Abstract
Fipronil (FPN) is an effective pesticide for veterinary and agricultural use; however, it can induce neurotoxic effects on non-target organisms after accidental exposure. Astaxanthin (AST) is a dark red carotenoid with antioxidant, anti-inflammatory, neuroprotective, and antiapoptotic effects. This study investigated the ameliorative impact of AST against FPN-induced brain damage in rats. Thirty-two adult Wistar rats were allocated into four groups (n = 8): Control, AST (20 mg/kg bwt/day), fipronil (FPN) (20 mg/kg bwt/day), and AST + FPN group. Acetylcholine (ACh), dopamine, malondialdehyde (MDA), and proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and inflammatory cytokine cyclooxygenase-2 (COX2) levels were enhanced in the FPN-administered group relative to the control group. In addition, a substantial reduction of acetylcholine esterase (AchE), gamma-aminobutyric acid (GABA), serotonin, reduced glutathione (GSH) levels, catalase (CAT), and total superoxide dismutase (T-SOD) enzyme activities were determined. FPN induced histopathological alterations in the cerebral and cerebellar tissues. Likewise, the histomorphometric image analysis of H and E-stained tissue sections was constant with FPN-induced neurotoxicity. Immunohistochemically, an intense positive immunohistochemical staining of apoptotic marker caspase-3 and astrocytes activation marker glial fibrillary acidic protein (GFAP) in the examined tissues was noticed. Inversely, the simultaneous administration of AST partially attenuated FPN impacts, ameliorating the severity of FPN-induced neuronal damage. These results were also established with the molecular docking findings. It could be suggested that AST has antioxidant, anti-inflammatory, and anti-apoptotic capabilities against FPN-induced neuronal damage via suppression of oxidative stress and pro-inflammatory cytokines, preservation of the neurotransmitters, and the cerebral and cerebellar histoarchitectures.
Keywords: Astaxanthin, Fipronil, Oxidative stress, Neurotransmitters, Proinflammatory cytokines, Immunohistochemistry
Subject terms: Biochemistry, Cell biology, Neuroscience, Physiology, Neurology
Introduction
Repeated exposure to environmental pollutants such as pesticides can lead to neurotoxicity in humans, animals, and rodents1 and negatively impact brain functions and histoarchitecture2–4. In public health, pesticides eliminate disease-carrying organisms, such as agricultural pests5. Due to their potential hazards to humans and other living organisms, pesticides must be applied cautiously and disposed of following proper safety protocols. Fipronil (FPN,4-trifluoromethylsulfinylpyrazole-3-arbonitrile) is a phenylpyrazole broad-spectrum insecticide categorized as a class II moderately dangerous pesticide by the World Health Organization (WHO)6. It is commonly consumed in agriculture, horticulture, and veterinary practices7. Understanding the neurological effects of FPN is essential since both animals and humans may experience chronic low-dose exposure or accidental high doses. Inside the insects’ central nervous systems, FPN blocks gamma-aminobutyric acid (GABA) chloride channels, effectively targeting those resistant to other insecticides3,7. FPN can cause neuronal dysfunctions in mammals by binding firmly to GABA chloride channels, which leads to hyperexcitability. Its primary metabolite, fipronil sulfone, has an even higher affinity for mammalian GABA receptors, indicating that FPN’s metabolites may harm non-target animals3. GABAergic interneurons linking the entorhinal cortex and hippocampus are crucial for spatial and perceptual memory. Their suppression in the prefrontal cortex delays cognitive activities8. It was also reported that in FPN-administered rats, serotonin levels decreased in the striatum, hippocampus, and hypothalamus7.
Numerous research studies indicated that FPN may disrupt the body’s natural antioxidant defense system, generating reactive oxygen species (ROS) and reducing antioxidant reserves in target cells9,10. Vascular changes such as congestion, ischemia, hypoxia, lipid peroxidation, and DNA damage are believed to result from these events6. Alterations in a cell’s redox balance often led to mitochondrial dysfunction, ultimately triggering apoptosis9. It is thought that FPN stimulates the production of ROS, which releases inflammatory and apoptotic markers, leading to neuronal cell death11. Proteins, lipids, and DNA are critical cellular macromolecules damaged by excessive ROS, causing cell dysfunction and disruption12. It was also reported that FPN inhibits the state 3 respiration in mitochondria energized with glutamate plus malate, substrates of complex I of the respiratory chain, and decreases the mitochondrial membrane potential, inhibiting ATP synthesis13.
Neuroinflammation and apoptosis are common causes of FPN-induced neuronal damage, indicated by caspase-3 activation, glial fibrillary acidic protein (GFAP) upregulation, inducible nitric oxide synthase overexpression, and neuronal degeneration2,14. In the SH-SY5Y cell line, FPN inhibits the energy supply, leading to mitochondrial malfunction and ATP depletion. These events could trigger apoptotic cell death by activation of caspase-3 signaling15.
Astaxanthin (AST; 3,3-dihydroxy-β, β-carotene-4,4-dione) is a lutein carotenoid in many micro- and marine species, including shrimp, crayfish, salmon, yeast, and trout. The hydroxyl groups in AST’s ββ-ionone rings are connected to two asymmetric carbons (3 and 3′)16,17. Because of its hydrophobic structure, which comprises terminal polar groups and a conjugated polyene, AST can pass through cell membranes and enter subcellular spaces18. AST impacts most organs and tissues’ biochemical activities19,20. AST, located in the cell membrane, scavenges free radicals, preserves membrane structure, enhances immune performance, and regulates gene expression, protecting cells from oxidative damage and maintaining tissue integrity21,22. AST, amid many carotenoids, has anti-inflammatory, antioxidant, anti-apoptotic, antiproliferative, neuroprotective, anti-diabetic, and possesses eye-, skin-, reno-, and hepato-protective abilities23. Numerous experiments showed that AST boosted the brain’s nuclear factor erythroid 2-related factor-2 (Nrf2)/antioxidant response elements system, directly reducing oxidative stress and indirectly lessening oxidative damage24. Furthermore, AST demonstrated multipotent biological properties, including enhanced immunity, anti-tumor, anti-inflammatory, and anti-apoptotic properties17. AST effectively combated apoptosis by blocking crucial components such as cytochrome c, caspase-3, and caspase-9 while modulating the Bcl-2 associated X-protein/BCL2 apoptosis regulator ratio. This decisive action underscores its potential to promote cell survival25. Furthermore, AST can cross the blood–brain barrier (BBB) and has no adverse side effects18. It has the potential to ameliorate brain damage in numerous neurological disorders such as dopaminergic neurodegeneration, Parkinson’s disease26traumatic brain injury, and cognitive impairment in Alzheimer’s disease20.
The current work was aimed to assess, for the first time, the possible ameliorative impact of AST against FPN-induced oxidative stress, inflammation, neurotransmitter disruption, histopathological changes, apoptosis, and astrogliosis in the brain tissues of male Wistar rats.
Materials and methods
Chemicals and reagents
AST was attained from Carbosynth Limited, UK, Code: FA18001), while dimethyl sulfoxide (DMSO) (0.25% v/v) was bought from SNL chem Co. FPN (Coash SC 20%), a preparation from Star Chem. Company (Wellford, SC, USA) and manufactured by Zhejiang Yongnong Chem. Co. (Shaoxing, China). Rat’s neurotransmitters, including acetylcholine (Ach), acetylcholinesterase (AchE), GABA, serotonin or 5-hydroxytryptamine (5-HT), and dopamine ELISA kits were obtained from Chongqing Biospes Co. Ltd. (Chongqing Shi, China).
Malondialdehyde (MDA), total superoxide dismutase (T-SOD), reduced glutathione (GSH), and catalase (CAT) kits were purchased from Biodiagnostic (Cat. Tahrir, Cairo, Egypt). Proinflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and inflammatory cytokine cyclooxygenase-2 (COX2) ELISA kits were attained from Anogen, Mississauga, Ontario, Canada. Rabbit polyclonal anti-cleaved caspase-3 antibody (AB3623) was obtained from Merck Millipore (Darmstadt, Germany), and rabbit polyclonal anti-GFAP antibody (ab7260) was bought from Abcam (Cambridge, UK). 3,3-diaminobenzidine tetrahydrochloride Kit (DAB) obtained from Thermo Fisher Scientific (Rockford, IL, USA). All chemicals used were of analytical grade and were consumed as received, lacking any additional purification.
Ethics statement
The Animal Care Review Committee of the Faculty of Veterinary Medicine at Alexandria University, Egypt, approved all experiments under the Ethical Committee Approval number of 2023/013/263. We followed the United States National Academy of Sciences’ "Guide for the Care and Use of Laboratory Animals, ensuring humane treatment and minimizing animal suffering.
Animals
Thirty-two male Wistar rats weighing 180–200 g and 7 weeks of age were used in this investigation. The rats were housed in plastic cages that allowed them unlimited access to water and a typical lab diet. Throughout the trials, rats were maintained at 22—25 °C in the natural room temperature range with a light cycle and humidity. Rats were obtained from the Medical Research Institute at Alexandria University in Egypt and were kept for two weeks to acclimate to the laboratory environment.
Experimental groups
Rats were randomly assigned into four equal groups of eight rats in each group as follows:
-
i.
The rats in group 1 (control group) were intra-gastrically administered with 0.5 ml of DMSO (0.25% v/v) by oral gavage.
-
ii.
