Abstract
The development of experimental models treated by chemotherapy is needed for elucidating the side effects of cancer treatments administered prior to puberty on male gonad function and the feasibility of restoring fertility from exposed testicular tissues. This study investigated for the first time the effects of cytarabine and daunorubicin administered before meiotic initiation on the first wave of mouse spermatogenesis under both in vivo or in vitro conditions. Prepubertal exposure to cytarabine did not exhibit immediate detrimental effects on testicular tissues, whereas daunorubicin administration resulted in a decreased spermatogonia-to-Sertoli cell ratio and diminished intratubular cell proliferation within three days post-treatment. While the completion of in vivo spermatogenesis was not hindered by chemotherapy exposure, a significant increase in the proportion of spermatozoa with fragmented DNA was observed in mice more than one month after treatment. In vitro spermatogenesis was also accomplished using prepubertal testicular tissues exposed to chemotherapy, indicating that neither cytarabine nor daunorubicin impeded the differentiation potential of spermatogonia into spermatozoa. However, in vitro conditions revealed an arrest in meiotic progression in a substantial proportion of seminiferous tubules and an elevated incidence of DNA double-strand breaks in intratubular cells compared to in vivo controls, irrespective of the treatment administered.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-98413-1.
Keywords: Chemotherapy, Cytarabine, Daunorubicin, Fertility restoration, In vitro spermatogenesis, Prepubertal mouse testis
Subject terms: Differentiation, Germline development
Introduction
Leukaemia is the most common paediatric cancer, accounting for almost one in every three reported cases in children. Thanks to advances in treatment, the five-year survival rate is now above 80% for children. In recent years, several studies have highlighted the sensitivity of prepubertal testicular tissue to chemotherapy treatments and demonstrated an increased risk of infertility in paediatric cancer survivors compared to the general population1–4. Cytarabine and daunorubicin are chemotherapy agents used in the treatment of pediatric cancers, including leukemia, and are considered to present a low risk to male fertility5,6. However, their effects on the prepubertal testis have been little studied or not studied at all.
Cytarabine (1-ß-D-arabinofuranosylcytosine, Ara-C) is an antimetabolite that specifically targets the S phase of the cell cycle. It is a pyrimidine nucleoside analogous to cytidine, in which the ribose has been replaced by arabinose. The cytotoxicity of Ara-C depends on its active metabolite, Ara-CTP, which competes with deoxycytidine triphosphate (dCTP) for incorporation into the DNA molecule. Once incorporated into the DNA, Ara-C acts as a terminator chain, blocking its elongation, synthesis and repair7. Exposure of prepubertal rats to 50, 100 and 200 mg/kg/day of Ara-C from 29 to 42 dpp resulted in testicular atrophy and impaired spermatogenesis due to reduced cell proliferation, and induced DNA damage in a dose-dependent manner8. Sperm concentration and the percentage of morphologically normal spermatozoa were significantly reduced 28 days after the last injection8. Recently, it was also shown that 14 dpp mice that received 140 mg/kg Ara-C showed a decrease in the testis weight to body weight ratio and an increase in the percentage of tubules with apoptotic cells 2 weeks post-injection, as well as a reduction in sperm concentration 5.5 weeks post-injection9. Moreover, a study conducted on testicular biopsies from patients aged 0 to 16 years reported that the cumulative dose of Ara-C was associated with a decrease in spermatogonia proliferation and severe alteration of the testicular tissue10.
Daunorubicin (DNR) belongs to the anthracycline family of antibiotics. Anthracyclines are capable of intercalating into the DNA double helix, distorting it and blocking transcription and replication. By intercalating between two DNA base pairs, they stabilize DNA-topoisomerase II complexes and cause DNA strand breaks. Anthracyclines can also induce the formation of free radicals that can damage the DNA molecule11. The effects of daunorubicin alone on the prepubertal testis have never been studied, unlike its analogue doxorubicin12. Doxorubicin has been reported to directly target spermatogonial stem cells (SSCs) in in vitro studies. In vitro exposure of 5 dpp mouse testes to 0.01–0.05 µg/mL doxorubicin for 24 h caused a decrease in the pool of SSCs and altered their ability to proliferate13,14. Furthermore, an increase in DNA breaks has been reported in rat GC-6spg, a cell line with SSC characteristics as well as in cultures of mouse 5 dpp testicular tissues, following a 16-hour exposure to 0.05 µg/mL doxorubicin13,15. In vivo exposure of rats to doxorubicin before puberty (3 mg/kg at 6 dpp) resulted in increased apoptosis in spermatogonia 48 h after treatment16, germ cell loss, decreased testicular and epididymal weights at 80 and 129 dpp, and persistent sterility17. Exposure to this molecule during prepuberty (5 mg/kg between 15 and 30 dpp) resulted in the depletion of germ cells associated with the presence of Sertoli cell-only seminiferous tubules and reduced testicular weight in late pubertal and adult rats (40, 64, 100 and 127 dpp)18–20. The administration of doxorubicin resulted in a decrease in sperm count and a higher rate of abnormal spermatozoa and DNA damage 9 to 10 weeks post-treatment19–21. Paternal treatment also led to a reduction in fertility indices and a significant increase in the number of post-implantation losses and fetal growth retardation20,21.
In France, fertility preservation procedures are generally proposed to prepubertal boys after the start of low or moderately gonadotoxic chemotherapy and before treatments at high risk of infertility. Current fertility preservation strategies in young boys with cancer involve the cryopreservation of the prepubertal testicular tissue, as sperm cryopreservation cannot be offered to these patients who are not yet producing spermatozoa22–24. One of the most common indications for fertility preservation in prepubertal patients is leukaemia, particularly acute lymphoblastic leukaemia.
In order to restore fertility in patients cured of paediatric cancer, fragments of prepubertal testicular tissue will need to be thawed and either matured in vivo or in vitro to produce spermatozoa for use in assisted reproduction techniques25. None of these fertility restoration approaches has yet been clinically validated in humans. In vivo maturation strategies (SSC transplantation, testicular tissue autografting) have limitations, such as the risk of reintroducing tumour cells into the patient. In vitro maturation of frozen/thawed tissues could instead be reserved for patients whose testicular tissue is contaminated with cancer cells. However, in vitro spermatogenesis from prepubertal testicular tissue is still at the experimental stage and is mainly developed in animal models25–27. In the mouse model, spermatozoa have been obtained from fresh or frozen/thawed immature testicular tissues using an organotypic gas-liquid interphase culture system28–30. The gametes produced in vitro exhibited nuclear quality comparable to that observed in vivo and were functionally competent, with viable and fertile offspring being obtained28,29,31. Another in vitro maturation approach, which involves the culture of dissociated testicular cells within a matrix (three-dimensional culture), can also support the differentiation of spermatogonia into spermatozoa in the mouse model, but the fertilization competence of the gametes has not yet been demonstrated32,33.
As mentioned above, in most cases, cancer treatment begins before cryopreservation of prepubertal testicular tissue due to the urgency of therapeutic management. As a result, testicular tissues may already have been exposed to low or moderately gonadotoxic doses of chemotherapy prior to fertility preservation and intensification of treatments34. Whether exposure of immature testicular tissue to chemotherapeutic agents could prevent its subsequent use for in vitro spermatogenesis has been poorly investigated. Recently, we have demonstrated for the first time that a complete in vitro spermatogenesis can be achieved using prepubertal mouse testicular tissues that have been pre-exposed in vivo to vincristine and/or cyclophosphamide at low gonadotoxic doses35. The aim of the present study was to assess for the first time (i) the effects of in vivo exposure to Ara-C or DNR on the prepubertal mouse testis before the initiation of meiosis and at the end of the first wave of spermatogenesis, as well as (ii) the ability of chemotherapy-exposed spermatogonia to differentiate in vivo or in vitro to generate spermatozoa. Specifically, the impact of these molecules was assessed in vivo to ascertain that they present a low gonadotoxic risk, and in vitro cultures of testicular tissues exposed to chemotherapy were performed to determine whether this approach could be a feasible fertility restoration procedure for sperm production. In the present study, testicular tissue integrity, cell proliferation, apoptosis and DNA strand breaks in seminiferous tubules, the progression of in vivo and in vitro spermatogenesis and sperm DNA integrity were investigated.
