Abstract
This study investigates the protective effects of Allicin (A), a bioactive compound from garlic, and Hesperidin (HSD), a citrus flavonoid, against reproductive damage induced by para-nonylphenol (p-NP), an environmental pollutant, in mice. Fifty 8- to 10-week-old NMRI mice were divided into five groups: a control group, a group exposed to p-NP, and three groups treated with p-NP plus either Allicin, Hesperidin, or both. Treatments lasted for 35 days. Researchers analyzed sperm count, viability, motility, DNA damage, and morphology, alongside testicular parenchyma markers like antioxidant capacity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GPx), and malondialdehyde (MDA) levels. Hormone levels (testosterone, LH, and FSH), testicular histopathology, apoptosis-related gene expression, and fertility indices were also evaluated. Results showed that mice treated with Allicin, Hesperidin, or both had reduced abnormal sperm morphology and DNA damage, with improvements in sperm motility, viability, and membrane integrity compared to the p-NP group. Antioxidant enzyme activities (TAC, SOD, GPx) and hormone levels increased, while MDA levels decreased, indicating reduced oxidative stress. Furthermore, expression of pro-apoptotic genes Bax and caspase-3 declined, while anti-apoptotic Bcl-2 expression rose. Treated mice demonstrated higher fertility indices and hormone levels. These findings suggest Allicin and Hesperidin mitigate p-NP-induced testicular damage by enhancing antioxidant defenses and regulating cell death pathways.
Keywords: Environmental toxicology, Reproductive health, Oxidative stress, Apoptosis, Fertility
Subject terms: Health care, Pathogenesis
Introduction
Infertility among males is a rising concern, particularly in industrial areas where various environmental factors compromise reproductive health. Common issues include disruptions in sperm production, maturation, and fertilization capabilities, with environmental toxins, notably alkylphenols, playing a substantial role1. Alkylphenol ethoxylates (APEs) serve as non-ionic surfactants, and their degradation product, para-nonylphenol (p-NP), is widely found in products like detergents, paints, phenolic resins, herbicides, and food packaging2,3. Humans are commonly exposed to p-NP via skin contact, inhalation, and ingestion of contaminated food or water3. P-NP displays mild estrogenic effects4,5, imitating estrogen by interacting with estrogen receptors at different life stages, from fetal to adult, thereby contributing to male reproductive issues6. Research shows that p-NP exposure during development can impact the male reproductive system in animal models, leading to reduced sperm production, undescended testicles, lower reproductive organ weight, and testicular anomalies7. Further studies have demonstrated that p-NP exposure at 250 mg/kg in adult rats causes hormonal disturbances, impacting testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) levels7. Like other environmental toxins, p-NP generates oxidative stress in the reproductive system. Research by Chitra et al.1 has demonstrated that p-NP elevates oxidative stress levels within the epididymal sperm of adult rats. Studies also highlight that vitamin E (Vit. E), a strong free radical scavenger, is effective in preventing free-radical-induced membrane damage8. Moreover, vitamin E’s antioxidant properties are known to alleviate oxidative stress in testicle6.
Allicin (A; diallylthiosulphinate), a bioactive compound found in garlic (Allium sativum L.), has various biological properties, with a chemical structure of C6H10OS2. This compound is produced when garlic tissue is disrupted, converting the amino acid alliin (S-allylcysteine sulphoxide) through the enzyme alliinase9. Allicin can neutralize free radicals, thereby providing cellular protection against stress10. Studies have shown Allicin’s hypoglycemic and hypolipidemic effects in poultry11, particularly in reducing liver enzymes such as ALT and AST, and enhancing immune responses by increasing antibody titers against pathogens like Newcastle disease virus in broiler chickens10. Recently, allicin’s status as a potent natural antioxidant has been highlighted, as it boosts the activity of internal antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), decreasing both inflammation and oxidative stress in male rabbits exposed to environmental toxins12. In addition, El‐Ratel et al.13 showed that Allicin increased antioxidant enzyme activity, including SOD and glutathione peroxidase (GPx), which further confirmed its protective role in the male reproductive system of rats treated with doxorubicin.
Hesperidin (HSD; 3′,5,7-trihydroxy 4′-methoxy flavanone 7-rutinoside, also known as hesperetin 7-rutinoside) is a glycoside flavanone predominantly present in citrus fruits from the Rutaceae family14. Recognized as a natural antioxidant, HSD is known for its ability to reduce oxidative stress (OS)15. Research indicates that HSD has numerous pharmacological properties, including anti-inflammatory, anticancer, and antimicrobial effects16,17. These applications are further supported by HSD’s high safety margin, non-accumulative nature, and minimal side effects18–20. Studies have particularly underscored HSD’s protective effects against testicular toxicity in animals, attributing this to its antioxidant and free-radical-neutralizing capacities21,22. Although various natural antioxidants have been studied for their roles in protecting against lead-induced testicular toxicity, which shares similar mechanisms of oxidative damage with p-NP, 23,24, HSD’s specific effects have not been comprehensively examined in the context of p-NP exposure. This study evaluates the protective effects of the non-toxic antioxidants HSD and A on p-NP-induced testicular toxicity, focusing on sperm traits, biochemical markers, and histopathology. It addresses a critical gap in understanding natural antioxidants like Allicin and Hesperidin against reproductive toxicity caused by p-NP. Identifying safe therapeutic agents is crucial due to the widespread impact of p-NP on male fertility. By analyzing the effects of Allicin and Hesperidin, this research sheds light on strategies for mitigating environmental toxin-related reproductive harm, contributing to reproductive health and toxicology.
Results
The analysis revealed that mice in the p-NP group displayed a notable reduction in sperm concentration and motility, along with decreased values for curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), amplitude of lateral head displacement (ALH), and beat cross frequency (BCF) compared to other groups (p ≤ 0.05; Table 1). Treatment with Allicin (A) and Hesperidin (HSD) resulted in improvements in both total and progressive sperm motility, with increases in VCL, VSL, VAP, ALH, and BCF compared to the p-NP group (p ≤ 0.05; Table 1). Additionally, the p-NP group exhibited diminished plasma membrane functionality (PMF) and sperm viability, which were significantly avoided with A and HSD administration (p ≤ 0.05; Table 2). DNA damage in sperm and the frequency of abnormal sperm morphology were significantly elevated in the p-NP group but were notably reduced by A and HSD treatments (p ≤ 0.05; Table 2). Notably, the combined administration of A and HSD led to enhancements in these parameters, closely matching those observed in the control group.
Table 1.
Epididymal sperm concentration, total and progressive motilities, and motility characteristics in different experimental groups. Values are expressed as mean ± SD.
| Analysis | Groups | ||||
|---|---|---|---|---|---|
| Control | p-NP | A + p-NP | HSD + p-NP | A + HSD + p-NP | |
| Epididymal sperm concentration (106/mL) | 32.17 ± 23.60a | 16.38 ± 21.09e | 24.29 ± 15.45d | 26.35 ± 27.88c | 29.45 ± 26.30b |
| Total motility (%) | 79.96 ± 2.15a | 45.88 ± 1.25e | 63.01 ± 2.58d | 68.96 ± 2.89c | 75.19 ± 2.13b |
| Progressive motility (%) | 40.85 ± 1.91a | 8.47 ± 1.28d | 29.24 ± 1.44c | 30.81 ± 1.85c | 34.67 ± 1.63b |
| VAP (μm/s) | 29.39 ± 1.47a | 14.99 ± 1.89d | 18.46 ± 1.38c | 19.43 ± 1.12c | 25.26 ± 1.56b |
| VCL (μm/s) | 83.80 ± 2.53a | 43.15 ± 2.34e | 68.30 ± 2.89d | 72.81 ± 1.50c | 78.48 ± 2.13b |
| VSL (μm/s) | 23.47 ± 1.43a | 11.32 ± 1.81d | 15.82 ± 1.39c | 17.26 ± 1.56bc | 20.82 ± 1.47ab |
| LIN (%) | 0.23 ± 0.04a | 0.21 ± 0.03a | 0.22 ± 0.03a | 0.22 ± 0.02a | 0.23 ± 0.03a |
| ALH (μm/s) | 2.70 ± 0.03a | 1.11 ± 0.02d | 1.84 ± 0.03c | 2.18 ± 0.02c | 2.51 ± 0.03b |
| STR (%) | 0.85 ± 0.03a | 0.83 ± 0.04a | 0.84 ± 0.03a | 0.84 ± 0.04a | 0.85 ± 0.02a |
| BCF (Hz) | 12.56 ± 1.26a | 6.40 ± 1.28c | 8.21 ± 1.13b | 8.98 ± 0.39b | 11.61 ± 1.30a |
p-NP, para-nonylphenol; A, allicin; HSD, hesperidin; VAP, Average path velocity; VCL, Curvilinear velocity; VSL, Straight line velocity; LIN, Linearity; ALH, Amplitude of lateral head displacement; STR, Straightness; BCF, Beat-cross frequency.
a-eDifferent superscripts within the same row demonstrate significant differences (p ≤ 0.05).