The rats in group 2 (AST group) received AST (20 mg/kg bwt /day) dissolved in DMSO (0.25% v/v)21 for four weeks by oral gavage. The dose was adjusted weekly to account for variations in body weight, ensuring a consistent dose for each kilogram of the rat’s body weight throughout the entire experimental period.
-
iii.
The rats in group 3 (FPN group) received FPN (20 mg/kg bwt /day) orally for 4 weeks27.
-
iv.
The rats in group 4 (AST + FPN group) were treated as groups 2 and 3.
Blood and tissue collection and preparation
Blood samples were taken straight from the heart in a clean dry tube at room temperature 24 h after the last previous dosing under ketamine/xylazine anesthesia (7.5 and 1.0 mg/kg ip). The samples were then centrifuged at 1,800 × g for 15 min to separate serum and investigate proinflammatory cytokines (IL-6, IL-1β, and TNF-α) and inflammatory cytokines (COX2). Following the collection of blood, and while the animals were still anesthetized, euthanasia was performed via decapitation and their brains were meticulously removed from their skulls and examined closely.
Each rat’s brain tissue was rinsed with physiological saline (NaCl 0.9%) and then cut into two halves. The left half was rinsed with deionized water, blotted dry, and perfused with a 50 mM sodium phosphate buffer (pH 7.4) comprising 0.1 mM ethylenediaminetetraacetic acid (EDTA) to remove red blood cells and platelets. The tissues were homogenized in ice-cold buffer, centrifuged for 30 min at 10,000 × g, and the supernatant was kept at -80°C for neurotransmitter (ACh, AchE, GABA, 5-HT, and dopamine), oxidative stress markers (MDA), and antioxidant status (GSH, CAT, and T-SOD) determinations. The right halves were kept in 10% neutral-buffered formalin for at least 24 h for further histopathological and immunohistochemical studies.
Neurotransmitters and their catabolizing enzyme determination
The brain contents of 5-HT, ACh, and dopamine were analyzed following the manufacturer’s instructions using 5-HT rat ELISA kit (catalog no. BYEK2838), ACh rat ELISA kit (catalog no. BYEK2989), and dopamine rat ELISA kit (catalog no. BYEK2898), respectively, from Chongqing Biospes Co. Ltd. The enzymatic activity of AchE in brain tissue homogenates was evaluated by the colorimetric technique of Ellman et al.28. GABA was evaluated by measurable HPLC utilizing the precolumn phenyl isothiocyanate (PITC, Edman’s Reagent) derivatization procedure method29.
Quantification of neural lipid peroxidation and antioxidants
The brain tissue homogenates were used for MDA30 and GSH31 levels and CAT32 and T-SOD33 activities determination. Protein levels in brain tissues were determined following the method of Lowry et al.34.
Examination of proinflammatory and anti-inflammatory cytokines
Proinflammatory cytokines (IL-6, TNF-α, and IL-1β) and inflammatory cytokine (COX2) were determined in serum following the manufacturer’s protocols of purchased ELISA kits35. All analyses were confirmed by an ELISA Plate Reader (Bio-Rad, Hercules, CA, USA).
Histopathological examination and lesion scoring
Following necropsy, brain tissue specimens (cerebrum and cerebellum) were obtained from different rat groups (n = 8 per group), rinsed in physiological saline solution (NaCl 0.9%), and then placed at least for 24 h in 10% neutral buffered formalin (pH 7.4). Fixed tissue specimens have been processed using the conventional paraffin embedding technique, sectioned at four µm, and stained with Mayer’s hematoxylin and eosin (H&E) stain following the method described by Bancroft et al.36. Stained tissue sections were inspected with a light microscope (Leica DM500). Photomicrographs were captured using a digital camera (Leica EC3, Leica, GmbH, Wetzlar, Germany).
The degree of severity of the observed histopathological findings was represented using a four-point semi-quantitative scoring system37 as follows: normal histology (-), mild ( +), moderate (+ +) and severe (+ + +) damage. Eight slides from eight different rats per group were evaluated to assess and grade the varied pathological alterations observed in the studied brain tissues. Each rat’s score was determined based on the area of the same slide. In addition, the grade was determined by calculating the median score for each group.
Histomorphometrical analysis
The quantitative analysis of the cerebral and cerebellar tissues was performed using Image J analysis software (Image J v1.46r, National Institute of Health, Bethesda, MD, USA). Images of H&E-stained cerebral cortex and cerebellum sections were used to quantify the number of different cell types using the cell counter plug-in available on Image J (manual computer-assisted cell counting, ImageJ plug-in-cell counter.jar). After applying a grid across the image, the number of various cell types in the respective brain regions was counted. To prevent bias, these morphometric measurements were conducted blindly for images of 10 fields per section for 8 rats per group (HPF, × 400).
Immunohistochemical studies and quantitative assessment
The localization of cleaved caspase-3 and GFAP was identified using immunohistochemical labeling38. The cerebral and cerebellar paraffin tissue sections of four µm thickness were taken on positively charged slides, then deparaffinized in xylene and rehydrated in descending ethanol concentrations. Microwave-assisted antigen retrieval was done to expose the antigen by boiling the slides with 10 mM citrate buffer, pH 6.0, for 10 min, then cooling for 20 min at room temperature. After washing with phosphate buffer saline, endogenous peroxidase was inactivated using 3% hydrogen peroxide in absolute methanol for 5 min at room temperature. Then, the non-specific reaction was blocked by incubating the slides with 2% bovine serum albumin in TBS for 60 min. The tissue sections were incubated overnight at 4 °C with rabbit polyclonal anti-cleaved caspase-3 antibody (1:100, AB3623 Merck Millipore, Darmstadt, Germany) and rabbit polyclonal anti-GFAP antibody (1:200, ab7260, Abcam, Cambridge, UK). After washing three times with Dako Tris-buffered saline, the tissue slices were treated with biotinylated goat anti-rabbit IgG antibody (AB132; Sigma Aldrich, Saint Louis, USA) for 30 min at 37 °C followed by incubation of the sections with the streptavidin-conjugated horseradish peroxidase reagent (18–152 Millipore, Merck, Darmstadt, Germany) and for 60 min at 37° C after being rinsed in Tris-buffered saline. To initiate a peroxidase reaction, the sections were rinsed with a washing buffer and subsequently incubated with 3,3-diaminobenzidine tetrahydrochloride (DAB, Thermo Fisher Scientific, Rockford, IL, USA). The tissue sections were counterstained with Mayer’s hematoxylin to improve nuclear staining, then cleared in xylene, dehydrated in absolute alcohol, and mounted with di-poly cysteine xylene (DPX). Immunoreactivity was visualized as dark brown staining of cleaved caspase-3 (brown coloration of nerve cells) and GFAP (brown coloration of the astrocytes, including their bodies and processes) using a light microscope. Micrographs of ten random fields from each section were captured at a magnification power of 400 × using a digital camera (EC3, Leica, Germany) linked to a Leica microscope (DM500) to quantify the immunoreactivity. ImageJ software (ImageJ software, v1.46r, National Institutes of Health, Bethesda, MD USA) was used to analyze these images and to estimate the area percentage (%) of cleaved caspase-3 and GFAP-positive brown immune-stained cells according to Schneider et al.39.
Molecular docking
The three-dimensional (3D) structures of caspase-3 (ID: P55213), AchE (ID: P37136), superoxide dismutase-1 (SOD1; ID: P07632), SOD2 (ID: P07895), and CAT (ID: P04762) proteins were obtained from the UniProt (https://www.uniprot.org/) database. The 3D structures of FPN (ID: 3352) and AST (ID: 5,281,224) were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/).
The 3D structures of protein and ligands were prepared for docking with energy minimization using Chimera 1.16 software40 while molecular docking was performed using InstaDock software41, and the molecular interactions between them were visualized by BIOVIA Discovery Studio Visualizer 2016 software.
Statistical analysis
GraphPad Prism v.9 (https://www.graphpad.com/) (GraphPad, San Diego, CA, USA) analyzed the data by a one-way ANOVA with Tukey’s post hoc multiple range testing. Data were presented as mean ± SD. All statements of significance were at P < 0.05.
Results
Neuronal neurotransmitters profile
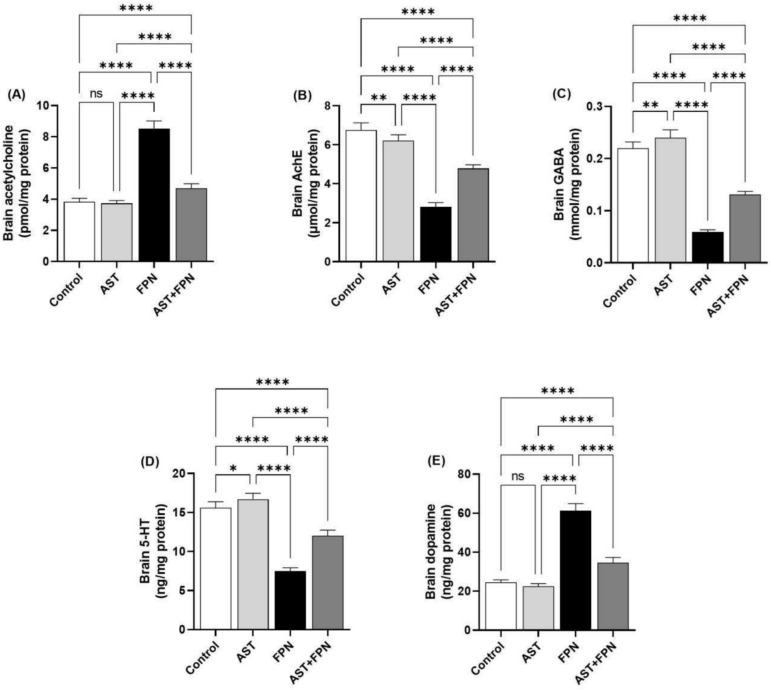
Our results showed that FPN caused a substantial (P < 0.001) enhancement in brain Ach and dopamine concentrations, in addition to a marked decrease in values of AchE, GABA, and serotonin relative to the control group (Fig. 1). AST + FPN treatment attenuated these effects by significantly (P < 0.001) reducing the neuronal Ach and dopamine and enhancing the AchE, GABA, and serotonin levels relative to the FPN group. A non-significant dissimilarity of Ach and dopamine levels was observed between the AST and the control groups; interestingly, a marked increase (P < 0.01) of the brain’s AchE, GABA, and serotonin levels was observed in the AST-only administered group relative to the control one.
