IVW, weighted median (WM), and MR Robust Adjusted Profile Score (MR-RAPS) showed positive associations. However, MR-Egger showed null associations with the MR-Egger intercept test suggesting the presence of directional pleiotropy. The MR-Egger I2GX ranged from 80 to 81% in both sexes.
Multivariable Mendelian randomization adjusted for body mass index and testosterone
We included 270 and 321 GVs predicting BMR, BMI and/or bioavailable/ total testosterone in men and women, respectively. The conditional F-statistics were 20, 13, and 4 for BMR, BMI, and bioavailable testosterone in men and 11, 9, and 4 for BMR, BMI, and total testosterone in women.
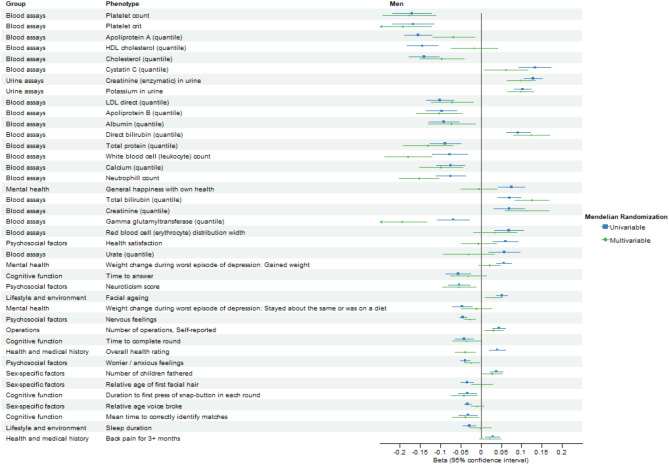
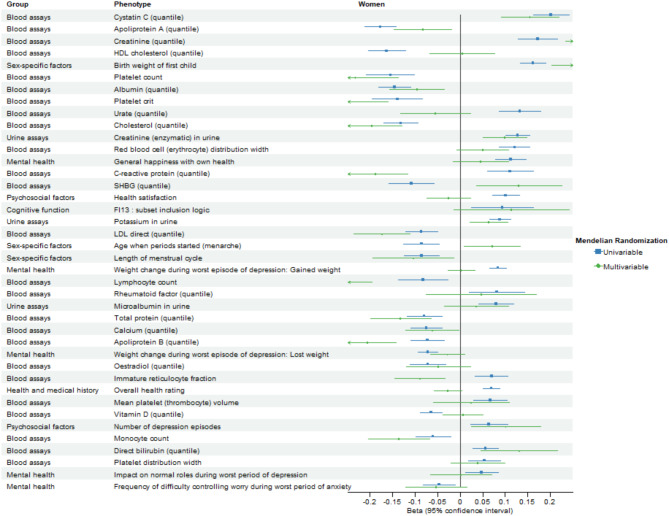
After excluding BMI, weight, and standing height, 187 and 267 phenotypes with significant FDR-corrected univariable IVW estimates were retained for multivariable analysis in men and women, respectively. Figures 4 and 5 compare the FDR-significant univariable MR associations and corresponding multivariable MR associations adjusted for BMI and bioavailable/ total testosterone using IVW in men and women, respectively.
Fig. 4.
Forest plot comparing false discovery rate-significant univariable Mendelian randomization associations and corresponding multivariable Mendelian randomization associations adjusted for body mass index and bioavailable testosterone using inverse-variance weighting in men. Only the top 40 associations excluding phenotypes in the group of physical measures are displayed, ranked by the magnitude of univariable Mendelian randomization associations.
Fig. 5.
Forest plot comparing false discovery rate-significant univariable Mendelian randomization associations and corresponding multivariable Mendelian randomization associations adjusted for body mass index and total testosterone using inverse-variance weighting in women. Only the top 40 associations excluding phenotypes in the group of physical measures are displayed, ranked by the magnitude of univariable Mendelian randomization associations.
After adjusting for BMI and testosterone, BMR remained associated with 123 phenotypes at FDR-corrected significance using multivariable IVW in men (Fig. 6 and Supplementary Table S7) and 122 phenotypes in women (Fig. 7 and Supplementary Table S8). BMR was associated with body composition attributes in both sexes. BMR was still positively associated with diseases of the circulatory system (ICD-10 IX) and diseases of the musculoskeletal system and connective tissue (ICD-10 XIII) in both sexes, diseases of the eye and adnexa (ICD-10 VII), diseases of the respiratory system (ICD-10 X), and diseases of the skin and subcutaneous tissue (ICD-10 XII) in men, and neoplasms (ICD-10 II) and endocrine, nutritional, and metabolic diseases (ICD-10 IV) in women. BMR was also positively associated with medical conditions in both sexes except for a few self-reported medical conditions, which showed inverse associations and except for mental health, which showed mixed associations. BMR was positively associated with cognitive function, hand grip strength, and spirometry in both sexes. BMR was associated with growth-related phenotypes including facial aging in both sexes and no balding in men. BMR was associated with reproduction-related phenotypes including more children fathered in men and higher birth weight of first child in women. BMR was associated with some biomarkers of renal disorders, liver disorders, and inflammation, lipids and related carriers, a sex hormone-related protein, nutrients, and blood cell-related parameters in men and/or women. Comparing estimates after excluding body composition-related phenotypes showed no directionally inconsistent results between men and women (Supplementary Table S9). Figure 8 compares the FDR-significant univariable MR associations and corresponding multivariable MR associations adjusted for BMI and bioavailable/ total testosterone using inverse-variance weighting in men and women.
Fig. 6.
Manhattan plot showing phenotypes with false discovery rate-significant multivariable Mendelian randomization associations adjusted for body mass index and bioavailable testosterone using inverse-variance weighting based on false discovery rate-significant phenotypes in univariable analysis using inverse-variance weighting in men. Phenotypes in the group of physical measures are omitted for better visualization. The red line corresponds to a p-value of 0.05. The triangles facing upwards represent positive associations, whereas the triangles facing downwards represent inverse associations. Some phenotypes are not labelled due to overlapping data points.
Fig. 7.
Manhattan plot showing phenotypes with false discovery rate-significant multivariable Mendelian randomization associations adjusted for body mass index and total testosterone using inverse-variance weighting based on false discovery rate-significant phenotypes in univariable analysis using inverse-variance weighting in women. Phenotypes in the group of physical measures are omitted for better visualization. The red line corresponds to a p-value of 0.05. The triangles facing upwards represent positive associations, whereas the triangles facing downwards represent inverse associations. Some phenotypes are not labelled due to overlapping data points.