Table 2.
Epididymal sperm plasma membrane functionality, DNA damage, viability and abnormal morphology in different experimental groups. Values are expressed as mean ± SD.
| Analysis | Groups | ||||
|---|---|---|---|---|---|
| Control | p-NP | A + p-NP | HSD + p-NP | A + HSD + p-NP | |
| Sperm plasma membrane functionality (%) | 82.96 ± 3.81a | 48.46 ± 3.69e | 65.51 ± 2.53d | 70.45 ± 1.27c | 77.82 ± 2.18b |
| Sperm viability (%) | 86.85 ± 3.24a | 55.45 ± 2.42e | 71.37 ± 2.37d | 75.09 ± 2.41c | 82.30 ± 2.53b |
| Sperm DNA damage (%) | 5.12 ± 0.20d | 28.95 ± 1.48a | 15.30 ± 1.09b | 11.42 ± 0.50c | 9.25 ± 1.09cd |
| Sperm abnormal morphology (%) | 7.27 ± 0.43 d | 34.77 ± 1.31 a | 18.43 ± 1.34b | 16.15 ± 0.39b | 11.92 ± 0.73c |
p-NP, para-nonylphenol; A, allicin; HSD, hesperidin.
a-eDifferent superscripts within the same row demonstrate significant differences (p ≤ 0.05).
In the p-NP group, serum testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) levels were significantly lower than in the control group (p ≤ 0.05). In contrast, groups treated with A, HSD, or both showed significant increases in these hormone levels compared to the p-NP group (p ≤ 0.05; Fig. 1).
Fig. 1.
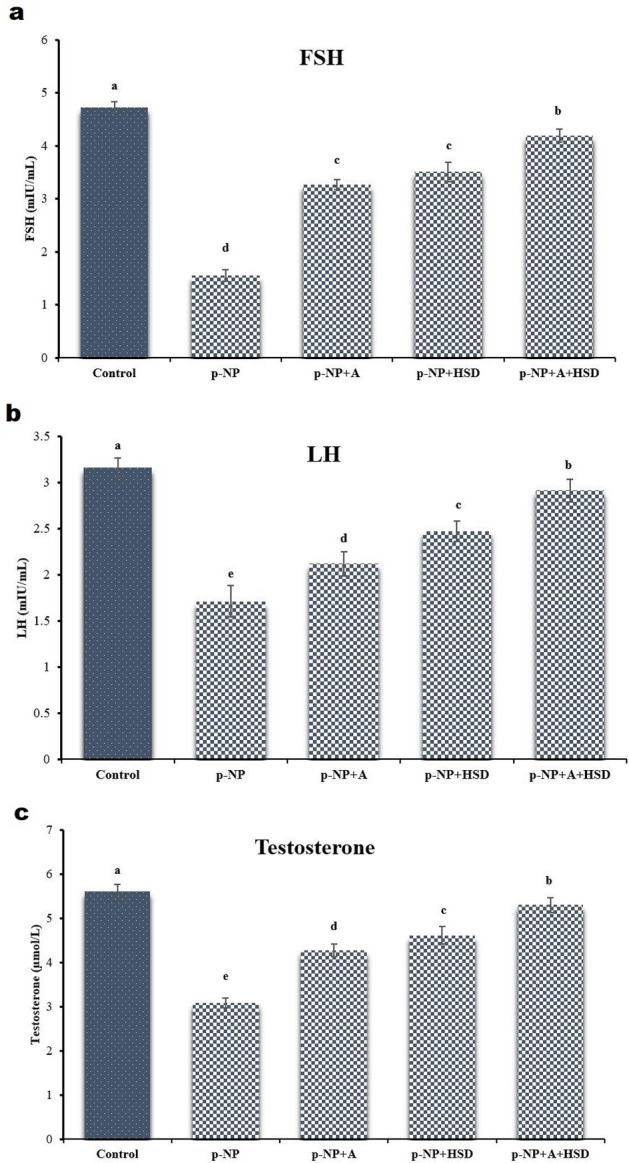
The hormonal assays of the (a) Follicle-stimulating hormone (FSH), (b) Luteinizing hormone (LH), and (c) testosterone for the treated groups and control. p-NP: para-nonylphenol; A: Allicin; HSD: Hesperidin. Different superscripts demonstrate significant differences (p ≤ 0.05; Mean ± SD).
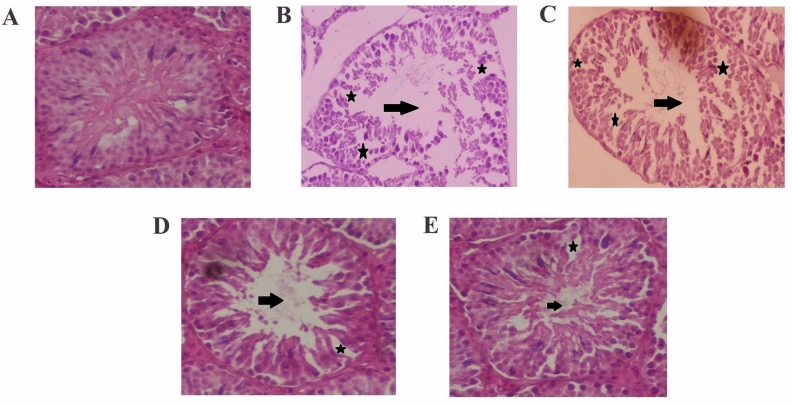
Histopathological analysis of the testes in the p-NP group revealed significant structural damage, including seminiferous tubule atrophy, intraepithelial vacuolization, and pronounced hypocellularity (Fig. 2). Tubular connective tissue exhibited lesions such as tears, empty spaces, blocked blood vessels, inflammatory infiltrations, fluid accumulation, and expanded interstitial spaces. Degeneration of Leydig cells with pyknotic nuclei and detachment of Sertoli cells from germ cells, alongside irregular, amorphous, and smaller nuclei, were also observed. Johnsen’s scoring confirmed germ cell degeneration, disorganization, and desquamation in the p-NP group (Table 3). These changes were significantly more severe compared to other groups (p ≤ 0.05; Table 3). Degeneration of germ cells during spermatogenesis in the p-NP group led to reduced SCI, RI, and STsD, adversely affecting the MI (p ≤ 0.05; Table 3). Additionally, a decrease in the SI and TDI was observed in the p-NP group, indicating impaired spermatogenic progression. Testicular histology parameters, including biopsy scores, spermiogenesis index (SPI), Leydig cell nuclear density (LCND), and TDI, were lower in the p-NP group but showed significant improvement with A, HSD, or combined A + HSD treatments (p ≤ 0.05; Table 3).
Fig. 2.
Testicular histo-architecture in different experimental groups. (A) control; (B) para-nonylphenol; (C) para-nonylphenol + Allicin; (D) para-nonylphenol + Hesperidin; (E) para-nonylphenol + Allicin + Hesperidin (hematoxylin and eosin staining, 400 ×). The black arrows represent the sperm density in the seminiferous tubule lumen, which was significantly lower in group B compared to the control. Groups C, D, and E showed improvements in sperm density compared to group B. The asterisks indicate disorganization and disturbance in the seminiferous tubules, with group B showing the most severe effects, while groups C, D, and E exhibited lesser degrees of disruption.
Table 3.