Fig. 1.
Effect of Astaxanthin (AST) (20 mg/kg bwt) on brain neurotransmitters profile of fipronil (FPN) (20 mg/kg bwt) administered male rats. (A) Acetylcholine. (B) Acetylcholine esterase (AchE). (C) Gamma-aminobutyric acid (GABA). (D) 5-hydroxytryptamine (5-HT = serotonin). (E) Dopamine. Data were analyzed with a one-way ANOVA followed by Tukey’s multiple comparison test. Data are expressed as the mean ± SD; n = 8. Means within columns carrying * are significantly different at P < 0.05, **P < 0.01, and ****P < 0.0001. ns = nonsignificant.
Brain oxidant-antioxidant status
The FPN-administered rats revealed a marked (P < 0.001) increase in MDA concentration associated with a significant decrease in T-SOD, GSH, and CAT activities compared with the control group. Nevertheless, AST treatment reduced the concentration of increased MDA relative to the FPN group; conversely, the antioxidant biomarkers were partially reestablished in the AST + FPN-treated rats relative to the FPN control (Fig. 2). Interestingly, MDA levels were significantly decreased (P < 0. 0.001) in AST-administrated rats relative to the control group; also, CAT activity was improved markedly (P < 0.0.01) in the AST-supplemented group compared with the control group. A non-significant difference in the brain’s GSH and T-SOD levels was recorded between the AST-supplemented and control groups.
Fig. 2.
Effect of Astaxanthin (AST) (20 mg/kg bwt) on brain oxidant-antioxidant status of fipronil (FPN) (20 mg/kg bwt) administered male rats. (A) Malondialdehydes (MDA). (B) Reduced glutathione (GSH). (C) Total superoxide dismutase (T-SOD). (D) Catalase (CAT). Data were analyzed with a one-way ANOVA followed by Tukey’s multiple comparison test. Data are expressed as the mean ± SD; n = 8. Means within columns carrying ** are significantly different at P < 0.01, ***P < 0.001, and ****P < 0.0001. ns = nonsignificant.
Serum pro-inflammatory and inflammatory cytokines
The pro-inflammatory and inflammatory biomarkers were evaluated to study the possible neuro-inflammatory impacts of FPN. The FPN group revealed a substantial (P < 0.001) increase in the serum content of IL-1β, TNF-α, IL-6, and COX2 compared with the control group. However, AST administration reduced the intensity of increased proinflammatory and inflammatory cytokines relative to FPN-administered rats (Fig. 3). Non-significant differences in the serum IL-1β, IL-6, and COX2 levels were found between AST-administrated and the control groups; in contrast, TNF-α was markedly (P < 0.05) decreased in the AST-supplemented rats relative to the control group.
Fig. 3.
Effect of Astaxanthin (AST) (20 mg/kg bwt) on brain serum pro-inflammatory and inflammatory cytokines level of fipronil (FPN) (20 mg/kg bwt) administered male rats. (A) Tumor necrosis factor-α (TNF-α). (B) Interleukin-1β (IL-1β). (C) Interleukin-6 (IL-6). (D) Cyclooxygenase-2 (COX2). Data were analyzed with a one-way ANOVA followed by Tukey’s multiple comparison test. Data are expressed as the mean ± SD; n = 8. Means within columns carrying * are significantly different at P < 0.05 and ****P < 0.0001. ns = nonsignificant.
Histopathological examination and lesion scoring
Table 1 shows the semiquantitative evaluations of different groups’ histopathological lesions in the cerebral cortex and cerebellum. Normal histological limits were visible in both the control and AST-treated groups. However, tissue sections from FPN-treated rats showed substantial histopathological changes and a high grade in the recorded criteria. Conversely, co-administration of AST with FPN alleviated most of these changes, revealing mild to moderate pathological alterations.
Table 1.
Lesions’ scoring in the cerebrum and cerebellum of the control, Astaxanthin (AST, 20 mg/kg bwt/day) and/or fipronil (FPN, 20 mg/kg bwt/day)-treated rats.
| Tissue | Lesions | Control | AST | FPN | AST + FPN |
|---|---|---|---|---|---|
| 1-Cerebrum | |||||
| Meninges | |||||
| -Vascular congestion | – | – | + + + | + | |
| -Edema | – | – | + + | + | |
| -Hemorrhage | – | – | + + | + | |
| -Inflammatory cells infiltrations | – | – | + + | + | |
| Cerebral cortex | |||||
| -Degenerated neurons | – | – | + + + | + + | |
| -Necrotic neurons | – | – | + + | + | |
| -Glial cells reactions | – | – | + + | + | |
| -Neuropil vacuolation | – | – | + + | + | |
| -Increased pericellular space | – | – | + + | + | |
| -Perivascular cuffing | – | – | + + | + | |
| -Vascular congestion | – | – | + + + | + | |
| -Hemorrhages | – | – | + + | - | |
| 2-Cerebellum | |||||
| Meninges | |||||
| -Vascular congestion | – | – | + + + | + + | |
| -Edema | – | – | + + | + | |
| -Hemorrhage | – | – | + + + | + | |
| -Inflammatory cells infiltrations | – | – | + + | + | |
| Cerebellar cortex | – | – | |||
| -Pyknosis of Purkinje cells | – | – | + + + | + + | |
| -Increased pericellular space | – | – | + + | + | |
| -Necrosis of Purkinje cells | – | – | + + | + | |
| -Loss of Purkinje cells | – | – | + + | + | |
| -Depletion of the granule cell layer | – | – | + + | + | |
| Cerebellar Medulla | – | – | + + | + | |
| -Gliosis | – | – | + | - | |
| -Vascular congestion | – | – | + + | + | |
| -Hemorrhages | – | – | + + | + | |
The control and AST-treated rats had almost normal cerebral cortices (Fig. 4A,B, respectively) and cerebellar (Fig. 5A,B, respectively) histological features. By contrast, in FPN-treated rats, the cerebral tissues exhibited meningitis, as evidenced by vascular congestion, edema, hemorrhage, and inflammatory mononuclear cells infiltrations (Fig. 4C). Many cortical neurons were degenerated, shrunken, and deeply stained with increased pericellular spaces. Necrotic neurons exhibited pyknotic nuclei and hypereosinophilic cytoplasm, which may or may not be associated with satellitosis and neuronophagia (Fig. 4D). The neuropil had varying-sized vacuoles (Fig. 4E) and focal malacic areas with gliosis (Fig. 4F). Perivascular cuffing, congestion (Fig. 4G), and hemorrhages (Fig. 4H) were also perceived in most sections.
Fig. 4.
Representative photomicrographs of rats, cerebrum (HE, × 400). (A) a control and (B) AST-treated rats showing normal histoarchitecture of the neurons (black arrows) and neuropil. (C–H) FPN-treated rats showing vascular congestion (arrowhead), inflammatory mononuclear cells infiltrations (green arrow), hemorrhage (yellow star), degenerated shrunken darkly stained neurons (blue arrow) and necrotic neurons associated with satellitosis (red arrow) and neuronophagia (yellow arrow), (D), varying sized vacuoles of the neuropil (black star) and malacic area with gliosis (white star). (I) AST + FPN- treated rat showing improvement of the cerebral tissue architecture with minimal degenerated darkly stained neurons (blue arrow) and necrotic neurons associated with satellitosis (red arrow) and neuronophagia (yellow arrow) as compared with normal neurons (black arrow).
Fig. 5.
Representative photomicrographs of rats, cerebellum (HE, × 400). (A) a control and (B) AST-treated rats showing normal histoarchitecture of molecular cell layer (MCL), Purkinje cell layer (PCL) with normal Purkinje cells (black arrow), and granule cell layer (GCL). (C–H) FPN-treated rats showing vascular congestion (arrowhead), inflammatory mononuclear cells infiltrations (green arrow), edema (*) and hemorrhage (yellow star) in the meninges (M), degenerated shrunken darkly stained Purkinje cells (blue arrow), and necrotic neurons associated with satellitosis (red arrow), and neuronophagia (yellow arrow), depletion of the granule cells (black star), hollow spaces in the granular cell layer (orange arrow), congestion (arrowhead) and hemorrhage (yellow star) in the cerebellar white matter. (I) AST + FPN-treated rat showing an improvement in the cerebellar tissue architecture with minimal degenerated darkly stained neurons (blue arrow) and necrotic neurons associated with neuronophagia (yellow arrow) as compared with normal neurons (black arrows).
