Abstract
Myelofibrosis (MF) is a myeloproliferative neoplasm characterized by marrow fibrosis, splenomegaly, constitutional symptoms and cytopenia with a proinflammatory and profibrotic cytokine phenotype involving the JAK-STAT pathway. Ruxolitinib is a JAK 1/2 inhibitor with proven efficacy on splenomegaly and constitutional symptoms, but it does not reverse fibrosis or the risk of leukemic transformation. While hematopoietic stem cell transplantation remains the only curative approach, it is still associated with a relatively high non-relapse mortality (NRM) rate, partly due to GVHD. The potential role of ruxolitinib or its withdrawal on NRM remains to be elucidated, and inflammatory cytokines might be implicated. In this report, we compared cytokine profiles in patients with myelofibrosis not treated with ruxolitinib (n = 18) or who received ruxolitinib and stopped it at conditioning regimen initiation (n = 53), at three different time points. At baseline, MF patients without ruxolitinib had increased inflammatory cytokine levels (CD25, REG3A, IL18 and ST2) as compared to MF patients on ruxolitinib. On day 0 and week 1 post-transplantation, levels of these cytokines were similar with and without ruxolitinib. On the other hand, cytokine levels at baseline did not predict grades 2–4 acute GVHD or hyperacute GVHD. These findings suggest that baseline cytokine profile in MF patients does not impact the risk of GVHD. Stopping ruxolitinib just before conditioning regimen may not influence GVHD risk more than in MF patients who have not received ruxolitinib. The potential benefit of a later ruxolitinib discontinuation on D0 or after transplantation ruxolitinib requires further investigation.
Electronic supplementary material
The online version of this article (10.1007/s00262-025-04046-8) contains supplementary material, which is available to authorized users.
Keywords: Myelofibrosis, Cytokines, Ruxolitinib, Graft-versus-host disease
Introduction
Myelofibrosis (MF) is a clonal disease with marrow fibrosis, splenomegaly, constitutional symptoms and cytopenia characterized by constitutive activation of the JAK/STAT pathway. Several driver mutations involved in this dysfunction have been identified, the JAK2 V617F mutation being the most frequent, followed by CALR and MPL. MF patients have an increased risk of transformation into acute myeloid leukemia. MF is also characterized by an increased proinflammatory and profibrotic cytokine phenotype [1]. Ruxolitinib, a JAK1/2 inhibitor, demonstrated efficacy in spleen size reduction and constitutional symptoms relief in two phase III trials, and is now approved for this indication [2]. However, even if overall survival (OS) may be improved by JAK inhibitors, the only curative approach to date is allogeneic hematopoietic stem cell transplantation (HSCT). Intermediate-2 and high-risk patients according to the Dynamic International Prognostic Scoring System (DIPSS) have been reported to have higher OS with transplantation, even if transplantation in this disease is associated with a substantial non-relapse mortality (NRM) rate of 15–40% [3–6]. Patients with MF are especially frail and have often comorbidities including liver, cardiac or renal impairment. In a previous SFGM-TC/FIM phase II prospective trial (JAK ALLO ClinicalTrials.gov with identifier NCT01795677), where all patients received ruxolitinib before transplantation, relative high rates of acute GVHD and of NRM were observed [7]. Furthermore, some patients had early complications after transplantation including cardiogenic shock in 4/59 (7%), tumor lysis syndrome (TLS) in 3 and ruxolitinib withdrawal syndrome (RWS) in 3, which were of uncertain origin but occurred in the setting of a systemic inflammatory syndrome. We thus wondered if these side effects could be attributed to ruxolitinib or ruxolitinib withdrawal syndrome or may have increased GVHD risk. After the first adverse events in the protocol, an amendment was written to modify ruxolitinib discontinuation. After this amendment, ruxolitinib was abruptly stopped on the 1st day of conditioning regimen instead of tapering over 15 days before this date, to try to limit a potential RWS during the conditioning regimen and the early phase of the transplantation. It is in this setting that prospective sample collections started to reflect a potential cytokine rebound in patients who stopped ruxolitinib before transplantation. These patients were compared to MF patients who did not receive ruxolitinib before transplantation.