Histological parameters and reproductive organ weights in different experimental groups. Values are expressed as mean ± SD.
| Analysis | Groups | ||||
|---|---|---|---|---|---|
| Control | p-NP | A + p-NP | HSD + p-NP | A + HSD + p-NP | |
| length of the right testis (cm) | 0.79 ± 0.03a | 0.65 ± 0.04d | 0.70 ± 0.03c | 0.74 ± 0.04b | 0.77 ± 0.03ab |
| length of the left testis (cm) | 0.80 ± 0.02a | 0.68 ± 0.03d | 0.72 ± 0.04c | 0.76 ± 0.03b | 0.78 ± 0.04ab |
| Diameter of the right testis (cm) | 0.50 ± 0.02a | 0.39 ± 0.03d | 0.43 ± 0.02c | 0.45 ± 0.03bc | 0.48 ± 0.03ab |
| Diameter of the left testis (cm) | 0.50 ± 0.03a | 0.38 ± 0.05d | 0.43 ± 0.04c | 0.46 ± 0.03bc | 0.49 ± 0.04ab |
| 1st-day body weight (g) | 25.20 ± 0.05a | 25.16 ± 0.04a | 25.09 ± 0.04a | 25.27 ± 0.05a | 25.22 ± 0.04a |
| 35-day body weight (g) | 26.54 ± 0.05a | 18.68 ± 0.05c | 21.96 ± 0.06bc | 22.43 ± 0.04b | 24.71 ± 0.04ab |
| Right testis weight (g) | 0.113 ± 0.001a | 0.070 ± 0.002b | 0.096 ± 0.003a | 0.105 ± 0.003a | 0.110 ± 0.004a |
| Left Testis weight (g) | 0.112 ± 0.003a | 0.069 ± 0.002b | 0.094 ± 0.001a | 0.103 ± 0.004a | 0.110 ± 0.002a |
| Epididymis weight (g) | 0.045 ± 0.003a | 0.031 ± 0.002d | 0.035 ± 0.003c | 0.037 ± 0.003c | 0.041 ± 0.004b |
| Testis/body weight (%) | 0.44 ± 0.003a | 0.28 ± 0.002c | 0.37 ± 0.004b | 0.38 ± 0.003b | 0.40 ± 0.004b |
| Johnsen score | 9.46 ± 0.23a | 5.63 ± 0.25d | 7.21 ± 0.24c | 7.45 ± 0.20c | 8.51 ± 0.23b |
| Cosentino score | 1.0 ± 0.02c | 3.41 ± 0.02a | 2.39 ± 0.01b | 2.21 ± 0.03b | 1.25 ± 0.02c |
| Seminiferous tubule diameter (µm) | 53.91 ± 1.31a | 33.52 ± 1.20d | 39.43 ± 1.31c | 42.65 ± 1.29c | 48.24 ± 1.41b |
| Sertoli cell index | 86.78 ± 2.87a | 70.89 ± 2.35d | 74.15 ± 2.93c | 77.34 ± 2.71c | 82.11 ± 2.82b |
| Repopulation index | 72.62 ± 2.81a | 45.31 ± 1.53e | 57.25 ± 2.85d | 61.47 ± 2.77c | 67.45 ± 2.48b |
| Miotic index | 2.74 ± 0.04a | 1.13 ± 0.02c | 1.84 ± 0.03b | 2.02 ± 0.02b | 2.46 ± 0.03a |
| Leydig cell nuclear diameter (µm) | 6.21 ± 0.26d | 8.19 ± 0.21a | 7.23 ± 0.34b | 7.14 ± 0.24b | 6.51 ± 0.27c |
| Tubular differentiation index (%) | 86.09 ± 2.70a | 70.47 ± 2.41d | 76.04 ± 2.64c | 78.19 ± 2.84c | 82.24 ± 2.49b |
| Spermiogenesis index (%) | 83.26 ± 2.26a | 58.89 ± 2.95d | 72.64 ± 2.12c | 76.68 ± 2.43b | 79.41 ± 3.15b |
p-NP, para-nonylphenol; A, allicin; HSD, hesperidin.
a–fDifferent superscripts within the same row demonstrate significant differences (p ≤ 0.05).
In the p-NP group, significant reductions in tissue total antioxidant capacity (TAC), glutathione peroxidase (GPx), and superoxide dismutase (SOD) were noted (p ≤ 0.05). These levels were significantly higher in the A and HSD groups, nearing those in the control group (p ≤ 0.05; Fig. 3). Malondialdehyde (MDA) levels, a marker of oxidative stress, were significantly elevated in the p-NP group compared to the other groups (p ≤ 0.05; Fig. 3). However, administration of A and HSD had a protective effect, significantly reducing MDA levels relative to the p-NP group (p ≤ 0.05). The combination of A and HSD avoided TAC, GPx, SOD, and MDA levels to values comparable to those in the control group (p ≤ 0.05; Fig. 3).
Fig. 3.
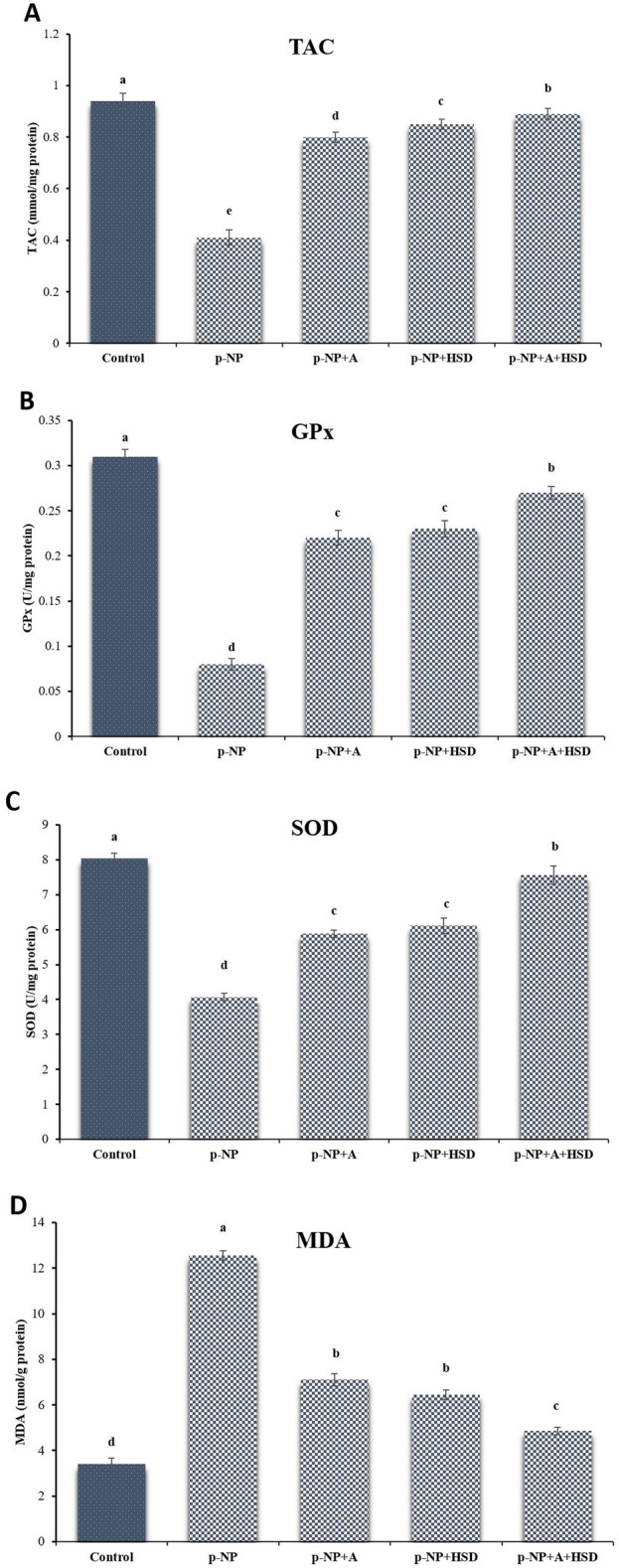
Biochemical findings in different experimental groups. p-NP: para-nonylphenol; A: Allicin; HSD: Hesperidin. (A) Total antioxidant capacity (TAC); (B) glutathione peroxidase (GPx); (C) superoxide dismutase (SOD); (D) Malondialdehyde (MDA). Different superscripts demonstrate significant differences (p ≤ 0.05; Mean ± SD).
No initial differences in body weight were observed between the groups. However, after 35 days, testicular weights in the p-NP group were significantly reduced compared to other groups (p ≤ 0.05). Epididymal weights in the p-NP group were also lower but showed notable improvement following A and HSD treatments (p ≤ 0.05). Additionally, the ratio of testis weight to body weight was significantly improved in the A and HSD groups relative to the control (p ≤ 0.05). Although testis diameter showed no significant differences across groups, the p-NP group had shorter testis lengths than the other groups (p ≤ 0.05; Table 3). The testis weight reduction correlated with a decline in spermatogenesis efficiency, as reflected in Johnsen’s and Cosentino’s scores (Table 3).