Concerning the cerebellar tissues of FPN-treated groups, the meninges displayed vascular congestion, edema (Fig. 5C), hemorrhage (Fig. 5D), and inflammatory mononuclear cells infiltrations. The cerebellar cortex showed a marked dissociation between the Purkinje cells layer and the other layers. Numerous shrunken Purkinje cells with pyknotic and hyperchromatic nuclei were visible (Fig. 5E). Others were necrotic and associated with satellitosis and neuronophagia with increased pericellular spaces. Also, there was a selective neuronal loss, and the granular cell layer showed cellular depletion and focal areas that showed hollow spaces (Fig. 5F). Additionally, focal areas of gliosis, congestion (Fig. 5G), and areas of hemorrhages (Fig. 5H) were evident in the cerebellar medulla.
Conversely, the cerebral (Fig. 4I) and cerebellar tissues (Fig. 5I) of AST + FPN-treated rats displayed improved histoarchitectures, with a considerable reduction in the severities and distributions of the earlier observed lesions (Table 1).
Histomorphometrical analysis
As demonstrated in Figs. 6 and 7, the image analysis of the control and AST-treated rat’s cerebral cortices showed no statistically significant changes in the mean values of the cerebral nerve cell size, degenerated and necrotic neuron counts, and mean glial cell count (Fig. 6A–D). Similarly, both groups exhibited no statistically significant differences in the mean values of the cerebellar Purkinje cell size, Purkinje, granule, and glial cell counts (Fig. 7A–D). Compared with the control group,s mean values, the FPN-treated group displayed a significant decrease in the mean cerebral neuron size and significant increases in the mean degenerated, necrotic, and glial cell counts (Fig. 6A–D). Also, the cerebellar tissues of this group exhibited significant decreases in the mean Purkinje cell size and count and the granule cell count. They showed a significant increase in the mean glial cell count (Fig. 7A–D).
Fig. 6.
Histomorphometrical analysis of the cerebral cortices’ images of the experimental groups (images of H&E-stained sections, 10 different fields per section, HPF, × 400 for 8 rats per group). Data were analyzed with a one-way ANOVA followed by Tukey’s multiple comparison test. Data are expressed as the mean ± SD; n = 8. Means within columns carrying * are significantly different at P < 0.05, ***P < 0.001, and ****P < 0.0001. ns = nonsignificant. FPN, fipronil; AST, astaxanthin.
Fig. 7.
Histomorphometrical analysis of the cerebellar cortices’ images of the experimental groups (images of H&E-stained sections, 10 different fields per section, HPF, × 400 for 8 rats per group). Data were analyzed with a one-way ANOVA followed by Tukey’s multiple comparison test. Data are expressed as the mean ± SD; n = 8. Means within columns carrying * are significantly different at P < 0.05, **P < 0.01, and ****P < 0.0001. ns = nonsignificant. FPN, fipronil; AST, astaxanthin.
On the contrary, the AST + FPN-treated group demonstrated a notable improvement of the previously mentioned parameters in the cerebrum and cerebellum compared with the FPN-treated group. Nevertheless, they were still comparable with the control group values (Fig. 6A-D and Fig. 7A–D).
Immunohistochemical studies and quantitative assessment
Figures 8 and 9 exemplify the immunohistochemical staining of the cleaved caspase-3 and GFAP in the cerebral and cerebellar tissues and the quantitative assessment of their levels depending on the area percentages of positive immune-stained cells.
Fig. 8.
Representative photomicrographs demonstrating the immunohistochemical staining (brown staining) of cleaved caspase-3 in the cerebral and cerebellar tissue sections of the experimental groups (IHC, × 400). (A) Control, (B) Astaxanthin (AST), (C) Fipronil (FPN) and (D) AST + FPN- treated rats. (E) Quantification of cleaved caspase-3 expression, the immunohistochemical staining of cleaved caspase-3 was measured as the area percentage (%) across 10 different fields/section, n = 8 rat/group. Data were analyzed with a one-way ANOVA followed by Tukey’s multiple comparison test. Data are expressed as the mean ± SD; n = 8. Means within columns carrying * are significantly different at P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. ns = nonsignificant.
Fig. 9.
Representative photomicrographs demonstrating the immunohistochemical staining (brown staining) of the glial fibrillary acidic protein (GFAP) in the cerebral and cerebellar tissue sections of the experimental groups (IHC, × 400). (A) Control, (B) Astaxanthin (AST), (C) Fipronil (FPN) and (D) AST + FPN-treated rats. (E) Quantification of GFAP expression, the immunohistochemical staining of GFAP was measured as the area percentage (%) across 10 different fields/sections, n = 8 rat/group. Data were analyzed with a one-way ANOVA followed by Tukey’s multiple comparison test. Data are expressed as the mean ± SD; n = 8. Means within columns carrying ** are significantly different at P < 0.01, ***P < 0.001, and ****P < 0.0001. ns = nonsignificant.
Concerning the cleaved caspase-3, the control (Fig. 8A) and AST-treated (Fig. 8B) groups revealed negative immunohistochemical staining of caspase-3 in the neurons and resident glial cells of the cerebral cortices and the Purkinje cells and granule cells in the cerebellar folia. There were no significant alterations in the mean area percentages of cleaved caspase-3 immunostained cells in AST-treated rats compared with the control group’s mean values (Fig. 8E).
The FPN-treated group (Fig. 8C) showed a strong positive immunoreactivity of cleaved caspase-3, as confirmed by prominent brown staining of the cerebral cortical neurons, Purkinje cells, and granule cells in the cerebellar folia. Relative to the control group,s mean values, the mean area percentages of cleaved caspase-3 positive immunostained cells showed significant increments in both cerebral and cerebellar tissues (Fig. 8E). Conversely, in AST + FPN-treated rats (Fig. 8D), most cerebral cortical neurons, cerebellar Purkinje, and granule cells exhibited weak to moderate immunoreactivity, and only a few cells showed intense immunopositive brown staining of cleaved caspase-3. Related to the FPN-treated group, the combination group showed significant decreases in the mean area percentages of cleaved caspase-3 positive immunostained cells in both cerebral and cerebellar tissues (Fig. 8E).
On the other hand, the immunohistochemical assessment showed that the GFAP immunoreactive-positive substances were observed in the astrocytes’ cell bodies and processes. The immunostained cells were widely distributed across the cerebral cortex and cerebellum in the control and the treated groups (Fig. 9). In the cerebral and cerebellum tissues of the control (Fig. 9A) and AST-treated (Fig. 9B) groups, the positive cells were brown, small, and had thin processes. The quantitative assessment showed no significant alterations in the GFAP immunostained cells’ mean area percentages between both groups (Fig. 9E). The FPN-treated rats had darker and relatively larger cells with thickened interdigitated processes in the examined tissues (Fig. 9C). Compared with the mean values of the control group, the FPN-treated group showed significant increments in the mean area percentages of GFAP-immunostained cells in both cerebral and cerebellar tissues (Fig. 9E). Meanwhile, in the AST + FPN-treated group, a substantial decrement in the astrocytic reaction was evident (Fig. 9D), with significant decreases in the mean area percentages of GFAP-immunostained cells in the cerebral and cerebellar tissues as compared with the FPN-treated group,s mean values (Fig. 9E).
Molecular docking
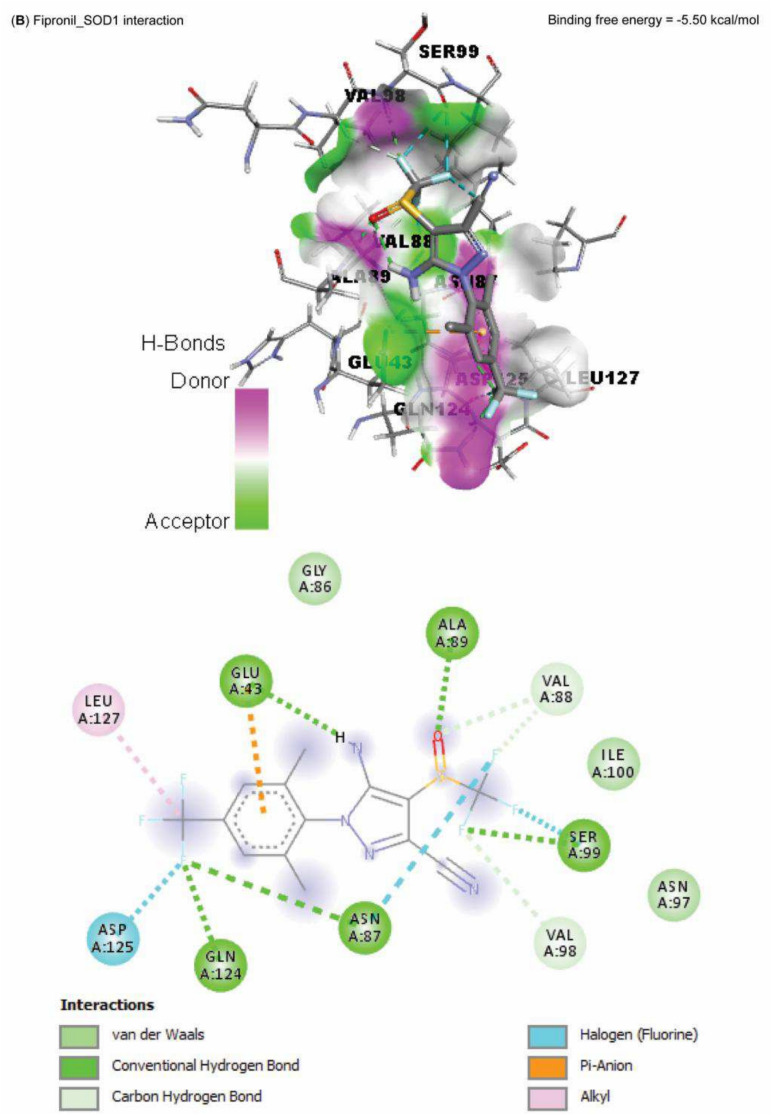
Data represented in Fig. 10 explored the molecular interaction of AST and caspase-3, with a binding free energy of -7.90 kcal/mol. On the other hand, FPN interacted with the binding site of acetylcholinesterase, SOD1, SOD2, and CAT, with binding free energies of -8.10, -5.50, -6.30, -5.90, and -7.60 kcal/mol, respectively (Fig. 11A–E).