Methods
Patient selection and sample collection
From December 2012 to October 2019, 82 patients undergoing HSCT were included in the study. There were 71 patients with MF and 11 patients with other hematological malignancies (OD, other diseases) that were used as comparators. Among the 71 patients with myelofibrosis, 53 received ruxolitinib before transplantation (MFR) at the dose of 15 mg twice a day and stopped it the 1st day of conditioning regimen, and 18 did not receive ruxolitinib. Forty-six patients were previously included in the JAK ALLO trial [7]. The remaining 36 patients consented to biological and clinical data collection. Cytokine exploration study was approved by ethical committee Paris Île-de-France (2014/43NICB).
Clinical data were collected from clinical medical reports and from a standardized data collection form in patients previously included in the JAK ALLO trial. Data regarding HSCT characteristics and post-transplantation outcomes such as GVHD, early toxicity before day (D) + 100 after HSCT and status on last news were collected. Samples were prospectively collected at three different time points: a) before conditioning regimen (day − 60 to day − 3) and on ruxolitinib for the MFR group (78 samples), referred as baseline period (BP), b) around the day of HSCT (day − 2 to day + 2) called D0 (63 samples) and c) after the transplantation (day + 3 to day + 9) called W1 (60 samples). Serum samples were stored and frozen until analysis.
All patients gave their informed consent for data and serum collection according to national regulations and in accordance with the Declaration of Helsinki. The study was approved by the ethical committee of Paris IV.
Definitions
Hyperacute GVHD was defined as acute GVHD occurring before day + 15 post-transplantation, grades 2–4 acute GVHD and chronic GVHD were defined according to previously published criteria [8–10].
Cytokine assays
Cytokine assays were performed in a central laboratory (Biologie Cellulaire, Saint-Louis Hospital, Paris). A total of 34 cytokines were considered: CCL11, CCL2, CCL4, CD25, CD40, CXCL1, CXCL10, EPO, HGF, IFNa, IL10, IL12p70, IL15, IL17, IL18, IL1b, IL1ra, IL21, IL22, IL27, IL33, IL4, IL5, IL6, IL7, LOXL2, MMP-3, REG3A, ST2, TNFa, TNFRI, TPO, Trappin2 and VEGF. Among these cytokines, only 27 were detectable in sufficient patient number to allow reliable comparisons. Panels previously reported as GVHD biomarkers were also tested [11]. Panel 2 included ST2 and REG3A; panel 3: ST2, REG3A and TNFR1; panel 4: CD25, TNFR1, HGF and IL-8 and panel 6: Trappin2, CD25, TNFR1, HGF, IL-8 and REG3A (Table 1S).
Cytokines were measured by three different multiplex Luminex-based assays (Reference: LXSAHM, from R&D system), and the lecture of the plates was performed on a Bio-Plex MAGPIX Multiplex Reader from Bio-Rad. All tests were performed according to the manufacturer instructions.
Statistical analysis
All analyses were performed using R version 4.3.3 [12]. A test was considered significant for p < 0.05, and multiple testing correction was used to control the false discovery rate (FDR; Benjamini–Hochberg correction). All test results are presented with raw p-values. Patients were classified into three groups: MF with (MFR) or without (MF) treatment by ruxolitinib and other disease (OD). Categorical data, including the quantifiable/not-quantifiable indicator for cytokine assays, were compared between the three groups using exact Fisher’s test for contingency tables. Cytokine levels were analyzed after log-transformation to ensure a distribution close to a Gaussian one (graphical check using normal quantile–quantile plots), and they were compared using two Aspin–Welch tests for the two comparisons of interest: MF vs OD and MFR vs MF.
Among cytokines, only those which were statistically significant after correction for multiple comparisons are reported. GvHD prediction was assessed with logistic regression, including both the cytokine and the group as independent predictors.
Results
Clinical characteristics
Median age in patients with MF was 59 years old for both MF and MFR and 58 years old in OD patients. All but one patient received a reduced intensity conditioning regimen. Most patients were transplanted from an HLA identical donor (Table 2S). Only patients with MF underwent splenectomy before transplantation (25%). Most patients (91.4%) received GVHD prophylaxis with cyclosporine and mycophenolate mofetil. Grades 2–4 acute GVHD was observed in 54.6%, 77.8% and 64.2% of the OD, MF and MFR patients (p-value: 0.4256). Grades 3–4 acute GVHD prevalence was 18.2%, 38.9% and 45.3% in OD, MF and MFR patients (p-value: 0.2865). The prevalence of hyperacute GVHD (occurring before day + 15) was 27.3%, 50.0% and 28.3% in OD, MF and MFR, respectively (p-value: 0.2558). Median follow-up was 592 days. At last follow-up, 64%, 50% and 49% of OD, MF and MFR patients were alive, and 18%, 17% and 11% had relapse or progression after transplantation.