Results
Impact of prepubertal exposure to Ara-C or DNR on testicular tissue in 6 dpp mice
Prepubertal 3 dpp mice received an intraperitoneal injection of either a saline solution (0.9% NaCl, control group) or a low gonadotoxic dose of Ara-C (20 mg/kg) or DNR (1 mg/kg). To determine the spermatogonia to Sertoli cells ratio three days post-treatment (i.e. before entry into meiosis), immunostaining for TRA98 (expressed in spermatogonia in 6 dpp testis) was performed (Fig. 1a). There was no significant change in the spermatogonia to Sertoli cells ratio in Ara-C-exposed mice compared to unexposed mice (0.39 vs. 0.50; P = 0.3396) (Fig. 1b). However, in DNR-exposed mice, the ratio was halved compared to control mice (0.27 vs. 0.50; P = 0.0047) (Fig. 1b). Exposure to DNR also resulted in a significantly lower testicular weight to body weight ratio in 6 dpp mice compared to the control mice (0.0006 vs. 0.0009; P = 0.0285) (Fig. 1c).
Fig. 1.
Testicular weight to body weight ratio, germ cell content, proliferation/apoptosis balance and DNA double-strand breaks in testicular tissues of 6 dpp mice pre-exposed or not to chemotherapy. (a) Representative images of TRA98 immunostaining in testicular tissues of 6 dpp mice treated or not with chemotherapy, red arrow: spermatogonia (cells with a spherical TRA98 + nucleus), yellow arrow: Sertoli cells (cells with an irregularly shaped TRA98- nucleus). (b) Spermatogonia to Sertoli cell ratios. (c) Mean testicular weight to body weight ratios. (d) Representative images of Ki67 immunostaining in testicular tissues of 6 dpp mice treated or not with chemotherapy. (e) Mean percentage of seminiferous tubules containing proliferating cells (Ki67-positive tubules). (f) Mean percentage of Ki67-positive (proliferating) cells per tubule. (g) Representative images of cleaved caspase 3 (CC3) immunostaining in testicular tissues of 6 dpp mice treated or not with chemotherapy. (h) Mean percentage of seminiferous tubules with apoptotic cells (CC3-positive tubules). (i) Mean percentage of CC3-positive (apoptotic) cells per tubule within testicular tissues. (j) Representative images of DDX4 (spermatogonia, green) and γH2A.X (DNA double-strand breaks, red) double immunostaining in testicular tissues of 6 dpp mice treated or not with chemotherapy. (k) Mean percentage of γH2A.X-positive spermatogonia per tubule. Data are presented as mean ± SEM with n = 4 biological replicates for each group. A value of *P < 0.05 or **P < 0.01 was considered statistically significant. Ara-C: cytarabine 20 mg/kg; CC3: cleaved caspase 3; DAPI: 4’,6-diamidino-2-phenylindole; DDX4: DEAD-Box Helicase 4; DNR: daunorubicin 1 mg/kg; dpp: days postpartum; γH2A.X: phosphorylated form of histone H2A.X; 0.9% NaCl: saline solution.
Cell proliferation was assessed by Ki67 immunostaining (Fig. 1d). The proportion of Ki67-positive seminiferous tubules did not vary significantly in 6 dpp tissues exposed to Ara-C (93.50% vs. 90.00%; P > 0.9999) or DNR (67.25% vs. 90.00%; P = 0.0942) relative to 0.9% NaCl (Fig. 1e). However, the data revealed a significant decrease in the mean percentage of Ki67 + cells per seminiferous tubule in DNR-treated mice compared to control mice (11.18% vs. 25.93%; P = 0.0482) (Fig. 1f). Cleaved caspase 3 immunostaining (CC3) was then performed to detect apoptotic cells (Fig. 1g). The proportion of CC3 + seminiferous tubules was not significantly different between the Ara-C (7.75% vs. 9.25%; P > 0.9999) and DNR (18.25% vs. 9.25%; P = 0.1646) groups and the control group (Fig. 1h). Additionally, the mean percentage of CC3 + cells per tubule was not significantly different in Ara-C (5.21% vs. 5.07%; P > 0.9999) and DNR-treated groups (4.42% vs. 5.07%; P = 0.6047) when compared with the 0.9% NaCl group (Fig. 1i).
Immunostaining for γH2A.X (phosphorylated form of histone H2A.X) was used to detect DNA double-strand breaks (Fig. 1j). The percentage of γH2A.X + spermatogonia per tubule was not significantly different in tissues exposed to Ara-C (63.35% vs. 66.94%; P > 0.9999) or DNR (53.04% vs. 66.94%; P = 0.3396) compared to control tissues (Fig. 1k).
Impact of prepubertal exposure to Ara-C or DNR on testicular tissue integrity in 36 dpp mice
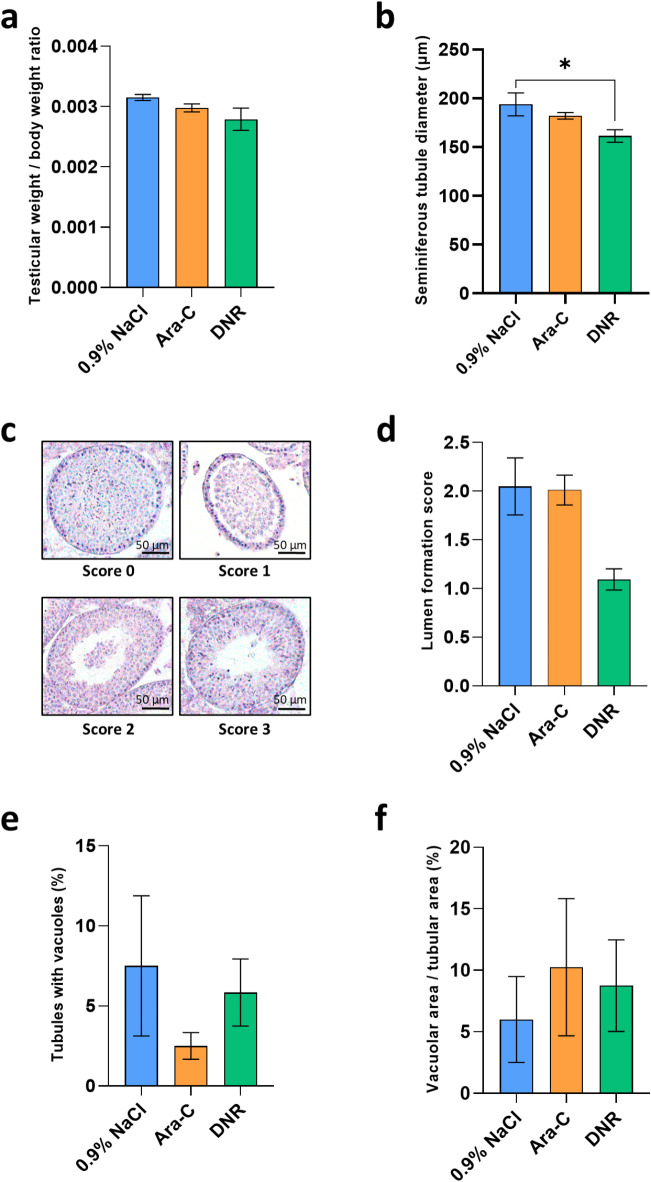
Thirty-three days post-treatment, at 36 dpp (i.e. at the end of the first spermatogenic wave), no significant changes in the testicular-body weight ratio were observed in mice exposed to Ara-C (0.00298 vs. 0.00315; P = 0.2333) or DNR (0.00279 vs. 0.00315; P = 0.1910) compared to control mice (Fig. 2a). Histological analyses were performed to assess the effect of chemotherapeutic agents on seminiferous tubules (diameter, lumen formation). In mice exposed to DNR, a significant decrease in mean seminiferous tubule diameter was observed (161.37 vs. 193.79; P = 0.0372) (Fig. 2b). Although a lumen was not observed in the centre of the majority of seminiferous tubules in DNR-exposed tissues, no significant difference in the lumen formation score was found between the Ara-C (2.01 vs. 2.05; P > 0.9999) and DNR groups (1.09 vs. 2.05; P = 0.0687) and control group (Fig. 2c-d). Seminiferous epithelium damage was also assessed by measuring the percentage of tubules presenting vacuolization and the area of the intraepithelial vacuoles. No significant differences were found for these two parameters between tissues exposed to Ara-C (2.50% vs. 7.50% and 10.25% vs. 6.00%; P = 0.6221 and P > 0.9999, respectively) or DNR (5.84% vs. 7.50% and 8.75% vs. 6.00%; P > 0.9999 for all comparison) and unexposed tissues (Fig. 2e-f).
Fig. 2.