Fig. 10.
Molecular docking interaction of Astaxanthin (AST) active compounds with rats’ caspase-3.
Fig. 11.
Molecular docking interaction of fipronil active compounds. Molecular docking interaction of fipronil with the binding site of rats’ (A) acetylcholinesterase, (B) superoxide dismutase-1 (SOD1), (C) superoxide dismutase-2 (SOD2), and (D) catalase.
Discussion
The brain, an essential organ in the body, serves as a system for coordinating and regulating all physiological functions21,42. The brain is a delicate tissue easily affected by ROS due to its elevated oxygen consumption, high peroxidizability of unsaturated fatty acids, and increased availability of highly peroxidizable substrates13,18. Furthermore, the antioxidant defenses of the brain are not very strong due to their limited capability to restore neurons that are more susceptible to pollutants43. The extensive use of pesticides in veterinary, agricultural, and domestic settings poses a significant risk to target species, the environment, and any living things that encounter them1,5. Due to their high invertebrate toxicity, systemic nature, and ease of administration, phenylpyrazoles are among the most frequently used insecticides in the world44. This ensures that the insecticides are effectively disseminated throughout the treated region10. Pesticide effects on non-target creatures are thought to be the main component of effective pest management strategies. Pesticide exposure can have a wide range of harmful impacts on non-target organisms, such as nephrotoxicity, hepatotoxicity, neurotoxicity, hematotoxicity, etc., either directly or indirectly9–11. FPN is extensively consumed in rice farms to kill pest insects. Throughout the last decade, there has been a cumulative concern regarding the human and animal health and environmental impacts related to the consumption of FPN45,46.
To our knowledge, the neuroprotective effects of AST against FPN-induced neuronal damage have not been studied before. This study examined AST’s protective effects in adult male rats exposed to FPN. Male rats were chosen due to the physiological hormonal variations that is associated with the reproductive cycle of females, which may alter their brains’ neurological state and impact the measured parameters. In contrast, the male physiology is characterized by a particular stable hormonal variation, which is more suitable for the brain research experiments2,3. The current study found that FPN causes oxidative brain damage, disrupting cellular membrane functions and producing excess inflammatory cytokines. These cytokines may inhibit neurotransmitter synthesis and result in neuronal necrosis47. However, these adverse effects were mostly reversed when AST was administered with FPN. AST is a xanthophyll carotenoid found in algae, microbes, and marine life, especially crustaceans. It has properties that modulate the immune system, combat cancer, reduce inflammation, and act as an antioxidant17. Oxidative stress, caused by pollution, occurs when an imbalance between oxidants and antioxidants in cells increases free oxygen radicals48,49. According to earlier research, ROS are crucial for FPN-induced neuronal damage and death in both the in-vitro and in-vivo models3,50. The BBB permeability is compromised by oxidative stress42, which mediates mitochondrial damage, encourages oxidative damage, and exacerbates neuro-inflammation51. Because of the brain’s high oxygen consumption, high values of polyunsaturated fatty acids, and higher concentrations of iron and ascorbate that promote radical generation, the brain tissues are especially susceptible to oxidative stress13. It also has fewer antioxidants and antioxidant enzymes than other tissues17. Specific defensive mechanisms may be triggered by tissue damage resulting from ROS production. Antioxidants such as CAT, T-SOD, and GSH are the most critical defenders against free radicals, helping to inhibit lipid peroxidation16,22.
The brain has a higher energy demand than other tissues and contains many mitochondria to supply this energy. It is also susceptible to oxidative stress, and mitochondrial dysfunction can lead to issues with ATP production, which relates to neurodegenerative diseases13,42. Lipid peroxidation arises from damage to lipid structures in cell membranes and was studied in this investigation using MDA52. In our study, as predicted, brain MDA levels increased considerably in the FPN group relative to the control. The AST + FPN-treated rats showed a substantial decrease in MDA values in this experimental condition relative to the FPN group. An increase in MDA levels indicates increased oxidative stress and lipid peroxidation20. Our results are consistent with previous research findings on this topic10,11,50.
Hafez et al.49 reported that SOD is an enzyme that changes superoxide ions into H2O2. Furthermore, Nishida et al.19 stated that one of the most significant defense enzymes, CAT, transforms hydrogen peroxide into water and oxygen in all living things. Antioxidative indicators (GSH, T-SOD, and CAT) reveal an improvement in AST + FPN-treated rats compared with the FPN group and a reduction in the FPN group relative to the control. The current research findings agree with those of Khalaf et al.2 and Mahmoud et al.3 about the rat brain’s markedly decreased amounts of antioxidant enzymes caused by FPN.
The harmful impact of FPN on the antioxidant defense enzymes was also established by the molecular docking results of our study, which reported an interaction between FPN and the binding site of SOD1, SOD2, and CAT. The cellular antioxidant mechanism’s malfunction increases the cellular vulnerability to FPN-induced free-radical oxidation10,11. Furthermore, GSH eliminates free radicals such as singlet oxygen, superoxide, and hydroxyl radicals in all cells as the primary endogenous antioxidant53. FPN metabolites may cause a drop in GSH levels by preventing the cellular absorption of cysteine required for GSH production9. Conversely, in the AST + FPN group, T-SOD, GSH, and CAT activities were much higher than in FPN-administered rats; this could be explained by the antioxidative effects of AST and its ability to scavenge free radicals17,20.
All mammalian neurons have serotonin and dopamine, and alterations in their expression are associated with neurological diseases20. Dopamine regulates animal aggression, while serotonin generally inhibits brain activity depending on the receptor subtype it binds to54. The present investigation verified that rats in the FPN group had significantly enhanced brain dopamine and decreased brain serotonin levels. These might be related to the effects of FPN’s neuronal malfunction55. The data obtained agrees with the findings of Mahmoud et al.3, who reported reduced serotonin levels in the brains of rats given FPN. Tryptophan, an amino acid considered a precursor for serotonin production56, may interact with FPN, potentially reducing serotonin levels54. FPN primarily acts at the nerve terminal, inhibiting membrane fusion, limiting neurotransmitter release, and contributing to degeneration. This exact mechanism was previously explained by Abdel-Daim and Abdeen27. Additionally, the results showed that administering FPN dramatically raised dopamine, which may cause anxious behavior11. FPN’s neurotoxic effects on the brain’s antioxidant system and microglial cells may harm dopaminergic and serotonergic neurotransmission57. Oxidative stress and neuroinflammation can deteriorate one another, compromising the brain’s defenses and causing neuronal degeneration52. Increased inflammation caused by FPN may lead to the gradual damage of nigrostriatal dopaminergic neurons and S-2A receptors8. Impaired adult neurogenesis and neural maturation hinder neural replacement, deteriorating the condition and depleting serotonergic and dopaminergic neurotransmission6. These effects provide strong evidence of the neurotoxic effects of FPN insecticide on creatures that are not targets. According to the current study, AST reduced the neuronal damage caused by FPN by modifying the release of serotonin and dopamine in the brains of rats treated with AST + FPN, consistent with earlier research of Si and Zhu21.
Numerous research studies showed that fipronil sulfone was the cause of FPN-related neurotoxicity9,11,50, which is a mainly prevalent FPN metabolite in the livers of rodents and humans27. In mice and humans, fipronil sulfone has a six-fold greater affinity for GABA receptors than FPN58. The amino acid GABA neurotransmitter facilitates fast inhibitory neurotransmission in the brain17. At cholinergic synapses, neurotransmission is stopped by the swift enzyme AchE56. Additionally, AchE is thought to be a possible biomarker of neuronal damage and is essential for transmitting neuromuscular impulses18. An increase in oxidative damage is frequently linked to AchE activity. The capacity of AchE to neutralize ROS may be responsible for its neuroprotective effects20. This study’s findings that FPN inhibited AchE activity are in line with those of Mahmoud et al.3, who found that FPN toxicity results in AchE reduction and that increased ACh appearance leads to increased ROS and inflammation. In addition, the molecular docking findings of our study revealed the interaction between FPN and the binding site of AchE, which confirmed the inhibition of AchE activity and the further reduction of its functions. GABA is an inhibitory neurotransmitter that calms when it binds to a GABA receptor24. According to this study, FPN significantly decreased GABA levels, indicating that the neuroendocrine mechanism that caused the neuronal damage caused by FPN was dysregulated57. Akkoyun et al.21 supported this study by showing that AST has a robust anxiolytic function facilitated via the GABAergic neurons. Our findings suggest modulating the oxidative/antioxidant balance positively affects neurotransmitter regulation. This improvement may enhance neurotransmitter production, release, and storage, reducing degenerative brain lesions. Thus, AST may offer neural protection against FPN through these mechanisms16,17,20.
