Abstract
Introduction
Unique from the other tumor cells, tumorigenic cancer stem cells (CSCs) manifest as a subpopulation of cells within the tumor that exhibit genetic and phenotypic features and signaling processes, which escape traditional anti-oncogenic treatments, thereby triggering metastases and relapses of cancers. Critical to cancer biology is the crosstalk between CSCs and tumor microenvironment (TME), implicating a CSC-based cancer immunotherapy. Cognizant of CSCs’ significant role in cancer pathology and treatment, finding a biological model that recapitulates CSCs and TME may allow a better understanding of tumor onset and progression for testing CSC-based therapies. In this review paper, we examined the CSC and TME characteristics of the human embryonal carcinoma NTERA-2 clonal cell line called NTERA-2 cl.D1 or NT2/D1 cells and discussed their potential utility for research and development of treatments for cancer and central nervous system (CNS) disorders.
Methods
To probe our hypotheses that NT2/D1 cells display CSC and TME properties key to tumor development, which can serve as a screening platform to test cancer and CNS therapeutics, we conducted a literature review over a 10-year period (2014–2024), focusing on PUBMED and Science Direct published articles on cellular models of cancer, with emphasis on milestone research discoveries on NT2/D1 cells relevant to CSCs and TME. We categorized the studies under pre-clinical and clinical investigations in supporting the existence of CSC and TME features in NT2/D1 cells and providing a laboratory-to-clinic translational basis for cancer and CNS therapeutics.
Conclusions
NT2/D1 cells stand as a feasible biological model that recapitulates the crosstalk of CSCs and TME, which may critically contribute to our understanding of cancer and CNS biology and therapeutics. Designing therapeutics against CSCs' distinct self-renewal and differentiation capacities within the TME opens new avenues for treating cancers and CNS disorders.
Graphical Abstract
Keywords: NT2 cells, NT2/D1 cells, Cancer stem cells, Tumor microenvironment, Cancer treatment, Stem cell therapy
Introduction
Cancer is one of the most common causes of premature death, affecting more than 20 million people worldwide [1, 2]. The number of cancer patients is predicted to double over the next 50 years due to population aging [3]. Amidst the search for effective cancer therapy, cancer stem cells (CSCs) have been proposed as a new model of tumor initiation and a potential target for novel treatment. CSCs refer to a subpopulation within the tumor mass that exhibits unique genetic and phenotypic features similar to traditional stem cells. CSCs largely resist conventional chemotherapy, resulting in relapses and metastases [4–6].
Therapeutics targeting CSCs'distinct self-renewal and differentiation capacities expand possibilities for cancer treatment [7, 8]. In the past decades, there has been accumulating evidence demonstrating CSCs'mechanisms [4]. Preclinical studies showing positive results from targeting CSCs’ signaling pathways have pushed forward the clinical translation of CSC-based therapies [9, 10]. Crosstalk between CSCs and the tumor microenvironment (TME), including extracellular matrix and immune cells, is critical to cancer biology [11–13]. Recognizing CSCs’ essential role in cancer development and their pivotal potential for therapeutic development, the establishment of a biological model that recapitulates CSCs and TME will facilitate a better understanding of tumor initiation and progression, while also serving as a screening platform for CSC-based therapies.
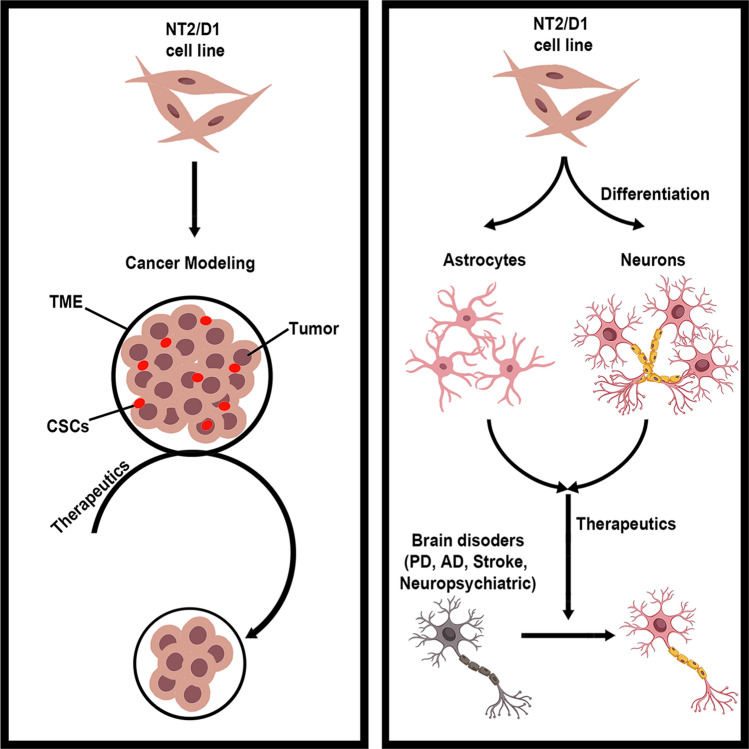
In this review article, we explore the potential of the human embryonal carcinoma NTERA-2 clonal cell line, known as NTERA-2 cl.D1 or NT2/D1 [14, 15], which satisfies both CSC and TME properties. Equally important, NT2/D1 cells have been demonstrated to differentiate into non-cancerous cells under proper post-mitotic treatments [16, 17], thereby providing novel insights and treatment strategies to induce cell cycle exit in CSCs. Indeed, compelling evidence indicates that NT2/D1 cells primed to commit towards the neural lineage can be used biological models as well as cell transplantation source for central nervous system (CNS) disorders [18–23]. This review paper addresses two key questions: (1) Do NT2/D1 cells display CSC and TME properties suitable for modeling tumor development? (2) Can NT2/D1 cells be utilized as a screening platform for cancer therapeutics? Moreover, we also review the application of NT2/D1 cells as biological models and treatment screening platforms for diseases beyond cancer, i.e., CNS disorders to fully elucidate the applications of the NT2/D1 cells (Fig. 1). Our paper’s outline consists of reviewing the biological models of CSCs, introducing NT2/D1 cells as potential platform in investigating the crosstalk between CSCs and TME, and discussing NT2/D1 cells as tool for developing cancer therapeutics. Throughout the paper, our main scope focuses on cancer biology and therapeutics, with the objective of advancing NT2/D1 cells as a potent biological model of CSCs and TME.
Fig. 1.
NT2/D1 cells as a biological model and treatment screening platforms for cancer and beyond. NT2/D1 cells possess the unique properties of CSCs and have been utilized as a treatment screening platform for CSC-based cancer therapy, particularly representing chemotherapy-resistant tumors. NT2/D1 cells are also able to differentiate into neurons and astrocytes under specific induction condition, serving as convenient biological models and drug screening platforms for multiple neuropsychiatric disorders
Biological models of CSCs
The pathogenesis of cancer consists of eight hallmarks: sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastasis, dysregulating cellular metabolism, and avoiding immune destruction [24]. Recently, two additional enabling characteristics, genome instability and tumor-promoting inflammation, have been included in this model [25]. CSCs, first discovered in 1877, are a subpopulation of tumor mass that originate from either differentiated cells or tissue-resident stem cells [26, 27]. CSCs drive tumor initiation as well as relapses, and they are frequently associated with aggressive, heterogeneous, and therapy-resistant tumor variants [28].
Widely recognized as a critical factor in tumorigenesis and progression, TME comprises diverse populations of cancer cells, including CSCs, and stromal cells [25]. Over the past decade, accumulating evidence has postulated mechanisms by which CSCs modify the TME, such as recruitment of macrophages, myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Treg) via chemokine (C–C motif) ligand 2 (CCL2)-CCR2, colony stimulating factor 1 (CSF1)-CSF1R and IDO1 signaling [25, 29]. Other cytokines involved in CSC-TME crosstalk include interleukin (IL)−1β, IL-6, transforming growth factor β (TGFβ), and signal transducer and activator of transcription 3 (STAT3) [29]. A reciprocal interaction exists between CSCs and infiltrating immune cells in the TME, suggesting that these immune cells may serve as specific markers for CSCs [29]. In tandem, CSC-immune cell communication appears as an attractive signaling pathway [29] for cancer biology.
The first CSC model was developed in human myeloid leukemias [30]. In the 1990 s, cancer cell subpopulations were found to demonstrate a functional hierarchy similar to regular stem cells [31, 32]. In acute myeloid leukemia (AML), only a small subpopulation of leukemic cells, characterized as [CD34 +, CD38-] cells and later termed leukemic stem cells (LSCs), can engraft in non-obese diabetic severe combined immune deficiency (NOD/SCID) mice. These LSCs, with surface markers resemble normal multipotent progenitor cells, reproduce the phenotypic heterogeneity of the original leukemia donor once engrafted. However, CSC populations reveal heterogeneity as not all AML subtypes show the same CSC markers. For instance, acute promyelocytic leukemia (APL or AML-M3) predominantly consists of [CD34 +, CD38 +] cells rather than typical [CD34 +, CD38-] LSCs, suggesting that APL may originate from different CSCs [33]. Self-renewal genes in normal stem cells may function differently in CSCs. For instance, knockout of the tumor suppressor phosphatase and tensin homolog (PTEN) gene reduced self-renewal capacity in hematopoietic stem cells (HSCs) but enhanced spontaneous development of acute leukemias [34, 35]. In contrast, the BMI1 oncogene maintains self-renewal capacity in both HSCs and leukemia models [36, 37]. Collectively, these findings suggest that the two hallmarks of cancer, namely sustaining proliferative signaling and aberrant tendency, are independent properties. To this end, probing the cell survival and proliferative genes and transcription factors, such as BM1 in CD34 +, CD38- cells may reveal novel markers and signaling pathways associated with tumor biology [33, 36].
CSCs have also been detected in diverse types of solid tumors, including breast cancer, brain cancer, prostate cancer, lung cancer, melanoma, and multiple myeloma [30]. In human breast cancer, only a small subpopulation, labeled as [CD44 +, CD24 −/low] cells, can sustain tumor growth when transplanted in NOD-SCID mice, replicating the phenotypic diversity of the original tumor [38]. CD133 + cells have been identified as tumorigenic cells in glioblastoma multiforme and medulloblastoma [39–42], while CD44 cells are proposed to be CSCs in prostate cancer [43, 44], and CD138- cells exhibit high engraftment capability in multiple myeloma [45]. Based on these studies, identifying CSCs with CD138- B phenotype may reveal stem cells with the capacity to replicate and eventually become malignant CD138 + tumor cells [45].