Testicular weight to body weight ratios and testicular tissue integrity of 36 dpp mice exposed or not to chemotherapy during the prepubertal period. (a) Mean testicular weight to body weight ratios of 36 dpp mice pre-exposed to Ara-C, DNR or 0.9% NaCl. (b) Mean diameter of seminiferous tubules in testicular tissues of control, Ara-C or DNR-treated mice. (c) Assessment of the formation of a lumen at the center of seminiferous tubules through a scale from 0 to 3, with 0 representing tubules without a lumen, 1 representing tubules without a lumen and in which the basal and adluminal compartments are loosely connected, 2 representing tubules with an accumulation of cells within the lumen and 3 representing tubules with an empty lumen. (d) Mean score of lumen formation for each group. (e) Percentage of seminiferous tubules with vacuolization in testicular tissues exposed to Ara-C, DNR or unexposed. (f) Proportion of vacuolar area to seminiferous tubule area for each condition. Data are presented as mean ± SEM with n = 4 biological replicates for each group. A value of *P < 0.05 was considered statistically significant. Ara-C: cytarabine 20 mg/kg; CC3: cleaved caspase 3; DNR: daunorubicin 1 mg/kg; dpp: days postpartum; 0.9% NaCl: saline solution.
Impact of prepubertal exposure to Ara-C or DNR after in vivo maturation (36 dpp) or organotypic culture (D30)
In the remainder of the study, the effect of prepubertal exposure to Ara-C or DNR on the progression of spermatogenesis, cell proliferation/apoptosis and DNA integrity was investigated both in vivo and in vitro thirty-three days after treatment.
The impact of chemotherapy exposure at 3 dpp was assessed in mice at the age of 36 dpp to ascertain that the doses administered did not alter the ability of spermatogonia to differentiate into spermatozoa. We then investigated whether administration of chemotherapy at 3 dpp could interfere with in vitro maturation of 6 dpp testicular tissues and with sperm production after 30 days (D30) of organotypic culture. Finally, we compared the efficacy of in vivo and in vitro spermatogenesis to assess the impact of organotypic culture conditions.
Impact of Ara-C and DNR on the progression of spermatogenesis
Immunostaining for TRA98 coupled to PAS reaction allowed the detection of spermatogonia, leptotene/zygotene and pachytene spermatocytes (TRA98 + cells) as well as the pink-labelled acrosome of round and elongated spermatids (PAS + cells) (Fig. 3a-b, Supplementary Fig. 1).
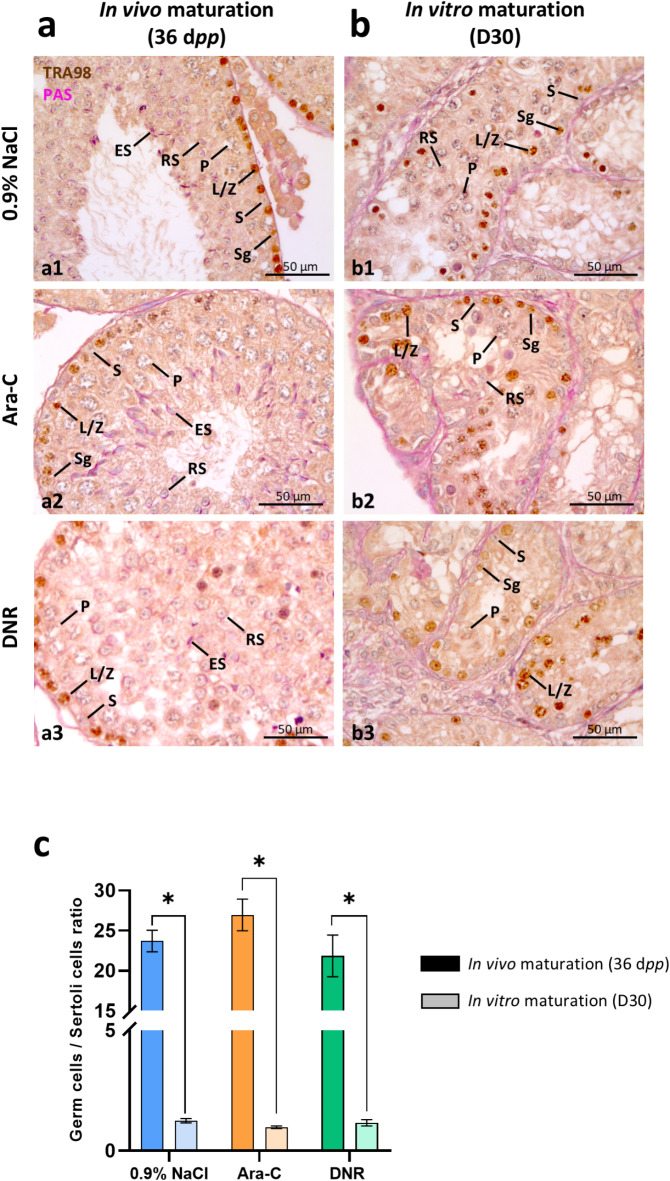
Fig. 3.
Germ cells to Sertoli cells ratio in mouse testicular tissues after exposure to chemotherapy and 30 days of in vitro (D30) or in vivo (36 dpp) maturation. (a-b) Representative images of TRA98 immunostaining coupled to PAS reaction in chemotherapy exposed or unexposed testicular tissues at 36 dpp (a, after in vivo maturation) and D30 (b, after in vitro maturation). Intratubular cells were identified as: S for Sertoli cells (cells with an irregularly-shaped TRA98- nucleus close to the basement membrane), Sg for spermatogonia (cells with a spherical TRA98 + nucleus close to the basement membrane), L/Z for leptotene/zygotene spermatocytes (cells with a spherical and condensed TRA98 + nucleus), P for pachytene spermatocytes (cells with a sexual vesicle and either a condensed TRA98 + nucleus for early pachytene or a TRA98- nucleus for late pachytene), RS for round spermatids (cells with a small round TRA98- nucleus and a PAS + acrosomal cap) or ES for elongated spermatids (cells with an elongated TRA98- nucleus and a PAS + acrosomal cap). (c) Germ cells to Sertoli cells ratios (per seminiferous tubule) in chemotherapy-exposed or unexposed testicular tissues at D30 and 36 dpp. Data are presented as mean ± SEM with n = 4 biological replicates for each group. A value of *P < 0.05 was considered statistically significant. Ara-C: cytarabine 20 mg/kg; D30: in vitro matured during 30 days; DNR: daunorubicin 1 mg/kg; dpp: days postpartum; 0.9% NaCl: saline solution; PAS: Periodic acid Schiff reaction.
No significant variation in the ratio of germ cells to Sertoli cells was observed in 36 dpp mice exposed to Ara-C (26.96 vs. 23.71; P = 0.4786) or DNR (21.85 vs. 23.71; P > 0.9999) compared to unexposed mice (Fig. 3c). After in vitro maturation (D30), the germ cells to Sertoli cells ratio was also not significantly different in testicular fragments from mice exposed to Ara-C (0.98 vs.1.25; P = 0.0997) or DNR (1.16 vs.1.25; P = 0.9849) with 0.9% NaCl (Fig. 3c). However, a comparison of in vivo and in vitro conditions revealed that this ratio was significantly lower after in vitro maturation than under physiological conditions, irrespective of the treatment (Fig. 3c). It was respectively 19-fold (1.25 vs. 23.70; P = 0.0286), 27.5-fold (0.98 vs. 26.95; P = 0.0286) and 18.7-fold (1.16 vs. 21.85; P = 0.0286) lower under in vitro than in vivo conditions for the 0.9% NaCl, Ara-C and DNR groups (Fig. 3c).
A complete spermatogenesis was observed at 36 dpp after prepubertal exposure to low gonadotoxic doses of Ara-C or DNR (Table 1). Moreover, the proportion of each cell type in the seminiferous tubules did not vary significantly between the treated and untreated groups (Table 1). In the context of using chemotherapy-exposed prepubertal tissues for in vitro maturation, we wondered whether prepubertal treatment with Ara-C or DNR might impair the progression of in vitro spermatogenesis. In 30-day organotypic cultures, spermatogenesis progressed to the elongated spermatid stage in both chemotherapy-exposed and unexposed tissue fragments (Table 1). A lower percentage of pachytene spermatocytes per seminiferous tubule was found at D30 after exposure to Ara-C than to 0.9% NaCl (14.2% vs. 22.4%; P = 0.0016) while no impact of DNR was observed (Table 1). The cellular composition of seminiferous tubules was dissimilar in in vivo and in vitro-matured tissues, with a higher percentage of Sertoli cells per seminiferous tubule in cultured tissues than in their respective in vivo controls (P = 0.0286 for all comparisons) (Table 1). Conversely, the percentage of round and elongated spermatids per tubule was lower in cultured tissues than in vivo, regardless of treatment (P = 0.0286 for all comparisons) (Table 1).