Fig. 8.
Forest plot comparing false discovery rate-significant univariable Mendelian randomization associations and corresponding multivariable Mendelian randomization associations adjusted for body mass index and bioavailable/ total testosterone using inverse-variance weighting in men and women. Only associations that are false discovery rate-significant in univariable Mendelian randomization in both men and women are displayed. Phenotypes in the group of physical measures are omitted for better visualization.
After adjusting for BMI and bioavailable testosterone in men, associations of BMR with self-reported and doctor-diagnosed diabetes, long-standing illness, disability, or infirmity, overall health rating and body fat percentages were reversed in direction (Supplementary Table S10). After adjusting for BMI and total testosterone in women, associations of BMR with self-reported hypertension, body fat percentages, heel broadband ultrasound attenuation, C-reactive protein, immature reticulocyte fraction, and sex hormone binding globulin were reversed in direction (Supplementary Table S10). Sensitivity analysis using different multivariable MR methods gave directionally consistent results in men (Supplementary Table S7) and in women (Supplementary Table S8).
Discussion
In the univariable MR-PheWAS, BMR was associated with a wide range of phenotypes, including anthropometric and body composition measures, biomarkers, diseases, and other health-related outcomes in both sexes. Sex differences were evident for several phenotypes, but usually affected magnitude rather than direction of the effects. To further assess the effect of BMR on different phenotypes independent of BMI, an established harmful risk factor, and independent of testosterone, which may influence BMR through muscle mass building52, we conducted multivariable MR adjusted for BMI and testosterone. We found that BMR remained associated with many different phenotypes.
Body composition, particularly muscle mass, is a driver of BMR53, but BMR may also affect muscle mass when energy intake does not equate to energy expenditure54, making it challenging to interpret associations adjusted for measures of body composition. However, obese people do not always have a lower BMR than other people, possibly due to higher energy requirements to support basic bodily functions55. This study showed that BMR was positively associated with body composition attributes such as muscle mass and fat mass and anthropometrics such as waist and hip circumference. Some associations could be a downstream effect of these attributes, such as greater grip strength, better lung function, and better exercise cardiac function.
Faster metabolism speeds up catabolism and increases metabolic waste, which may impact health. BMR was associated with biomarkers possibly related to these processes. Specifically, BMR was associated with lower cholesterol and lower direct low-density lipoprotein, which could be due to faster metabolism and hence breakdown. BMR was also associated with higher levels of substances that are generally considered as metabolic waste, such as cystatin C, bilirubin, and creatinine.
The effects of BMR on clinical diseases have not been extensively considered. However, several previous MR studies have suggested BMR might increase the risk of cancer35,37–40, which is in line with our study results. MR studies have found BMR possibly reduces risk of Alzheimer’s disease56,57, consistent with our finding of a positive association of BMR with cognitive function. However, Alzheimer’s disease typically occurs in old age, so the observed associations are open to selection bias. A previous MR study found BMR might reduce risk of self-reported osteoporosis45, which is not consistent with our null finding. A sex-specific analysis for osteoporosis may clarify the discrepancy because osteoporosis is more relevant in women, especially following menopause, when estrogen falls58. An experimental study in mice showed that lower BMR increased the risk of insulin resistance and type 2 diabetes59. We also found an inverse association of BMR with type 2 diabetes after adjusting for BMI and testosterone in men
MR studies have also reported that higher BMR might increase the risk of multiple sclerosis34 and COVID-1941, where inflammation could play a role60. However, we did not find multiple sclerosis associated with inflammatory-related biomarkers, possibly because of lack of power. BMR was associated with lower C-reactive protein in women, a marker of inflammation. It has been suggested that obesity causes low-grade inflammation61. MR studies have suggested that obesity might be causally associated with multiple sclerosis62–64.
BMR was associated with diseases of the circulatory system, such as atrial fibrillation and venous thromboembolism in both sexes. Higher BMI may increase risk of atrial fibrillation65,66, possibly by modifying ventricular architecture in response to increased perfusion pressure from fat tissue accumulation65 and to obesity-related inflammation66. However, the positive associations were robust to adjusting for BMI and testosterone, suggesting BMR might increase risk of atrial fibrillation via alternative pathways. Mechanical pressure from greater body size preventing blood from returning properly66–71 and obesity-related inflammation66–70,72 have been suggested as causes of venous thromboembolism. BMR was inversely associated with various cardiometabolic phenotypes, including total cholesterol and direct low-density lipoprotein in both sexes, type 2 diabetes in men, and hypertension in women, after adjusting for BMI and testosterone. A recent MR study has suggested that higher BMI might reduce low-density lipoprotein cholesterol73, although the reasons remain unclear. The inverse associations of BMR with type 2 diabetes and hypertension also suggest that higher BMR might increase the risk of diseases of the circulatory system through causal risk factors not traditionally recognized. A recent MR study reported higher BMR might have different effects on the risk of CVD subtypes74, including an increased risk of atrial fibrillation but a decreased risk of myocardial infarction, which partly agrees with the current results. However, that study was not sex-specific. The current sex-specific study is limited by small sample sizes for CVD subtypes.
Unexpectedly, BMR was associated with diseases of the musculoskeletal system and connective tissue, in particular prolapsed/slipped discs in both sexes. The underlying mechanism is unclear because disc degeneration is mainly thought to be the result of mechanical force generated by obesity75. Other novel findings include the varied association with mental health-related measures, inverse association with corneal power, conflicting associations with heel bone density, arterial stiffness measures and resting electrocardiogram parameters by sex, as well as null associations with liver and kidney disorders despite associations with some of their biomarkers. The associations of BMR with blood cell parameters related to clinical diseases also warrants further investigation.