Biological models accurately representing CSCs are indispensable to understanding intratumor heterogeneity, metastasis, and treatment-resistant tumors [30]. CSCs establish a new perspective in cancer biology, that the heterogeneous subpopulations of tumor cells may arise from CSCs at various differentiation stages, not only from genetic variation and environmental influences [46, 47]. Targeting pathways mediating CSC function, proliferation, and differentiation may be pivotal for effective tumor eradication. The conventional model of cancer proposes that metastases arise from monoclonal expansions of distinctive tumor subclones, resulting in metastatic tumors with significant differences in genotype and phenotype compared to the primary tumors. On the contrary, the CSC model posits that since primary and metastatic tumors have consistent intratumor heterogeneity and differentiation antigen expression, they should experience a similar differentiation paradigm possibly from CSCs [46, 48, 49]. CSCs have significant implications for cancer treatment design by introducing novel targets that are a small subset of cancer cells with stem cell-like properties [50, 51]. Treatments that fail to effectively eradicate CSCs may be unable to prevent relapse or metastasis. In addition, the CSC model may also explain why some novel cancer therapies, despite promising preclinical results, show limited clinical efficacy, possibly due to unsuccessful CSC targeting [30]. CSC-based therapy may suppress the tumors’ proliferation capacity, resulting in gradual tumor shrinkage, and can be used in combination with conventional treatments that target tumor bulk [30]. Accordingly, CSC-targeting using stem cell markers and signaling pathways may pose as a potent cancer therapeutic.
With CSCs’ pivotal role in cancer biology and their potential as a target for novel treatment development, establishing a biological model that recapitulates CSCs and TME will enable a better understanding of tumor initiation and progression, while also serving as a screening platform for CSC-based therapies. However, despite the promising potential of CSC-based treatments, biological models of CSCs are not yet fully established, warranting further exploration to find suitable platforms.
NTERA-2 clonal D1 (NT2/D1) cells
NT2/D1 cells are a human teratocarcinoma cell line derived from a testicular germ cell tumor [52, 53]. NT2/D1 cells possess the unique property among germ cell tumors that they differentiate exclusively into postmitotic, neuron-like cells after treatment with retinoic acid. Given the ease of differentiation, NT2/D1-derived neurons (NT2/D1-N) have been widely utilized for modeling neurological disorders. Interestingly, since the only differentiated phenotype of NT2/D1 cells is neuronal, undifferentiated NT2/D1 cells have been proposed to represent neuronal progenitor cells [54], providing a valuable tool for the investigation of CNS development.
As NT2/D1 cells are derived from germ cell tumors and exhibit embryonic markers such as nestin and SOX2, they can also be utilized as a biological model of CSCs [55, 56]. NT2/D1 cells are also capable of interacting with TME, an ability essential for CSCs. Examples of crosstalk between NT2/D1 cells and TME include extracellular matrix modification, recruitment of macrophages and fibroblasts, along with enhanced angiogenesis [57–59].
NT2/D1 cells, satisfying properties of both CSCs and TME, are a promising biological model to represent CSCs. Investigating the signaling pathways underlying NT2/D1 cells, particularly self-renewal and differentiation, will provide insights into CSC-based therapy, namely, inducing CSCs to exit the cell cycle through differentiation. Equally important, experiments focusing on the crosstalk between NT2/D1 cells and TME members will elucidate how CSCs interact with the surrounding environments and how to attenuate the modification. This timely field of research may reveal novel therapeutic targets that could reduce the rate of tumor growth and invasion.
NT2/D1 cells as a biological model of CSCs
In the past decade, laboratory investigations have demonstrated multiple signaling mechanisms in the NT2/D1 cells, which may also represent those in CSCs (Table 1). Transcription factors SOX2 and OCT3/4 play critical roles in tumor cell proliferation, migration, and invasion [82, 83]. Both in vitro and in vivo studies have shown elevated expression levels of SOX2 and SOX2 overlapping transcript (SOX2OT) long noncoding RNAs (lncRNAs) in NT2/D1 cells [76, 83]. Another In vitro study further demonstrated that SOX2 influenced NT2/D1 cells’ migration speed, mode, and path, but not cell adhesion [82]. Anterior gradient-2 (AGR2) was also found upregulated in NT2/D1 cells, due to hypomethylation of the AGR2 gene, promoting cell proliferation, invasion, and glycolysis [65]. Knockdown of AGR2 reduced tumor proliferation, invasion, and glycolysis in vitro while lessening tumor growth in vivo. Mechanically, AGR2 binds to annexin A2 (AnXA2), subsequently increasing epidermal growth factor receptor (EGFR) expression. Additionally, the transcription factor homeobox A10 (HOXA10), essential for testis development, exhibited less expression in NT2/D1 cells [84]. Under physiological conditions, HOXA10 inhibits cell proliferation and delays cell cycle progression at the G2/M phase through TP53, c-Kit, STAT3, AKT, and ERK signaling pathways. Reduced HOXA10 expression in NT2/D1 cells may contribute to the self-renewal properties of CSCs. Genome-wide association studies have identified SPRY4 as another potential oncogene in NT2/D1 cells, functioning through activation of the PI3 K/AKT pathway [78]. The proliferation of NT2/D1 cells also relies on hormonal signaling, particularly estrogen. Estradiol (E2) activates estrogen receptor 1/2 (ESR1/ESR2) in NT2/D1 cells, promoting cell proliferation and viability [67]. Human epidermal growth factor receptor 2 (HER2) is endogenously produced by NT2/D1 cells and can suppress the Wnt signaling pathway, one of the crucial differentiation mechanisms of NT2/D1 cells [68]. Moreover, demographic characteristics, such as biological gender, age, microbiome, and metabolic factors, also influence the treatment resistance of CSCs. For instance, the DLK1-MEG3 locus, containing paternally imprinted gene DLK1 and maternally imprinted gene IGF2, has been found to drive tumorigenicity of NT2/D1 cells [80].
Table 1.
NT2/D1 cells as a biological model of CSCs
| Intervention/animal model | Characterization | References |
|---|---|---|
| In vitro | ||
| 17β-estradiol, ERβ-selective agonist (DPN, PPT) | - ↑Migration, invasion and anchorage‑independent growth | [60] |
| Activin A, BMP4 |
- Activin A → ↑pluripotency, TGF-β, Notch, TP53, and Hippo signalling - BMP4 → ↑TGF-β, pluripotency, Hippo and Wnt signalling |
[61] |
| piR-36249, DHX36 | - piR-36249 and DHX36 inhibit the malignant phenotype of testicular cancer cells by ↑OAS2 protein | [62] |
| phorbol 12-myristate 13-acetate (PMA) | - ↑miR-630 → ↓NANOG | [63] |
| Retinoic acid | - Opposite Insulin-like growth factor 1 receptor (IGF-1R) and miR-6165 pattern | [64] |
| No intervention | - ↑Anterior gradient-2 (AGR2) → ↑proliferation, ↑invasion, ↑glycolysis | [65] |
| - SOX9/TRPC3 promotes proliferation and controls cell morphology | [66] | |
| - E2 activate ESR1/ESR2 → ↑number and viability | [67] | |
| - Endogenous HER2-miR1 found in NT2 → ↓Wnt signaling | [68] | |
| - ↓miR-196a-5p | [69] | |
|
- ↓TIG1 and SPINK2 - TIG1 inhibited cell invasion, migration, and epithelial–mesenchymal transition (EMT) through uPA/uPAR signaling pathway - SPINK2 augments TIG1 functions |
[70] | |
|
- Short-term activation of WNT signaling induced a differentiation - WNT signaling is distinct among types of germ cell tumors |
[71] | |
|
- ↑HOTTIP → ↑cell proliferation - HOTTIP binds to miR-128-3p to regulate HOXA13 expression |
[72] | |
|
- ↑Citric acid cycle/mitochondrial oxidative phosphorylation - ↑Sphingolipid biosynthesis |
[73] | |
| - Loss of functions of HOXA10 → ↑proliferation of testicular cancer cells | [74] | |
| - DNA methylation promotes PIWI-LIKE 2 expression | [75] | |
| - ↑SOX2OT expression | [76] | |
| - Oxidative stress increased LRWD1 expression through a Nrf2-dependent mechanism | [77] | |
| - SPRY4 and SPRY4-IT1 may be oncogenes through activation of the PI3 K/Akt pathway | [78] | |
|
- OCT4 A and OCT4B2 have similar expression - ↑OCT4B2 transcription after heat shock |
[79] | |
|
- DLK1–MEG3 locus drives the tumorigenicity - 5-azaD suppresses DLK1 |
[80] | |
| - Activation of PPARβ/δ inhibits RAR/RXR dimerization and tumor proliferation | [81] | |
|
- SOX2 overexpression promote speed, mode and path of cell migration, but not the adhesion ability - ↑Expressions of tumor suppressor protein TP53 and the HDM2 oncogene |
[82] | |
|
- ↑SOX2 expression - Down-regulation of SOX2 promotes apoptosis - Inhibition of OCT3/4 induces differentiation |
[83] | |
| - NF-Y-induced inhibition of cell growth is p53-independent | [56] | |
| In vivo | ||
| Mouse | - Keratin, vimentin, neurofilament proteins and desmin | [15] |
| - ↑SOX2 expression | [83] | |
NT2/D1 cells interact with TME through diverse mechanisms. NT2/D1 cells exhibited increased extracellular matrix metalloproteinase inducer (EMMPRIN) mRNA expression, leading to elevated MMP-2 production in the surrounding fibroblasts and contributing to matrix-mediated tumor progression [57]. NT2/D1 cells also interact with macrophages and cancer-associated fibroblasts (CAFs) to remodel the extracellular matrix and evade immune destruction. CAFs promote tumor cell proliferation, expression of cisplatin resistance factors, and modification of macrophage polarization through effector molecules such as IGFBP1, LGALS3BP, LYVE1, and PTX3 [59]. The CXCR4/CXCR7/CXCL12 axis represents another way of communication between NT2/D1 cells and TME, influencing tumor cell proliferation, migration, metastasis, and resistance to conventional treatment [58]. In addition, increased stromal CD44 expression induced by NT2/D1 cells promotes angiogenesis to support tumor growth and metastasis [85].
NT2/D1 cells serve as a promising biological model of CSCs, satisfying both stem cell properties and TME modification capabilities. However, studies on signaling mechanisms in NT2/D1 cells are limited, with a major focus on neural differentiation rather than cancer pathogenesis. Moreover, nearly all experiments are conducted in vitro, lacking the influence of TME in actual in vivo conditions. Novel CSC-based interventions, such as AGR2 and HOXA10 modification, remain in the preclinical stage. Further investigations to identify potential therapeutic targets based on the CSC model are warranted.
NT2/D1 cells as a screening platform for cancer therapeutics
Over the past decades, numerous chemotherapies and immunotherapies have been developed against increasing cancer prevalence. While these conventional therapeutic strategies can eradicate the majority of tumor mass, they are not effective enough to deplete CSCs, potentially due to the remodeling capacity of CSCs to evade immune destruction [29]. For instance, acquired resistance to receptor tyrosine kinase inhibitors occurs at the epigenetic level and can be reversed with histone deacetylase inhibition [86]. The crosstalk between CSCs and TME also contributes to chemotherapy resistance through paracrine signaling. Moreover, CSCs stimulate macrophages to secrete lactadherin and IL-6, which in turn activate STAT3 signaling [87]. Given these unique mechanisms of chemotherapy resistance in CSCs, using biological models of CSCs to identify therapeutic targets and evaluate treatment efficacy will enable us to sequester cancer more effectively.