Table 1.
Germ cell content in mouse testicular tissues after exposure to chemotherapy and 30 days of in vitro (D30) or in vivo (36 dpp) maturation.
| Percentage of each cell type / tubule (%) | 0.9% NaCl | Ara-C | DNR | |||
|---|---|---|---|---|---|---|
| 36 dpp | D30 | 36 dpp | D30 | 36 dpp | D30 | |
| Sertoli cells | 4.8 ± 0.4 | 47.0 ± 1.7* | 3.7 ± 0.1 | 54.9 ± 1.7* | 5.9 ± 0.9 | 51.0 ± 3.3* |
| Spermatogonia | 9.5 ± 2.3 | 17.5 ± 2.3 | 7.7 ± 0.8 | 16.0 ± 1.5* | 9.0 ± 1.7 | 18.7 ± 1.0* |
| Leptotene/zygotene spermatocytes | 10.1 ± 2.0 | 12.3 ± 3.2 | 8.9 ± 1.5 | 13.9 ± 1.5 | 16.3 ± 2.7 | 12.6 ± 1.8 |
| Pachytene spermatocytes | 23.1 ± 1.9 | 22.4 ± 1.0 | 20.3 ± 1.1 | 14.2 ± 1.0* a | 20.6 ± 2.4 | 17.2 ± 1.9 |
| Round spermatids | 34.3 ± 2.8 | 0.8 ± 0.3* | 36.3 ± 2.2 | 0.9 ± 0.5* | 30.9 ± 2.2 | 0.5 ± 0.3* |
| Elongated spermatids | 18.6 ± 1.5 | 0.1 ± 0.1* | 22.5 ± 0.6 | 0.1 ± 0.1* | 17.3 ± 2.5 | 0.1 ± 0.05* |
Data are presented as mean ± SEM with n = 4 biological replicates for each group.
aP < 0.05 vs. in vitro matured control (pre-exposed to 0.9% NaCl) testes; * P < 0.05 vs. age-matched in vivo controls.
In addition, the progression of spermatogenesis was assessed by determining the proportion of seminiferous tubules containing the most differentiated germ cells (Table 2). In both exposed and unexposed 36 dpp mice, the majority of seminiferous tubules analyzed were at the round or elongated spermatid stage, again showing that the treatments did not disturb the progression of the first wave of spermatogenesis (Table 2). In in vitro matured tissues, we observed that a greater proportion of seminiferous tubules were arrested at the leptotene/zygotene spermatocyte stage after Ara-C exposure in comparison with controls (19.2% vs. 5.8%; P = 0.0393), whereas no significant differences were found between DNR-exposed and control tissues (6.8% vs. 5.8%; P > 0.9999) (Table 2). However, the majority of seminiferous tubules were arrested at the pachytene spermatocyte stage after 30 days of organotypic culture (Table 2).
Table 2.
Percentage of seminiferous tubules at the most advanced differentiation stage of spermatogenesis in mouse testicular tissues after exposure to chemotherapy and 30 days of in vitro (D30) or in vivo (36 dpp) maturation.
| Seminiferous tubules at the most advanced stage (%) |
0.9% NaCl | Ara-C | DNR | |||
|---|---|---|---|---|---|---|
| 36 dpp | D30 | 36 dpp | D30 | 36 dpp | D30 | |
| Sertoli cell only | 0.0 ± 0.0 | 0.8 ± 0.8 | 0.0 ± 0.0 | 1.5 ± 0.9 | 0.0 ± 0.0 | 0.8 ± 0.8 |
| Spermatogonia | 0.0 ± 0.0 | 4.8 ± 2.8 | 0.0 ± 0.0 | 8.5 ± 3.5 | 0.0 ± 0.0 | 14.3 ± 6.0* |
| Leptotene/zygotene spermatocytes | 0.0 ± 0.0 | 5.8 ± 3.8 | 0.0 ± 0.0 | 19.3 ± 1.4* a | 0.0 ± 0.0 | 6.8 ± 2.4 |
| Pachytene spermatocytes | 3.3 ± 1.4 | 75.0 ± 8.2* | 0.8 ± 0.8 | 50.0 ± 5.8* | 1.5 ± 0.9 | 67.8 ± 6.4* |
| Round spermatids | 20.8 ± 3.7 | 11.8 ± 4.4 | 15.8 ± 1.7 | 18.5 ± 7.5 | 18.0 ± 2.9 | 8.3 ± 2.1 |
| Elongated spermatids | 75.3 ± 2.8 | 1.5 ± 0.9* | 83.5 ± 2.4 | 2.3 ± 0.8* | 81.0 ± 2.4 | 2.3 ± 0.8* |
Data are presented as mean ± SEM with n = 4 biological replicates for each group.
aP < 0.05 vs. in vitro matured control (pre-exposed to 0.9% NaCl) testes; * P < 0.05 vs. age-matched in vivo controls.
Impact of Ara-C and DNR on cell proliferation and apoptosis
The cytotoxicity of Ara-C and DNR was evaluated by analyzing cell proliferation and apoptosis (Fig. 4ab, ef). The proportion of Ki67 + seminiferous tubules was not significantly different in 36 dpp Ara-C-exposed tissues and in controls (P = 0.0997) (Fig. 4c). However, the percentage of Ki67 + tubules was significantly decreased in 36 dpp DNR-treated tissues compared to controls (28.25% vs. 83.25%; P = 0.0121) (Fig. 4c). In contrast, there was not significant difference in the percentage of Ki67 + cells per seminiferous tubule at 36 dpp across all conditions (P > 0.9999 for all comparisons) (Fig. 4d). After 30 days of organotypic culture, the proportion of Ki67 + seminiferous tubules was not significantly different in Ara-C (47.5% vs. 50.7%; P = 0.9849) and DNR (54.0% vs. 50.7%; P > 0.9999) and control group (Fig. 4c). Similarly, no significant differences were found for Ki67 + cells per tubule between tissues exposed to Ara-C (14.9% vs. 12.0%; P = 0.9194) or DNR (15.8% vs. 12.0%; P = 0.5615) and unexposed tissues (Fig. 4d).
Fig. 4.
Cell proliferation and apoptosis in chemotherapy exposed testicular tissues after organotypic culture (D30) or in vivo maturation (36 dpp). (a-b) Representative images of Ki67 immunostaining in chemotherapy exposed or unexposed testicular tissues at 36 dpp (a) and D30 (b). (c) Mean percentage of seminiferous tubules containing proliferating cells (Ki67-positive tubules). (d) Mean percentage of Ki67-positive (proliferating) cells per tubule. (e-f) Representative images of cleaved caspase 3 (CC3) immunostaining in chemotherapy exposed or unexposed testicular tissues at 36 dpp (e) and D30 (f). (g) Mean percentage of seminiferous tubules with apoptotic cells (CC3-positive tubules). (h) Mean percentage of CC3-positive (apoptotic) cells per tubule. Data are presented as mean ± SEM with n = 4 biological replicates for each group. A value of *P < 0.05 was considered statistically significant. Ara-C: cytarabine 20 mg/kg; CC3: cleaved caspase 3; D30: in vitro matured during 30 days; DAPI: 4’,6-diamidino-2-phenylindole; DNR: daunorubicin 1 mg/kg; dpp: days postpartum; 0.9% NaCl: saline solution.
At 36 dpp, the proportion of CC3 + seminiferous tubules was not significantly different between the Ara-C (5.3% vs. 14.2%; P = 0.3984) and DNR (22.6% vs. 14.2%; P > 0.9999) groups and the control group (Fig. 4g). Similarly, the mean percentage of CC3 + cells per tubule showed no significant difference in Ara-C (0.4% vs. 0.6%; P = 0.7550) and DNR- treated groups (0.9% vs. 0.6%; P = 0.2827) compared to the 0.9% NaCl group (Fig. 4h). After 30 days of in vitro maturation (D30), no significant differences were found for these two parameters between tissues exposed to Ara-C (14.3% vs. 24.3% and 3.7% vs. 5.2%; P = 0.3058 and P = 0.5615, respectively) or DNR (25.8% vs. 24.3% and 6.6% vs. 5.2%; P > 0.9999 and P = 0.8655, respectively) and unexposed tissues (Fig. 4g-h). However, the percentage of CC3 + tubules was higher in Ara-C-exposed tissues after in vitro maturation (D30) than after in vivo maturation (36 dpp) (14.17% vs. 5.00%; P = 0.0286) (Fig. 4g). Moreover, the percentage of CC3 + cells per tubule was higher in in vitro matured tissues than in in vivo controls, regardless of treatment (P = 0.0286 for all comparisons) (Fig. 4h).