Quantitative RT-PCR analysis of mRNA expression for Bax, caspase-3, and Bcl-2 genes revealed elevated levels of caspase-3 mRNA and Bax in the p-NP group, in contrast to other groups (p ≤ 0.05; Fig. 4). In the p-NP group, expression of pro-apoptotic genes Bax and caspase-3 was significantly elevated, while anti-apoptotic Bcl-2 expression was reduced compared to the control group (p ≤ 0.05). Consequently, the Bax/Bcl-2 ratio was significantly higher in the p-NP group (p ≤ 0.05). Treatment with Allicin, Hesperidin, or both led to a significant decrease in Bax and caspase-3 expression and an increase in Bcl-2 expression, resulting in a lower Bax/Bcl-2 ratio compared to the p-NP group (p ≤ 0.05; Fig. 4).
Fig. 4.
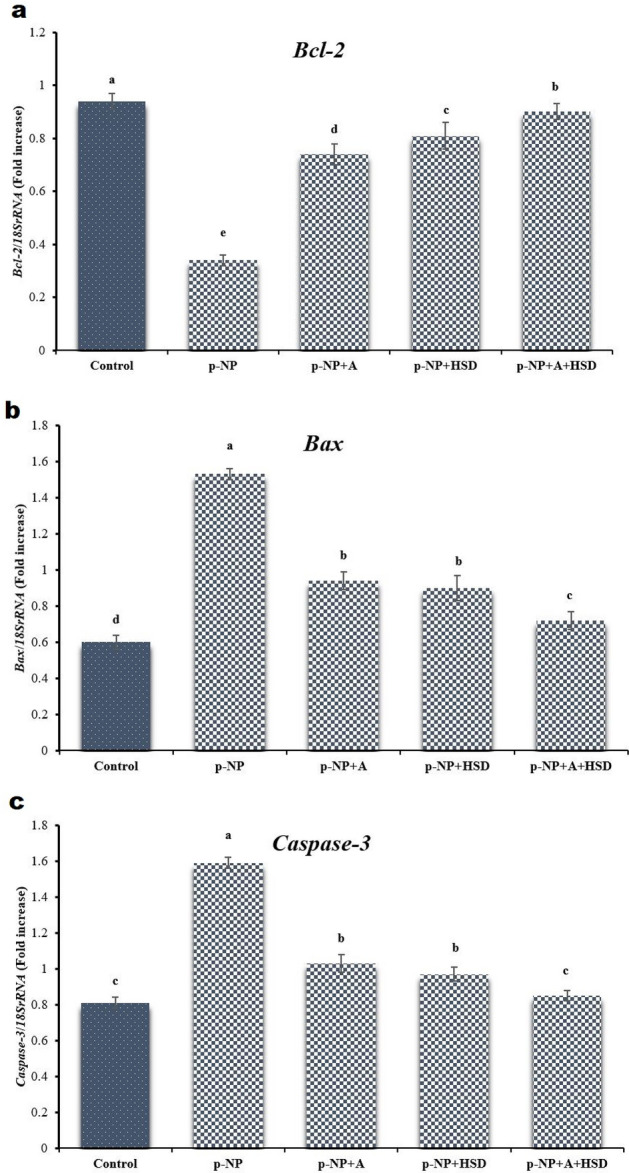
Reverse transcription-polymerase chain reaction findings in different experimental groups. p-NP: para-nonylphenol; A: Allicin; HSD: Hesperidin. The densities of Bcl-2 (a), Bax (b), and Caspase-3 (c) mRNA levels in testicular parenchyma were measured by densitometry and normalized to the 18SrRNA mRNA expression level. Significant differences between groups are indicated by different superscripts (p ≤ 0.05; Mean ± SD).
All animals displayed mating ability, as indicated by positive mating indices with untreated female partners. However, a lower number of males in the p-NP group succeeded in impregnating females (p ≤ 0.05; Table 4). Administration of A, HSD, and A + HSD significantly increased the number of males achieving successful fertilization compared to the p-NP group (p ≤ 0.05; Table 4). Both male fertility and pregnancy indices were significantly lower in the p-NP group (p ≤ 0.05; Table 4), but these parameters were substantially improved in groups receiving A, HSD, and the combined A + HSD treatment relative to the p-NP group (p ≤ 0.05; Table 4). Additionally, the number of pregnant females per group closely matched improvements in male fertility indices, suggesting a direct relationship between sperm quality and reproductive success.
Table 4.
Fertility indexes of adult male rats exposed to heat stress after natural mating with non-exposed females.
| Analysis | Groups | ||||
|---|---|---|---|---|---|
| Control | p-NP | A + p-NP | HSD + p-NP | A + HSD + p-NP | |
| Number of females | 20 | 20 | 20 | 20 | 20 |
| Number of females mated | 20 | 20 | 20 | 20 | 20 |
| Number of males mated | 10 | 10 | 10 | 10 | 10 |
| Number of males impregnating females | 10 | 1 | 4 | 5 | 8 |
| Number of females pregnant | 20 | 1 | 5 | 7 | 15 |
| Female mating index (%) | 100a | 100a | 100a | 100a | 100a |
| Male mating index (%) | 100a | 100a | 100a | 100a | 100a |
| Pregnancy index (%) | 100a | 5d | 25c | 35c | 75b |
| Male fertility index (%) | 100a | 5d | 40c | 50c | 80b |
p-NP, para-nonylphenol; A, allicin; HSD, hesperidin. Female mating index = number of females mated/number of females × 100; Male mating index = number of males mated/number of males × 100; Pregnancy index = number of females pregnant/number of females mated × 100; Male fertility index = number of males impregnating females/number of males mated × 100.
Discussion
Nonylphenol is an environmental toxin known for its harmful effects on various organs, including the testes. Its toxicity primarily arises from its ability to generate reactive oxygen species (ROS), which disrupt the endocrine and reproductive systems25. By interfering with hormonal balance, nonylphenol contributes to structural damage in the testes, negatively affecting sperm production and inducing cell death in the germinal layers26. One of the main mechanisms underlying its detrimental effects is the induction of oxidative stress. Previous research has shown that nonylphenol exposure lowers the activity of critical antioxidant enzymes such as glutathione reductase and superoxide dismutase, leading to oxidative damage in testicular parenchyma27. The resulting oxidative stress promotes free radical formation, which attacks cellular membranes and damages testicular cells. The estrogen-like properties of para-nonylphenol, combined with its oxidative stress-inducing potential, significantly contribute to the observed damage in testicular parenchyma25. Numerous studies have demonstrated the effectiveness of antioxidants in countering the harmful effects of this pollutant28–30. This research assesses how Allicin (A) and Hesperidin (HSD) impact fertility indices, testicular structure, and spermatogenesis in mice exposed to para-nonylphenol. Results show that A and HSD enhance sperm quality, reduce testicular damage and apoptosis, restore oxidant-antioxidant balance, and improve fertility after exposure. Additionally, the Bax/Bcl-2 ratio, linked to apoptosis, decreased significantly with A and HSD, offering protection to testicular cells. These findings align with Azizi and Mehranjani28, which found that antioxidants like green tea extract mitigate p-NP-induced damage, extending this knowledge to Allicin and Hesperidin. Although our study primarily investigates male reproductive parameters, future studies need to ascertain the effect on female reproductive health and offspring outcomes to facilitate clearer appreciation of the benefits afforded by such antioxidants.
Sperm, being rich in polyunsaturated fatty acids, are particularly prone to damage caused by ROS, which can impair membrane integrity and DNA stability. These factors contribute to the toxicity associated with para-nonylphenol exposure31. Previous findings have reported a significant decline in sperm count in animals subjected to para-nonylphenol32. Similar to other environmental toxins, para-nonylphenol disrupts the delicate equilibrium between antioxidants and free radicals, resulting in elevated oxidative stress and altered sperm function1. Furthermore, lipid peroxidation, triggered by oxidative stress, can adversely affect sperm motility32. The decline in motility is linked to a reduction in the mitochondrial density within the sperm midpiece, which is essential for generating movement33. Research also indicates that low doses of para-nonylphenol inhibit ATP synthesis in the mitochondria, which may further impair motility34. In this study, it was observed that exposure to para-nonylphenol reduced sperm motility and led to mitochondrial dysfunction, while A, HSD, or their combination improved motility and prevented damage. The decline in sperm motility and viability observed in the p-NP group can be attributed to oxidative stress-induced damage to sperm membranes and mitochondrial dysfunction. Allicin and Hesperidin, through their antioxidant properties, mitigated these effects by reducing lipid peroxidation and enhancing mitochondrial function, as evidenced by improved sperm motility and viability. The doses of Allicin (30 mg/kg) and Hesperidin (100 mg/kg) were selected based on their proven efficacy in previous studies, offering physiologically relevant antioxidant protection against oxidative stress in rodent models. These results are consistent with the findings of Abdel-Daim et al.35, who reported that Allicin improves mitochondrial function in doxorubicin-treated rats, and Olayinka & Adewole21, who observed that Hesperidin restores sperm motility in finasteride toxicity.