Cytokine profiles by disease
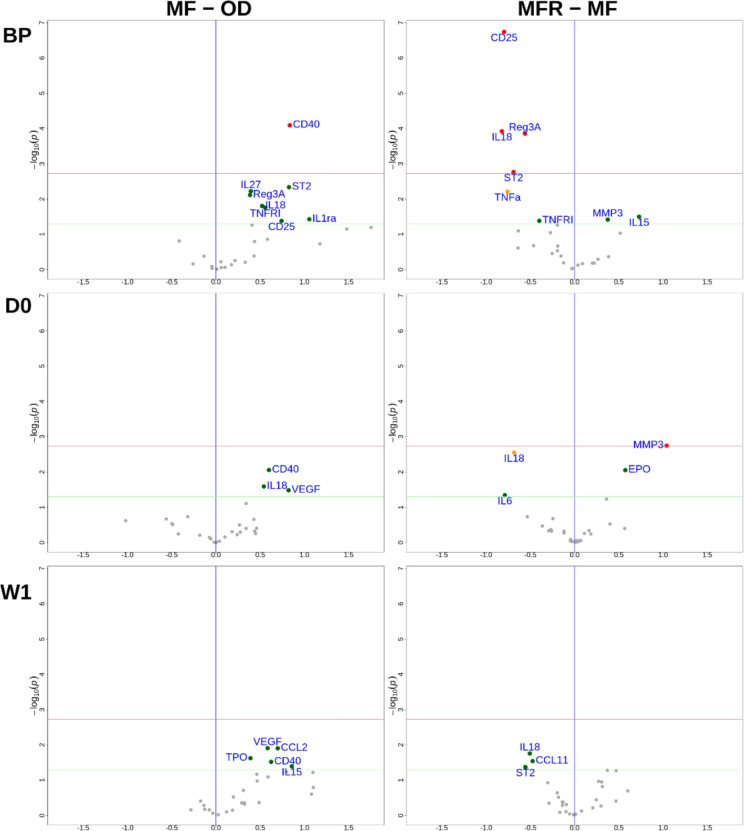
At baseline (BP), 22 of the 27 quantifiable cytokines were increased in MF patients compared to OD patients (p = 0.0015, exact binomial test). Among all increased cytokines, CD40 was the most significantly increased in MF patients (Figs. 1 and 2). When comparing MFR to MF, 18/27 cytokines were decreased in ruxolitinib-treated patients (p = 0.1221) (Fig. 1). Among these reduced cytokines, CD25, IL18, ST2 and REG3A were significantly decreased in MFR patients as compared to MF patients (Figs. 1 and 2).
Fig. 1.
Cytokine expression according to the disease at baseline (BP), day 0 (D0) and after the transplantation (W1). Comparison of mean cytokine level in OD versus MF and in MF versus MFR is shown by volcano plot at different time points. Green points are values with p < 0.05 without multiplicity correction, red points are values with p < 0.05 after multiplicity correction
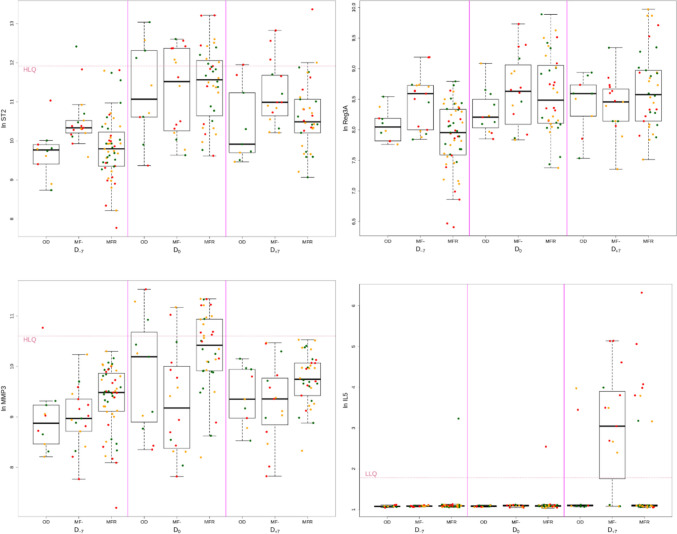
Fig. 2.