Impact of Ara-C and DNR on DNA integrity
The presence of DNA double-strand breaks in intratubular cells was also examined (Fig. 5a-b). At 36 dpp, there was also no difference in the mean percentage of γH2A.X + cells per tubule between Ara-C (29.1% vs. 34.8%; P = 0.0997) and DNR (27.5% vs. 34.8%; P = 0.0620) treated animals and control animals (Fig. 5c). At D30 however, the percentage of γH2A.X + cells per tubule was significantly higher in DNR-exposed tissues than in control tissues (76.56% vs. 66.04%; P = 0.0285) (Fig. 5c). Moreover, a significant increase in the percentage of γH2A.X + cells per tubule was observed in in vitro matured tissues compared to 36 dpp testes (P = 0.0286) (Fig. 5c).
Fig. 5.
DNA double-strand breaks in mouse testicular tissues and DNA fragmentation in spermatozoa extracted from chemotherapy-exposed testicular tissues after in vitro (D30) or in vivo maturation (36 dpp). (a-b) Representative images of DDX4 (germ cells, green) and γH2A.X (DNA double-strand breaks, red) double immunostaining in chemotherapy exposed or unexposed testicular tissues at 36 dpp (a) and D30 (b). (c) Mean percentage of γH2A.X-positive germ cells per tubule at D30 and 36 dpp. (d) Representative images of testicular spermatozoa (from 36 dpp mice) presenting DNA fragmentation (TUNEL+, green) or not (TUNEL-, detection with DAPI counterstaining). Red arrow: TUNEL- spermatozoon, yellow arrow: TUNEL + spermatozoon. (e) Mean percentage of in vitro and in vivo-produced spermatozoa showing DNA fragmentation. Data are presented as mean ± SEM with n = 4 biological replicates for each group. A value of *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001 was considered statistically significant. Ara-C: cytarabine 20 mg/kg; D30: in vitro matured during 30 days; DAPI: 4’,6-diamidino-2-phenylindole; DDX4: DEAD-Box Helicase 4; DNA: deoxyribonucleic acid; DNR: daunorubicin 1 mg/kg; dpp: days postpartum; γH2A.X: phosphorylated form of histone H2A.X; 0.9% NaCl: saline solution; TUNEL: terminal deoxynucleotidyl transferase dUTP Nick End Labeling.
Finally, TUNEL assays performed on spermatozoa extracted from 36 dpp mice or 30-day organotypic cultures to assess the impact of chemotherapy and in vitro culture on sperm DNA integrity (Fig. 5d). At 36 dpp, an increase in the percentage of spermatozoa with DNA fragmentation was observed in the Ara-C and DNR groups compared to the 0.9% NaCl group (27.25% vs. 8.25%; P = 0.0004) (Fig. 5e). Nevertheless, the percentage of TUNEL + spermatozoa extracted from in vitro matured testicular tissues at D30 was not significantly different between Ara-C (48% vs. 39%; P = 0.1993) or DNR (35% vs. 39%; P = 0.5580) groups and control group (Fig. 5e). Finally, a higher proportion of TUNEL + spermatozoa was extracted from in vitro matured tissues pre-exposed to 0.9% NaCl (39.00% vs. 8.25%; P < 0.0001) or Ara-C (48.00% vs. 27.25%; P = 0.0022) than from their respective in vivo controls (Fig. 5e).
Discussion
During the infancy, the testis is the site of crucial developmental processes necessary for normal adult function36. Any exposure to gonadotoxic substances during this period may cause damage affecting the patient’s future reproductive outcome, potentially leading to fertility problems. Cytarabine (Ara-C) and daunorubicin (DNR), two chemotherapeutic agents considered to present a low risk to fertility, can be administered to young boys with cancer (including leukaemia) before testicular tissue cryopreservation, a fertility preservation option offered before treatment intensification34. Because of the scarcity of testicular tissues from children treated with chemotherapy available for research and the difficulty of assessing the effect of each drug individually in human tissues, prepubertal animal models treated by mono-chemotherapy are being developed to better understand the side effects of the treatments administered. In the present study, we assessed in a juvenile mouse model whether exposure to Ara-C or DNR could impair the initiation of a spermatogenic wave and sperm production, particularly in the context of fertility restoration by in vitro maturation of prepubertal testicular tissues.
The effects of Ara-C or DNR were first evaluated at 6 dpp (3 days post-injection, before the initiation of meiosis). The administration of 20 mg/kg Ara-C had no short-term impact on the testes, as no differences were observed in testicular/body weight ratio, spermatogonia/Sertoli cells ratio, cell proliferation, apoptosis and DNA strand breaks at 6 dpp compared with unexposed mice. The individual effect of DNR on the prepubertal testis has never been studied. Here, we show for the first time that prepubertal exposure to 1 mg/kg DNR resulted in a decrease in the testicular/body weight ratio as early as 3 days post-injection, with a halving of the spermatogonia/Sertoli cells ratio and a reduction in intratubular cell proliferation. Because of their high mitotic activity, spermatogonia are known to be particularly sensitive to chemotherapy. A reduction in the number and proliferative capacity of SSCs in 5 dpp mouse testes and of rat GC-6spg cells had previously been reported after their in vitro exposure to 0.01–0.05 µg/mL doxorubicin, a DNR analogue13–15. Moreover, an increase in the number of apoptotic spermatogonia was observed 48 h after the injection of a 3 mg/kg dose of doxorubicin to 6 dpp rats16.
We then explored the effects of Ara-C or DNR at 36 dpp (over a month after injection, at the end of the first spermatogenic wave) to ascertain that the chemotherapeutic agents exhibit a low gonadotoxicity by not interfering with the initiation and progression of spermatogenesis. At 36 dpp, no impact of Ara-C on testicular/body weight ratio, testicular tissue integrity, germ cells/Sertoli cells ratio, cell proliferation, apoptosis and germ cell DNA integrity was found. Additionally, a complete spermatogenesis was observed in more than 80% of seminiferous tubules. In another study, repeated injections of Ara-C (50, 100 and 200 mg/kg/day) to rats between 29 and 42 dpp resulted in decreased testicular and epididymal weights, reduced cell proliferation, increased apoptosis and DNA damage in a dose-dependent manner8. Ara-C was also found to be gonadotoxic when a dose higher than ours (140 mg/kg) was administered to 14 dpp mice9. The gonadotoxicity reported in these two studies is probably a consequence of the doses used or their daily injections8,9. The dose of Ara-C used in our study (20 mg/kg) is equivalent to 60 mg/m² in humans, which corresponds to the concentrations typically used in the induction treatment of leukemia. Moreover, it is important to point out that our animal model of chemotherapy exposure better mimics the clinical scenario encountered in the treatment of childhood cancers, unlike the aforementioned studies in which Ara-C was administered after the onset of meiosis. In our study, the only negative impact of Ara-C was an increase in the proportion of testicular spermatozoa with fragmented DNA compared with controls at the end of the first spermatogenic wave. This result suggests a likely incorporation of the active metabolite Ara-CTP into spermatogonial DNA, which would persist and cause DNA damage. Indeed, whereas the high-fidelity replicative polymerase δ can be blocked from DNA replication by Ara-C insertion and lead to cell death, the lower-fidelity translesion synthesis DNA polymerase is able to continue DNA replication from the Ara-C residue, leading to persistence of damaged DNA throughout spermatogenesis37,38.
In DNR-exposed tissues, although a significant reduction in seminiferous tubule diameter was found at 36 dpp, no difference was observed in testicular/body weight ratio and germ cell content. Moreover, lumen formation in the seminiferous tubules was not significantly affected, although we cannot exclude that statistical significance would have been reached with additional replicates. Furthermore, prepubertal exposure to DNR did not interfere with the progression of the first wave of spermatogenesis, and had no impact on intratubular cell proliferation or apoptosis. However, more spermatozoa with fragmented DNA were extracted from DNR-exposed tissues than from unexposed tissues at 36 dpp. Anthracyclines are indeed known to cause DNA damage through the formation of reactive oxygen species39. For DNR, no comparison can be made with the literature as our study is the first to assess its individual effect on the prepubertal testis. In a previous study in which the impact of doxorubicin was studied, exposure of rats to a dose of 3 mg/kg before the onset of meiosis severely damaged testicular morphology, impaired spermatogenesis and induced persistent sterility17. The dose of DNR used in this study (1 mg/kg) is equivalent to 3 mg/m² in humans, which is lower than that administered to children with leukemia in the clinics. However, it corresponds to the maximum dose tolerated by mouse pups. Higher chemotherapy concentrations lead to excessive cardiotoxicity in the neonatal mouse40,41.