When oxidative damage occurs due to an imbalance favoring free radicals over antioxidants, various biological mechanisms and molecules can be affected. Sperm rely on both enzymatic and non-enzymatic antioxidant defenses to mitigate this damage36. We observed reduced redox enzyme activity in mice exposed to para-nonylphenol. Treatment with A and HSD was found to counteract the para-nonylphenol-induced decrease in enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) within testicular parenchyma. Para-nonylphenol also significantly elevated levels of malondialdehyde (MDA), a marker of lipid peroxidation, in testicular parenchyma, which indicates oxidative damage affecting cellular membranes and leading to cell death37,38. Previous studies also linked toxic exposure to increased lipid peroxidation39–41. In fact, our observations are consistent with previous ones, while treatment with A and HSD reduced MDA levels previously increased by exposure to para-nonylphenol, which showed a protective effect against lipid peroxidation and supported antioxidant defense. Taken together, these findings support the findings of Bisht et al.42, who have attributed oxidative stress to infertility in rodent models and are expanding the use of antioxidants such as A and HSD in clinics.
Spermatogenesis generates significant free radicals, requiring a balance with antioxidants to maintain the testes’ oxidative stability, as they are susceptible to oxidative stress43, and can suffer considerable damage if the antioxidant defense system is compromised. Nonylphenol (NP) has been shown to impair cell connectivity by lowering connexin protein expression, which can lead to cell death and defects in both spermatogenic and Sertoli cells, potentially reducing the epithelial cell layer29. This effect can also impair the blood testis barrier, increasing the risk of tissue swelling44. Additionally, NP exposure can interrupt B-type spermatogonia in the G1 phase of cell division by inhibiting cyclin 1 protein expression, which is essential for mitosis29. In a study by Kim et al.45, exposure to NP at 50 μg/L reduced the seminiferous tubules’ size, thickened the basement membrane, and caused testicular degeneration in mice. Azizi and Mehranjani28 found that 200 mg/kg of para-nonylphenol reduced key testicular parameters for 56 days. However, green tea extract improved these histological parameters. Our findings support these findings and show that A and HSD mitigated the damage caused by para-nonylphenol exposure. It is important to note that the paraffin embedding technique used in this study may have caused tissue retraction, potentially affecting the interpretation of histopathological findings. Future studies should consider using alternative embedding methods, such as HistoResin, to preserve testicular morphology better.
Excess free radicals in the testes can damage germ cells, spermatozoa, somatic cells, and the testicular microenvironment by disrupting DNA, RNA, and protein integrity36. Such damage interferes with the testis’s endocrine functionality, ultimately stalling spermatogenesis and spermiogenesis46. Testosterone plays a pivotal role in spermatogenesis, acting indirectly by fostering interactions between Sertoli cells47. Evidence indicates that exposure to toxic chemicals in rats results in a decline in testosterone synthesis, accompanied by a substantial reduction in circulating sex hormone levels37. The estrogen-like effect of p-NP may inhibit FSH because estrogen negatively controls FSH secretion48. Estrogen regulates inhibin expression49, an important factor that regulates FSH secretion by Sertoli cells50. Therefore, one possibility is that p-NP may interfere with FSH levels by influencing inhibin expression, which is responsible for its role in low sperm production32. p-NP also blocks testosterone production by inhibiting 17α-HSD and disrupting the cAMP pathway in Leydig cells51. Our results in this study show that A and HSD eliminate this inhibition. We suggest that these compounds protect testicular cells from p-NP-induced oxidative stress, thus enhancing interactions between testosterone, LH, FSH, Sertoli cells, and spermatogenesis. Future studies are needed to find out whether A and HSD can reverse damage in chronic exposure scenarios.
Excessive free radicals contribute to various pathologies, inducing cell death and linking oxidative stress to spermatogenic cell apoptosis52. Apoptosis, a form of programmed cell death, occurs under both physiological and pathological conditions and is triggered by specific intracellular or extracellular signals that activate genetic pathways53. Certain chemicals have been identified as inducers of apoptosis via the activation of caspase-354. Nonylphenol promotes oxidative stress by generating reactive oxygen species such as hydrogen peroxide26. This leads to increased Bax activity and the release of cytochrome c from mitochondria, which activates the Apaf1/Caspase-9 complex33,55. Nonylphenol exposure induces apoptosis in germ and Leydig cells, reducing sperm count and production29,32. In this study, p-NP treatment increased caspase-3 and Bax while reducing Bcl-2 levels, suggesting increased apoptosis in testicular parenchyma. Antioxidants A and HSD counteracted these effects by inhibiting P-NP-triggered genes associated with apoptosis. The lower Bax/Bcl-2 ratio underscores the anti-apoptotic effects of A and HSD and thus supports previous findings on citrus flavonoids14.
Infertility is associated with oxidative damage, reduced antioxidant defenses, and increased ROS production56. Reduced testosterone and sperm quality, often with lower testicular weight, was associated with infertility57. P-NP exposure significantly reduced fertility rates in rats58 and mice59, and certain antioxidants were unable to mitigate these effects. Exogenous antioxidants such as vitamins C, A, E, and trace minerals improved oxidative stres60 and embryonic survival rates61. In this study, fertility indices were significantly reduced in the p-NP group, where only 5% of males were able to successfully impregnate females. This drastic decline in fertility can be attributed to observing sperm DNA damage, abnormal morphology, and reduced motility, all essential for successful fertilization. Our results show that daily A and HSD supplementation increased fertility indices in mice, which is due to better membrane stability, better mitochondrial function, and higher levels of antioxidants, which had a positive effect on sperm mobility and viability62. This study confirms that A and HSD improve sperm quality, integrity maintain testicular parenchyma, and improve fertility rates. Treatments with Allicin and Hesperidin improved fertility indices, likely due to their protective effects on sperm quality and testicular health. Additionally, post-testicular events, such as impaired sperm maturation in the epididymis, may have further contributed to the reduced fertility in the p-NP group, which warrants further investigation.
Conclusion
In summary, this study represents the first investigation into the preventive effects of A and HSD against testicular damage caused by p-NP exposure. Both A and HSD effectively prevent the morphological and histological damage induced by p-NP, thereby preserving testicular functionality. Their combined administration improves endocrine balance, boosts antioxidant defenses, enhances fertility parameters, and promotes sperm quality. The reduction in the Bax/Bcl-2 ratio further underscores their anti-apoptotic effects. These findings contribute to the understanding of testicular protection mechanisms and suggest antioxidant-based approaches as potential strategies to mitigate the harmful impact of p-NP on reproductive health. Future studies should explore the long-term effects of these compounds and their possible applications in human reproductive health.
Methods
Ethics statements
This study followed the ethical guidelines provided by the Animal Ethics Committee of the Islamic Azad University, Iran, under approval number IR-IAU-2/24/40.
Chemicals
Essential chemical reagents were also purchased from long-standing and renowned suppliers such as Sigma-Aldrich (St. Louis, Missouri, USA) and Merck (Darmstadt, Germany) to achieve high quality and reliability. Allicin (CAS number: 539-86-6, PubChem CID: 65036), Hesperidin (CAS number: 520-26-3, PubChem CID: 10621), p-nonylphenol (p-NP) (CAS number: 104-40-5, PubChem CID: 1752), and p-nonylphenol (CAS number: 104-40-5, PubChem CID: 1752) were purchased from Sigma-Aldria. The remaining chemicals were of analytical quality and were purchased from Merck.
Animals
Fifty adult male mice, with an average weight of 27.0 ± 2.0 g and aged between 8 and 10 weeks, were obtained from the Animal Resource Center of Urmia University of Medical Sciences in Urmia, Iran. The mice were kept in standard plastic cages within a regulated environment with a 12-h light/dark cycle, temperature maintained at 20–22 °C, and relative humidity of 50 ± 10%. For one week before the experiment, the mice had unrestricted access to standard laboratory chow and tap water.
Experimental protocol
Following one week of acclimatization, mice were distributed randomly into five groups containing n = 10 animals each. Sample size was calculated using G*Power analysis (v3.1.9.7) with parameters of an effect size = 0.8, α = 0.05, and power = 0.95. The doses of p-NP (200 mg/kg)63, Allicin (30 mg/kg)64, and Hesperidin (100 mg/kg)65 were selected based on previous studies demonstrating their efficacy in inducing reproductive toxicity and providing antioxidant protection, respectively. The p-NP dose reflects environmentally relevant exposure levels known to cause testicular damage, while Allicin and Hesperidin doses were chosen based on their established antioxidant effects in rodent models. The duration of 35 days was chosen to cover a significant portion of the spermatogenic cycle in mice, which is approximately 35–40 days, allowing for assessing effects on spermatogenesis52. Groups are specified as follows:
Group 1 (control): Received a daily oral dose of corn oil (0.1 mL/kg) administered by intragastric gavage63.