Cytokine levels according to the disease at baseline (BP), day 0 (D0) and after the transplantation (W1). Box plot comparing cytokines with significantly different expression levels according to the disease at baseline, day 0 and after the transplantation. Points are red if hyperacute GVHD, orange if acute GVHD and green if no GVHD occur
At transplantation (D0), there were less differences between the three groups (OD, MF and MFR) (Fig. 1). Seventeen of the 27 quantifiable cytokines were increased in MF patients but did not reach significance (p = 0.2478). Levels of CD40, IL18 and VEGF were not significantly higher in MF than OD. MFR patients had significantly lower levels of IL18 than in the MF group. Levels of MMP-3 and EPO were higher in MFR than MF, but only MMP-3 was significant after correction for multiple comparisons (Figs. 1 and 2).
After transplantation (W1), 21/27 cytokines were increased in MF patients as compared to OD (p = 0.0059) (Fig. 1). However, no individual cytokine was significantly increased in MF patients. There was no significant difference in cytokine expression in MFR patients as compared to MF patients, even if IL18, CCL11 and ST2 were under-expressed in MFR, it did not reach significance after multiplicity correction (Figs. 1 and 2).
GVHD biomarker panels by disease
At baseline, MF patients had a higher proportion of high-risk GVHD cytokine panels than OD patients, but that was only significant for panel 6 (88% higher risk versus 40%) (Table 1). On the other hand, MFR patients less frequently had high-risk profiles than MF patients in the same period, which was significant for panel 2 (53% versus 94%) (Table 1). On D0 and W1, high-risk panels were equally distributed in the three groups.
Table 1.
High-risk GVHD panel profiles by disease
| Other disease | Myelofibrosis (no ruxolitinib) | Myelofibrosis-ruxolitinib | |
|---|---|---|---|
|
Baseline Proportion of high-risk panel |
|||
| Panel 2 | 70% | 94.12% | 52.94% |
| p = 0.1282 | p = 0.0028 | ||
| Panel 3 | 0% | 17.65% | 7.84% |
| p = 0.2735 | p = 0.3546 | ||
| Panel 4 | 10% | 23.53% | 5.26% |
| p = 0.6210 | p = 0.1672 | ||
| Panel 6 | 40% | 88.24% | 63.16% |
| p = 0.0248 | p = 0.1279 | ||
|
D0 Proportion of high-risk panel |
|||
| Panel 2 | 90% | 93.75% | 97.22% |
| p = 1.0000 | p = 0.5249 | ||
| Panel 3 | 30% | 43.75% | 47.22% |
| p = 0.6834 | p = 1.000 | ||
| Panel 4 | 36.36% | 42.86% | 31.25% |
| p = 1.0000 | p = 0.7065 | ||
| Panel 6 | 54.55% | 78.57% | 56.25% |
| p = 0.3892 | p = 0.2602 | ||
|
W1 Proportion of high-risk panel |
|||
| Panel 2 | 77.78% | 100% | 88.24% |
| p = 0.1304 | p = 0.2983 | ||
| Panel 3 | 11.11% | 53.33% | 32.35% |
| p = 0.0803 | p = 0.2103 | ||
| Panel 4 | 22.22% | 53.33% | 53.85% |
| p = 0.2099 | p = 1.000 | ||
| Panel 6 | 88.89% | 80% | 84.62% |
| p = 1.0000 | p = 1.0000 | ||
p-value in myelofibrosis column is the p-value of the test comparing MF to other disease, under myelofibrosis-ruxolitinib is the p-value comparing MFR to MF. Italic means significant after multiplicity correction
Cytokine profile and GVHD probability
Based on these results, we sought potential associations between cytokine data and GVHD. At baseline, none of the inflammatory cytokines significantly associated with disease group (CD40, CD25, IL18, ST2 or REG3A) were associated with grades 2–4 acute or hyperacute GVHD risk (Fig. 3a, b). On D0, IL18 was associated with the occurrence of hyperacute GVHD, while no cytokine was associated with grades 2–4 acute GVHD (Fig. 3a, b). On W1, IL5, TNFR1, VEGF, ST2, TNFa, IL10 and IL18 were significantly associated with hyperacute GVHD (Fig. 3a, b), while only IL5 was associated with grades 2–4 acute GVHD.
Fig. 3.