Consistent with our findings, it was demonstrated that FPN treatment for four weeks raised neuroinflammatory markers2,3,11. It has been shown that FPN increases oxidative stress and triggers inflammatory cascades, leading to neuronal dysfunction9,27. TNF-α, IL-1β, IL-6, and COX2 have all been demonstrated to rise in response to FPN poisoning in the tissues of the hippocampus50. These increased levels could result from inflammation in the brain and neuronal damage48. FPN caused hippocampal microglia and astrocytes to proliferate and become more active, potentially increasing inflammatory cytokine expression14, such as IL-6, TNF-α, and IL-1β, which could exacerbate oxidative stress and inflammation3. TNF-α induced inducible nitric oxide synthase and peroxynitrite intracellular expression, which resulted in either neuronal death or a change in synaptic plasticity57. Following brain injury, COX2 activation results in increased prostaglandin E2 (PGE2) levels59. Since IL-6 can cross the BBB, it may also contribute to producing PGE222,60. Consequently, a positive relationship exists between elevated PGE2 and elevated values of IL-6 and COX2. These are likely reasons for the observed neuronal dysfunction after 4 weeks of FPN treatment. Conversely, the AST + FPN group’s IL-1β, IL-6, and TNF-α levels returned to normal following AST treatment, possibly related to AST’s anti-inflammatory properties16,25,61.
The histopathological, histomorphometrical, and immunohistochemical findings were consistent with the biochemical results. In the FPN-treated group, we observed various histopathological alterations, including neuronal degeneration, apoptosis, necrosis, glial cells reactions, and increased pericellular spaces in the cerebral and cerebellar tissues. These changes were likely a result of FPN-induced oxidative stress, generation of ROS, mitochondrial dysfunction, and cytoskeleton disruption, which consequently led to cell death62,63. The ROS disrupt essential cellular processes by interacting with DNA, RNA, and protein synthesis, which may result in inflammation, cell necrosis, or apoptosis14. They also target polyunsaturated fatty acids in cell membranes, leading to membrane breakdown and cellular vacuolization64. Additionally, ROS can disrupt brain vascular function, explaining the edema and hemorrhages found in the neural tissues with eventual cell damage and death65. The reported cerebral and cerebellar lesions were aligned with those stated by Mahmoud et al.3, Awad et al.63, Abou-Zeid et al.66, and Bakr et al.55. Conversely, the concurrent administration of AST with FPN partially ameliorated these histopathological findings, as the histopathological scoring shows. This effect was likely due to AST’s ability to cross the BBB, scavenge free radicals, and alleviate FPN-induced oxidative stress17. So, it has the potential to treat neurological diseases and mitigate the histological changes which occurred in the brain tissue21, as reported in previous studies such as the mouse model of vascular cognitive impairment induced by repeated cerebral ischemia/reperfusion injury45, acute cerebral infarction in rats67, traumatic brain injury in mice68 and many neurodegenerative disorders, including Alzheimer’s69,70, Parkinson’s71, and autoimmune diseases72 in which oxidative stress mechanisms play a significant role in the disease pathogenesis26,73.
On the other hand, the histomorphometrical analysis indicated that compared with the control group's mean values, the cerebral cortices of FPN-treated rats revealed a substantial reduction in mean nerve cell size in addition to significant increases in the mean degenerated and necrotic neuron and glial cells, counts. Furthermore, the cerebellar cortex of this group exhibited significant decreases in the mean Purkinje cells’ size and count and the granule cells’ count, as well as a significant increase in the mean glial cells’ count. These results may be related to oxidative damage, neuronal inflammation, necrosis, and progressive loss of neurons48,74. Contrariwise, the simultaneous administration of AST with FPN partially improved these alterations. These effects could be attributed to the ability of AST to mitigate oxidative damage, reduce lipid peroxidation and inflammation, and maintain the cell’s integrity73.
Apoptosis, or programmed cell death, is a cellular mechanism that eliminates injured and infected cells or those that have reached the end of their lifespan75. It involves the expression of specific genes and the regulation of several proteins76 including anti-apoptotic protein B cell lymphoma 2 family proteins, which are mainly located in the mitochondrial membrane and play an essential role in reducing apoptosis77 and apoptotic genes, including caspases78. The detection of active caspase-3 offers a valuable and accurate way to identify apoptotic cells in tissues before all morphological signs of apoptosis appear79. There are many diseases and neurodegenerative disorders characterized by the upregulation of caspases80. Since FPN and its metabolites can cross BBB and the neuronal membrane81,82. They provoke the production of ROS, which mediate mitochondrial injury and interfere with the permeability of the mitochondria7 leading to the production of key apoptotic proteins, including caspase-3 and cytochrome-c44.
In the current experiment, the immunohistochemical examination displayed that the FPN-treated group showed a strong positive immunoreactivity of cleaved caspase-3, as confirmed by prominent brown staining of the cerebral cortical neurons, Purkinje, and granule cells in the cerebellar folia. These findings were affirmed by quantitative analysis, which demonstrated substantial increases in the mean area percentages of cleaved caspase-3 positive immunostained cells in the examined tissues. Our results followed those reported by Awad et al.63 and Elshony et al.57, which proposed that FPN triggered the apoptotic signal via mitochondrial damage and upregulation of caspase-3 expression.
Concurrent treatment of AST with FPN modulated the cleaved caspase-3 immunohistochemical staining in the examined tissues, as most cerebral cortical neurons, cerebellar Purkinje, and granule cells exhibited weak to moderate immunoreactivity. In contrast, only a few cells displayed intense immunopositive brown staining of cleaved caspase-3 with substantial decreases in the mean area percentages of cleaved caspase-3 positive immunostained cells in both cerebral and cerebellar tissues. The immunohistochemical findings were consistent with the molecular docking findings of our study. The anti-apoptotic activity of AST could be due to its anti-inflammatory and antioxidant properties, as recorded by Fang et al.83 and Zhang et al.84.
Astrocytes, the most prevalent cells in neural tissue, respond to central nervous system injuries through reactive astrogliosis. It is considered a key marker of structural changes in CNS. In reactive astrogliosis, astroglia cells experience cellular hypertrophy (increased size and GFAP protein expression) and hyperplasia (increased number of glial cells)85,86. FPN’s overproduction of ROS may trigger the progression of reactive astrogliosis and the corresponding inflammatory and apoptotic reactions. GFAP, a specific marker for mature astrocytes, is astrocytes’ key intermediate filament III protein. It is essential for maintaining the cytoskeleton structure and mechanical strength of astrocytic processes and for supporting neighboring neurons. GFAP corroborates BBB integrity, white matter architecture, and myelination85. Furthermore, it preserves the structural integrity of astrocytes, especially when these cells experience hypertrophy and hyperplasia in response to CNS damage, which is associated with neuroinflammation and neurodegenerative diseases. GFAP serves as an early and sensitive biomarker of neurotoxicity, and its upregulation precedes the perceptible histological alterations in the brain87,88.
GFAP-immunoreactive substances were observed in the cell bodies and processes of the astrocytes across astrocytes of the cerebral cortex and cerebellum. In the FPN-treated group, we observed darker, larger cells with thickened interdigitated processes and a significant increase in the mean area percentages of GFAP-positive immunostained cells in cerebral and cerebellar tissues relative to the control rats. This showed that FPN was linked to brain injury and neuroinflammation. These findings were agreed with those recorded by Awad et al.63.
The co-administration of AST with FPN substantially decreased the astrocytic reaction, evident in the significant decreases in the mean area percentages of GFAP in cerebral and cerebellar tissues relative to FPN-administered rats’ mean values. The neuroprotective effect of AST could be explained by its proven capability to alleviate oxidative stress, improve resistance to the free radicals, attacks20 that were triggered by FPN and to boost the total antioxidant defenses and regulation of redox homeostasis89, which in turn limited oxidative stress-driven neuroinflammation and modulated the immunohistochemical staining of the GFAP, as reported by Ying et al.46.
Conclusion
AST emerges as a promising therapeutic option in countering the oxidative stress and inflammatory responses triggered by FPN exposure in the rat brain. This study marks a significant milestone, revealing for the first time that AST can substantially alleviate the neuronal damage caused by FPN. Although the precise mechanisms through which AST exerts its protective effects remain uncertain, it is plausible to surmise that its neuromodulatory properties play a crucial role. Specifically, AST appears to influence the balance of neurotransmitters, modulate oxidative stress levels, and impact the expression of pro-inflammatory cytokines and the markers indicative of neuronal apoptosis, such as cleaved caspase-3 and astrogliosis as GFAP which were evident through immunohistochemical staining. Despite these encouraging findings, further investigation at the molecular level is essential to fully elucidate the intricate pathways through which AST mitigates FPN-induced neuronal injury.