Germ cell tumors, the original source of NT2/D1 cells, are primarily treated with chemotherapy, particularly cisplatin. However, some patients develop resistance to cisplatin through multiple mechanisms. NT2/D1 cells are utilized as a treatment screening platform to overcome such resistance (Table 2). Cyclin-dependent kinase 5 (CDK5), nuclear factor erythroid 2-related factor 2 (NRF2), and regulatory factor X1 (RFX1) contribute to cisplatin resistance in NT2/D1 cells. Combination therapies with dinaciclib or palbociclib have been shown to reduce cisplatin resistance along with suppressing tumor cell viability and proliferation in NT2/D1 cells [88, 91]. NRF2 upregulates multidrug resistance (MDR) genes in NT2/D1 cells, while RFX1 downregulates them. RFX1 expression is low in undifferentiated NT2/D1 cells but significantly elevates after differentiation with retinoic acid. Bexarotene, the retinoid X receptor (RXR) agonist that also inhibits the NRF2-ARE signaling pathway, augments RFX1 transcription, resulting in attenuated CSC properties and MDR protein expressions in NT2/D1 cells [89]. CSCs’ chemotherapy resistance is partially attributed to epigenetic modifications. Histone deacetylase inhibitors, belinostat and panobinostat, synergist with cisplatin to trigger cell cycle arrest and apoptosis in NT2/D1 cells [92]. Likewise, the demethylating agent guadecitabine upregulates TP53 target genes and downregulates pluripotency genes, leading to tumor regression and cisplatin sensitization [101]. Notably, cisplatin can induce resistance to itself and paclitaxel in NT2/D1 cells by triggering differentiation, suggesting another mechanism of chemotherapy resistance in CSCs [100].
Table 2.
NT2/D1 cells as a treatment screening platform for CSC-based therapy
| Culture environment/animal model | Intervention | Outcomes | References |
|---|---|---|---|
| In vitro | |||
| No modification | sh-AGR2, sh-AnXA2, and sh-EGFR |
- AGR2 knockdown ↓teratoma growth - AGR2 functions through AnXA2/EGFR signaling |
[65] |
| Dinaciclib, cisplatin |
- Dinaciclib enhances the cisplatin effect - ↑CDK5 expression in the cisplantin-resistant model |
[88] | |
| Regulatory factor X1 (RFX1), Bexarotene (RXR agonist) |
- ↓Drug resistance proteins - ↑Cisplatin sensitivity |
[89] | |
| bis-bibenzyls isolated from liverworts | - Perrottetin E and perrottetin F is cytotoxic against cancer cells | [90] | |
| Cisplatin, palbociclib |
- ↓Cancer cell recovery - Additive effect when combined |
[91] | |
| Belinostat and panobinostat |
- ↑cell cycle arrest, Ki67 index, apoptosis - Effective in cisplatin-resistant cells |
[92] | |
| miR-196a-5p | - miR-196a-5p inhibits NR6 A1/E-cadherin signaling axis → ↓cell proliferation, migration, invasion, and tumor neurogenesis | [69] | |
| SOX2OT siRNA | - ↓SOX2OT | [93] | |
| pTIG1-myc-his vector, pSPINK2-flag vector |
- ↓TIG1 and SPINK2 - TIG1 inhibited cell invasion, migration, and epithelial–mesenchymal transition (EMT) through uPA/uPAR signaling pathway - SPINK2 augments TIG1 functions |
[70] | |
| Benzothiazole-based carbamates and amides | - ↓Cell proliferation, migration and invasiveness | [94] | |
| imsnc761 and DDX6 |
- ↑Cell apoptosis - ↓Proliferation via the p53 pathway - ↓Mitochondrial function |
[95] | |
| lipoplexes, nioplexes, and polyplexes | - Lipid-based vectors have a faster intracellular transportation | [96] | |
| Rana catesbeiana ribonuclease-6 (RC-6) |
- ↑Cytotoxicity, caspase 9/3, and cellular senescence - ↑Neuron-like morphology |
[97] | |
| Quercetin |
- Block Wnt/β-catenin pathway - ↓SOX2, Oct4 and Nanog - ↓Proliferation, adhesion and migration |
[98] | |
| MigR1-eGFP-hPPARβ/δ vector | - Activation of PPARβ/δ inhibits RAR/RXR dimerization and tumor proliferation | [81] | |
| Synthetic lipid A analog (ALA) | - Activate TLR4/IFNγ/NOS II pathway for tumor cell death induction | [99] | |
| Retinoic acid, cisplatin, paclitaxel |
- ↓NANOG and POU5 F1 (Oct3/4) - Tetinoic acid ↑Resistance to both cisplatin and paclitaxel - Cisplatin induces resistance to itself and paclitaxel by triggering a differentiation response |
[100] | |
| Guadecitabine |
- Cisplatin-resistant tumor is sensitive to guadecitabine - The hypersensitivity depends on high levels of DNA methyltransferase 3B - ↑p53 target genes and ↓pluripotency genes |
[101] | |
| Clathrodin | - ↑Apoptosis | [102] | |
| In vivo | |||
| Mouse | sh-AGR2 | - AGR2 knockdown ↓teratoma growth | [65] |
| MigR1-eGFP-hPPARβ/δ vector |
- Activation of PPARβ/δ inhibits RAR/RXR dimerization, tumor proliferation, and migration - ↑Tumor necrosis |
[81] | |
| Synthetic lipid A analog (ALA) | - Activate TLR4/IFNγ/NOS II pathway for tumor cell death induction | [99] | |
| Guadecitabine |
- Cisplatin-resistant tumor is sensitive to guadecitabine - ↑MAGE-A3 and MAGE-A1 expression |
[101] | |
| Zebrafish | Dinaciclib, cisplatin | - Dinaciclib enhances the cisplatin effect | [88] |
Novel therapeutic targets have been proposed based on preclinical experiments on NT2/D1 cells. Knockdown of AGR2 and AnXA2 has been shown to inhibit the EGFR signaling pathway in NT2/D1 cells, interrupting TME modification by CSCs [65]. In addition, TIG1 and SPINK2, downregulated in NT2/D1 cells compared to normal testicular cells, have been demonstrated to inhibit cell invasion, migration, and epithelial-mesenchymal transition (EMT) [70]. Another potential treatment is miR-196a-5p, usually downregulated in NT2/D1 cells, but can inhibit the NR6 A1/E-cadherin pathway to attenuate tumor cell proliferation and migration [69]. Intermediate-sized non-coding RNA 761 (imsnc761) interacts with DEAD-box helicase 6 (DDX6) to trigger apoptosis and suppress cell proliferation in NT2/D1 cells through the TP53 signaling pathway [95]. Other substances with cytotoxic activity against NT2/D1 cells include bis-bibenzyl compounds from liverworts, benzothiazole-based carbamates, RC-6 ribonuclease, quercetin, and synthetic lipid A analogs (ALA) [90, 94, 97–99].
NT2/D1 cells offer a promising treatment screening platform representing chemotherapy-resistant cancer cells, particularly CSCs. However, studies focusing on cancer treatments in NT2/D1 cells remain limited. For instance, some key mediators in the crosstalk between CSCs and TME, such as STAT3, have not been studied in NT2/D1 cells [29]. Comprehensive analysis, such as whole-genome sequencing, may facilitate identifying additional mediators of CSCs’ signaling mechanisms and potential targets for the eradication of CSCs. Furthermore, most experiments with NT2/D1 cells are in vitro, lacking the component of TME interaction which is critical in cancer pathogenesis. In vivo or in vitro studies with cocultured cells representing TME may demonstrate more practical outcomes of CSC-based therapy.
NT2/D1 cells as biological models and treatment screening platforms beyond cancer
With the ease of neuronal differentiation in NT2/D1 cells, NT2/D1 cells and NT2/D1-Ns have been widely utilized as a neurodevelopmental model (Table 3). Retinoic acid is the well-established method for inducing neuronal differentiation in NT2/D1 cells. Diverse mechanisms have been shown to underly neuronal differentiation, involving both genetic and epigenetic levels. The Gαq/phospholipase C-β (PLCβ) signaling pathway triggers neuronal differentiation in NT2/D1 cells, as transfecting PLCβ1 into undifferentiated NT2/D1 cells can induce differentiation without external agents [105]. Activation of PPARα accelerates neuronal differentiation in NT2/D1 cells compared to only retinoic acid treatment, functioning through the ERK and p38 MAPK pathways [126]. In addition, BRCA1-associated ATM activator 1 (BRAT1) interacts with the integrator complex subunit 11 (INTS11) and INTS9 subunits, forming a trimeric complex essential for activating RE1 Silencing Transcription Factor (REST)-responsive genes required for differentiation [104, 109]. Epigenetic modifications play a crucial role in NT2/D1 cell differentiation, particularly histone H3 tail trimming and SMAD3/EZH2-based regulation [103, 124]. Beyond neurodevelopmental studies, NT2/D1 cells are also utilized as biological models for traumatic brain injury (TBI), neurological malignancies, and astrocytic senescence [64, 106, 137, 138].
Table 3.