The association of BMR with growth- and reproduction-related phenotypes is consistent with the evolutionary biology perspective, where metabolism, growth, and reproduction may interact with each other to allow organisms to maximize fitness76. Higher BMR was associated with phenotypes related to growth, specifically facial aging in both sexes and retinal detachment in men, where excessive axial length growth is an established factor77. Higher BMR was associated with greater reproductive investment, specifically higher birth weight of first child in women and more children fathered in men. Additionally, we found that higher BMR was associated with worse health in general and more medical conditions in both sexes. This is consistent with the perspective from evolutionary biology that suggests a tradeoff between reproduction and maintenance of the body, thereby compromising health and longevity78,79 and an MR study reporting higher BMR might reduce lifespan44. An MR-PheWAS reported that higher BMR might increase the risk of erectile dysfunction43, which was not evident here, possibly due to fewer cases of erectile dysfunction in the current study. Such a finding is also inconsistent with higher BMR favoring reproductive investment. However, not all reproduction-related phenotypes are the same, some may represent overall reproductive investment in early life or a specific aspect of reproductive health at a particular age. In multivariable analysis BMR was positively associated with sex hormone binding globulin, a protein that binds to and reduces bioavailable sex hormones80, in women but not in men. Due to the strong correlation between sex hormone binding globulin and total testosterone in men and bioavailable testosterone in women81, we adjusted for bioavailable testosterone and total testosterone respectively. In addition, BMR was associated with no balding in men. These inconsistent findings require further investigation.
Despite the comprehensive nature of this study generating several novel findings, this study has limitations. First, it relies on the assumptions of MR, which we addressed carefully. Specifically, we used GVs strongly associated with BMR. As a sensitivity analysis, we employed various MR methods that are robust to horizontal pleiotropy or capable of detecting and correcting horizontal pleiotropy by statistical means, including WM, MR-Egger, and MR-RAPS, which are based on different assumptions, to address possible violations of the assumption that the GVs should be independent of the outcome given the exposure in general. One significant concern is that the sex-specific GVs associated with BMR might also be related to sex hormones, particularly testosterone. However, the sex-specific analysis was based on the fact that men have much higher level of testosterone than women and testosterone is considered the main sex hormone in men, so it should not pose an issue. Nonetheless, we accounted for this by including bioavailable testosterone and total testosterone in the multivariable analysis for men and women, respectively, which also considered the influence of sex hormone binding globulin81. The directionally consistent results also suggest that bias due to horizontal pleiotropy is minimal. We also limited the analysis to people of European descent to avoid confounding by population stratification. However, survival bias due to missing potential recruits who have already died from BMR or its genetic predictors or who have already died of the outcome of interest or a competing risk of the outcome of interest could not be addressed82. Second, we used completely overlapping samples for BMR and BMI, which may have biased the estimates towards the observational associations if weak instrument bias was present83. However, the sample size of the UK Biobank means such bias is likely minimal84. The MR-Egger I2GX ranged from 80 to 81%, which is suggestive of an overall weak set of instruments85. Simulation suggests that a value ≥ 97% is needed in an MR study with completely overlapping samples to give an unbiased MR-Egger estimate84. However, most MR-Egger estimates were directionally consistent with the IVW estimates. Also, MR-Egger was only one of several sensitivity analyses. In multivariable MR, weak instrument bias could bias to any direction86. While the conditional F-statistics for bioavailable testosterone and total testosterone were < 10, the F-statistics for BMR and the conditional F-statistics for BMR and BMI were > 10, indicating potential weak instrument bias was still possible86,87. Third, in the multivariable analysis, we adjusted for BMI as a potential genetic confounder. However, BMI does not differentiate between muscle mass and fat mass. Future studies could consider using metrics that more comprehensively reflect body composition. Fourth, participants from the UK Biobank do not represent the general population. The findings in this study may not be generalized to populations beyond those of European descent. However, causes should be consistent across populations, although relevance is an issue88, especially as body composition varies across populations. Also, a previous comparison of associations from the UK Biobank with those obtained from a more representative UK population study yielded similar results89. Fifth, some phenotypes with a small sample size were excluded to avoid low statistical power. No power calculations were performed because of the exploratory nature of this study. Sixth, multiple comparisons can result in false positive results. However, we applied an FDR correction to control for it. We did not use a Bonferroni correction to determine the critical value for significance due to its conservativeness, and because we aimed to discover novel associations using a less stringent threshold. Seventh, we did not assess any bidirectional associations. A bidirectional MR study could be conducted to confirm whether the phenotypes affect BMR when most of the genetic associations with the phenotypes are estimated from a large sample to ensure GVs strongly predicting the phenotypes as exposures. Alternatively, Steiger filtering can be used to omit GVs having a stronger genetic association with the outcome than the exposure90. Eighth, replication was not performed because of the exploratory nature of this study and no other studies offer such a wealth of sex-specific genetic associations as the UK Biobank. Ninth, MR estimates reflect the lifelong impact of BMR on the outcomes. Whether changing BMR in actuality would produce the same effect is unclear.
Here, we considered health consequences of BMR. However, even using MR we cannot exclude the possibility that BMR is a biomarker of more fundamental physiological causes. A such, the effects of intervening on BMR, say via diet or exercise, could depend on the extent to which the intervention changes that fundamental underlying cause. A similar issue arises for BMI. For instance, semaglutide, a glucagon-like peptide 1 receptor agonist, has been very effective in reducing BMI, but some of its effects on health appear to go beyond the effects on BMI91–93, suggesting semaglutide is addressing a precursor of BMI, which has been suggested as calorie restriction and the central driver of cell metabolism, AMP-activated protein kinase94–96. AMP-activated protein kinase also controls cell metabolism97,98. Whether interventions that reduce BMR, such as lower energy or protein intake99–101, lower muscle strength or muscle mass102,103, and higher temperature104, also operate by the same mechanism, i.e., AMP-activated protein kinase, has not previously been considered, but provides a unifying explanation.
Overall, this study showed that BMR likely affects a wide range of health-related outcomes. We highlighted some robust associations after adjusting for BMI and testosterone, including the positive association of BMR with diseases of the circulatory system and diseases of the musculoskeletal system and connective tissue in both sexes. Further studies are needed to investigate the underlying mechanism, especially the role of biomarkers in these disease groups. The impact of higher BMR being associated with more growth and reproductive investment on disease development and aging is also worth further investigation.
Methods
We conducted a sex-specific MR-PheWAS of BMR by applying sex-specific GVs predicting BMR in the UK Biobank to over 1,000 phenotypes within the same study using two-sample summary MR methods. MR relies on three key assumptions corresponding to the assumptions of instrumental variable analysis. First, the GVs must be strongly associated with the exposure. Second, the GV-outcome associations must not be confounded. Third, the GVs must be independent of the outcome given the exposure. We further conducted multivariable MR adjusted for BMI and testosterone by applying GVs strongly predicting BMR, BMI, and testosterone to selected phenotypes within the same study. Assumptions of multivariable MR extend those of univariable MR to multiple exposures86.