Group 2 (c group): Administered p-NP orally at a daily dose of 200 mg/kg (dissolved in corn oil) through intragastric gavage63.
Group 3 (A + p-NP): Received p-NP as previously described and an additional daily oral dose of Allicin (A) at 30 mg/kg (dissolved in corn oil) via gavage64.
Group 4 (HSD + p-NP): Treated with p-NP as described, alongside a daily oral dose of Hesperidin (HSD) at 100 mg/kg (dissolved in corn oil) via gavage65.
Group 5 (A + HSD + p-NP): Received the p-NP treatment, along with daily oral doses of both A (30 mg/kg) and HSD (100 mg/kg), all administered via gavage.
After the 35-day experimental period, male mice were mated with untreated female mice to assess reproductive parameters. The male mice were sacrificed on the third day after mating to ascertain the existence of mature sperm in the epididymal cauda. Euthanasia was carried out using an intraperitoneal (IP) injection of a solution containing ketamine (80 mg/kg) and xylazine (10 mg/kg). The two anesthetics were obtained from Alfasan, the Netherlands66, 36.
Fertility indexes
Natural mating tests were carried out with 10 treated male mice (n = 10 per treatment) and 20 untreated female mice BALB/c (n = 20 per treatment; average weight of 20.0–25.0 g and aged between 8 and 12 weeks) in a ratio of 1:2 males to females in separate cages for up to 72 h. Vaginal smears were collected 24, 48, and 72 h after pairing to detect the presence of sperm, which signaled successful mating and was marked as gestational day 0 (GD0). At the end of this period, males were separated from females. Pregnant females at GD17 were anesthetized with a combination of ketamine (10% at 80 mg/kg/IP) and xylazine (2% at 10 mg/kg/IP) from Alfasan, Netherlands, before euthanasia36.
Levels of plasma reproductive hormone
Following euthanasia on day 35, blood was collected via cardiac puncture into plain sample bottles for hormone level analysis. Euthanasia was conducted using an intraperitoneal (IP) injection of ketamine (80 mg/kg) and xylazine (10 mg/kg), sourced from Alfasan, Netherlands36. Serum samples were analyzed for follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T) using a radioimmunoassay technique provided by DIA source. For testosterone extraction, serum was mixed with diethyl ether, vortexed, and the organic phase was collected and evaporated. The dried extract was reconstituted in assay buffer. The antibody used for testosterone showed cross-reactivity of less than 0.01% with other steroids. The intra-assay and inter-assay coefficients of variation for FSH were 3.5% and 5.2%, respectively, and for LH were 4.1% and 6.3%, respectively52.
Sperm collection
At the end of the experiment, all mice were weighed before euthanasia. Euthanasia was performed via intraperitoneal (IP) injection of ketamine (80 mg/kg) and xylazine (10 mg/kg), sourced from Alfasan, Netherlands36. Subsequently, the epididymis and testes were carefully excised by dissection, and their absolute and relative weights were recorded. The sperm was extracted from the epididymis of the cauda by dissecting it into a petri dish containing 1 ml of human tube fluid (HTF). This medium, which contained sperm-carrying tissue, was incubated at 37°C in a 5% CO₂-rich environment for 30 minutes36.
Sperm analysis
Count of sperm
Sperm concentration was assessed with a Brand (Germany) Neubauer hemocytometer after diluting devitalized samples (1:5) with distilled water67,36.
Motility of sperm
Sperm motility, including progressive and total motility and motility characteristics, was evaluated at room temperature using Test Sperm 3.2 (Videotest, Russia) (Table 5). A 10 μL sample was examined with an Olympus BX41 microscope (Japan)52.
Table 5.
Parameter settings for the computer-assisted semen analysis.
| Parameter | Setting |
|---|---|
| Frame rate | 60-Hz |
| Number of frames | 30 |
| Duration of capture | 1 s |
| Stage temperature rate | 37 °C |
| Minimum cell size | 4 pixels |
| Cell size | 13 pixels |
| Minimum contrast | 30 |
| Cell intensity | 75 pixels |
| Chamber type | Slide-Coverslip (22*22 mm) |
| Volume per slide | 7 μL |
| Chamber depth | ≈ 20 μm |
| Minimum number of field analysis | 500 cells |
| Image type | Phased contrast |
| Average path velocity (VAP) | 50.0 μm/s |
| Straightness ( | 50% |
| VAP cutoff | 10.0 μm/s |
| Straight line velocity cutoff | 0.0 μm/s |
| Curvilinear velocity | > 180 μm/s |
| Amplitude of lateral head | > 9.5 μm |
| Mean linearity | < 38% |
Sperm viability and morphology
The morphology and viability of sperm were examined using eosin nigrosin stain in accordance with World Health Organization (WHO) guidelines68.
DNA damage of sperm
Concentrated sperm smears were exposed to a 1:3 methanol and acetic acid solution for two hours, air-dried and then subjected to an acridine orange solution. The stained sperm samples were examined under fluorescence microscopy52.
Plasma membrane functionality (PMF) of sperm
The hypoosmotic swelling test was conducted to evaluate sperm PMF. A 10 μL sperm sample was combined with 100 μL of a hypoosmotic solution containing fructose and sodium citrate, followed by incubation at 37 °C for one hour. PMF was assessed at 400 × with an Olympus BX41, identifying curled or swollen tails52.
Enzymatic antioxidant activity assessment
Left testicle from each mouse was homogenized in 1000 μL of lysis buffer. After centrifuging the homogenates at 9000 rpm for 15 min, the supernatant was collected and used for subsequent biochemical analyses, as per the methods described by Sadeghi Rad et al.36. Total protein concentration was determined using the Bradford method, and all enzymatic activities were normalized to the total protein content. Testicular TAC, GPx, and SOD activities were measured using Naxifer, Nagpix™, and Nasdox kits (Navand Salamat, Iran). MDA levels were assessed using the Nalondi™ kit (Navand Salamat, Iran) at 535 nm and reported as nmol/g protein36.
Testicular histopathology and histomorphometry
Testicular parenchyma was fixed in 10% formalin, dehydrated through graded ethanol, and embedded in paraffin. Sections 7 μm thick were prepared with a microtome and stained using hematoxylin and eosin (H&E). The spermiogenesis index (SPI) was calculated as the ratio of sperm-containing tubules to those without. Other indices, including Sertoli cell index (SCI), meiotic index (MI), tubular differentiation index (TDI), and repopulation index (RI), were analyzed following standard protocols52. Johnsen’s score was applied to assess the quality of seminiferous tubules (Table 6). Testicular injury was graded based on the Cosentino system: Grade 1 (normal), Grade 2 (mild disruption), Grade 3 (moderate disorganization), and Grade 4 (severe damage with necrosis)36.
Table 6.
Johnsen scoring system used for testicular damage evaluation.
| Johnsen score | Description of histological criteria |
|---|---|
| 10 | Full spermatogenesis |
| 9 | Slightly impaired spermatogenesis, many late spermatids, disorganized epithelium |
| 8 | Less than five spermatozoa per tubule, few late spermatids |
| 7 | No spermatozoa, no late spermatids, many early spermatids |
| 6 | No spermatozoa, no late spermatids, few early spermatids |
| 5 | No spermatozoa or spermatids, many spermatocytes |
| 4 | No spermatozoa or spermatids, few spermatocytes |
| 3 | Spermatogonia only |
| 2 | No germinal cells, Sertoli cells only |
| 1 | No seminiferous epithelium |
qRT-PCR
Bax, caspase-3, and Bcl-2 gene expression were analyzed via qRT-PCR using SinaSyber Blue HF-qPCR mix (CinnaGen, Iran) on a StepOne system (Applied Biosystems, USA), with 18SrRNA as the reference (Table 7). The experiment was carried out in biological form for each group. The cycle conditions included 35 cycles at 94 °C, 55 °C, and 72 °C, which were preceded by initial denaturation at 95°C. In order to calculate the relative expression levels of the genes in the samples, expression was determined using a formula provided: The relative expression can be measured using the formula 2−(SΔct-CΔct), where sΔct is obtained by subtracting the cycle threshold (ct) of the reference gene from that of the target gene and CΔct is the ct values from the internal control samples. The values were then transformed logarithmically with base 10 and analyzed to determine the normal distribution of the relative expression values52.