NT2/D1 cells a biological models of diseases beyond cancer
| Model | Differentiation agents | Characterization | References |
|---|---|---|---|
| In vitro | |||
| Neurodevelopment |
Retinoic acid retinal, retinol, 5-bro- mouracil 2'deoxyribose (BUdR), 5-iodouracil 2'deoxyri- hose (IUdR), hexamethylene bisacetamide (HMBA), di- methylacetamide (DMA), and dimethylsulfoxide (DMSO), CAMP,butyrate, TPA, CDDP, or cytosine arabin- oside |
- Neuronal morphology - ↑HCMV susceptibility - SSEA-3, A2B5, ALPp/Sp2/3, ALPp/Sp2/5, neurofilaments, GFP, desmin |
[16] |
| Retinoic acid |
- ↓EGFR expression - SSEA-3 |
[14] | |
| Retinoic acid |
- Neuronal morphology - ↑Cytokeratin - ↓Neurofilaments, glial filaments, vimentin |
[17] | |
| Retinoic acid | - ↑Chromatin-associated histone H3 tail trimming due to ↑cathepsin B and cathepsin D | [103] | |
| Retinoic acid, shBRAT1 | - BRAT1/INTS11/INTS9 involves in neuronal differentiation | [104] | |
| Retinoic acid, AraC, PLCβ1 shRNAs transfection | - PLCβ1 promote differentiation | [105] | |
| Retinoic acid | - SOX2 and SOX9 overexpression ↑proliferation | [106] | |
| Retinoic acid | - NeuroD, GluR, Tau | [107] | |
| Retinoic acid, CRISPR/Cas9 | - GA-repeat/GPM6B reduce neuronal differentiation | [108] | |
| Retinoic acid | - BRAT1/INTS11/INTS9 complex involves in neuron differentiation | [109] | |
| AraC | - ↓pre-mir-106b and pre-mir-19b | [110] | |
| Flavonoids | - ↑HIF-1α → hypoxia response element (HRE) → ↑erythropoietin | [111] | |
| Retinoic acid | - ↓linc-ROR spliced variants | [112] | |
| Retinoic acid, hypoxia, nitric oxide | - Mitochondrial biogenesis is unchanged in differentiated cells | [113] | |
| Retinoic acid | - ↑DQ582359, ↑DQ596268 piRNAs → ↑MAP2 and TUBB3 | [114] | |
| Retinoic acid, vitreous humor |
- ↑SSEA3, TRA-1–81, OCT4 - ↑Growth rate and S phase |
[115] | |
| Retinoic acid, siRNA (Nup93, Nup188, Nup205, CTCF) | - Nup93 and CTCF modulate HOXA gene locus during differentiation | [116] | |
| Retinoic acid |
- TrkC-miR2 is reversely correlated to NGFR/TrkC in differentiation - ↓TrkC-miR2 down-regulation → ↓neural differentiation |
[117] | |
| Retinoic acid | - ↓OCT4 A and ←→OCT4B5 after differentiation | [118] | |
| Retinoic acid |
- ↑PIWIL4 - PIWIL4 regulates PTN and NLGN3 in glioma suppression - PIWIL4-silenced NT2 culture media ↓proliferation of glioma cells |
[119] | |
| Neuron induction medium, TIAM2S shRNA |
- TIAM2S is produced in NT2/N - TIAM2S-KD reduces serotonin level |
[120] | |
| Retinoic acid, glucocorticoids | - PSORS1 C3 is regulated by glucocorticoids and could modulate OCT4 expression by acting as an enhancer | [121] | |
| Retinoic acid |
- H2 AK119ub1 E3 ligase CUL4B is required for retinoic acid-inducible HOX gene - CUL4B suppresses RORγ activity |
[122] | |
| Retinoic acid, BMP-2, UV irradiation |
- ↑Nucleotide excision repair during differentiation - ↑Xeroderma pigmentosum (XP) complementation |
[123] | |
| Retinoic acid | - Smad3 and EZH2 control epigenetic regulation of differentiation | [124] | |
| Retinoic acid |
- Extended PHD domain (ePHD6) and zinc finger in MLL3/4 specifically recognizes an H4H18-containing histone H4 fragment - ePHD6 of MLL3/4 and histone H4 interact in nucleosomal methylation and MLL4-mediated neuronal differentiation |
[74] | |
| Retinoic acid | - PPM1D maintains the undifferentiation state and suppresses retinoic acid-induced ERK regulation in cell differentiation | [125] | |
| Retinoic acid, Wy14643 |
- PPARα activation → ↑neuronal differentiation - ↑NeuroD, MAP2, and CDK5 - ↓Nestin |
[126] | |
| Retinoic acid, 5-aminoimidazole-4-carboxamide-1-β-D-ribotide (AICAR) |
- ↑MAP2, NRG1, NRP1, NRP2, NEUROG1 and EN1 by retinoic acid - ↑SHH, WNT2 and WNT7B by AICAR |
[127] | |
| Retinoic acid, paraquat |
- ROS decreases stemness-related genes (NANOG, OCT4 and TDGF1) - ROS promotes neural differentiation through MAPK-ERK1/2 pathway |
[128] | |
| Retinoic acid, measles virus | - Neuron-to-neuron spreading of measles virus is dependent on hyperfusogenic F, hemagglutinin, and putative neuronal receptor for measles virus | [129] | |
| Retinoic acid, 1% O2 (hypoxia) | - Hypoxia promotes regression to stem cells with HIF-2α pathway | [130] | |
| Retinoic acid | - hsa-miR-6165 affects the cell cycle progression and increases apoptosis | [131] | |
| Ara-C | - ↑Differentiation efficiency (glutamatergic/cholinergic neurons) | [132] | |
| Retinoic acid | - Differentiation efficiency depends on the cell density at differentiation-initiation and the type of cell culture plastic ware | [133] | |
| Retinoic acid, paraoxon, mipafox |
- Paraoxon disrupts chromatin assembly and nucleosome integrity - Mipafox teratogenicity is unclear |
[134] | |
| Retinoic acid, methylmercury chloride, sodium arsenite, sodium valproate, and methylazoxymethanol |
- ↓Differentiation - ↓Migration |
[135] | |
| Retinoic acid | - SOX2 overexpression ↓MAP2-positive neurons while no difference in the number of GFAP-positive astrocytes | [136] | |
| TBI | Retinoic acid |
- SOX2 regulates proliferation of mature astrocytes - SOX9 overexpression promote migration and glutamate uptake |
[106] |
| Neurological malignancy | Retinoic acid | - Opposite Insulin-like growth factor 1 receptor (IGF-1R) and miR-6165 pattern | [64] |
| Retinoic acid | - PML gene expression changes depending on the stage of differentiation | [137] | |
| Senescence | Retinoic acid, anti-miR-21 | - miR-21 downregulation → Arrest of cell growth and premature cellular senescence | [138] |
Neurons differentiated from NT2/D1 cells are also frequently used for neuropsychiatric drug screening, especially for bipolar disorder, schizophrenia, and Parkinson’s disease (Table 4). Furthermore, both undifferentiated NT2/D1 cells and NT2/D1-Ns are routinely used to evaluate the efficacy and safety of biological substances and drug delivery vehicles in preclinical studies [107, 139–141, 145–149].
Table 4.
NT2/D1 cells as a treatment screening platform for diseases beyond cancer
| Model | Differentiation agent | Intervention | Outcomes | References |
|---|---|---|---|---|
| In vitro | ||||
| Neuroprotection | Retinoic acid | Baicalin |
- ↑Neurite outgrowth - ↑Glycolysis pathway |
[107] |
| No differentiation | Tectorigenin | - ↑Hypoxia-inducible factor (HIF)−1α → ↑EPO mRNA | [139] | |
| Retinoic acid | Nlackberry-digested polyphenols (BDP) and BDP major aglycones (hBDP) | - Neuroprotective effects through mTOR signaling and ASNS/ATF5 genes | [140] | |
| Retinoic acid | Bravo de Esmolfe extraction | - ↓Free radical production | [141] | |
| Insecticide toxicity | Retinoic acid, rotenone, fipronil | Rho-kinase inhibitor Y-27632, n-acetyl cysteine | - ↑cell migration and neuronal differentiation | [142] |
| Glioma | Retinoic acid | PIWIL4 knockdown |
- PIWIL4 regulates PTN and NLGN3 in glioma suppression - PIWIL4-silenced NT2 culture media ↓proliferation of glioma cells |
[119] |
| Radiation-induced neuron injury | Retinoic acid | Radiation | - ↑Apoptosis, senescence and cell cycle arrest | [143] |
| Chemotherapy-induced peripheral neuropathy | Retinoic acid, cisplatin | Sodium azide, amifostine |
- ↑Cell viability - ↓Oxidative stress and apoptosis |
[144] |
| Drug-delivery vehicle | No differentiation | Niosome formulations without and with poloxamer 188 | - ↑Cellular uptake and viability | [145] |
| No differentiation | Niosomes, nioplexes | - Effective drug vehicles with low cytotoxicity | [146] | |
| No differentiation | Niosomes/nioplexes | - Stable transfection efficiency | [147] | |
| No differentiation | superparamagnetic iron oxide (SPION) | - Low cytotoxicity | [148] | |
| No differentiation | PEI-functionalized graphene oxide (PEI-GO) | - Low cytotoxicity with effective siRNA delivery | [149] | |
| Psychiatric disorders | Retinoic acid | Amisulpride, aripiprazole, clozapine, and risperidone |
- ↑Circadian rhythm and vascular endothelial growth factor signaling - ↓Adherens junction and cell cycle pathways |
[150] |
| Retinoic acid | Lithium chloride, valproate, lamotrigine and quetiapine |
- Trimetazidine as a potential drug - ↑ATP production |
[151] | |
| Retinoic acid | Amisulpride, aripiprazole, clozapine, risperidone |
- Mechanisms involve Wnt signaling and action potential regulation - Protein–protein interaction (PPI) networks have clusters of PDCD10, ANK2, and AKT3 |
[152] | |
| Retinoic acid | Amisulpride, aripiprazole, clozapine, lamotrigine, lithium, quetiapine, risperidone, valproate |
- ↓Ribosomal gene expression and protein synthesis - Propose protein synthesis inhibitors as novel agents for neuropsychiatric disorders |
[153] | |
| Retinoic acid | Lamotrigine, lithium, quetiapine, valproate | - GAS6-AS1 and MIR100HG lncRNAs are key drivers in synaptic vesicle cycle, endoplasmic reticulum-related functions and neurodevelopment | [154] | |
| Retinoic acid | Aripiprazole, amisulpride, risperidone, quetiapine, clozapine | - Quetiapine ↑SREBF1 and SREBF2 | [116] | |
| Retinoic acid | Amisulpride, aripiprazole, clozapine, lamotrigine, lithium, quetiapine, risperidone, valproate |
- ↓Hippo pathway - ↓MAPK and NFκB pro-inflammatory signaling |
[155] | |
| Retinoic acid | Lamotrigine, lithium, quetiapine, valproate | - ↓CHDH mitochondrial protein from quetiapine and lamotrigine | [156] | |
| Retinoic acid | Amisulpride, aripiprazole, clozapine, lamotrigine, lithium, quetiapine, risperidone, valproate |
- ↓SOX2 and target genes (CCND1, BMP4, and DKK1) - ↓Neurogenesis |
[157] | |
| Retinoic acid | Lithium, valproate, quetiapine, and lamotrigine |
- Valproate: ↑OXPHOS genes, mitochondrial uncoupling and maximal respiratory capacity - Quetiapine: ↓OXPHOS genes and respiration - Lamotrigine: ↓OXPHOS genes, no effect on respiration - Lithium: ↓citric acid cycle |
[158] | |
| Retinoic acid | Lithium, valproate, lamotrigine and quetiapine |
- ↑Cholesterol biosynthesis pathway when combined medications - ↑Intracellular cholesterol transport genes - ↓Cholesterol efflux gene |
[159] | |
| Retinoic acid | Lithium, valproate, quetiapine and lamotrigine | - Gene expression signature (GES) and connectivity map (Cmap) can be used as tools to repurpose drugs for BD | [160] | |
| Parkinson’s disease | 1-methyl-4-phenylpyridinium (MPP +), retinoic acid | Hexagonal boron nitride nanoparticles (hBNs) |
- ↑Cell viability - ↑Antioxidant capacity - ↓Apoptosis |
[161] |
| Retinoic acid | Polysaccharide alginate capsulation | - Effective dopamine production capability | [162] | |
| Alzheimer's disease, Parkinson's disease, and stroke | No differentiation | Tripeptide H-Gly-Pro-Glu-OH (GPE) | - Low cytotoxicity, oxidative, genotoxicity, and embryotoxicity | [163] |
Future challenges
The extensive application of NT2/D1 cells beyond the field of cancer may yield valuable cross-disciplinary insights. For instance, some antipsychotic medications affect the Wnt signaling pathway, SOX expression, and intercellular protein synthesis, which may be applicable in cancer therapy [152–154, 157]. On the contrary, substances that promote the viability of NT2/D1 cells, such as hexagonal boron nitride nanoparticles, may require careful assessment of the safety profiles in cancer patients [161]. Additional factors, including lactylation index [164] and long non-coding RNAs [165], which affect CSCs and TME will need to be considered when contemplating NT2/D1 cells as a biological model. In the end, establishing cancer models and oncologic treatment should incorporate laboratory-to-clinical translational paradigms with emphasis on safety and efficacy in addressing CSCs and TME [166, 167].