Data source: the UK biobank
The UK Biobank is an ongoing population-based, prospective cohort study designed to recruit over half a million participants aged 40 to 69 years in Great Britain from 2006 to 2010105, with a mean age of 57 years (https://biobank.ndph.ox.ac.uk/ukb/field.cgi?id=21022). It has gathered wide-ranging and in-depth information on baseline characteristics, including family history, diseases, and health attributes such as lifestyle, measures of mental health, and physical measures. Biological samples were also collected, with blood samples genotyped. Data collected during follow-up included imaging studies, electronic health records of primary care and hospital admissions, and cancer and death registries.
Neale Lab conducted GWAS of the UK Biobank and provided sex-specific genetic summary statistics from the UK Biobank for over 4,000 phenotypes using linear regression adjusted for age, age × age, and first 20 principal components in up to 361,194 unrelated individuals (54% women) of white British ancestry (http://www.nealelab.is/uk-biobank/). Quality control procedures were applied to various aspects, such as sample quality (e.g., sample relatedness and population stratification) and marker quality (e.g., Hardy-Weinberg equilibrium). Previous MR studies of BMR have also utilized the sex-specific data provided by Neale Lab36,44.
Ruth et al.81 conducted GWAS of the UK Biobank and provided sex-specific genetic summary statistics from the UK Biobank for testosterone, including total testosterone and bioavailable testosterone using linear mixed model adjusted for age, first 10 principal components, fasting time, and genotyping chips in 178,782 men and 230,454 women of white European ancestry who were not on hormone replacement therapy medication (https://www.ebi.ac.uk/gwas/publications/32042192). Quality control procedures were applied to various aspects, such as sample quality (e.g., sample relatedness and population structure) and exclusion of individuals with discordant self-reported ancestry based on the “K-means clustering” approach. Previous MR studies of metabolic-related disorders have also utilized the sex-specific data provided by Ruth et al.106–108.
Genetic predictors of basal metabolic rate and body mass index
We obtained sex-specific GVs strongly (p < 5 × 10-8) predicting BMR and BMI from the UK Biobank provided by Neale Lab as instruments (http://www.nealelab.is/uk-biobank/). BMR was estimated using a body composition analyzer (Tanita BC418MA Body Composition Analyzer) (https://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=1421), which calculates body composition measurements based on the data obtained from dual-energy X-ray absorptiometry (https://www.tanita.com/es/.downloads/download/?file=855638086&fl=en_US) and provides BMR derived mainly from fat-free mass using a proprietary regression formula. The estimated BMR has been shown to be highly correlated with that measured using indirect calorimetry (r = 0.9, p < 0.0001) (https://www.tanita.com/es/.downloads/download/?file=855638086&fl=en_US). BMI was estimated using the same body composition analyzer. BMR in kilojoules and BMI in kg/m2 were transformed into effect sizes using inverse-rank normalization (phenotype code, 23105_irnt and 23104_irnt, respectively).
Non-biallelic or rare (minor allele frequency < 0.01) GVs were excluded. GVs that did not fulfil the assumptions of Hardy-Weinberg equilibrium (p for Chi-squared test < 0.05) or those without a strong association with BMR or BMI (genetic association with p ≥ 5 × 10− 8) were also excluded. We then obtained the uncorrelated GVs, i.e., GVs not in linkage disequilibrium, with r2 < 0.001 using the “TwoSampleMR” R package109.
Genetic predictors of testosterone
We obtained sex-specific GVs strongly (p < 5 × 10-8) predicting bioavailability testosterone for men and total testosterone for women from the UK Biobank provided by Ruth et al.81 (https://www.ebi.ac.uk/gwas/publications/32042192), given their lower correlation with sex hormone binding globulin81. Serum total testosterone was analyzed using an immunoassay analyzer (Beckman Coulter UniCel Dxl 800) (https://biobank.ctsu.ox.ac.uk/~bbdatan/biomarkers.pdf). Bioavailable testosterone was calculated from the total testosterone, accounting for concentration of sex hormone binding globulin and albumin. Testosterone levels in nmol/L were transformed into effect sizes using inverse-rank normalization81.
Non-biallelic or rare (minor allele frequency < 0.01) GVs were excluded. GVs without a strong association with bioavailable testosterone for men or total testosterone for women (genetic association with p ≥ 5 × 10− 8) were also excluded. We then obtained the uncorrelated GVs, i.e., GVs not in linkage disequilibrium, with r2 < 0.001 using the “TwoSampleMR” R package109.
Genetic associations with health-related outcomes
We obtained sex-specific genetic associations with the phenotypes from the UK Biobank provided by Neale Lab (http://www.nealelab.is/uk-biobank/). Of the 11,934 available phenotypes, we excluded summary files, inferred sex, combined-sex phenotypes if sex-specific data were available, and phenotypes in natural units if inverse-rank normalized data were available. We removed duplicated phenotypes and binary phenotypes without a phenotype code in the UK Biobank or an ICD-10 code. We excluded phenotypes that are unlikely to be health effects of BMR, such as behavior and external causes, using classifications recommended by the UK Biobank. To ensure sufficient statistical power, we also excluded binary phenotypes with fewer than 200 cases and continuous and ordinal phenotypes with a sample size less than 1,000110. The phenotype selection process is shown in Supplementary Figure S1.
Binary phenotypes with an ICD-10 code were categorized by ICD-10 chapter, as (I) infectious diseases, (II) neoplasms, (III) diseases of the hematopoietic system and blood disorders, (IV) endocrine and metabolic diseases, (V) psychological disorders, (VI) diseases of the nervous system, (VII and VIII) diseases of the sensory system (eyes and ear), (IX) diseases of the circulatory system, (X) diseases of the respiratory system, (XI) diseases of the digestive system, (XII) diseases of skin and subcutaneous tissue, (XIII) diseases of the musculoskeletal system and connective tissue, (XIV) diseases of the genitourinary system, (XV) pregnancy, childbirth, and the puerperium, and (XVIII) symptoms.
Statistical analysis
We calculated GV-specific Wald estimates as the ratio of the GV-outcome associations to GV-exposure associations111,112. In the main analysis, these estimates were meta-analyzed using IVW with multiplicative random effects113, which assumes no measurement error for the GV-exposure associations85 or directional pleiotropy114. The Cochran’s Q statistic quantifies the heterogeneity among the instruments and serves as an indicator of potential horizontal pleiotropy109,115. As a sensitivity analysis to address horizontal pleiotropy, we also used the WM116, MR-Egger85,117, and MR-RAPS118, from the “MendelianRandomization” and “mr.raps” R packages118–120. WM provides a valid estimate when > 50% of weight is from valid GVs116. MR-Egger provides a valid estimate assuming no measurement error85 and instrument strength is independent of any pleiotropic effects85,117. A non-zero MR-Egger intercept indicates the presence of directional pleiotropy, which biases the IVW estimate85. The MR-Egger I2GX reflects the overall strength of the instrument set and a value close to unity minimizes regression dilution bias arising from any measurement error85. MR-RAPS assumes that the pleiotropic effects of GVs, except idiosyncratic outliers, are independent and normally distributed around zero118. MR-RAPS takes weak instruments into account and provides a valid estimate in the presence of systematic and idiosyncratic pleiotropy118.