Table 7.
Nucleotide sequences and product size of primers used in reverse transcription-polymerase chain reaction.
| Gene name | Primer | Band size |
|---|---|---|
| Bcl-2 |
Forward: 5-CTCGTCGCTACCGTCGTCACT TCG-3 Reverse: 5-CAGATGCCGGTTCAGGTACTCAGTC-3 |
242 bp |
| Bax | Forward: 5-ACC AGC TCTGAACAGATC ATG-3 | 424 bp |
| Forward: 5-TGG TCT TGGATCCAGACAAG-3 | ||
| caspase-3 | Forward: 5-GTACAGAGCTGGACTGCGGTATTG-3 | 84 bp |
| Reverse: 5-AGTCGGCCTCCACTGGTATCTTC-3 | ||
| 18SrRNA |
Forward: 5-GCAATTATTCCCCATGAACG-3 Reverse: 5-GGCCTCACTAAACCATCCAA-3 |
123 bp |
Statistical analysis
The data analysis was carried out using SPSS (version 26.0; IBM, USA). The normality of the data was verified by the Shapiro–Wilk test, while the Levene test was used to ensure the homogeneity of the variance. A one-way ANOVA was performed for comparison between groups, followed by Tukey’s post-hoc analysis. Nonparametric data were compared using the Kruskal–Wallis test and then with Dunn’s post-hoc analysis. Statistical significance was set at p ≤ 0.05.
Acknowledgements
The authors would like to sincerely thank the members of the Faculty of Veterinary Medicine, Islamic Azad University Urmia Branch Research Council, for the approval and support of this research.
Abbreviations
- A
Allicin
- HSD
Hesperidin
- Ca2+
Calcium
- TM
Total motility
- PM
Progressive motility
- VCL
Curvilinear velocity
- VSL
Straight-line velocity
- VAP
Average path velocity
- STR
Straightness
- LIN
Linearity
- ALH
Amplitude of lateral head displacement
- BCF
Beat-cross frequency
- PMF
Plasma membrane functionality
- TAC
Total antioxidant capacity
- MDA
Malondialdehyde
- GPx
Glutathione peroxidase
- SOD
Superoxide dismutase
- AO
Acridine orange
- ROS
Reactive oxygen species
- H&E
Hematoxylin and eosin
- CAT
Catalase
- NO
Nitric oxide
Author contributions
Tohid Mohammadi and Golsa Behnejad contributed to the conception, design, data collection, statistical analysis, and manuscript drafting. Golsa Behnejad, Tohid Mohammadi, and Ali Soleimanzadeh contributed to the conception, design, supervision of the study, and drafting of the manuscript. All authors approved the final version for submission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All protocols were approved by the Faculty of Veterinary Medicine’s Committee on the Ethics of Animal Experiments at the Islamic Azad University. Every procedure was carried out by the relevant laws and standards. The study was conducted in compliance with the ARRIVE standards. The owner(s) of the animals gave their informed consent for us to use them in the study.
Competing interests
The authors declare no competing interests.
Declaration of generative AI in scientific writing
While preparing this work the author(s) used ChatGPT to make the text antive. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the publication’s content.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chitra, K., Latchoumycandane, C. & Mathur, P. Effect of nonylphenol on the antioxidant system in epididymal sperm of rats. Arch. Toxicol.76, 545–551 (2002). [DOI] [PubMed] [Google Scholar]
- 2.White, R., Jobling, S., Hoare, S. A., Sumpter, J. P. & Parker, M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology135, 175–182 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Toppari, J. et al. Male reproductive health and environmental xenoestrogens. Environ. Health Perspect.104, 741–803 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe, H. et al. Tissue-specific estrogenic and non-estrogenic effects of a xenoestrogen, nonylphenol. J. Mol. Endocrinol.33, 243–252 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Sikka, S. C. & Wang, R. Endocrine disruptors and estrogenic effects on male reproductive axis. Asian J. Androl.10, 134–145 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Kutlubay, R. et al. Vitamin E protection from testicular damage caused by intraperitoneal aluminium. Int. J. Toxicol.26, 297–306 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Han, X. D. et al. The toxic effects of nonylphenol on the reproductive system of male rats. Reprod. Toxicol.19, 215–221 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Gurel, A., Coskun, O., Armutcu, F., Kanter, M. & Ozen, O. A. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: Biochemical and histological studies. J. Chem. Neuroanat.29, 173–178 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Borlinghaus, J., Albrecht, F., Gruhlke, M. C. H., Nwachukwu, I. D. & Slusarenko, A. J. Allicin: Chemistry and biological properties. Molecules19, 12591–12618 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-katcha, M. I., Soltan, M. A., Sharaf, M. M. & Hasen, A. Growth performance, immune response, blood serum parameters, nutrient digestibility and carcass traits of broiler chicken as affected by dietary supplementation of garlic extract (Allicin). Alex. J. Vet. Sci.49, 50–64 (2016). [Google Scholar]
- 11.Singh, J. et al. Effect of sun dried whole bulb garlic powder on nutrient utilization, blood parameters, duodenum morphology and faecal microbial load in broiler chickens. Indian J. Anim. Sci.87, 195–198 (2017). [Google Scholar]
- 12.Alam, R. T. M. et al. Anti-inflammatory, immunomodulatory, and antioxidant activities of allicin, norfloxacin, or their combination against Pasteurella multocida infection in male New Zealand rabbits. Oxid. Med. Cell Longev.2018, 1780956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Ratel, I. T., Abdel-Khalek, A. E., Gabr, S. A., Hammad, M. E. & El-Morsy, H. I. Influence of allicin administration on reproductive efficiency, immunity and lipid peroxidation of rabbit does under high ambient temperature. J. Anim. Physiol. Anim. Nutr. (Berl.)104, 539–548 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Tejada, S. et al. Potential anti-inflammatory effects of hesperidin from the genus citrus. Curr. Med. Chem.25, 4929–4945 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Selmi, S., Rtibi, K., Grami, D., Sebai, H. & Marzouki, L. Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat. Lipids Health Dis.16, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato, M., Okuno, A., Ishisono, K., Yajima, Y. & Toyoda, A. Dietary hesperidin suppresses lipopolysaccharide-induced inflammation in male mice. Int. J. Tryptophan Res.15, 11786469221128696 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Y. et al. Exploring the potential pharmacological mechanism of hesperidin and glucosyl hesperidin against COVID-19 based on bioinformatics analyses and antiviral assays. Am. J. Chin. Med. (Gard City N Y)50, 351–369 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Nagasako-Akazome, Y. Safety of high and long-term intake of polyphenols. In Polyphenols in Human Health and Disease 747–756 (Elsevier, 2014).
- 19.Garg, A., Garg, S., Zaneveld, L. J. D. & Singla, A. K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res.15, 655–669 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Rabe, E., Agus, G. B. & Roztocil, K. Analysis of the effects of micronized purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: From a prospective randomized trial. Int. Angiol.34, 428–436 (2015). [PubMed] [Google Scholar]
- 21.Olayinka, E. T. & Adewole, K. E. In vivo and in silico evaluation of the ameliorative effect of hesperidin on finasteride-induced testicular oxidative stress in Wistar rats. Toxicol. Mech. Methods31, 81–89 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Aksu, E. H., Kandemir, F. M. & Küçükler, S. Ameliorative effect of hesperidin on streptozotocin-diabetes mellitus-induced testicular DNA damage and sperm quality degradation in Sprague-Dawley rats. J. Food Biochem.45, e13938 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Hassan, E., Kahilo, K., Kamal, T., Hassan, M. & Elgawish, M. S. The protective effect of epigallocatechin-3-gallate on testicular oxidative stress in lead-induced toxicity mediated by Cyp19 gene/estradiol level. Toxicology422, 76–83 (2019). [DOI] [PubMed] [Google Scholar]
- 24.AL-Megrin, W. A. et al. Luteolin protects against testicular injury induced by lead acetate by activating the Nrf2/HO-1 pathway. IUBMB Life72, 1787–1798 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Shalaby, K. A. & Saleh, E. M. Ameliorative effect of honey bee propolis on the nonylphenol induced-reproductive toxicity in male Albino rats. Aus. J. Basic Appl. Sci.5, 918–927 (2011). [Google Scholar]
- 26.McClusky, L. M., De Jager, C. & Bornman, M. S. Stage-related increase in the proportion of apoptotic germ cells and altered frequencies of stages in the spermatogenic cycle following gestational, lactational, and direct exposure of male rats to p-nonylphenol. Toxicol. Sci.95, 249–256 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Chitra, K. C. & Mathur, P. P. Vitamin E prevents nonylphenol-induced oxidative stress in testis of rats (2004). [PubMed]
- 28.Azizi, P. & Mehranjani, M. S. The effect of green tea extract on the sperm parameters and histological changes of testis in rats exposed to para-nonylphenol. Int. J. Reprod. Biomed.17, 717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malmir, M., Faraji, T., Noreini, N. S. & Mehranjani, S. M. Protective antioxidant effects of N-acetylcysteine on testicular tissue and serum testosterone in paranonylphenol-treated mice (a stereological analysis). Reprod. Syst. Sex Disord.7, 2 (2018). [Google Scholar]
- 30.Malmir, M., Soleimani Mehranjani, M., Naderi Noreini, S. & Faraji, T. Protective antioxidant effects of N-acetylcysteine against impairment of spermatogenesis caused by paranonylphenol. Andrologia50, e13114 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Ezejiofor, A. N. & Orisakwe, O. E. The protective effect of Costus afer Ker Gawl aqueous leaf extract on lead-induced reproductive changes in male albino Wistar rats. JBRA Assist. Reprod.23, 215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moumeni, H. R., Soleymani, M. M., Abnousi, M. H. & Mahmoudi, M. Effects of vitamin E on sperm parameters and reproductive hormones in developing rats treated with para-nonylphenol (2009).