Conclusion
Cancer is one of the most dominant contributors to premature death globally. CSCs pose a major challenge in cancer therapy due to their resistance to conventional chemotherapy, driving metastases and relapses [4–6]. Targeting CSCs opens new therapeutic avenues, particularly focusing on their self-renewal capacities and interactions with TME. NT2/D1 cells can be utilized as a biological model that accurately recapitulates CSCs and the TME, enhancing our understanding of tumor initiation and progression as well as serving as a valuable platform for screening CSC-based therapies. In addition, the ability of NT2 s/D1 cells to differentiate into non-cancerous cells provides novel insights into potential therapeutic strategies targeting CSCs. Furthermore, NT2/D1 cells have been employed as biological models and treatment screening platforms for CNS disorders. However, significant gaps remain in our understanding of CSCs’ signaling mechanisms. A major limitation of this report is it lacks original experimental data and relies solely on a literature review. Clearly, primary research experiments are warranted to probe these CSC signaling pathways, as well as the application of the insights gained from NT2/D1 cells in cancer and beyond, will expand the boundaries of CSC-based therapy for clinical indications.
Acknowledgements
Not applicable.
Abbreviations
- AGR2
Anterior gradient-2
- ALA
Lipid A analogs
- AML
Acute myeloid leukemia
- AnXA2
Annexin A2
- APL
Acute promyelocytic leukemia
- BRAT1
BRCA1-associated ATM activator 1
- CAFs
Cancer-associated fibroblasts
- CCL2
Chemokine (C–C motif) ligand 2
- CCR2
Chemokine (C–C motif) ligand 2 receptor
- CDK5
Cyclin-dependent kinase 5
- CNS
Central nervous system
- CSCs
Cancer stem cells
- CSF1
Colony stimulating factor 1
- CSF1R
Colony stimulating factor 1 receptor
- DDX6
DEAD-box helicase 6
- E2
Estradiol
- EGFR
Epidermal growth factor receptor
- EMMPRIN
Extracellular matrix metalloproteinase inducer
- EMT
Epithelial-mesenchymal transition
- ESR1
Estrogen receptor 1
- ESR2
Estrogen receptor 2
- HER2
Human epidermal growth factor receptor 2
- HOXA10
Homeobox A10
- HSCs
Hematopoietic stem cells
- IL
Interleukin
- Imsnc
Intermediate-sized non-coding RNA
- lncRNAs
Long noncoding RNAs
- LSCs
Leukemic stem cells
- MDR
Multidrug resistance
- MDSCs
Myeloid-derived suppressor cells
- NOD/SCID
Non-obese diabetic severe combined immune deficiency
- NRF2
Nuclear factor erythroid 2-related factor 2
- NT2/D1
NTERA-2 clonal D1 cell line
- PLCβ
Phospholipase C-β
- PTEN
Phosphatase and tensin homolog
- REST
RE1 silencing transcription factor
- RFX1
Regulatory factor X1
- RXR
Retinoid X receptor
- SOX2OT
SOX2 overlapping transcript
- STAT3
Signal transducer and activator of transcription 3
- TBI
Traumatic brain injury
- TGFβ
Transforming growth factor β
- TME
Tumor microenvironment
- Treg
Regulatory T cells
Author contributions
MCB, TR, NP, HW and JYL drafted and finalized the manuscript. HW and JYL provided guidance and supervision. All authors read and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mia C. Borlongan, Thomas Rodriguez and Napasiri Putthanbut contributed equally.
References
- 1.Deo SVS, Sharma J, Kumar S. GLOBOCAN 2020 report on global cancer burden: challenges and opportunities for surgical oncologists. Ann Surg Oncol. 2022;29:6497–500. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 3.Soerjomataram I, Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat Rev Clin Oncol. 2021;18:663–72. [DOI] [PubMed] [Google Scholar]
- 4.Borlongan MC, Wang H. Profiling and targeting cancer stem cell signaling pathways for cancer therapeutics. Front Cell Dev Biol. 2023;11:1125174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francescangeli F, De Angelis ML, Rossi R, Cuccu A, Giuliani A, De Maria R, et al. Dormancy, stemness, and therapy resistance: interconnected players in cancer evolution. Cancer Metastasis Rev. 2023;42(1):197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh AH, Samuel N, Juraschka K, Saleh MH, Taylor MD, Fehlings MG. The biology of ependymomas and emerging novel therapies. Nat Rev Cancer. 2022;22(4):208–22. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosini G, Cordani M, Zarrabi A, Alcon-Rodriguez S, Sainz RM, Velasco G, et al. Transcending frontiers in prostate cancer: the role of oncometabolites on epigenetic regulation, CSCs, and tumor microenvironment to identify new therapeutic strategies. Cell Commun Signal. 2024;22(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquale EB. Eph receptors and ephrins in cancer progression. Nat Rev Cancer. 2024;24(1):5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massagué J, Ganesh K. Metastasis-initiating cells and ecosystems. Cancer Discov. 2021;11(4):971–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razi S, Haghparast A, Chodari Khameneh S, Ebrahimi Sadrabadi A, Aziziyan F, Bakhtiyari M, et al. The role of tumor microenvironment on cancer stem cell fate in solid tumors. Cell Commun Signal. 2023;21(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbasi-Malati Z, Azizi SG, Milani SZ, Serej ZA, Mardi N, Amiri Z, et al. Tumorigenic and tumoricidal properties of exosomes in cancers; a forward look. Cell Commun Signal. 2024;22(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlongan MC, Saha D, Wang H. Tumor microenvironment: a niche for cancer stem cell immunotherapy. Stem Cell Rev Rep. 2024;20(1):3–24. [DOI] [PubMed] [Google Scholar]
- 13.Guha A, Goswami KK, Sultana J, Ganguly N, Choudhury PR, Chakravarti M, et al. Cancer stem cell-immune cell crosstalk in breast tumor microenvironment: a determinant of therapeutic facet. Front Immunol. 2023;14:1245421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlin CR, Andrews PW. Human embryonal carcinoma cells express low levels of functional receptor for epidermal growth factor. Exp Cell Res. 1985;159(1):17–26. [DOI] [PubMed] [Google Scholar]
- 15.Damjanov I, Clark RK, Andrews PW. Cytoskeleton of human embryonal carcinoma cells. Cell Differ. 1984;15(2–4):133–9. [DOI] [PubMed] [Google Scholar]
- 16.Andrews PW, Gönczöl E, Plotkin SA, Dignazio M, Oosterhuis JW. Differentiation of TERA-2 human embryonal carcinoma cells into neurons and HCMV permissive cells. Induction by agents other than retinoic acid. Differentiation. 1986;31(2):119–26. [DOI] [PubMed] [Google Scholar]
- 17.Lee VM, Andrews PW. Differentiation of NTERA-2 clonal human embryonal carcinoma cells into neurons involves the induction of all three neurofilament proteins. J Neurosci. 1986;6(2):514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149(2):310–21. [DOI] [PubMed] [Google Scholar]
- 19.Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, et al. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol. 2008;85(3):318–34. [DOI] [PubMed] [Google Scholar]
- 20.Hara K, Matsukawa N, Yasuhara T, Xu L, Yu G, Maki M, et al. Transplantation of post-mitotic human neuroteratocarcinoma-overexpressing Nurr1 cells provides therapeutic benefits in experimental stroke: in vitro evidence of expedited neuronal differentiation and GDNF secretion. J Neurosci Res. 2007;85(6):1240–51. [DOI] [PubMed] [Google Scholar]
- 21.Kleppner SR, Robinson KA, Trojanowski JQ, Lee VM. Transplanted human neurons derived from a teratocarcinoma cell line (NTera-2) mature, integrate, and survive for over 1 year in the nude mouse brain. J Comp Neurol. 1995;357(4):618–32. [DOI] [PubMed] [Google Scholar]
- 22.Trojanowski JQ, Mantione JR, Lee JH, Seid DP, You T, Inge LJ, et al. Neurons derived from a human teratocarcinoma cell line establish molecular and structural polarity following transplantation into the rodent brain. Exp Neurol. 1993;122(2):283–94. [DOI] [PubMed] [Google Scholar]
- 23.Miyazono M, Lee VM, Trojanowski JQ. Proliferation, cell death, and neuronal differentiation in transplanted human embryonal carcinoma (NTera2) cells depend on the graft site in nude and severe combined immunodeficient mice. Lab Invest. 1995;73(2):273–83. [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. [DOI] [PubMed] [Google Scholar]
- 26.Walcher L, Kistenmacher A-K, Suo H, Kitte R, Dluczek S, Strauß A, et al. Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. 2020;11:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capp J-P. Cancer stem cells: from historical roots to a new perspective. J Oncol. 2019;2019:5189232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer. 2021;21:526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. [DOI] [PubMed] [Google Scholar]
- 31.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. [DOI] [PubMed] [Google Scholar]
- 32.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. [DOI] [PubMed] [Google Scholar]
- 33.Turhan AG, Lemoine FM, Debert C, Bonnet ML, Baillou C, Picard F, et al. Highly purified primitive hematopoietic stem cells are PML-RARA negative and generate nonclonal progenitors in acute promyelocytic leukemia. Blood. 1995;85:2154–61. [PubMed] [Google Scholar]
- 34.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–82. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441(7092):518–22. [DOI] [PubMed] [Google Scholar]
- 36.Park I-K, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–5. [DOI] [PubMed] [Google Scholar]
- 37.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–60. [DOI] [PubMed] [Google Scholar]
- 38.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. [DOI] [PubMed] [Google Scholar]
- 40.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 41.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. [DOI] [PubMed] [Google Scholar]
- 42.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–36. [DOI] [PubMed] [Google Scholar]
- 43.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. [DOI] [PubMed] [Google Scholar]
- 44.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–708. [DOI] [PubMed] [Google Scholar]
- 45.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. [DOI] [PubMed] [Google Scholar]