We computed the F-statistic for each GV to assess instrument strength, approximated by dividing the squared effect size by the squared standard error of the GV-exposure association85. GVs with an F-statistic > 10 are less likely to be weak instruments87.
To account for multiple comparisons, we used the FDR to determine the critical value for significance121, with an FDR threshold of 5%. Specifically, we ranked the p-values of the associations from the smallest to largest and compared them to the corresponding FDR-corrected critical value for significance, as given by FDR threshold × rank / number of associations tested.
For phenotypes with significant results using IVW at FDR-corrected significance in univariable analysis, we carried out multivariable MR adjusted for BMI and testosterone to assess the effect of BMR on these phenotypes independent of BMI and testosterone. To perform multivariable MR, we assembled all GVs predicting BMR, BMI, or testosterone from univariable analysis and selected between correlated GVs with r2 < 0.001 using the “TwoSampleMR” R package109, which itself uses the lower of the associated p-value for BMR, BMI, or testosterone. We used multivariable IVW as the primary analysis and multivariable median and multivariable MR-Egger as a sensitivity analysis, using the “MendelianRandomization” R package119,120. Since the MR-Egger intercept test is sensitive to orientation of the GVs122, which can be further complicated in multivariable MR-Egger123, we oriented the GVs with respect to the exposure of primary interest, i.e., BMR. We used the conditional F-statistic to assess the instrument strength of the combined set of instruments and adjusted Cochran’s Q statistics to assess the extent of residual horizontal pleiotropy using the “MVMR” R package86. We assumed a phenotypic correlation of 0.509 between BMR and BMI in the UK Biobank51 and 0 between testosterone and BMR/BMI as related data is unavailable when computing the conditional F-statistics.
To visualize the results, we produced Manhattan plots to display the distribution of -log (FDR-corrected p-values), where the corrected p-value was computed by p-value × number of associations tested / rank, for each phenotype group using the “PheWAS” R package124. We also produced forest plots to compare the results between univariable MR and multivariable MR and between men and women using the “forestploter” R package (https://github.com/adayim/forestploter).
All statistical analyses were conducted using R (Version 4.0.3, The R Foundation for Statistical Computing Platform, Vienna, Austria).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Neale Lab and NHGRI-EBI Catalog of human genome-wide association studies for providing the summary-level data. We also thank Guoyi Yang for checking part of the results.
Abbreviations
- BMI
Body mass index
- BMR
Basal metabolic rate
- FDR
False discovery rate
- GV
Genetic variant
- GWAS
Genome-wide association study
- IVW
Inverse-variance weighting
- MR
Mendelian randomization
- MR-RAPS
Mendelian Randomization Robust Adjusted Profile Score
- PheWAS
Phenome-wide association study
- WM
Weighted median
Author contributions
JCMN: formal analysis, writing-original draft, writing-review and editing. CMS: Conceptualization, formal analysis, supervision, writing-review and editing.
Data availability
The datasets generated during and/or analyzed during the current study are available from the website of Neale Lab (http://www.nealelab.is/uk-biobank/) and the NHGRI-EBI Catalog of human genome-wide association studies (https://www.ebi.ac.uk/gwas/publications/32042192). The statistical codes needed to reproduce the results in the article are publicly accessible.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol was not pre-registered. We used only publicly available summary-level data and did not collect any original data in this study. Ethics approval and consent from individual participants can be found in the original publications.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heymsfield, S. B. et al. Resting energy expenditure: from cellular to Whole-Body level, a mechanistic historical perspective. Obes. (Silver Spring). 29, 500–511 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Rose, K. L., Evans, E. W., Sonneville, K. R. & Richmond, T. The set point: weight destiny established before adulthood? Curr. Opin. Pediatr.33, 368–372 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Busetto, L. et al. Mechanisms of weight regain. Eur. J. Intern. Med.93, 3–7 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Walczak, K. & Sieminska, L. Obesity and thyroid Axis. Int. J. Environ. Res. Public. Health18 (2021). [DOI] [PMC free article] [PubMed]
- 5.Caron, N., Peyrot, N., Caderby, T., Verkindt, C. & Dalleau, G. Energy expenditure in people with diabetes mellitus: A review. Front. Nutr.3, 56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purcell, S. A., Elliott, S. A., Baracos, V. E., Chu, Q. S. & Prado, C. M. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur. J. Clin. Nutr.70, 1230–1238 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Tisdale, M. J. Mechanisms of cancer cachexia. Physiol. Rev.89, 381–410 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Van Soom, T. et al. Perspective: towards personalised metabolic coaching in Cancer. Facts Views Vis. ObGyn. 10, 125–130 (2018). [PMC free article] [PubMed] [Google Scholar]
- 9.Jouinot, A., Vazeille, C. & Goldwasser, F. Resting energy metabolism and anticancer treatments. Curr. Opin. Clin. Nutr. Metab. Care. 21, 145–151 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Seyfried, T. N. & Shelton, L. M. Cancer as a metabolic disease. Nutr. Metab. (Lond). 7, 7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper, J. A. et al. Longitudinal change in energy expenditure and effects on energy requirements of the elderly. Nutr. J.12, 73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfonzo-González, G., Doucet, E., Bouchard, C. & Tremblay, A. Greater than predicted decrease in resting energy expenditure with age: cross-sectional and longitudinal evidence. Eur. J. Clin. Nutr.60, 18–24 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Luhrmann, P. M., Edelmann-Schafer, B. & Neuhauser-Berthold, M. Changes in resting metabolic rate in an elderly German population: cross-sectional and longitudinal data. J. Nutr. Health Aging. 14, 232–236 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Zampino, M., AlGhatrif, M., Kuo, P. L., Simonsick, E. M. & Ferrucci, L. Longitudinal changes in resting metabolic rates with aging are accelerated by diseases. Nutrients12 (2020). [DOI] [PMC free article] [PubMed]
- 15.Kim, S. & Jazwinski, S. M. Quantitative measures of healthy aging and biological age. Healthy Aging Res. 4 (2015). [DOI] [PMC free article] [PubMed]