Abbreviations
- 5-HT
5-Hydroxytryptamine
- AchE
Acetylcholinesterase
- AST
Astaxanthin
- BBB
Blood–brain barrier
- CAT
Catalase
- COX2
Cyclooxygenase-2
- DAB
3,3-diaminobenzidine tetrahydrochloride
- DMSO
Dimethyl sulfoxide
- DPX
Di-poly cysteine xylene
- EDTA
Ethylenediamine tetra-acetic acid
- FPN
Fipronil
- GABA
Gamma-aminobutyric acid
- GFAP
Glial fibrillary acidic protein
- GSH
Reduced glutathione
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- MDA
Malondialdehyde
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor-α
- T-SOD
Total superoxide dismutase
Author contributions
Conceptualization M.H.H and S.S.E; formal analysis, M.H.H, A.H.E.F, and S.S.E; investigation, M.H.H and S.S.E; software, M.H.H, A.H.E.F, and S.S.E; validation, M.H.H, A.H.E.F, and S.S.E; visualization, M.H.H and S.S.E; writing—original draft, M.H.H and S.S.E; writing—review and editing, M.H.H, A.H.E.F, and S.S.E. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data will be made available on reasonable request from the corresponding author (M.H.H.)
Declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The Animal Care Review Committee of the Faculty of Veterinary Medicine at Alexandria University, Egypt, approved all experiments under the Ethical Committee’s 2023/013/263 approval number. We followed the United States National Academy of Sciences’ "Guide for the Care and Use of Laboratory Animals, ensuring humane treatment and minimizing animal suffering. Also, all animal experiments conducted in this study follow the ARRIVE guidelines (https://arriveguidelines.org).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richardson, J. R., Fitsanakis, V., Westerink, R. H. S. & Kanthasamy, A. G. Neurotoxicity of pesticides. Acta Neuropathol.138, 343–362 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalaf, A. A. et al. The Terminalia laxiflora modulates the neurotoxicity induced by fipronil in male albino rats. Biosci Rep39, (2019). [DOI] [PMC free article] [PubMed]
- 3.Mahmoud, Y. K. et al. Neurotoxic Effect of Fipronil in Male Wistar Rats: Ameliorative Effect of L-Arginine and L-Carnitine. Biology (Basel)10, 682 (2021). [DOI] [PMC free article] [PubMed]
- 4.Utomo, N. P., Pinzon, R. T., Latumahina, P. K. & Damayanti, K. R. S. Astaxanthin and improvement of dementia: A systematic review of current clinical trials. Cereb. Circ. Cogn. Behav.7, 100226 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parrilla Vázquez, P. et al. Large multiresidue analysis of pesticides in edible vegetable oils by using efficient solid-phase extraction sorbents based on quick, easy, cheap, effective, rugged and safe methodology followed by gas chromatography–tandem mass spectrometry. J Chromatogr A1463, 20–31 (2016). [DOI] [PubMed]
- 6.Kartheek, R. M. & David, M. Assessment of fipronil toxicity on wistar rats: A hepatotoxic perspective. Toxicol. Rep.5, 448–456 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt, P. et al. Insights into the toxicity and biodegradation of fipronil in contaminated environment. Microbiol. Res.266, 127247 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Bharatiya, R. et al. Chronic Administration of Fipronil Heterogeneously Alters the Neurochemistry of Monoaminergic Systems in the Rat Brain. Int. J. Mol. Sci.21, 5711 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleh, H. et al. Chemo-Protective Potential of Cerium Oxide Nanoparticles against Fipronil-Induced Oxidative Stress, Apoptosis, Inflammation and Reproductive Dysfunction in Male White Albino Rats. Molecules25, 3479 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae, J.-W. & Kwon, W.-S. Proteomic analysis of fipronil-induced molecular defects in spermatozoa. Sci. Rep.14, 7668 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koslowski, S., Latapy, C., Auvray, P., Blondel, M. & Meijer, L. Long-Term Fipronil Treatment Induces Hyperactivity in Female Mice. Int. J. Environ. Res. Public Health17, 1579 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, J. et al. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J.10, 1532–1538 (1996). [PubMed] [Google Scholar]
- 13.Cenini, G., Lloret, A. & Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell Longev.2019, 1–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awad, M. A., Ahmed, Z. S. O., AbuBakr, H. O., Elbargeesy, G. A. E.-F. H. & Moussa, M. H. G. Oxidative stress, apoptosis and histopathological alterations in brain stem and diencephalon induced by subacute exposure to fipronil in albino rats. Environmental Science and Pollution Research29, 936–948 (2022). [DOI] [PubMed]
- 15.Romero, A. et al. Fipronil sulfone induced higher cytotoxicity than fipronil in SH-SY5Y cells: Protection by antioxidants. Toxicol. Lett.252, 42–49 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Si, P. & Zhu, C. Biological and neurological activities of astaxanthin (Review). Mol. Med. Rep.26, 300 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baburina, Y. et al. The Improvement of Functional State of Brain Mitochondria with Astaxanthin in Rats after Heart Failure. Int. J. Mol. Sci24, 31 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng, X. et al. The Neuroprotective Effect of Astaxanthin on Pilocarpine-Induced Status Epilepticus in Rats. Front Cell Neurosci13, (2019). [DOI] [PMC free article] [PubMed]
- 19.Nishida, Y., Nawaz, A., Hecht, K. & Tobe, K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients14, 107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hafez, H. A. et al. Ameliorative effects of astaxanthin on brain tissues of alzheimer’s disease-like model: cross talk between neuronal-specific microRNA-124 and related pathways. Mol. Cell. Biochem.476, 2233–2249 (2021). [DOI] [PubMed] [Google Scholar]
- 21.AKKOYUN, H. T. et al. Effect Of Astaxanthin On Rat Brains Against Oxidative Stress Induced By Cadmium:Biochemical, Histopathological Evaluation. Iğdır Üniversitesi Fen Bilimleri Enstitüsü Dergisi8, 33–39 (2018).
- 22.Wei, N. et al. Astaxanthin Rescues Memory Impairments in Rats with Vascular Dementia by Protecting Against Neuronal Death in the Hippocampus. Neuromolecular. Med.26, 29 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Zhou, P. et al. Directed Coevolution of β-Carotene Ketolase and Hydroxylase and Its Application in Temperature-Regulated Biosynthesis of Astaxanthin. J. Agric. Food Chem.67, 1072–1080 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Grimmig, B., Kim, S.-H., Nash, K., Bickford, P. C. & Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: a potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience39, 19–32 (2017). [DOI] [PMC free article] [PubMed]
- 25.Fakhri, S., Yosifova Aneva, I., Farzaei, M. H. & Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules24, 2640 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen, D.-F. et al. Astaxanthin suppresses endoplasmic reticulum stress and protects against neuron damage in Parkinson’s disease by regulating miR-7/SNCA axis. Neurosci. Res.165, 51–60 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Daim, M. M. & Abdeen, A. Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem. Toxicol.114, 69–77 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Ellman, G. L., Courtney, K. D., Andres, V. & Featherstone, R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.7, 88–95 (1961). [DOI] [PubMed] [Google Scholar]
- 29.Heinrikson, R. L. & Meredith, S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: Precolumn derivatization with phenylisothiocyanate. Anal Biochem.136, 65–74 (1984). [DOI] [PubMed] [Google Scholar]
- 30.Buege, J. A. & Aust, S. D. [30] Microsomal lipid peroxidation. 302–310 (1978). [DOI] [PubMed]
- 31.Glatzle, D., Vuilleumier, J. P., Weber, F. & Decker, K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia30, 665–667 (1974). [DOI] [PubMed] [Google Scholar]
- 32.Aebi, H. C. Methods of Enzymatic Analysis (Elsevier, 1974). [Google Scholar]
- 33.Nishikimi, M., Appaji Rao, N. & Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun46, 849–854 (1972). [DOI] [PubMed]
- 34.Lowry, OliverH., Rosebrough, NiraJ., Farr, A. L. & Randall, RoseJ. PROTEIN MEASUREMENT WITH THE FOLIN PHENOL REAGENT. Journal of Biological Chemistry193, 265–275 (1951). [PubMed]
- 35.Seriolo, B., Paolino, S., Ferrone, C. & Cutolo, M. Effects of etanercept or infliximab treatment on lipid profile and insulin resistance in patients with refractory rheumatoid arthritis. Clin. Rheumatol.26, 1799–1800 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Bancroft, J. D. & Gamble, M. The Hematoxylin and Eosin. In: Suvarna, S.K., Layton, C., Bancroft, J.D. (Eds.), Theory and Practice of Histological Techniques. (Churchill Livingstone, Edinburgh; New York, 2013).