Overall, this in vivo part of the study gives reassuring results, indicating that the chemotherapeutic agents administered before puberty were indeed of low gonadotoxicity. For patients who received highly gonadotoxic treatments (resulting in no spermatogenesis in vivo), the use of cryopreserved prepubertal testicular tissues pre-exposed to low-risk chemotherapy could therefore be a valuable option for restoring fertility.
In the context of using chemotherapy-exposed prepubertal testicular tissues to produce spermatozoa in vitro, we assessed for the first time the possibility of obtaining a full wave of spermatogenesis in organotypic cultures after exposure to Ara-C or DNR. To this end, we reproduced in the mouse model the sequence that could be proposed to certain patients: in vivo administration of low doses of chemotherapy before puberty initiation, followed by in vitro maturation of prepubertal testicular tissues.
We show that prepubertal exposure to Ara-C did not interfere with the completion of in vitro spermatogenesis. No changes in germ cells/Sertoli cells ratio, cell proliferation and apoptosis were observed after in vitro maturation in Ara-C-treated tissues compared to controls. However, a more pronounced arrest in meiosis was found in Ara-C-exposed tissues, with a greater percentage of seminiferous tubules arrested at the leptotene/zygotene spermatocyte stage and a decrease in the content of pachytene spermatocytes. A comparable proportion of spermatozoa with fragmented DNA was extracted after a 1-month culture period from Ara-C-exposed or unexposed tissues. Similarly, prepubertal DNR exposure did not alter the ability of spermatogonia to differentiate in vitro into spermatozoa. Indeed, DNR exposure did not affect germ cell content, cell proliferation and apoptosis, and elongated spermatids were found in similar proportions after in vitro maturation in DNR-exposed and unexposed tissues. Although a higher proportion of intratubular cells with DNA strand breaks (γH2A.X+) was found in DNR-treated tissues than in control tissues after 30 days of culture, a similar proportion of spermatozoa with DNA damage was extracted from DNR-exposed and unexposed tissues. One of the mechanisms responsible for anthracycline cytotoxicity is the induction of DNA strand breaks through inhibition of topoisomerase II11. These lesions induce the phosphorylation of histone H2A.X into γH2A.X, the first step in the recruitment of DNA repair proteins42. This in vitro study therefore provides encouraging evidence that mouse spermatozoa can be produced in vitro in tissues that have been exposed to low gonadotoxic doses of Ara-C or DNR, showing that in vitro spermatogenesis could indeed be a potential fertility restoration option.
In order to assess the impact of organotypic culture conditions, we compared in vivo and in vitro-matured tissues. The organotypic culture conditions had an impact on the progression of in vitro spermatogenesis, regardless of treatment. Indeed, as previously described30,43,44, a complete spermatogenesis can be achieved under our in vitro culture conditions, albeit with a drastic reduction in spermatid production compared to age-matched in vivo controls. In the present study, the majority of seminiferous tubules were arrested at the pachytene spermatocyte stage, with a greater proportion of cells showing DNA strand breaks and undergoing apoptosis per seminiferous tubule, and the proportion of spermatids did not exceed 2% of intratubular cells. The proportion of spermatozoa with DNA fragmentation recovered from in vitro matured testicular tissues ranged from 35 to 48%, in line with a previous study31, irrespective of the treatment administered. Efforts are therefore essential to optimize sperm production in organotypic cultures. The use of KSR will have to be avoided in the future as this component has been linked to DNA instability45. KSR has also been shown to block the entry into mitotic arrest important for the differentiation of Sertoli cells and germ cells45. Its use in our study could thus explain, at least in part, the higher proportions of intratubular cells with DNA strand breaks in organotypic cultures than in 36 dpp testes.
An alternative approach to restore fertility in pediatric cancer survivors could be the development of optimal procedures for the detection and removal of malignant cells from frozen/thawed prepubertal testicular biopsies, the isolation of SSCs and their propagation in vitro before autotransplantation.
In addition to the necessity of gaining a better understanding of the repercussions of chemotherapy administered prior to puberty and the imperative need to develop optimal procedures of fertility restoration, more attention also needs to be paid to the impact of the cancer itself on the prepubertal testis. Indeed, the disease has been found to cause a reduction in the quantity of spermatogonia in the testicular tissue of patients before any gonadotoxic treatment was introduced46.
In conclusion, our results demonstrate for the first time the possibility of achieving complete in vitro spermatogenesis in testicular tissues after prepubertal exposure to low gonadotoxic doses of Ara-C or DNR. These findings are promising for patients who have received chemotherapy prior to cryopreservation of their prepubertal germinal tissue and for whom the in vitro maturation approach could be proposed. However, further research is needed to optimize the organotypic culture system and analyze the quality of in vitro produced spermatozoa before this approach can be transposed to the clinic.
Methods
Ethical approval
All the experimental procedures were approved by the Institutional Animal Care and Use Committee of University of Rouen Normandy under the protocol number APAFiS #18.208 and were carried out in accordance with relevant guidelines, regulations, and recommendations, including the ARRIVE guidelines.
Mice and collection of testes
CD-1 mice (Charles River Laboratories, L’Arbresle, France) were housed in a temperature-controlled room (22–23 °C) under a 12-hour light/dark cycle. To adhere to the 3Rs principle, we have reduced our groups to n = 4 mice per group.
The animals were randomly divided into three different groups: 0.9% NaCl, Ara-C and DNR groups. Prepubertal 3-day-old mice received either a saline solution (0.9% NaCl group) or a low gonadotoxic dose of cytarabine (Ara-C group) or daunorubicin (DNR group). The treatments were administered intraperitoneally in a single dose of 20 mg/kg for cytarabine (Aracytine 2 g, Pfizer, Paris, France) or 1 mg/kg for daunorubicin (Cérubidine 20 mg, Sanofi Aventis, Paris, France). The drugs used in our study are the same as those employed in cancer treatment in humans, as well as the composition of the excipients. The doses used have been adapted to the mouse model with a standard factor to interspecies doses conversion, as recommended by the Food and Drug Administration47. The dose of DNR corresponds to the highest dose that could be administered to 3 dpp pups while respecting the limit points. Higher doses of DNR resulted in the death of prepubertal mice.
Prepubertal 6-day post-partum (dpp) male mice were euthanized by decapitation and underwent a bilateral orchidectomy. The testes were transferred to Petri dishes containing α-MEM (Gibco by Life Technologies, Saint-Aubin, France) and the tunica albuginea was completely removed using two needles under a binocular magnifier (S8AP0, Leica Microsystems GmbH, Wetzlar, Germany). The testes were then either cultured immediately (culture from fresh tissues), or fixed with Bouin’s solution (Sigma-Aldrich, Saint-Quentin Fallavier, France) or paraformaldehyde (4% PFA, Sigma-Aldrich) to analyze testicular tissue prior to organotypic culture. Moreover, mice aged 36 dpp were euthanized by CO2 asphyxiation and their testes were used as the in vivo controls for 30 days of culture. The testes of these mice were fixed as previously described.
A total of 54 mice were used in this study to evaluate the effects of the chemotherapeutic molecules in the short and medium term, specifically 3 and 33 days after treatment.
Organotypic cultures at a gas–liquid interphase
In vitro tissue cultures were performed at a gas-liquid interphase following the methods described previously28,30. Briefly, 6 dpp mouse testes containing only spermatogonia as germ cells were first cut into four fragments. They were placed on top of 1.5% (w/v) agarose gels (Sigma-Aldrich) half soaked in medium. The culture medium contained α-MEM, 10% KSR (KnockOut Serum Replacement, Gibco by Life Technologies) and 5 µg/mL gentamycin (Sigma-Aldrich). The medium was replaced every 4 days and retinol (10− 6 M, Sigma-Aldrich) was added from the second day of culture and then every 8 days in order to respect the cycle of meiotic entry of spermatogonia30. Testicular tissues were cultured under 5% CO2-95% air at 34 °C for 30 days.
Histological and immunohistochemical analyses
Tissue fixation, processing and sectioning
Testicular tissues were fixed in Bouin’s solution or 4% PFA at room temperature. They were then dehydrated in ethanol in the Citadel 2000 tissue processor (Shandon, Cheshire, UK) and embedded in paraffin. Tissue Sect. (3 μm thick) were cut on an RM2125 RTS microtome (Leica) and were mounted on polysine slides (Thermo Fisher Scientific, Waltham, MA, USA). Two sections -separated by 36 μm- were analysed for each technique and for each replicate.