GVHD prediction according to cytokine levels at baseline (BP), day 0 (DO) and after the transplantation (W1). Volcano plot showing cytokines significantly associated with GVHD at BP, DO and W1. a Acute II–IV GVHD and b hyperacute GVHD. Volcano plot showing impact of cytokine evolution associated with GVHD. c Acute II–IV GVHD and b hyperacute GVHD
Thus, we analyzed if a change on cytokine levels between baseline and D0 may influence further acute GVHD, but none of the cytokines were significantly associated with acute or hyperacute GVHD (Fig. 3c, d). Similarly, the cytokine evolution between D0 and W1 was not associated with any GVHD. However, from baseline to W1, an increase in IL5 was associated with acute GVHD while IL5 and CCL2 were associated with hyperacute GVHD (Fig. 3c, d). When we look at Supplementary Fig. 1 for IL5, it appears that this cytokine is not detectable at baseline or at D0 but is detectable at W1 mainly in patients who developed acute GVHD. Cytokine trajectories are available in supplementary data.
GVHD cytokine panels were also analyzed. At baseline, no panel was associated with GVHD (Table 2). On D0, panels were not associated with grades 2–4 acute GVHD or hyperacute GVHD (Table 2). On W1, panels 3, 4 and 6 were associated with grades 2–4 acute GVHD, and the four panels were associated with hyperacute GVHD (Table 2).
Table 2.
Cytokine and GVHD panels predicting acute GVHD
| Biomarkers | Odds ratio [95%CI] | p-value | Odds ratio [95%CI] | p-value |
|---|---|---|---|---|
|
Baseline Prediction of grades 2–4 GVHD |
Baseline Prediction of hyperacute GVHD |
|||
| Panel 2 | 0,6607[0,3339; 1,249] | 0.2038 | 0,841[0,4338; 1,593] | 0.5949 |
| Panel 3 | 0,9651[0,5051; 1,801] | 0.9114 | 0,9724[0,5176; 1,858] | 0.9306 |
| Panel 4 | 1[0.9998; 1] | 0.5781 | 1[0,9999; 1] | 0.2158 |
| Panel 6 | 0,9625[0,5086; 1,783] | 0.9027 | 1,139[0,6407; 2,085] | 0.6581 |
| Biomarkers | Odds ratio [95%CI] | p-value | Odds ratio [95%CI] | p-value |
|---|---|---|---|---|
|
D0 Prediction of grades 2–4 GVHD |
D0 Prediction of hyperacute GVHD |
|||
| Panel 2 | 1,037[0,5637; 1,896] | 0.9056 | 1,11[0,5991; 2,104] | 0.7402 |
| Panel 3 | 1,917[1,01; 3,973] | 0.04629 | 2,281[1,119; 5,211] | 0.02197 |
| Panel 4 | 1[1; 1] | 0.1077 | 1 [1; 1] | 0.04182 |
| Panel 6 | 2,268[1,124; 5,348] | 0.02095 | 2,667[1,223; 6,944] | 0.01203 |
| Biomarkers | Odds ratio [95%CI] | p-value | Odds ratio [95%CI] | p-value |
|---|---|---|---|---|
|
W1 Prediction of grades 2–4 GVHD |
W1 Prediction of hyperacute GVHD |
|||
| Panel 2 | 1,674[0,8325; 3,671] | 0.1521 | 3,996[1,764; 10,8] | 0.0004991 |
| Panel 3 | 3,62[1,522; 10,32]] | 0.0025 | 7,289[2,624; 27,43] | < 0.0001 |
| Panel 4 | 1 [1; 1] | 0.002333 | 1 [1; 1.001] | < 0.0001 |
| Panel 6 | 5,338[1,65; 27,62] | 0.002532 | 18,2[3,211; 271,4] | < 0.0001 |
Bold means significant only before multiplicity correction, and italic means significant after multiplicity correction
Discussion
The current study investigated if MF patients who stopped ruxolitinib the 1st day of conditioning regimen before transplantation were at higher risk of post-transplant cytokine rebound than patients of ruxolitinib and subsequently at higher risk of acute GVHD. First, the prevalence of acute GVHD was higher in patients with MF, but without significant differences regarding pre-transplant ruxolitinib treatment or when comparing with patients with other diseases. However, grades 3–4 acute GVHD in the MF group was twice than that observed in patients with other disease, suggesting a possibly higher risk of severe GVHD in patients with MF. MF patients who received ruxolitinib had less hyperacute GVHD than patients who did not (22% versus 50%), which is not in favor of cytokine rebound and increased risk of GVHD related to ruxolitinib. However, numbers were possibly too low to reach statistical significance for clinical analysis.