- 47.Losi L, Baisse B, Bouzourene H, Benhattar J. Evolution of intratumoral genetic heterogeneity during colorectal cancer progression. Carcinogenesis. 2005;26:916–22. [DOI] [PubMed] [Google Scholar]
- 48.Weigelt B, Glas AM, Wessels LFA, Witteveen AT, Peterse JL, van’t Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci U S A. 2003;100:15901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weigelt B, Peterse JL, Van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. [DOI] [PubMed] [Google Scholar]
- 50.Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci U S A. 2002;99:16220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–7. [DOI] [PubMed] [Google Scholar]
- 52.Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–93. [DOI] [PubMed] [Google Scholar]
- 53.Andrews PW. Human teratocarcinomas. Biochim Biophys Acta. 1988;948:17–36. [DOI] [PubMed] [Google Scholar]
- 54.Pleasure SJ, Lee VM. NTera 2 cells: a human cell line which displays characteristics expected of a human committed neuronal progenitor cell. J Neurosci Res. 1993;35:585–602. [DOI] [PubMed] [Google Scholar]
- 55.Guillemain I, Fontès G, Privat A, Chaudieu I. Early programmed cell death in human NT2 cell cultures during differentiation induced by all-trans-retinoic acid: apoptosis in differentiating NT2 cells. J Neurosci Res. 2003;71:38–45. [DOI] [PubMed] [Google Scholar]
- 56.Mojsin M, Topalovic V, Marjanovic Vicentic J, Stevanovic M. Transcription factor NF-Y inhibits cell growth and decreases SOX2 expression in human embryonal carcinoma cell line NT2/D1. Biochemistry (Mosc). 2015;80:202–7. [DOI] [PubMed] [Google Scholar]
- 57.Milia-Argeiti E, Mourah S, Vallée B, Huet E, Karamanos NK, Theocharis AD, et al. EMMPRIN/CD147-encriched membrane vesicles released from malignant human testicular germ cells increase MMP production through tumor-stroma interaction. Biochim Biophys Acta. 2014;1840:2581–8. [DOI] [PubMed] [Google Scholar]
- 58.Wakileh GA, Bierholz P, Kotthoff M, Skowron MA, Bremmer F, Stephan A, et al. Molecular characterization of the CXCR4 / CXCR7 axis in germ cell tumors and its targetability using nanobody-drug-conjugates. Exp Hematol Oncol. 2023;12(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stephan A, Suhrmann JH, Skowron MA, Che Y, Poschmann G, Petzsch P, et al. Molecular and epigenetic ex vivo profiling of testis cancer-associated fibroblasts and their interaction with germ cell tumor cells and macrophages. Matrix Biol. 2024;132:10–23. [DOI] [PubMed] [Google Scholar]
- 60.Macheroni C, Leite GGF, Souza DS, Vicente CM, Lacerda JT, Moraes MN, et al. Activation of estrogen receptor induces differential proteomic responses mainly involving migration, invasion, and tumor development pathways in human testicular embryonal carcinoma NT2/D1 cells. J Steroid Biochem Mol Biol. 2024;237: 106443. [DOI] [PubMed] [Google Scholar]
- 61.Radhakrishnan K, Luu M, Iaria J, Sutherland JM, McLaughlin EA, Zhu HJ, et al. Activin and BMP signalling in human testicular cancer cell lines, and a role for the nucleocytoplasmic transport protein importin-5 in their crosstalk. Cells. 2023;12(7):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, Chen P, Wang X, Wu Y, Xia K, Mu X, et al. piR-36249 and DHX36 together inhibit testicular cancer cells progression by upregulating OAS2. Noncoding RNA Res. 2023;8(2):174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chu WK, Hung LM, Hou CW, Chen JK. MicroRNA 630 represses NANOG expression through transcriptional and post-transcriptional regulation in human embryonal carcinoma cells. Int J Mol Sci. 2021;23(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanisavljevic D, Popovic J, Petrovic I, Davidovic S, Atkinson MJ, Anastasov N, et al. Radiation effects on early phase of NT2/D1 neural differentiation in vitro. Int J Radiat Biol. 2019;95(12):1627–39. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Zhang J. AGR2 facilitates teratoma progression by regulating glycolysis via the AnXA2/EGFR axis. Exp Cell Res. 2024;442(1): 114228. [DOI] [PubMed] [Google Scholar]
- 66.Ming Z, Bagheri-Fam S, Frost ER, Ryan JM, Harley VR. A role for TRPC3 in mammalian testis development. Front Cell Dev Biol. 2024;15(12):1337714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macheroni C, Gameiro Lucas TF, Souza DS, Vicente CM, Pereira GJDS, Junior IDSV, et al. Activation of estrogen receptor ESR1 and ESR2 induces proliferation of the human testicular embryonal carcinoma NT2/D1 cells. Mol Cell Endocrinol. 2022;20(554): 111708. [DOI] [PubMed] [Google Scholar]
- 68.Shabaninejad Z, Mowla SJ, Yousefi F, Soltani BM. A novel miRNA located in the HER2 gene shows an inhibitory effect on Wnt signaling and cell cycle progression. Biomed Res Int. 2022;14(2022):7216758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X, Fan Z, Li Y, Li Z, Zhou Z, Yu X, et al. microRNA-196a-5p inhibits testicular germ cell tumor progression via NR6A1/E-cadherin axis. Cancer Med. 2020;9(23):9107–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shyu RY, Wang CH, Wu CC, Wang LK, Chen ML, Kuo CY, et al. Tazarotene-induced gene 1 (TIG1) interacts with serine protease inhibitor Kazal-Type 2 (SPINK2) to inhibit cellular invasion of testicular carcinoma cells. Biomed Res Int. 2019;25(2019):6171065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atlasi Y, van Dorsten RT, Sacchetti A, Joosten R, Oosterhuis JW, Looijenga LHJ, et al. Ectopic activation of WNT signaling in human embryonal carcinoma cells and its effects in short- and long-term in vitro culture. Sci Rep. 2019;9(1):11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eskandarian Boroujeni M, Aliaghaei A, Maghsoudi N, Gardaneh M. Complementation of dopaminergic signaling by Pitx3-GDNF synergy induces dopamine secretion by multipotent Ntera2 cells. J Cell Biochem. 2020;121(1):200–12. [DOI] [PubMed] [Google Scholar]
- 73.Batool A, Chen SR, Liu YX. Distinct metabolic features of seminoma and embryonal carcinoma revealed by combined transcriptome and metabolome analyses. J Proteome Res. 2019;18(4):1819–26. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Qin S, Chen TY, Lei M, Dhar SS, Ho JC, et al. Structural insights into trans-histone regulation of H3K4 methylation by unique histone H4 binding of MLL3/4. Nat Commun. 2019;10(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giebler M, Greither T, Behre HM. Differential regulation of PIWI-LIKE 2 expression in primordial germ cell tumor cell lines by promoter methylation. Front Genet. 2018;20(9):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farhangian P, Jahandoost S, Mowla SJ, Khalili M. Differential expression of long non-coding RNA SOX2OT in gastric adenocarcinoma. Cancer Biomark. 2018;23(2):221–5. [DOI] [PubMed] [Google Scholar]
- 77.Hung JH, Wee SK, Omar HA, Su CH, Chen HY, Chen PS, et al. Nuclear factor erythroid-2-related factor regulates LRWD1 expression and cellular adaptation to oxidative stress in human embryonal carcinoma cells. Biochimie. 2018;148:99–106. [DOI] [PubMed] [Google Scholar]
- 78.Das MK, Furu K, Evensen HF, Haugen ØP, Haugen TB. Knockdown of SPRY4 and SPRY4-IT1 inhibits cell growth and phosphorylation of Akt in human testicular germ cell tumours. Sci Rep. 2018;8(1):2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poursani EM, Mehravar M, Soltani BM, Mowla SJ. OCT4B2, a novel alternative spliced variant of OCT4, is significantly upregulated under heat-stress condition and downregulated in differentiated cells. Tumour Biol. 2017;39(10):1010428317724280. [DOI] [PubMed] [Google Scholar]
- 80.Sellers ZP, Schneider G, Maj M, Ratajczak MZ. Analysis of the paternally-imprinted DLK1-MEG3 and IGF2-H19 tandem gene loci in NT2 embryonal carcinoma cells identifies DLK1 as a potential therapeutic target. Stem Cell Rev Rep. 2018;14(6):823–36. [DOI] [PubMed] [Google Scholar]
- 81.Yao P-L, Chen LP, Dobrzański TP, Phillips DA, Zhu B, Kang B-H, et al. Inhibition of testicular embryonal carcinoma cell tumorigenicity by peroxisome proliferator-activated receptor-β/δ- and retinoic acid receptor-dependent mechanisms. Oncotarget. 2015;6:36319–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drakulic D, Vicentic JM, Schwirtlich M, Tosic J, Krstic A, Klajn A, et al. The overexpression of SOX2 affects the migration of human teratocarcinoma cell line NT2/D1. An Acad Bras Cienc. 2015;87:389–404. [DOI] [PubMed] [Google Scholar]
- 83.Eini R, Stoop H, Gillis AJM, Biermann K, Dorssers LCJ, Looijenga LHJ. Role of SOX2 in the etiology of embryonal carcinoma, based on analysis of the NCCIT and NT2 cell lines. PLoS ONE. 2014;9: e83585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen R, Li H, Li Y, Fazli L, Gleave M, Nappi L, et al. Loss of nuclear functions of HOXA10 is associated with testicular cancer proliferation. Front Oncol. 2018;7(8):594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Labropoulou VT, Manou D, Ravazoula P, Alzahrani FM, Kalofonos HP, Theocharis AD. Expression of CD44 is associated with aggressiveness in seminomas. Mol Biol Rep. 2024;51:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rossini E, Tamburello M, Abate A, Zini S, Ribaudo G, Gianoncelli A, et al. The cdk inhibitor dinaciclib improves cisplatin response in nonseminomatous testicular cancer: a preclinical study. Cells. 2024;13(5):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Issac J, Raveendran PS, Kunnummal M, Angelin M, Ravindran S, Basu B, et al. RXR agonist, bexarotene, effectively reduces drug resistance via regulation of RFX1 in embryonic carcinoma cells. Biochim Biophys Acta Mol Cell Res. 2023;1870(7): 119510. [DOI] [PubMed] [Google Scholar]
- 90.Ivković I, Novaković M, Veljić M, Mojsin M, Stevanović M, Marin PD, et al. Bis-Bibenzyls from the liverwort pellia endiviifolia and their biological activity. Plants (Basel). 2021;10(6):1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossini E, Bosatta V, Abate A, Fragni M, Salvi V, Basnet RM, et al. Cisplatin cytotoxicity in human testicular germ cell tumor cell lines is enhanced by the CDK4/6 inhibitor Palbociclib. Clin Genitourin Cancer. 2021;19(4):316–24. [DOI] [PubMed] [Google Scholar]