- 16.Fabbri, E. et al. Energy metabolism and the burden of Multimorbidity in older adults: results from the Baltimore longitudinal study of aging. J. Gerontol. Biol. Sci. Med. Sci.70, 1297–1303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han, F. et al. Association between basal metabolic rate and All-Cause mortality in a prospective cohort of Southern Chinese adults. Front. Physiol.12, 790347 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jumpertz, R. et al. Higher energy expenditure in humans predicts natural mortality. J. Clin. Endocrinol. Metab.96, E972–976 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggiero, C. et al. High basal metabolic rate is a risk factor for mortality: the Baltimore longitudinal study of aging. J. Gerontol. Biol. Sci. Med. Sci.63, 698–706 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitazoe, Y., Kishino, H., Tanisawa, K., Udaka, K. & Tanaka, M. Renormalized basal metabolic rate describes the human aging process and longevity. Aging Cell.18, e12968 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen, S. W. et al. Human longevity is characterised by high thyroid stimulating hormone secretion without altered energy metabolism. Sci. Rep.5, 11525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzo, M. R. et al. Resting metabolic rate and respiratory quotient in human longevity. J. Clin. Endocrinol. Metab.90, 409–413 (2005). [DOI] [PubMed] [Google Scholar]
- 23.MacKenzie-Shalders, K., Kelly, J. T., So, D., Coffey, V. G. & Byrne, N. M. The effect of exercise interventions on resting metabolic rate: A systematic review and meta-analysis. J. Sports Sci.38, 1635–1649 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Ahuja, K. D., Robertson, I. K., Geraghty, D. P. & Ball, M. J. The effect of 4-week Chilli supplementation on metabolic and arterial function in humans. Eur. J. Clin. Nutr.61, 326–333 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Belza, A., Toubro, S. & Astrup, A. The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur. J. Clin. Nutr.63, 57–64 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Stohs, S. J. et al. Effects of p-synephrine alone and in combination with selected bioflavonoids on resting metabolism, blood pressure, heart rate and self-reported mood changes. Int. J. Med. Sci.8, 295–301 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell, B. I. et al. The effects of a fat loss supplement on resting metabolic rate and hemodynamic variables in resistance trained males: a randomized, double-blind, placebo-controlled, cross-over trial. J. Int. Soc. Sports Nutr.13, 14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo, E. et al. Dietary caffeine and polyphenol supplementation enhances overall metabolic rate and lipid oxidation at rest and after a bout of sprint interval exercise. J. Strength. Cond Res.30, 1871–1879 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Cameron, M., Camic, C. L., Doberstein, S., Erickson, J. L. & Jagim, A. R. The acute effects of a multi-ingredient pre-workout supplement on resting energy expenditure and exercise performance in recreationally active females. J. Int. Soc. Sports Nutr.15, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell, B. I. et al. A commercially available thermogenic dietary supplement increases resting metabolic rate in physically active males: A randomized, Double-Blind, Placebo-Controlled investigation. J. Diet. Suppl.17, 150–160 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Didelez, V. & Sheehan, N. Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res.16, 309–330 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Smith, G. D. & Ebrahim, S. Mendelian randomization’: can genetic epidemiology contribute to Understanding environmental determinants of disease? Int. J. Epidemiol.32, 1–22 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Smith, G. D. et al. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med.4, e352 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, C. et al. Basal metabolic rate and risk of multiple sclerosis: a Mendelian randomization study. Metab. Brain Dis.37, 1855–1861 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Cornish, A. J. et al. Modifiable pathways for colorectal cancer: a Mendelian randomisation analysis. Lancet Gastroenterol. Hepatol.5, 55–62 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng, J. C. M. & Schooling, C. M. Effect of basal metabolic rate on cancer: a Mendelian randomization study. Front. Genet.12, 735541 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, E., Ni, J., Tao, L. & Xie, T. A bidirectional Mendelian randomization study supports the causal effects of a high basal metabolic rate on colorectal cancer risk. PLoS One. 17, e0273452 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, H., Qiu, J., Meng, F. & Shu, X. Insight into the causality between basal metabolic rate and endometrial and ovarian cancers: analysis utilizing systematic Mendelian randomization and genetic association data from over 331,000 UK biobank participants. Eur. J. Clin. Invest.53, e13971 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Du, Q., Zheng, Z., Wang, Y., Yang, L. & Zhou, Z. Genetically predicted thyroid function and risk of colorectal cancer: a bidirectional Mendelian randomization study. J. Cancer Res. Clin. Oncol.149, 14015–14024 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Si, S. et al. Identifying causality, genetic correlation, priority and pathways of large-scale complex exposures of breast and ovarian cancers. Br. J. Cancer. 125, 1570–1581 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baranova, A., Song, Y., Cao, H. & Zhang, F. Causal associations between basal metabolic rate and COVID-19. Diabetes72, 149–154 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Baranova, A., Cao, H., Teng, S. & Zhang, F. A phenome-wide investigation of risk factors for severe COVID-19. J. Med. Virol.95, e28264 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong, Y. et al. Insights into modifiable risk factors of erectile dysfunction, a wide-angled Mendelian randomization study. J. Adv. Res. (2023). [DOI] [PMC free article] [PubMed]
- 44.Ng, J. C. M. & Schooling, C. M. Effect of basal metabolic rate on lifespan: a sex-specific Mendelian randomization study. Sci. Rep.13, 7761 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, J. et al. Effect of basal metabolic rate on osteoporosis: A Mendelian randomization study. Front. Public. Health. 11, 1096519 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arciero, P. J., Goran, M. I. & Poehlman, E. T. Resting metabolic rate is lower in women than in men. J. Appl. Physiol. (1985). 75, 2514–2520 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Denny, J. C. et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics26, 1205–1210 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorling, J. L., Martin, C. K. & Redman, L. M. Calorie restriction for enhanced longevity: the role of novel dietary strategies in the present obesogenic environment. Ageing Res. Rev.64, 101038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tresierras, M. A. & Balady, G. J. Resistance training in the treatment of diabetes and obesity: mechanisms and outcomes. J. Cardiopulm. Rehabil Prev.29, 67–75 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Sodini, S. M., Kemper, K. E., Wray, N. R. & Trzaskowski, M. Comparison of genotypic and phenotypic correlations: Cheverud’s conjecture in humans. Genetics209, 941–948 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng, J. et al. PhenoSpD: an integrated toolkit for phenotypic correlation Estimation and multiple testing correction using GWAS summary statistics. Gigascience 7 (2018). [DOI] [PMC free article] [PubMed]