Next, we monitored peri-transplantation cytokines to investigate cytokine profile in each group from baseline to the early post-transplantation period. At baseline, only CD40 was significantly over-expressed in MF patients as compared to OD patients, but most cytokines (22/27) were higher in MF patients. MFR patients had a significant decrease in several cytokines (CD25, REG3a, IL18 and ST2) as compared to MF at baseline, but these differences were no longer observed on D0 and W1. Furthermore, at baseline GVHD, high-risk biomarker panels were more frequently high in MF than in MFR reaching significance only for panel 2, while these differences were no more observed on D0 and W1. We did not observe at any time point an increase in cytokine levels or a higher risk for GVHD panel in MFR as compared to MF. These findings are not in favor of an increased risk of GVHD due to ruxolitinib effect or of its withdrawn before transplantation, per se.
At baseline, several cytokines were detected at higher levels in MF patients as compared to controls. Indeed, as reported by others, inflammatory cytokines are over-expressed in MF patients and explain at least in part patient symptoms [13]. Among cytokines which were especially high among MF patients, CD40 was the most significant one. CD40 plays an essential role in antigen presentation as a co-stimulation molecule, favoring immune system activation, as well as endothelial activation [14, 15]. CD40 overexpression may be repressed by ruxolitinib; indeed, we previously reported that CD40 levels in ruxolitinib-treated patients decreased especially in patients who responded to ruxolitinib [7].
In our study, we observed that ruxolitinib decreased multiple inflammatory cytokines and significantly IL18, CD25, ST2 and REG3A expression. This effect was major at baseline and less significant on D0 and W1. It is possible that the conditioning regimen and all conditions related to the transplantation induce some cytokine release rapidly changing the initial profile.
IL18 is a proinflammatory cytokine involved in IFN-gamma production and Th1 responses, and is part of the inflammasome [16]. IL18 is known to be released in excess in MPN patients and contributes to activate the inflammasome [17].
CD25, the IL2 receptor alpha, is a widely recognized biomarker of immune system activation (notably T-cells) and is associated with cytokine storm situations. It has also been recently reported as a risk factor for MPN [18]. In our study, CD25 was decreased in MFR at baseline, suggesting that ruxolitinib represses CD25 expression.
REG3A is produced in the pancreas and small intestine, and its expression is increased during inflammatory processes, endothelial damage, and notably is a known biomarker for GHVD [19]. At baseline, REG3A was strongly repressed by ruxolitinib, which is in line with the immunosuppressive effect of ruxolitinib and its use for GVHD treatment. This effect was transient.
We also tested the distribution of high-risk GVHD biomarker panels in the three groups of patients [20–24]. At baseline, MF patients who did not receive ruxolitinib before transplantation had more frequently a panel 2 high-risk profile than patients who received ruxolitinib. This was no longer observed on D0 and W1, meaning that after conditioning regimen and immunosuppressive therapy, GVHD profile became similar between the three groups. This may suggest that ruxolitinib given before transplantation does not increase the risk of acute GVHD, and it might even decrease the risk of GVHD as suggested in small series [25–27]. Our study was, however, not designed to show such an association.
The other relevant finding was that MMP-3 levels were significantly increased in patients receiving ruxolitinib, especially at day 0. MMP-3 is a matrix metalloproteinase of stromelysins sub-family involved in extracellular matrix degradation (collagen types III, IV and V, fibronectine, elastin, proteoglycans and laminin) and produced by mesenchymal stem cells (MSC) and megakaryocytes [28]. Bone marrow MSC secrete MMP-3 under IL-1β stimulation, then MMP-3 promotes MSC migration by extracellular matrix degradation [29]. In megakaryocytes, MMP-3 displays an intra-nuclear localization and could act as a transcription factor [30]. Decreased plasma levels of MMP-3 were described in primary myelofibrosis versus healthy controls. Moreover, MMP-3 levels were negatively correlated to the degree of bone marrow fibrosis [31]. These data suggest that increased MMP-3 associated with ruxolitinib treatment could display a protective role in bone marrow fibrosis process.
Finally, we also tested inflammatory cytokines and panels which characterized MF for their association with GVHD. At baseline, none of the cytokines were associated with the occurrence of GVHD. That confirms that even if some baseline cytokines and panels were variable among the three groups, they were not involved in further acute GVHD. However, cytokines and GVHD panels measured on D0 and moreover on W1, close to GVHD onset, were associated with acute and hyperacute GVHD. As previously mentioned, on W1, MFR had not specific cytokine profile suggesting that their risk to develop acute GVHD is similar to MF patients.