TRA98 immunohistochemical staining and periodic acid schiff (PAS) reaction
Tissue sections were deparaffinized in xylene and rehydrated in a graded series of decreasing concentrations of ethanol. Endogenous peroxidases were blocked with HP Block (Dako, Les Ulis, France) for 5 min and non-specific binding sites were blocked with Ultra-V Block solution (Thermo Scientific) for 5 min at RT. Tissue sections were then incubated at RT with anti-TRA98 antibodies (Table 3) for 30 min. After three 5-minute washes in PBS, they were incubated for 30 min at RT with rabbit anti-rat biotinylated secondary antibodies (Table 3) and then for 30 min at RT with goat anti-rabbit biotinylated tertiary antibodies (Table 3). After three 5-minute washes in PBS, incubation with peroxidase-conjugated streptavidin (UltraVision Detection System HRP kit, Thermo Scientific) was performed for 5 min at RT. Labeling was revealed after application of a chromogenic substrate (3,3’-diaminobenzidine tetrahydrochloride, Thermo Scientific) for 1 min at RT. A PAS reaction was then carried out. The slides were immersed in 0.5% periodic acid (Thermo Scientific) for 10 min, rinsed for 5 min in water and then placed for 30 min in Schiff’s reagent (Merck, Darmstadt, Germany). After three 2-minute washes in water, sections were counterstained with Mayer’s haematoxylin and mounted with Eukitt (CML, Nemours, France). Images were acquired on a DM4000B microscope (Leica).
Table 3.
Detailed list of antibodies used in this study.
| Manufacturer | Reference | Dilution | ||
|---|---|---|---|---|
| IHC | IF | |||
| Primary antibody | ||||
| TRA98 | Abcam | ab-82,527 | 1:200 | |
| Ki67 | Abcam | ab16667 | 1:200 | |
| CC3 | Abcam | ab49822 | 1:200 | |
| DDX4 | Abcam | ab13840 | 1:1000 | |
| γH2A.X | Merck | JBW301 | 1:1000 | |
| Secondary antibody | ||||
| Biotinylated rabbit anti-rat | Abcam | ab6733 | 1:200 | |
| Biotinylated goat anti-rabbit | Abcam | ab6720 | 1:200 | |
| Biotinylated goat anti-mouse | Abcam | ab6788 | 1:200 | |
| Alexa 594-conjugated streptavidin | Invitrogen | S32356 | 1:500 | |
| Alexa 488-conjugated goat anti-rabbit | Abcam | ab150077 | 1:200 | |
IF: immunofluorescence; IHC: immunohistochemistry.
TRA98 immunostaining allowed the identification of spermatogonia, leptotene, zygotene and early pachytene spermatocytes. In addition, the PAS reaction performed on tissues at 36 dpp and D30 allowed the identification of the acrosome of round and elongated spermatids and thus to evaluate the progression of in vivo and in vitro spermatogenesis. In in vitro matured testicular tissue fragments, it was assessed in seminiferous tubules located at the periphery (i.e. outside the central necrotic zone). The structural integrity of testicular tissues was assessed through the measurement of the diameter of seminiferous tubules with the LAS Module Interactive Measurements software and of the vacuolar area with FIJI (version 2.3.0). Seminiferous tubule lumen formation was scored semi-quantitatively on a scale from 0 to 3 as follows: 0 (absence of a lumen), 1 (absence of lumen and loose contact between the basal and adluminal compartments in seminiferous tubule), 2 (tubule with accumulation of cells within the lumen), 3 (complete achievement of the lumen formation).
Ki67, cleaved caspase 3, γH2A.X and DDX4 Immunofluorescence staining
Proliferation and apoptosis in intratubular cells were assessed using immunofluorescence staining for Ki67 and cleaved caspase 3, respectively. Germ cells with DNA double-strand breaks were identified through the combined detection of γH2A.X and DDX4. Tissue sections were deparaffinized, rehydrated, and washed in PBS with 0.05% Tween-20 (PBST) for 3 min before being boiled in 10 mM citrate buffer pH 6.0 (Diapath, Martinengo, Italy) for 40 min at 96 °C. The samples were cooled at RT for 20 min and then rinsed with distilled water for 5 min. To perform Ki67 immunostaining, a permeabilization step was carried out with 0.1% (v/v) Triton X-100 (Sigma-Aldrich) at RT for 15 min. Non-specific binding sites were blocked with 5% (w/v) bovine serum albumin (BSA, Sigma-Aldrich) and 5% (v/v) horse serum (Sigma-Aldrich) or 20% (v/v) goat serum for dual γH2A.X/DDX4 immunostaining. The slides were incubated with primary antibodies (Table 3), rinsed 3 times in PBST, and then incubated with the appropriate secondary antibodies (Table 3). The sections were washed, dehydrated with ethanol and mounted in Vectashield with DAPI (4’,6-diamidino-2-phenylindole). Tissue sections were observed with a fluorescence microscope (DM6B, Leica) coupled with LAS X + THUNDER software. For each tissue, the analysis was performed on two levels and 30 cross-sectioned tubules (located outside the necrotic area for in vitro matured tissues). The percentage of seminiferous tubules containing at least one Ki67 + or CC3 + positive cell was calculated by dividing the number of positive tubules by the total number of tubules analysed. To determine the percentage of positive cells per positive tubule, the number of Ki67 + or CC3 + positive cells was divided by the number of intratubular cells. Similarly, the percentage of germ cells with DNA double-strand breaks per tubule was calculated by dividing the number of γH2A.X+/DDX4 + cells by the number of DDX4 + cells.
TUNEL assay
The central necrotic area of in vitro matured testicular fragments was removed in 1 mL of α-MEM using two 27-gauge needles under binocular magnifying glasses. The tissues were then dilacerated to obtain a suspension of testicular cells. 36 dpp testes were also dilacerated in 1 mL of α-MEM after removal of the albuginea. The resulting cell suspensions were centrifuged for 10 min at 300×g. The testicular cells were fixed for 1 h at 4 °C in a methanol/acetic acid mixture. They were then spread on SuperFrost Plus slides (Thermo Fisher). After a 10-minute bath in PBS, the slides were immersed in acetone (Carlo Erba Reagents, Val-de-Reuil, France) for 2 min to allow permeabilization. After two rinses in PBS, DNA fragmentation was detected by the TUNEL technique using the In Situ Cell Death Detection POD kit (Roche, Mannheim, Germany) following the manufacturer’s instructions. The slides were then mounted with VectaShield containing DAPI after two 10-minute baths in PBS and ethanol baths of increasing concentrations. A positive control was performed by preincubating testicular cells with 50 µg/mL DNase I (Sigma-Aldrich) for 15 min at 37 °C before the TUNEL assay. The slides were examined using a fluorescence microscope (Leitz DMRB, Leica). For each 36 dpp testis, 100 spermatozoa were counted, totalling 400 spermatozoa for each in vivo group (0.9% NaCl, Ara-C, DNR). Additionally, 100 spermatozoa from a pool of 24 in vitro matured fragments were analysed.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 8 software (GraphPad Software Inc., La Jolla, CA, USA). The data were presented as mean and standard error of the mean (mean ± SEM). Non-parametric tests were used due to the small sample size (n = 4), as they are more robust and less likely to give biased results with small samples. The non-parametric Kruskall Wallis test followed by Dunn’s post hoc tests were used to compare Ara-C and DNR groups with the control 0.9% NaCl group. The non-parametric Mann–Whitney test was used to compare in vitro and in vivo conditions for each group to determine the potential impact of organotypic culture on spermatogenesis. The data obtained from the TUNEL assays were compared using the χ2 test. A P value < 0.05 was considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by a Ph.D. grant from Région Normandie and Ligue Nationale Contre le Cancer (awarded to Marion Delessard), a Ph.D. grant from Région Normandie (awarded to Laura Moutard) and by a funding from Agence de la Biomédecine (#19AMP010 awarded to Christine Rondanino). The funding sources had no role in the design of the study, collection, analysis and interpretation of data, and in writing the manuscript.
Author contributions
M.D., L.M., C.C. led experiments, acquired, analysed and interpreted data, and drafted the manuscript. M.D. and C.R. conceived the design of the study. L.D., N.R. and C.R. supervised the project, obtained funding and corrected the manuscript. All the authors read and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Marion Delessard, Laura Moutard and Coline Charnay.