- 37.Chaâbane, M. et al. Penconazole alters redox status, cholinergic function, and membrane-bound ATPases in the cerebrum and cerebellum of adult rats. Hum. Exp. Toxicol.36, 854–866 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Mohamed, A.A.-R., Galal, A. A. A. & Elewa, Y. H. A. Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem.117, 649–658 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem.25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Mohammad, T., Mathur, Y. & Hassan, M. I. InstaDock: A single-click graphical user interface for molecular docking-based virtual high-throughput screening. Brief Bioinform22, (2021). [DOI] [PubMed]
- 42.Angelova, P. R. & Abramov, A. Y. Role of mitochondrial <scp>ROS</scp> in the brain: from physiology to neurodegeneration. FEBS Lett.592, 692–702 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Ataie, A., Ataie, R. & Shadifar, M. Polyphenolic Antioxidants and Neuronal Regeneration. Basic and Clinical Neuroscience Journal7, (2016). [DOI] [PMC free article] [PubMed]
- 44.Zhang, B. et al. Fipronil induces apoptosis through caspase-dependent mitochondrial pathways in Drosophila S2 cells. Pestic Biochem. Physiol119, 81–89 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Xue, Y. et al. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res. Bull.131, 221–228 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Ying, C. et al. Anti-inflammatory Effect of Astaxanthin on the Sickness Behavior Induced by Diabetes Mellitus. Cell Mol. Neurobiol.35, 1027–1037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tingle, C. C. D., Rother, J. A., Dewhurst, C. F., Lauer, S. & King, W. J. Fipronil: Environmental Fate, Ecotoxicology, and Human Health Concerns. 1–66 (2003). 10.1007/978-1-4899-7283-5_1. [DOI] [PubMed]
- 48.Elblehi, S. S., El Euony, O. I. & El-Sayed, Y. S. Apoptosis and astrogliosis perturbations and expression of regulatory inflammatory factors and neurotransmitters in acrylamide-induced neurotoxicity under ω3 fatty acids protection in rats. Neurotoxicology76, 44–57 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Hafez, M. H., Elblehi, S. S. & El-Sayed, Y. S. Date palm fruit extract ameliorated pancreatic apoptosis, endocrine dysfunction and regulatory inflammatory cytokines in Streptozotocin-induced diabetes in rats. Environ. Sci. Pollut. Res.27, 43322–43339 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Kuo, J.-F., Cheng, Y.-H., Tung, C.-W. & Wang, C.-C. Fipronil disturbs the antigen-specific immune responses and GABAergic gene expression in the ovalbumin-immunized BALB/c mice. BMC Vet. Res.20, 30 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goda, A. et al. Astaxanthin and Docosahexaenoic Acid Reverse the Toxicity of the Maxi-K (BK) Channel Antagonist Mycotoxin Penitrem A. Mar. Drugs14, 208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He, J., Zhu, G., Wang, G. & Zhang, F. Oxidative Stress and Neuroinflammation Potentiate Each Other to Promote Progression of Dopamine Neurodegeneration. Oxid. Med. Cell. Longev.2020, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elblehi, S. S., Hafez, M. H. & El-Sayed, Y. S. L-α-Phosphatidylcholine attenuates mercury-induced hepato-renal damage through suppressing oxidative stress and inflammation. Environ. Sci. Pollut. Res.26, 9333–9342 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Yang, R. et al. Serotonin and dopamine depletion in distinct brain regions may cause anxiety in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice as a model of early Parkinson’s disease. NeuroReport34, 551–559 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakr, A. A., Ali, M. & Ibrahim, K. Garlic and allopurinol alleviate the apoptotic pathway in rats’ brain following exposure to fipronil insecticide. Environ. Anal. Health Toxicol.37, e2022037 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nimgampalle, M. et al. Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing. Res. Rev.89, 101994 (2023). [DOI] [PubMed] [Google Scholar]
- 57.Elshony, N. et al. Ameliorative Role of Cerium Oxide Nanoparticles Against Fipronil Impact on Brain Function, Oxidative Stress, and Apoptotic Cascades in Albino Rats. Front Neurosci15, (2021). [DOI] [PMC free article] [PubMed]
- 58.Das, P. C., Cao, Y., Cherrington, N., Hodgson, E. & Rose, R. L. Fipronil induces CYP isoforms and cytotoxicity in human hepatocytes. Chem. Biol. Interact.164, 200–214 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Strauss, E. J., Weil, W. M., Jordan, C. & Paksima, N. A Prospective, Randomized, Controlled Trial of 2-Octylcyanoacrylate Versus Suture Repair for Nail Bed Injuries. J. Hand Surg. Am.33, 250–253 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Ethell, I. M. & Pasquale, E. B. Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol.75, 161–205 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Masoudi, A. et al. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav. Brain Res.329, 104–110 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Wang, X. et al. Fipronil insecticide toxicology: oxidative stress and metabolism. Crit. Rev. Toxicol.46, 876–899 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Awad, M. A., Ahmed, Z. S. O., AbuBakr, H. O., Elbargeesy, G.A.E.-F.H. & Moussa, M. H. G. Fipronil induced oxidative stress in neural tissue of albino rat with subsequent apoptosis and tissue reactivity. Acta Histochem.123, 151764 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Readnower, R. D. et al. Increase in blood–brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res.88, 3530–3539 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeWitt, D. S. & Prough, D. S. Blast-Induced Brain Injury and Posttraumatic Hypotension and Hypoxemia. J. Neurotrauma26, 877–887 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Abou-Zeid, S. M., Tahoun, E. A. & AbuBakr, H. O. Ameliorative effects of jojoba oil on fipronil-induced hepatorenal- and neuro-toxicity: the antioxidant status and apoptotic markers expression in rats. Environ. Sci. Pollut. Res.28, 25959–25971 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Yang, B., Zou, M., Zhao, L. & Zhang, Y.-K. Astaxanthin attenuates acute cerebral infarction via Nrf-2/HO-1 pathway in rats. Curr. Res. Transl. Med.69, 103271 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Zhang, X. et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br. J. Pharmacol.178, 1114–1132 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Liu, N. et al. Astaxanthin attenuates cognitive deficits in Alzheimer’s disease models by reducing oxidative stress via the SIRT1/PGC-1α signaling pathway. Cell Biosci.13, 173 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shehata, M. K., Ismail, A. A. & Kamel, M. A. Combined Donepezil with Astaxanthin via Nanostructured Lipid Carriers Effective Delivery to Brain for Alzheimer’s Disease in Rat Model. Int. J. Nanomedicine18, 4193–4227 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang, L. et al. Astaxanthin ameliorates dopaminergic neuron damage in paraquat-induced SH-SY5Y cells and mouse models of Parkinson’s disease. Brain Res. Bull.202, 110762 (2023). [DOI] [PubMed] [Google Scholar]
- 72.Lotfi, A., Soleimani, M. & Ghasemi, N. Astaxanthin Reduces Demyelination and Oligodendrocytes Death in A Rat Model of Multiple Sclerosis. Cell J.22, 565–571 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adıgüzel, E. & Ülger, T. G. A marine-derived antioxidant astaxanthin as a potential neuroprotective and neurotherapeutic agent: A review of its efficacy on neurodegenerative conditions. Eur. J. Pharmacol.977, 176706 (2024). [DOI] [PubMed] [Google Scholar]
- 74.Morrison, H., Young, K., Qureshi, M., Rowe, R. K. & Lifshitz, J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci. Rep.7, 13211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuchs, Y. & Steller, H. Programmed Cell Death in Animal Development and Disease. Cell147, 742–758 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oppenheim, R. W., Prevette, D., Tytell, M. & Homma, S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: Evidence for the role of cell death genes. Dev. Biol.138, 104–113 (1990). [DOI] [PubMed] [Google Scholar]
- 77.Qian, S. et al. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol12, (2022). [DOI] [PMC free article] [PubMed]
- 78.Prokhorova, E. A., Kopeina, G. S., Lavrik, I. N. & Zhivotovsky, B. Apoptosis regulation by subcellular relocation of caspases. Sci. Rep.8, 12199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nichani, K., Li, J., Suzuki, M. & Houston, J. P. Evaluation of Caspase-3 Activity During Apoptosis with Fluorescence Lifetime-Based Cytometry Measurements and Phasor Analyses. Cytometry A97, 1265–1275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Julien, O. & Wells, J. A. Caspases and their substrates. Cell. Death Differ.24, 1380–1389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cravedi, J. P., Delous, G., Zalko, D., Viguié, C. & Debrauwer, L. Disposition of fipronil in rats. Chemosphere93, 2276–2283 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Qu, H. et al. The effect of biochar on the mitigation of the chiral insecticide fipronil and its metabolites burden on loach (Misgurnus.anguillicaudatus). J Hazard Mater360, 214–222 (2018). [DOI] [PubMed]
- 83.Fang, J., Bai, W. & Yang, L. Astaxanthin inhibits oxidative stress and apoptosis in diabetic retinopathy. Acta Histochem.125, 152069 (2023). [DOI] [PubMed] [Google Scholar]
- 84.Zhang, Q. et al. Astaxanthin activates the Nrf2/Keap1/HO-1 pathway to inhibit oxidative stress and ferroptosis, reducing triphenyl phosphate (TPhP)-induced neurodevelopmental toxicity. Ecotoxicol. Environ. Saf.271, 115960 (2024). [DOI] [PubMed] [Google Scholar]
- 85.Eng, L. F., Ghirnikar, R. S. & Lee, Y. L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res.25, 1439–1451 (2000). [DOI] [PubMed] [Google Scholar]
- 86.Yang, Z. & Wang, K. K. W. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci.38, 364–374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ho, G., Zhang, C. & Zhuo, L. Non-invasive fluorescent imaging of gliosis in transgenic mice for profiling developmental neurotoxicity. Toxicol. Appl. Pharmacol.221, 76–85 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Schiff, L., Hadker, N., Weiser, S. & Rausch, C. A literature review of the feasibility of glial fibrillary acidic protein as a biomarker for stroke and traumatic brain injury. Mol. Diagn. Ther.16, 79–92 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Medoro, A. et al. Dietary Astaxanthin: A Promising Antioxidant and Anti-Inflammatory Agent for Brain Aging and Adult Neurogenesis. Mar. Drugs21, 643 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request from the corresponding author (M.H.H.)