- 52.Welle, S., Jozefowicz, R., Forbes, G. & Griggs, R. C. Effect of testosterone on metabolic rate and body composition in normal men and men with muscular dystrophy. J. Clin. Endocrinol. Metab.74, 332–335 (1992). [DOI] [PubMed] [Google Scholar]
- 53.Jagim, A. R. et al. Sex differences in resting metabolic rate among athletes. J. Strength. Cond Res.33, 3008–3014 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Hill, J. O., Wyatt, H. R. & Peters, J. C. Energy balance and obesity. Circulation126, 126–132 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carneiro, I. P. et al. Is obesity associated with altered energy expenditure?? Adv. Nutr.7, 476–487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou, Y., Wang, Q. & Cheng, X. Causal relationship between basal metabolic rate and Alzheimer’s disease: A bidirectional Two-sample Mendelian randomization study. Neurol. Therapy. 12, 763–776 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen, S. D. et al. A Phenome-wide association and Mendelian randomization study for Alzheimer’s disease: A prospective cohort study of 502,493 participants from the UK biobank. Biol. Psychiatry. 93, 790–801 (2023). [DOI] [PubMed] [Google Scholar]
- 58.Levin, V. A., Jiang, X. & Kagan, R. Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int.29, 1049–1055 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Maciak, S. et al. Low basal metabolic rate as a risk factor for development of insulin resistance and type 2 diabetes. BMJ Open. Diabetes Res. Care8 (2020). [DOI] [PMC free article] [PubMed]
- 60.Foulkes, A. S. et al. Understanding the link between obesity and severe COVID-19 outcomes: causal mediation by systemic inflammatory response. J. Clin. Endocrinol. Metab.107, e698–e707 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khanna, D., Khanna, S., Khanna, P., Kahar, P. & Patel, B. M. Obesity: A chronic Low-Grade inflammation and its markers. Cureus14, e22711 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Almramhi, M. M. et al. The role of body fat in multiple sclerosis susceptibility and severity: A Mendelian randomisation study. Mult Scler.28, 1673–1684 (2022). [DOI] [PubMed] [Google Scholar]
- 63.Vandebergh, M., Becelaere, S., Group, C. I. W., Dubois, B. & Goris, A. Body mass index, Interleukin-6 signaling and multiple sclerosis: A Mendelian randomization study. Front. Immunol.13, 834644 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan, S., Xiong, Y. & Larsson, S. C. An atlas on risk factors for multiple sclerosis: a Mendelian randomization study. J. Neurol.268, 114–124 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma, M., Zhi, H., Yang, S., Yu, E. Y. & Wang, L. Body mass index and the risk of atrial fibrillation: A Mendelian randomization study. Nutrients 14 (2022). [DOI] [PMC free article] [PubMed]
- 66.Larsson, S. C., Back, M., Rees, J. M. B., Mason, A. M. & Burgess, S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK biobank: a Mendelian randomization study. Eur. Heart J.41, 221–226 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klovaite, J., Benn, M. & Nordestgaard, B. G. Obesity as a causal risk factor for deep venous thrombosis: a Mendelian randomization study. J. Intern. Med.277, 573–584 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Tan, J. S., Liu, N. N., Guo, T. T., Hu, S. & Hua, L. Genetically predicted obesity and risk of deep vein thrombosis. Thromb. Res.207, 16–24 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Wang, J. et al. Genetic predisposition of both waist circumference and hip circumference increased the risk of venous thromboembolism. Thromb. Haemost. 123, 347–361 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindstrom, S. et al. Assessing the causal relationship between obesity and venous thromboembolism through a Mendelian randomization study. Hum. Genet.136, 897–902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roetker, N. S. et al. Taller height as a risk factor for venous thromboembolism: a Mendelian randomization meta-analysis. J. Thromb. Haemost. 15, 1334–1343 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang, C. et al. Causal associations of obesity related anthropometric indicators and body compositions with knee and hip arthritis: A large-scale genetic correlation study. Front. Endocrinol. (Lausanne). 13, 1011896 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell, J. A. et al. Effects of general and central adiposity on Circulating lipoprotein, lipid, and metabolite levels in UK biobank: A multivariable Mendelian randomization study. Lancet Reg. Health Europe. 21, 100457 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li, Y. et al. Causal association between basal metabolic rate and risk of cardiovascular diseases: a univariable and multivariable Mendelian randomization study. Sci. Rep.13, 12487 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou, J., Mi, J., Peng, Y., Han, H. & Liu, Z. Causal associations of obesity with the intervertebral degeneration, low back pain, and sciatica: A Two-Sample Mendelian randomization study. Front. Endocrinol. (Lausanne). 12, 740200 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White, C. R., Alton, L. A., Bywater, C. L., Lombardi, E. J. & Marshall, D. J. Metabolic scaling is the product of life-history optimization. Science377, 834–839 (2022). [DOI] [PubMed] [Google Scholar]
- 77.Shah, R., Vlasak, N. & Evans, B. J. W. High myopia: reviews of myopia control strategies and myopia complications. Ophthalmic Physiological Optics: J. Br. Coll. Ophthalmic Opticians (Optometrists). 44, 1248–1260 (2024). [DOI] [PubMed] [Google Scholar]
- 78.Wells, J. C. K., Nesse, R. M., Sear, R., Johnstone, R. A. & Stearns, S. C. Evolutionary public health: introducing the concept. Lancet390, 500–509 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Jasienska, G., Bribiescas, R. G., Furberg, A. S. & Helle, S. Nunez-de La Mora, A. Human reproduction and health: an evolutionary perspective. Lancet390, 510–520 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Rosner, W., Hryb, D. J., Khan, M. S., Nakhla, A. M. & Romas, N. A. Sex hormone-binding Globulin: anatomy and physiology of a new regulatory system. J. Steroid Biochem. Mol. Biol.40, 813–820 (1991). [DOI] [PubMed] [Google Scholar]
- 81.Ruth, K. S. et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med.26, 252–258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schooling, C. M. et al. Use of multivariable Mendelian randomization to address biases due to competing risk before recruitment. Front. Genet.11, 610852 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burgess, S., Davies, N. M. & Thompson, S. G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol.40, 597–608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Minelli, C. et al. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int. J. Epidemiol.50, 1651–1659 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bowden, J. et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol.45, 1961–1974 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanderson, E., Davey Smith, G., Windmeijer, F. & Bowden, J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int. J. Epidemiol.48, 713–727 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawlor, D. A., Harbord, R. M., Sterne, J. A. & Timpson, N. Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med.27, 1133–1163 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Lopez, P. M., Subramanian, S. V. & Schooling, C. M. Effect measure modification conceptualized using selection diagrams as mediation by mechanisms of varying population-level relevance. J. Clin. Epidemiol.113, 123–128 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Batty, G. D., Gale, C. R., Kivimaki, M., Deary, I. J. & Bell, S. Comparison of risk factor associations in UK biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ368, m131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hemani, G. & Tilling, K. Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet.13, e1007081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl. J. Med.389, 2221–2232 (2023). [DOI] [PubMed] [Google Scholar]