- 33.Agarwal, A. Role of oxidative stress in male infertility and antioxidant supplementation. US Kidney Urol. Dis.122, 65 (2005). [Google Scholar]
- 34.Bragadin, M. & Dell’Antone, P. Mitochondrial bioenergetics as affected by cationic detergents. Arch. Environ. Contam. Toxicol.30, 280–284 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Daim, M. M., Kilany, O. E., Khalifa, H. A. & Ahmed, A. A. M. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer Chemother. Pharmacol.80, 745–753 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Sadeghirad, M., Soleimanzadeh, A., Shalizar-Jalali, A. & Behfar, M. Synergistic protective effects of 3, 4-dihydroxyphenylglycol and hydroxytyrosol in male rats against induced heat stress-induced reproduction damage. Food Chem. Toxicol.8, 114818 (2024). [DOI] [PubMed] [Google Scholar]
- 37.Fouad, A. A., Al-Sultan, A. I. & Yacoubi, M. T. Coenzyme Q10 counteracts testicular injury induced by sodium arsenite in rats. Eur. J. Pharmacol.655, 91–98 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Reddy, P. S., Rani, G. P., Sainath, S. B., Meena, R. & Supriya, C. H. Protective effects of N-acetylcysteine against arsenic-induced oxidative stress and reprotoxicity in male mice. J. Trace Elem. Med. Biol.25, 247–253 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Uygur, R. et al. Effects of quercetin and fish n-3 fatty acids on testicular injury induced by ethanol in rats. Andrologia46, 356–369 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Das, J., Ghosh, J., Manna, P., Sinha, M. & Sil, P. C. Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol. Lett.187, 201–210 (2009). [DOI] [PubMed] [Google Scholar]
- 41.García-Chávez, E., Segura, B., Merchant, H., Jiménez, I. & Del Razo, L. M. Functional and morphological effects of repeated sodium arsenite exposure on rat peripheral sensory nerves. J. Neurol. Sci.258, 104–110 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Bisht, S., Faiq, M., Tolahunase, M. & Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol.14, 470–485 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Anbara, H., Shahrooz, R., Razi, M., Malekinejad, H. & Najafi, G. The effect of vitamin C on mice hemolytic anemia induced by phenylhydrazine: An animal model study using histological changes in testis, pre-implantation embryo development, and biochemical changes. Iran J. Basic Med. Sci.21, 668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Retamal, M. A. Connexin and Pannexin hemichannels are regulated by redox potential. Front. Physiol.5, 80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim, Y.-B., Cheon, Y.-P., Choi, D. & Lee, S.-H. Adverse effect of nonylphenol on the reproductive system in F2 male mice: A qualitative change?. Dev. Reprod.23, 255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozyurt, H. et al. Oxidative stress in testicular tissues of rats exposed to cigarette smoke and protective effects of caffeic acid phenethyl ester. Asian J. Androl.8, 189–193 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Walker, W. H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis1, 116–120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’donnell, L., Robertson, K. M., Jones, M. E. & Simpson, E. R. Estrogen and spermatogenesis. Endocr. Rev.22, 289–318 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Charpentier, A. H. et al. Effects of estrogen on global gene expression: identification of novel targets of estrogen action. Cancer Res.60, 5977–5983 (2000). [PubMed] [Google Scholar]
- 50.Burger, H. G. & Robertson, D. M. inhibin in the male—Progress at last. Endocrinology138, 1361–1362 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Gong, Y. & Han, X. D. Nonylphenol-induced oxidative stress and cytotoxicity in testicular Sertoli cells. Reprod. Toxicol.22, 623–630 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Soleimanzadeh, A., Karvani, N., Davoodi, F., Molaie, R. & Raisi, A. Efficacy of silver-doped carbon dots in chemical castration: A rat model study. Sci. Rep.14, 24132 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.İlter, K. U. Ş et al. Deneysel formaldehit zehirlenmesinde omega-3 yağ asitlerinin testislerdeki antiapopitotik etkileri: Immunohistokimyasal bir çalışma. Fırat Tıp Dergisi13, 162–166 (2008). [Google Scholar]
- 54.Wang, H., Zhai, N., Chen, Y., Xu, H. & Huang, K. Cadmium induces Ca2+ mediated, calpain-1/caspase-3-dependent apoptosis in primary cultured rat proximal tubular cells. J. Inorg. Biochem.172, 16–22 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Sayed, A.E.-D.H., Kotb, A. M., Oda, S., Kashiwada, S. & Mitani, H. Protective effect of p53 knockout on 4-nonylphenol-induced nephrotoxicity in medaka (Oryzias latipes). Chemosphere236, 124314 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Casao, A. et al. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod. Biol. Endocrinol.8, 1–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shokoohi, M. et al. Investigating the effects of onion juice on male fertility factors and pregnancy rate after testicular torsion/detorsion by intrauterine insemination method. Int. J. Womens Health Reprod. Sci.6, 499–505 (2018). [Google Scholar]
- 58.De Jager, C., Bornman, M. S., Wandrag, S. & Sharp, V. W. Lethal dose and reproductive parameters of p-nonylphenol in rats. Arch. Androl.46, 183–187 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Kyselova, V., Peknicova, J., Buckiova, D. & Boubelik, M. Effects of p-nonylphenol and resveratrol on body and organ weight and in vivo fertility of outbred CD-1 mice. Reprod. Biol. Endocrinol.1, 1–10 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jan, M. H. et al. Follicular attributes and intra-follicular nitric oxide and ascorbic acid concentrations in cyclic and acyclic buffaloes during summer season. Theriogenol. Insight Int. J. Reprod. Anim.1, 83–88 (2011). [Google Scholar]
- 61.De Rensis, F. & Scaramuzzi, R. J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology60, 1139–1151 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Lu, X. et al. Mitochondria-targeted antioxidant MitoTEMPO improves the post-thaw sperm quality. Cryobiology80, 26–29 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Aly, H. A. A., Domènech, Ò. & Banjar, Z. M. Effect of nonylphenol on male reproduction: Analysis of rat epididymal biochemical markers and antioxidant defense enzymes. Toxicol. Appl. Pharmacol.261, 134–141 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Huang, H. et al. Protective effects of allicin on streptozotocin-induced diabetic nephropathy in rats. J. Sci. Food Agric.97, 1359–1366 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Abu-Khudir, R., Almutairi, H. H., Abd El-Rahman, S. S. & El-Said, K. S. The palliative and antioxidant effects of hesperidin against lead-acetate-induced testicular injury in male Wistar rats. Biomedicines11, 2390 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zolfaghari, S., Soleimanzadeh, A and Baqerkhani, M. The synergistic activity of fisetin on quercetinimproves testicular recover in ischemia-reperfusion injury in rats. Sci. Rep.15(1), 12053 (2025). [DOI] [PMC free article] [PubMed]
- 67.Soleimanzadeh, A., and Saberivand, A. Effect of curcumin on rat sperm morphology after the freeze-thawing process. In Veterinary Research Forum, 4(3), 185 (2013). [PMC free article] [PubMed]
- 68.Organization, W. H. Department of reproductive health and research. WHO Lab. Manual Exam. Process. Hum. Semen5, 21–22 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.