- 92.Lobo J, Guimarães-Teixeira C, Barros-Silva D, Miranda-Gonçalves V, Camilo V, Guimarães R, et al. Efficacy of HDAC inhibitors belinostat and panobinostat against cisplatin-sensitive and cisplatin-resistant testicular germ cell tumors. Cancers (Basel). 2020;12(10):2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sadeghi Z, Dodange F, Maleki P, Zarei M, Taheri M, Raheb J. Evaluating the effect of siRNA on SOX2OT expression in the human neuron-committed teratocarcinoma NT2 cell line. Hum Antibodies. 2020;28(4):299–303. [DOI] [PubMed] [Google Scholar]
- 94.Videnović M, Mojsin M, Stevanović M, Opsenica I, Srdić-Rajić T, Šolaja B. Benzothiazole carbamates and amides as antiproliferative species. Eur J Med Chem. 2018;5(157):1096–114. [DOI] [PubMed] [Google Scholar]
- 95.Duan Z, Ping P, Wang G, Zhang X, Sun F. Imsnc761 and DDX6 synergistically suppress cell proliferation and promote apoptosis via p53 in testicular embryonal carcinoma cells. Biosci Rep. 2018;38(4):BSR20180271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agirre M, Ojeda E, Zarate J, Puras G, Grijalvo S, Eritja R, et al. New insights into gene delivery to human neuronal precursor NT2 cells: a comparative study between lipoplexes, nioplexes, and polyplexes. Mol Pharm. 2015;12(11):4056–66. [DOI] [PubMed] [Google Scholar]
- 97.Yiang G-T, Tsai H-F, Chen J-R, Chou P-L, Wu T-K, Liu H-C, et al. RC-6 ribonuclease induces caspase activation, cellular senescence and neuron-like morphology in NT2 embryonal carcinoma cells. Oncol Rep. 2014;31:1738–44. [DOI] [PubMed] [Google Scholar]
- 98.Mojsin M, Vicentic JM, Schwirtlich M, Topalovic V, Stevanovic M. Quercetin reduces pluripotency, migration and adhesion of human teratocarcinoma cell line NT2/D1 by inhibiting Wnt/β-catenin signaling. Food Funct. 2014;5:2564–73. [DOI] [PubMed] [Google Scholar]
- 99.Lamrani M, Sassi N, Paul C, Yousfi N, Boucher JL, Gauthier N, et al. TLR4/IFNγ pathways induce tumor regression via NOS II-dependent NO and ROS production in murine breast cancer models. Oncoimmunology. 2015;5(5): e1123369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abada PB, Howell SB. Cisplatin induces resistance by triggering differentiation of testicular embryonal carcinoma cells. PLoS ONE. 2014;9: e87444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Albany C, Hever-Jardine MP, von Herrmann KM, Yim CY, Tam J, Warzecha JM, et al. Refractory testicular germ cell tumors are highly sensitive to the second generation DNA methylation inhibitor guadecitabine. Oncotarget. 2017;8(2):2949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nabergoj D, Vrbek S, Zidar N, Tomašić T, Kikelj D, Mašič LP, et al. Synthetic analogues of marine alkaloid clathrodin differently induce phosphatidylserine exposure in monocytic cancer cells then in cancer stem cell lines. Medchemcomm. 2016;7:1546–54. [Google Scholar]
- 103.Oliviero G, Wynne K, Andrews D, Crean J, Kolch W, Cagney G. Expression proteomics and histone analysis reveal extensive chromatin network changes and a role for histone tail trimming during cellular differentiation. Biomolecules. 2024;14(7):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dokaneheifard S, Gomes Dos Santos H, Guiselle Valencia M, Arigela H, Edupuganti RR, Shiekhattar R. Neuronal differentiation requires BRAT1 complex to remove REST from chromatin. Proc Natl Acad Sci U S A. 2024;121(23):2318740121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.González-Burguera I, Lin G, López de Jesús M, Saumell-Esnaola M, Barrondo S, García Del Caño G, et al. PLCβ1 by-passes early growth response -1 to induce the differentiation of neuronal cells. Cell Death Discov. 2024;10(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Balint V, Peric M, Dacic S, Stanisavljevic Ninkovic D, Marjanovic J, Popovic J, et al. The role of SOX2 and SOX9 transcription factors in the reactivation-related functional properties of NT2/D1-derived astrocytes. Biomedicines. 2024;12(4):796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu ZSJ, Truong TTT, Bortolasci CC, Spolding B, Panizzutti B, Swinton C, et al. The potential of baicalin to enhance neuroprotection and mitochondrial function in a human neuronal cell model. Mol Psychiatry. 2024;29:2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bayat H, Mirahmadi M, Azarshin Z, Ohadi H, Delbari A, Ohadi M. CRISPR/Cas9-mediated deletion of a GA-repeat in human GPM6B leads to disruption of neural cell differentiation from NT2 cells. Sci Rep. 2024;14(1):2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dokaneheifard S, Gomes Dos Santos H, Valencia MG, Arigela H, Shiekhattar R. BRAT1 associates with INTS11/INTS9 heterodimer to regulate key neurodevelopmental genes. BioRxiv. 2023. 10.1101/2023.08.10.552743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaneko Y, Takahashi T. AraC-induced neuron-like differentiation of human NTERA2/D1 cells and quantification of endogenous pre-mir-106b and 19b levels. MicroPubl Biol. 2023. 10.17912/micropub.biology.000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng ZX, Liu EY, Wu QY, Wu JH, Dong TTX, Tsim KWK. The flavonoids induce the transcription of mRNA encoding erythropoietin in cultured embryonic stem cells via the accumulation of hypoxia-inducible factor-1α. Chem Biol Interact. 2023;1(382): 110609. [DOI] [PubMed] [Google Scholar]
- 112.Mirzadeh Azad F, Taheri Bajgan E, Naeli P, Rudov A, Bagheri Moghadam M, Sadat Akhtar M, et al. Differential expression pattern of linc-ROR spliced variants in pluripotent and non-pluripotent cell lines. Cell J. 2022;24(10):569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sandhu JK, Sodja C, Ribecco-Lutkiewicz M, Wu YT, Ma YS, Wei YH, et al. Effects of tolerance-induced preconditioning on mitochondrial biogenesis in undifferentiated and differentiated neuronal cells. Front Biosci (Landmark Ed). 2022;27(4):115. [DOI] [PubMed] [Google Scholar]
- 114.Subhramanyam CS, Cao Q, Wang C, Heng ZS, Zhou Z, Hu Q. piRNAs interact with cold-shock domain-containing RNA binding proteins and regulate neuronal gene expression during differentiation. Mol Neurobiol. 2022;59(2):1285–300. [DOI] [PubMed] [Google Scholar]
- 115.Rezaeian Z, Bahrami AR, Matin MM, Hosseiny SS. Investigation the effects of vitreous humor on proliferation and dedifferentiation of differentiated NTERA2 cells. Braz J Biol. 2021;22(84): e250151. [DOI] [PubMed] [Google Scholar]
- 116.Panizzutti B, Bortolasci CC, Spolding B, Kidnapillai S, Connor T, Richardson MF, et al. Biological mechanism(s) underpinning the association between antipsychotic drugs and weight gain. J Clin Med. 2021;10(18):4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dokaneheifard S, Soltani BM. Implication of TrkC-miR2 in neurotrophin signalling pathway regulation through NGFR transcript targeting. J Cell Mol Med. 2021;25(7):3381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mehravar M, Poursani EM. Novel variant of OCT4, mamed OCT4B5, is highly expressed in human pluripotent cells. Stem Cell Rev Rep. 2021;17(3):1068–73. [DOI] [PubMed] [Google Scholar]
- 119.Subhramanyam CS, Cao Q, Wang C, Heng ZSL, Zhou Z, Hu Q. Role of PIWI-like 4 in modulating neuronal differentiation from human embryonal carcinoma cells. RNA Biol. 2020;17(11):1613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chu CH, Chen JS, Chuang PC, Su CH, Chan YL, Yang YJ, et al. TIAM2S as a novel regulator for serotonin level enhances brain plasticity and locomotion behavior. FASEB J. 2020;34(2):3267–88. [DOI] [PubMed] [Google Scholar]
- 121.Mirzadeh Azad F, Malakootian M, Mowla SJ. lncRNA PSORS1C3 is regulated by glucocorticoids and fine-tunes OCT4 expression in non-pluripotent cells. Sci Rep. 2019;9(1):8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin Y, Yu J, Wu J, Wang S, Zhang T. Abnormal level of CUL4B-mediated histone H2A ubiquitination causes disruptive HOX gene expression. Epigenetics Chromatin. 2019;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li W, Liu W, Kakoki A, Wang R, Adebali O, Jiang Y, et al. Nucleotide excision repair capacity increases during differentiation of human embryonic carcinoma cells into neurons and muscle cells. J Biol Chem. 2019;294(15):5914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Andrews D, Oliviero G, De Chiara L, Watson A, Rochford E, Wynne K, et al. Unravelling the transcriptional responses of TGF-β: Smad3 and EZH2 constitute a regulatory switch that controls neuroretinal epithelial cell fate specification. FASEB J. 2019;33(5):6667–81. [DOI] [PubMed] [Google Scholar]
- 125.Ogasawara S, Chuman Y, Michiba T, Kamada R, Imagawa T, Sakaguchi K. Inhibition of protein phosphatase PPM1D enhances retinoic acid-induced differentiation in human embryonic carcinoma cell line. J Biochem. 2019;165(6):471–7. [DOI] [PubMed] [Google Scholar]
- 126.Lin C, Chen PY, Chan HC, Huang YP, Chang NW. Peroxisome proliferator-activated receptor alpha accelerates neuronal differentiation and this might involve the mitogen-activated protein kinase pathway. Int J Dev Neurosci. 2018;71:46–51. [DOI] [PubMed] [Google Scholar]
- 127.Torres RJ, Puig JG. Aicar effect in early neuronal development. Nucleosides Nucleotides Nucleic Acids. 2018;37(5):261–72. [DOI] [PubMed] [Google Scholar]
- 128.Hu Q, Khanna P, Ee Wong BS, Lin Heng ZS, Subhramanyam CS, Thanga LZ, et al. Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget. 2017;9(3):4223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sato Y, Watanabe S, Fukuda Y, Hashiguchi T, Yanagi Y, Ohno S. Cell-to-cell measles virus spread between human neurons is dependent on hemagglutinin and hyperfusogenic fusion protein. J Virol. 2018;92(6):e02166-e2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martín-Aragón Baudel MAS, Rae MT, Darlison MG, Poole AV, Fraser JA. Preferential activation of HIF-2α adaptive signalling in neuronal-like cells in response to acute hypoxia. PLoS ONE. 2017;12(10): e0185664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hassanlou M, Soltani BM, Mowla SJ. Expression and function of hsa-miR-6165 in human cell lines and during the NT2 cell neural differentiation process. J Mol Neurosci. 2017;63(2):254–66. [DOI] [PubMed] [Google Scholar]