- 92.Fairbank, R. Ozempic keeps wowing: trial data show benefits for kidney disease. Nature630, 16–17 (2024). [DOI] [PubMed] [Google Scholar]
- 93.Reis-Barbosa, P. H., Marcondes-de-Castro, I. A., Marinho, T. S., Aguila, M. B. & Mandarim-de-Lacerda, C. A. The mTORC1/AMPK pathway plays a role in the beneficial effects of semaglutide (GLP-1 receptor agonist) on the liver of obese mice. Clin. Res. Hepatol. Gastroenterol.46, 101922 (2022). [DOI] [PubMed] [Google Scholar]
- 94.Schooling, C. M., Yang, G., Soliman, G. A. & Leung, G. M. A Hypothesis That Glucagon-like Peptide-1 Receptor Agonists Exert Immediate and Multifaceted Effects by Activating Adenosine Monophosphate-Activate Protein Kinase (AMPK). Life (Basel, Switzerland). 15 (2025). [DOI] [PMC free article] [PubMed]
- 95.Stone, C. et al. Semaglutide improves myocardial perfusion and performance in a large animal model of coronary artery disease. Arterioscler. Thromb. Vasc Biol.45, 285–297 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balteau, M. et al. AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol.307, H1120–1133 (2014). [DOI] [PubMed] [Google Scholar]
- 97.Kahn, B. B., Alquier, T., Carling, D. & Hardie, D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern Understanding of metabolism. Cell. Metab.1, 15–25 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Langer, H. T., Rohm, M., Goncalves, M. D. & Sylow, L. AMPK as a mediator of tissue preservation: time for a shift in dogma? Nat. Rev. Endocrinol.20, 526–540 (2024). [DOI] [PubMed] [Google Scholar]
- 99.Laurens, C. et al. Is muscle and protein loss relevant in long-term fasting in healthy men? A prospective trial on physiological adaptations. J. Cachexia Sarcopenia Muscle. 12, 1690–1703 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soares, M. J., Kulkarni, R. N., Piers, L. S., Vaz, M. & Shetty, P. S. Energy supplementation reverses changes in the basal metabolic rates of chronically undernourished individuals. Br. J. Nutr.68, 593–602 (1992). [DOI] [PubMed] [Google Scholar]
- 101.Kouda, K. et al. Metabolic response to short-term 4-day energy restriction in a controlled study. Environ. Health Prev. Med.11, 89–92 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnstone, A. M., Murison, S. D., Duncan, J. S., Rance, K. A. & Speakman, J. R. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and Circulating thyroxine but not sex, Circulating leptin, or Triiodothyronine. Am. J. Clin. Nutr.82, 941–948 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Oh, S. K., Son, D. H., Kwon, Y. J., Lee, H. S. & Lee, J. W. Association between basal metabolic rate and handgrip strength in older Koreans. Int. J. Environ. Res. Public. Health16 (2019). [DOI] [PMC free article] [PubMed]
- 104.Luna, F., Naya, H. & Naya, D. E. Understanding evolutionary variation in basal metabolic rate: an analysis in subterranean rodents. Comp. Biochem. Physiol. Mol. Integr. Physiol.206, 87–94 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med.12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu, M. et al. Lifestyle factors, serum parameters, metabolic comorbidities, and the risk of kidney stones: a Mendelian randomization study. Front. Endocrinol. (Lausanne). 14, 1240171 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xia, L. et al. A Mendelian randomization study between metabolic syndrome and its components with prostate cancer. Sci. Rep.14, 14338 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu, S., Mu, Z., Chen, X. & Xu, Y. The impact of sex hormones on metabolic syndrome: univariable and multivariable Mendelian randomization studies. Diabetol. Metab. Syndr.16, 215 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife7, e34408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verma, A. et al. A simulation study investigating power estimates in phenome-wide association studies. BMC Bioinform.19, 120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wald, A. The fitting of straight lines if both variables are subject to error. Ann. Math. Stat.11, 284–300 (1940). [Google Scholar]
- 112.Palmer, T. M. et al. Instrumental variable Estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am. J. Epidemiol.173, 1392–1403 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol.37, 658–665 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bowden, J. et al. A framework for the investigation of Pleiotropy in two-sample summary data Mendelian randomization. Stat. Med.36, 1783–1802 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greco, M. F. D., Minelli, C., Sheehan, N. A. & Thompson, J. R. Detecting Pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med.34, 2926–2940 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent Estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol.40, 304–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect Estimation and bias detection through Egger regression. Int. J. Epidemiol.44, 512–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao, Q., Wang, J., Hemani, G., Bowden, J. & Small, D. S. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann. Stat.48, 1742–1769 (2020). [Google Scholar]
- 119.Yavorska, O. O. & Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol.46, 1734–1739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Broadbent, J. R. et al. MendelianRandomization v0.5.0: updates to an R package for performing Mendelian randomization analyses using summarized data. Wellcome Open. Res.5, 252 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R Stat. Soc. Ser. B Stat. Methodol.57, 289–300 (1995). [Google Scholar]
- 122.Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol.32, 377–389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rees, J. M. B., Wood, A. M. & Burgess, S. Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured Pleiotropy. Stat. Med.36, 4705–4718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carroll, R. J., Bastarache, L. & Denny, J. C. R PheWAS: data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinformatics30, 2375–2376 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the website of Neale Lab (http://www.nealelab.is/uk-biobank/) and the NHGRI-EBI Catalog of human genome-wide association studies (https://www.ebi.ac.uk/gwas/publications/32042192). The statistical codes needed to reproduce the results in the article are publicly accessible.