References
- 1.Wasilewski-Masker, K. et al. Male infertility in long-term survivors of pediatric cancer: a report from the childhood cancer survivor study. J. Cancer Surviv Res. Pract.8, 437–447 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brougham, M. F. H. & Wallace, W. H. B. Subfertility in children and young people treated for solid and haematological malignancies. Br. J. Haematol.131, 143–155 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Benedict, C., Shuk, E. & Ford, J. Fertility issues in adolescent and young adult cancer survivors. J. Adolesc. Young Adult Oncol.5, 48–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenney, L. B. et al. Improving male reproductive health after childhood, adolescent, and young adult cancer: progress and future directions for survivorship research. J. Clin. Oncol.36, 2160–2168 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Wyns, C., Curaba, M., Vanabelle, B., Van Langendonckt, A. & Donnez, J. Options for fertility preservation in prepubertal boys. Hum. Reprod. Update. 16, 312–328 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Kaplan, J. A. Leukemia in children. Pediatr. Rev.40, 319–331 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Kufe, D. W., Munroe, D., Herrick, D., Egan, E. & Spriggs, D. Effects of 1-beta-D-arabinofuranosylcytosine incorporation on eukaryotic DNA template function. Mol. Pharmacol.26, 128–134 (1984). [PubMed] [Google Scholar]
- 8.Namoju, R. C. et al. Pre-pubertal exposure of cytarabine-induced testicular atrophy, impaired spermatogenesis and germ cell DNA damage in SD rats. Toxicol. Mech. Methods. 24, 703–712 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Khaleel, B., Lunenfeld, E., Kapelushnik, J. & Huleihel, M. Effect of granulocyte colony-stimulating factor on the development of spermatogenesis in the adulthood of juvenile AML mice model treated with cytarabine. Int. J. Mol. Sci.24, 12229 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medrano, J. V. et al. Histologic analysis of testes from prepubertal patients treated with chemotherapy associates impaired germ cell counts with cumulative doses of cyclophosphamide, Ifosfamide, cytarabine, and asparaginase. Reprod. Sci.28, 603–613 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz, D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and Daunorubicin. Biochem. Pharmacol.57, 727–741 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Allen, M., Lopes, F. & Mitchell, R. T. & Spears, N. How does chemotherapy treatment damage the prepubertal testis? Reproduction 156, R209–R233 (2018). [DOI] [PMC free article] [PubMed]
- 13.Smart, E. et al. Chemotherapy drugs cyclophosphamide, cisplatin and doxorubicin induce germ cell loss in an in vitro model of the prepubertal testis. Sci. Rep.8, 1773 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes, F. et al. Chemotherapy induced damage to spermatogonial stem cells in prepubertal mouse in vitro impairs long-term spermatogenesis. Toxicol. Rep.8, 114–123 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaud, H., van Pelt, A. & Delbes, G. Doxorubicin and vincristine affect undifferentiated rat spermatogonia. Reprod. Camb. Engl.153, 725–735 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Hou, M. et al. Doxorubicin induces apoptosis in germ line stem cells in the immature rat testis and amifostine cannot protect against this cytotoxicity. Cancer Res.65, 9999–10005 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Bechter, R., Haebler, R., Ettlin, R. A., Haseman, J. K. & Dixon, R. L. Differential susceptibility of immature rat testes to doxorubicin at critical stages of maturation. Biochemical and functional assessment. Arch. Toxicol.60, 415–421 (1987). [DOI] [PubMed] [Google Scholar]
- 18.Brilhante, O., Okada, F. K., Sasso-Cerri, E., Stumpp, T. & Miraglia, S. M. Late morfofunctional alterations of the Sertoli cell caused by doxorubicin administered to prepubertal rats. Reprod. Biol. Endocrinol.10, 79 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabral, R. E. L., Mendes, T. B., Vendramini, V. & Miraglia, S. M. Carnitine partially improves oxidative stress, acrosome integrity, and reproductive competence in doxorubicin-treated rats. Andrology6, 236–246 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Abdelaziz, M. H., Salah EL-Din, E. Y., El‐Dakdoky, M. H. & Ahmed, T. A. The impact of mesenchymal stem cells on doxorubicin‐induced testicular toxicity and progeny outcome of male prepubertal rats. Birth Defects Res.111, 906–919 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Vendramini, V., Robaire, B. & Miraglia, S. M. Amifostine–doxorubicin association causes long-term prepubertal spermatogonia DNA damage and early developmental arrest. Hum. Reprod.27, 2457–2466 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Tran, K. T. D., Valli-Pulaski, H., Colvin, A. & Orwig, K. E. Male fertility preservation and restoration strategies for patients undergoing gonadotoxic therapies. Biol. Reprod.107, 382–405 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goossens, E. et al. Fertility preservation in boys: recent developments and new insights. Hum. Reprod. Open hoaa016 (2020). (2020). [DOI] [PMC free article] [PubMed]
- 24.Stukenborg, J. B. & Wyns, C. Fertility sparing strategies for pre- and peripubertal male cancer patients. Ecancermedicalscience14, 1016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ntemou, E., Alexandri, C., Lybaert, P., Goossens, E. & Demeestere, I. Oncofertility: Pharmacological protection and immature testicular tissue (ITT)-based strategies for prepubertal and adolescent male cancer patients. Int. J. Mol. Sci.20, 5223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pampanini, V. et al. Fertility preservation for prepubertal patients at risk of infertility: present status and future perspectives. Horm. Res. Paediatr.93, 599–608 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Wyns, C., Kanbar, M., Giudice, M. G. & Poels, J. Fertility preservation for prepubertal boys: lessons learned from the past and update on remaining challenges towards clinical translation. Hum. Reprod. Update. 27, 433–459 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Sato, T. et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature471, 504–507 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Yokonishi, T. et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat. Commun.5, 4320 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Arkoun, B. et al. Retinol improves in vitro differentiation of pre-pubertal mouse spermatogonial stem cells into sperm during the first wave of spermatogenesis. PLoS One. 10, e0116660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oblette, A. et al. Assessment of sperm nuclear quality after in vitro maturation of fresh or frozen/thawed mouse pre-pubertal testes. Mol. Hum. Reprod.23, 674–684 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Abu Elhija, M., Lunenfeld, E., Schlatt, S. & Huleihel, M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J. Androl.14, 285–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bashiri, Z. et al. In vitro production of mouse morphological sperm in artificial testis bioengineered by 3D printing of extracellular matrix. Int. J. Biol. Macromol.217, 824–841 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Valli-Pulaski, H. et al. Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum. Reprod.34, 966–977 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delessard, M. et al. Achievement of complete in vitro spermatogenesis in testicular tissues from prepubertal mice exposed to mono- or polychemotherapy. Sci. Rep.12, 7407 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chemes, H. E. Infancy is not a quiescent period of testicular development. Int. J. Androl.24, 2–7 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Rechkoblit, O. et al. Structural insights into mutagenicity of anticancer nucleoside analog cytarabine during replication by DNA polymerase η. Sci. Rep.9, 16400 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon, J. H., Choudhury, R., Prakash, J., Prakash, S. & L. & Translesion synthesis DNA polymerases Η, Ι, and Ν promote mutagenic replication through the anticancer nucleoside cytarabine. J. Biol. Chem.294, 19048–19054 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinstein, E., Canaani, E. & Weiner, L. M. Dependence of nucleic acid degradation on in situ free-radical production by adriamycin. Biochemistry32, 13156–13161 (1993). [DOI] [PubMed] [Google Scholar]
- 40.Huang, C. et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation121, 675–683 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, W., Shou, W., Payne, R. M., Caldwell, R. & Field, L. J. A mouse model for juvenile doxorubicin-induced cardiac dysfunction. Pediatr. Res.64, 488–494 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuo, L. J. & Yang, L. X. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. Vivo22, 305–309 (2008). [PubMed] [Google Scholar]
- 43.Dumont, L. et al. Throughout in vitro first spermatogenic Wave: Next-generation sequencing gene expression patterns of fresh and cryopreserved prepubertal mice testicular tissue explants. Front. Endocrinol.14, 1112834 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moutard, L. et al. Steroidogenesis and Androgen/estrogen signaling pathways are altered in in vitro matured testicular tissues of prepubertal mice. eLife12, RP85562 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogg, K. & Western, P. S. Differentiation of fetal male germline and gonadal progenitor cells is disrupted in organ cultures containing knockout serum replacement. Stem Cells Dev.24, 2899–2911 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Masliukaite, I. et al. Childhood cancer and hematological disorders negatively affect spermatogonial quantity at diagnosis: a retrospective study of a male fertility preservation cohort. Hum. Reprod.38, 359–370 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FDA. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article.