- 132.González-Burguera I, Ricobaraza A, Aretxabala X, Barrondo S, García del Caño G, López de Jesús M, et al. Highly efficient generation of glutamatergic/cholinergic NT2-derived postmitotic human neurons by short-term treatment with the nucleoside analogue cytosine β-D-arabinofuranoside. Stem Cell Res. 2016;16:541–51. [DOI] [PubMed] [Google Scholar]
- 133.Abolpour Mofrad S, Kuenzel K, Friedrich O, Gilbert DF. Optimizing neuronal differentiation of human pluripotent NT2 stem cells in monolayer cultures. Dev Growth Differ. 2016;58:664–76. [DOI] [PubMed] [Google Scholar]
- 134.Pamies D, Sogorb MA, Fabbri M, Gribaldo L, Collotta A, Scelfo B, et al. Genomic and phenotypic alterations of the neuronal-like cells derived from human embryonal carcinoma stem cells (NT2) caused by exposure to organophosphorus compounds paraoxon and mipafox. Int J Mol Sci. 2014;15:905–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stern M, Gierse A, Tan S, Bicker G. Human Ntera2 cells as a predictive in vitro test system for developmental neurotoxicity. Arch Toxicol. 2014;88:127–36. [DOI] [PubMed] [Google Scholar]
- 136.Klajn A, Drakulic D, Tosic M, Pavkovic Z, Schwirtlich M, Stevanovic M. SOX2 overexpression affects neural differentiation of human pluripotent NT2/D1 cells. Biochemistry (Mosc). 2014;79:1172–82. [DOI] [PubMed] [Google Scholar]
- 137.Karbalaie K, Vallian S, Lachinani L, Tanhaei S, Baharvand H, Nasr-Esfahani MH. Analysis of promyelocytic leukemia in human embryonic carcinoma stem cells during retinoic acid-induced neural differentiation. Iran J Biotechnol. 2016;14(3):169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balint V, Ninkovic DS, Anastasov N, Lazic S, Kovacevic-Grujicic N, Stevanovic M, et al. inhibition of miR-21 promotes cellular senescence in NT2-derived astrocytes. Biochemistry (Mosc). 2021;86(11):1434–45. [DOI] [PubMed] [Google Scholar]
- 139.Liu EY, Zheng ZX, Zheng BZ, Xia Y, Guo MS, Dong TT, et al. Tectorigenin, an isoflavone aglycone from the rhizome of Belamcanda chinensis, induces neuronal expression of erythropoietin via accumulation of hypoxia-inducible factor-1α. Phytother Res. 2020;34(6):1329–37. [DOI] [PubMed] [Google Scholar]
- 140.Figueira I, Tavares L, Jardim C, Costa I, Terrasso AP, Almeida AF, et al. Blood-brain barrier transport and neuroprotective potential of blackberry-digested polyphenols: an in vitro study. Eur J Nutr. 2019;58(1):113–30. [DOI] [PubMed] [Google Scholar]
- 141.Bordalo M, Seabra IJ, Silva AB, Terrasso AP, Brito C, Serra M, et al. Using high-pressure technology to develop antioxidant-rich extracts from Bravo de Esmolfe apple residues. Antioxidants (Basel). 2021;10(9):1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schmitz A, Dempewolf S, Tan S, Bicker G, Stern M. Developmental neurotoxicity of Fipronil and Rotenone on a human neuronal in vitro test system. Neurotox Res. 2021;39(4):1189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hassanlou M, Soltani BM, Medlej A, Kay M, Mowla SJ. Hsa-miR-6165 downregulates insulin-like growth factor-1 receptor (IGF-1R) expression and enhances apoptosis in SW480 cells. Biol Chem. 2020;401(4):477–85. [DOI] [PubMed] [Google Scholar]
- 144.Popović J, Klajn A, Paunesku T, Ma Q, Chen S, Lai B, et al. Neuroprotective role of selected antioxidant agents in preventing cisplatin-induced damage of human neurons in vitro. Cell Mol Neurobiol. 2019;39(5):619–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Attia N, Mashal M, Soto-Sánchez C, Martínez-Navarrete G, Fernández E, Grijalvo S, et al. Gene transfer to rat cerebral cortex mediated by polysorbate 80 and poloxamer 188 nonionic surfactant vesicles. Drug Des Devel Ther. 2018;16(12):3937–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mashal M, Attia N, Soto-Sánchez C, Martínez-Navarrete G, Fernández E, Puras G, et al. Non-viral vectors based on cationic niosomes as efficient gene delivery vehicles to central nervous system cells into the brain. Int J Pharm. 2018;552(1–2):48–55. [DOI] [PubMed] [Google Scholar]
- 147.Mashal M, Attia N, Grijalvo S, Eritja R, Puras G, Pedraz JL. Stability of polymeric cationic niosomes and their plasmid DNA-based complexes as gene delivery carriers. Drug Deliv. 2023;30(1):2219420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sadeghi Z, Maleki P, Shahabi F, Bondarkhilli SAM, Masoumi M, Taheri M, et al. Surface modification of superparamagnetic iron oxide (SPION) and comparison of cytotoxicity effect of mPEG2000-PEI-SPION and mPEG750-PEI-SPION on the human embryonic carcinoma stem cell, NTERA2 cell line. Hum Antibodies. 2020;28(2):159–67. [DOI] [PubMed] [Google Scholar]
- 149.Sadeghi Z, Maleki P, Mohammadi Bondarkhilli SA, Mohammadi M, Raheb J. Dataset on cytotoxicity effect of polyethylenimine-functionalized graphene oxide nanoparticles on the human embryonic carcinoma stem cell, NTERA2 cell line. Data Brief. 2019;6(26): 104487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bortolasci CC, Jaehne EJ, Hernández D, Spolding B, Connor T, Panizzutti B, et al. Metergoline shares properties with atypical antipsychotic drugs identified by gene expression signature screen. Neurotox Res. 2023;41(6):502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bortolasci CC, Kidnapillai S, Spolding B, Truong TTT, Connor T, Swinton C, et al. Use of a gene expression signature to identify trimetazidine for repurposing to treat bipolar depression. Bipolar Disord. 2023;25(8):661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Truong TTT, Bortolasci CC, Kidnapillai S, Spolding B, Panizzutti B, Liu ZSJ, et al. Integrative analyses of transcriptomes to explore common molecular effects of antipsychotic drugs. Int J Mol Sci. 2022;23(14):7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu ZSJ, Truong TTT, Bortolasci CC, Spolding B, Panizzutti B, Swinton C, et al. Effects of psychotropic drugs on ribosomal genes and protein synthesis. Int J Mol Sci. 2022;23(13):7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Truong TT, Bortolasci CC, Spolding B, Panizzutti B, Liu ZS, Kidnapillai S, et al. Co-expression networks unveiled long non-coding RNAs as molecular targets of drugs used to treat bipolar disorder. Front Pharmacol. 2022;8(13): 873271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Panizzutti B, Bortolasci CC, Spolding B, Kidnapillai S, Connor T, Richardson MF, et al. Transcriptional modulation of the Hippo signaling pathway by drugs used to treat bipolar disorder and schizophrenia. Int J Mol Sci. 2021;22(13):7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Truong TT, Bortolasci CC, Kidnapillai S, Spolding B, Panizzutti B, Liu ZS, et al. Common effects of bipolar disorder medications on expression quantitative trait loci genes. J Psychiatr Res. 2022;150:105–12. [DOI] [PubMed] [Google Scholar]
- 157.Bortolasci CC, Spolding B, Kidnapillai S, Connor T, Truong TTT, Liu ZSJ, et al. Transcriptional effects of psychoactive drugs on genes involved in neurogenesis. Int J Mol Sci. 2020;21(21):8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bortolasci CC, Spolding B, Kidnapillai S, Richardson MF, Vasilijevic N, Martin SD, et al. Effects of psychoactive drugs on cellular bioenergetic pathways. World J Biol Psychiatry. 2021;22(2):79–93. [DOI] [PubMed] [Google Scholar]
- 159.Kidnapillai S, Bortolasci CC, Panizzutti B, Spolding B, Connor T, Bonifacio K, et al. Drugs used in the treatment of bipolar disorder and their effects on cholesterol biosynthesis—a possible therapeutic mechanism. World J Biol Psychiatry. 2019;20(10):766–77. [DOI] [PubMed] [Google Scholar]
- 160.Kidnapillai S, Bortolasci CC, Udawela M, Panizzutti B, Spolding B, Connor T, et al. The use of a gene expression signature and connectivity map to repurpose drugs for bipolar disorder. World J Biol Psychiatry. 2020;21(10):775–83. [DOI] [PubMed] [Google Scholar]
- 161.Küçükdoğru R, Türkez H, Arslan ME, Tozlu ÖÖ, Sönmez E, Mardinoğlu A, et al. Neuroprotective effects of boron nitride nanoparticles in the experimental Parkinson’s disease model against MPP+ induced apoptosis. Metab Brain Dis. 2020;35(6):947–57. [DOI] [PubMed] [Google Scholar]
- 162.Cacciotti I, Ceci C, Bianco A, Pistritto G. Neuro-differentiated Ntera2 cancer stem cells encapsulated in alginate beads: first evidence of biological functionality. Mater Sci Eng C Mater Biol Appl. 2017;1(81):32–8. [DOI] [PubMed] [Google Scholar]
- 163.Turkez H, Ozdemir Tozlu O, Tatar A, Arslan ME, Cadirci K, Marinelli L, et al. Toxicity of glycyl-l-prolyl-l-glutamate pseudotripeptides: cytotoxic, oxidative, genotoxic, and embryotoxic perspectives. J Toxicol. 2022;19(2022):3775194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhai X, Liu J, Xiao J, Zhang T, Wang J, Li J, et al. The lactylation index predicts the immune microenvironment and prognosis of pan-cancer patients. Biocell. 2024;48(8):1223–39. [Google Scholar]
- 165.Lin Q, Zhu J, Hu Y. Involvement of lncRNAs in the tumor microenvironment: a new property of tumor immunity. Cancer Biol Fedicine. 2023;20(8):553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang B, Chen K, Liu X, Liu W, Ma Y, Tian X, Yang Y. Advance in tumor immunotherapy: establishing a new paradigm for oncological treatment. Transl Surg Oncol. 2023;1(2):30–43. [Google Scholar]
- 167.Lee JH, Kim I, Kim SW, Lim S, Lee S, Hwang K, et al. Microenvironmental regulation of stem cells injected in the area at risk of neurodegenerative diseases. Biocell. 2022;46(10):2231–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.