Our study has several limitations. The first one is the low number of patients which might limit the achievement of definitive conclusions, especially from a clinical point of view, there was no significant difference in GVHD rate among the three groups. There was also some heterogeneity in the population, for instance regarding GVHD prophylaxis which preclude any conclusions regarding specific subgroups. Some cytokines were significant in univariate analysis and did not reach significance after multiplicity correction, which may be due to an insufficient number of patients. However, MFR had a trend to have under-expression of cytokines (except for MMP-3 which has a role in stroma modeling), while GVHD risk was associated with an increase in cytokines, which does not suggest a higher cytokine release augmented by ruxolitinib.
We can recommend ruxolitinib discontinuation on the 1st day of conditioning regimen because it may not increase cytokine release more than in patients who have never received ruxolitinib. However, that would be also useful to analyze cytokine profile in patients who continued ruxolitinib until the transplantation or even later on. Several prospective studies raised the question of post-transplantation ruxolitinib at least 30 days usually at small doses [25, 32–34]. Except for one study where ruxolitinib was associated with post-transplantation cyclophosphamide [33], engraftment and graft function was not impaired, but all these studies included a small number of patients (from 12 to 26 patients). The study conducted by Kröger et al. confirmed that for five patients received ruxolitinib at conditioning regimen start, cytokine levels were decreased on D0, as compared to a control group who did not receive ruxolitinib [25]. One trial is still ongoing and will probably provide more data [34]. There are no data currently available which compare post-transplantation clinical outcome with or without ruxolitinib.
In conclusion, we did not observe a deleterious effect of pre-transplant ruxolitinib treatment in MF patients. We did not observe an early post-transplantation biological inflammatory rebound in patients who stopped ruxolitinib before transplantation. In contrast, we were able to show that the inflammatory cytokine repression induced by ruxolitinib was partially maintained up to 7 days after transplantation for some of them.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge all centers, investigators, data managers, patients and their families for their participation in this study. We thank Romuald Peux for his skillful technical work.
Author contributions
MR designed the study. MR, SV, MHS and NB wrote the manuscript. EC performed the statistical analysis. MR, AC, MTR, PT, HL, JOB, JC, LV, CO and JJK recruited the patients. All authors reviewed and approved the final manuscript.
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ghosh K, Shome DK, Kulkarni B, Ghosh MK, Ghosh K (2023) Fibrosis and bone marrow: understanding causation and pathobiology. J Transl Med 21:703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF et al (2012) A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366:799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cipkar C, Kumar S, Thavorn K, Kekre N (2022) Optimal timing of allogeneic stem cell transplantation for primary myelofibrosis. Transplant Cell Ther 28:189–194 [DOI] [PubMed] [Google Scholar]
- 4.Gagelmann N, Ditschkowski M, Bogdanov R, Bredin S, Robin M, Cassinat B et al (2019) Comprehensive clinical-molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood 133:2233–2242 [DOI] [PubMed] [Google Scholar]
- 5.Gupta V, Kosiorek HE, Mead A, Klisovic RB, Galvin JP, Berenzon D et al (2019) Ruxolitinib therapy followed by reduced-intensity conditioning for hematopoietic cell transplantation for myelofibrosis: myeloproliferative disorders research consortium 114 study. Biol Blood Marrow Transplant 25:256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernández-Boluda J-C, Pereira A, Kröger N, Cornelissen JJ, Finke J, Beelen D et al (2021) Allogeneic hematopoietic cell transplantation in older myelofibrosis patients: a study of the chronic malignancies working party of EBMT and the Spanish Myelofibrosis registry. Am J Hematol 96:1186–1194 [DOI] [PubMed] [Google Scholar]
- 7.Robin M, Porcher R, Orvain C, Bay J-O, Barraco F, Huynh A et al (2021) Ruxolitinib before allogeneic hematopoietic transplantation in patients with myelofibrosis on behalf SFGM-TC and FIM groups. Bone Marrow Transplant 56:1888–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. (1995) 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant, 15: 825–828 [PubMed]
- 9.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E et al (2006) Histopathologic diagnosis of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. pathology working group report. Biol Blood Marrow Transplant 12:31–47 [DOI] [PubMed] [Google Scholar]
- 10.Saliba RM, de Lima M, Giralt S, Andersson B, Khouri IF, Hosing C et al (2007) Hyperacute GVHD: risk factors, outcomes, and clinical implications. Blood 109:2751–2758 [DOI] [PubMed] [Google Scholar]
- 11.Robin M, Porcher R, Michonneau D, Taurines L, de Fontbrune FS, Xhaard A et al (2022) Prospective external validation of biomarkers to predict acute graft-versus-host disease severity. Blood Adv 6:4763–4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 13 Nov 2024
- 13.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A (2011) Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol 29:1356–1363 [DOI] [PubMed] [Google Scholar]
- 14.Tang T, Cheng X, Truong B, Sun L, Yang X, Wang H (2021) Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther 219:107709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piechutta M, Berghoff AS (2019) New emerging targets in cancer immunotherapy: the role of cluster of differentiation 40 (CD40/TNFR5). ESMO Open 4:e000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu J, Wu H (2023) Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol 41:301–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longhitano L, Li Volti G, Giallongo C, Spampinato M, Barbagallo I, Di Rosa M et al (2020) The role of inflammation and inflammasome in myeloproliferative disease. J Clin Med 9:2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong H, Zhang H, Bai J, Li Y, Li L, Zhang L (2024) Associations of the circulating levels of cytokines with the risk of myeloproliferative neoplasms: a bidirectional mendelian-randomization study. BMC Cancer 24:531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrara JLM, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T et al (2011) Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood 118:6702–6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H et al (2015) A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol 2:e21-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinagesh HK, Özbek U, Kapoor U, Ayuk F, Aziz M, Ben-David K et al (2019) The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood Adv 3:4034–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartwell MJ, Özbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P et al (2017) An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight 2:e89798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine JE, Logan BR, Wu J, Alousi AM, Bolaños-Meade J, Ferrara JLM et al (2012) Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a blood and marrow transplant clinical trials network study. Blood 119:3854–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R et al (2009) A biomarker panel for acute graft-versus-host disease. Blood 113:273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kröger N, Abd Kadir SSS, Zabelina T, Badbaran A, Christopeit M, Ayuk F et al (2018) Peritransplantation ruxolitinib prevents acute graft-versus-host disease in patients with myelofibrosis undergoing allogenic stem cell transplantation. Biol Blood Marrow Transplant 24:2152–2156 [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Jin X, Zeng J, Xiong Z, Chen X (2024) The application of JAK inhibitors in the peri-transplantation period of hematopoietic stem cell transplantation for myelofibrosis. Ann Hematol. 10.1007/s00277-024-05703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim U, Petrone GEM, Mascarenhas J, Keyzner A (2020) Peritransplantation use of ruxolitinib in myelofibrosis. Biol Blood Marrow Transplant 26:2177–2180 [DOI] [PubMed] [Google Scholar]
- 28.Wang JC (2005) Importance of plasma matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinase (TIMP) in development of fibrosis in agnogenic myeloid metaplasia. Leuk Lymphoma 46:1261–1268 [DOI] [PubMed] [Google Scholar]
- 29.Chang C-H, Lin Y-L, Tyan Y-S, Chiu Y-H, Liang Y-H, Chen C-P et al (2021) Interleukin-1β-induced matrix metalloproteinase-3 via ERK1/2 pathway to promote mesenchymal stem cell migration. PLoS ONE 16:e0252163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malara A, Ligi D, Di Buduo CA, Mannello F, Balduini A (2018) Sub-cellular localization of metalloproteinases in megakaryocytes. Cells 7:80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JC, Novetsky A, Chen C, Novetsky AD (2002) Plasma matrix metalloproteinase and tissue inhibitor of metalloproteinase in patients with agnogenic myeloid metaplasia or idiopathic primary myelofibrosis. Br J Haematol 119:709–712 [DOI] [PubMed] [Google Scholar]
- 32.Ali H, Tsai N-C, Synold T, Mokhtari S, Tsia W, Palmer J et al (2022) Peritransplantation ruxolitinib administration is safe and effective in patients with myelofibrosis: a pilot open-label study. Blood Adv 6:1444–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morozova EV, Barabanshikova MV, Moiseev IS, Shakirova AI, Barhatov IM, Ushal IE et al (2021) A prospective pilot study of graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and ruxolitinib in patients with myelofibrosis. Acta Haematol 144:158–165 [DOI] [PubMed] [Google Scholar]
- 34.Hobbs G, Kim HT, Bottoms AS, Byrne MT, Schroeder MA, Tamari R et al (2021) A phase II study of ruxolitinib pre-, during- and post-hematopoietic celltransplantation for patients with primary or secondary myelofibrosis. Blood 138:169 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.