Abstract
Cutaneous T-cell lymphomas (CTCLs), particularly Mycosis fungoides (MF), frequently exhibit deletions and reduced expression of HNRNPK in CD4 + T cells. To enable in vivo studies, we developed a conditional Hnrnpk knockout mouse that thrives, facilitating the investigation of HNRNPK’s role in CTCL onset. We generated mice with a floxed Hnrnpk allele, then crossbred them with Cd4CreERT2 mice to generate Hnrnpk flox Cd4CreERT2 mice, all in BL6 background. PCR confirmed the targeted deletion of Hnrnpk in CD4 + T cells after tamoxifen i.p. injection. Skin allergic reactions were induced with oxazolone, and Cre was activated in skin-infiltrating CD4 + T cells using tamoxifen topically after the first allergic skin reaction. The mice exhibited no immediately obvious phenotype. Flow cytometry and histopathological analysis were conducted on blood and skin samples collected throughout the experiment. Following 20 weeks of sustained allergic reactions, inflammation persisted over 20 weeks after challenges ceased, demonstrating early CTCL characteristics such as chronic skin inflammation, CD3 + CD4 + T cell infiltration, and stable peripheral blood parameters. This mouse model provides experimental access to the complex microenvironment and immune responses involved in early inflammatory stages, providing opportunities for further research into the role of HNRNPK in CTCL and the development of effective therapeutic interventions for this challenging malignancy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-98640-6.
Keywords: Cutaneous T-cell lymphomas, Mycosis fungoides, CD4 + T cells, HNRNPK, Transgenic mouse
Subject terms: Skin cancer, Non-hodgkin lymphoma, Experimental models of disease, Cancer models, Oncogenesis
Introduction
Cutaneous T-cell lymphomas (CTCL) are a diverse group of non-Hodgkin’s lymphomas (NHL) characterized by the localization of neoplastic T lymphocytes to the skin1,2. Recent advancements have significantly contributed to our understanding of the molecular mechanisms underlying CTCL, revealing the involvement of genes such as heterogenous nuclear ribonucleoprotein K (HNRNPK) and Suppressor of cytokine signaling 1(SOCS1)3–5. However, a comprehensive understanding of CTCL requires the development of appropriate model systems that can capture the complex nature of the disease and facilitate the identification of effective therapeutic strategies6.
The role of the tumor microenvironment within the lesions in the development, progression and treatment resistance of CTCL has become an important area of research due to its complicated pathogenesis7. In vitro models, although valuable, have limitations in simulating essential factors such as the tumor microenvironment and intact immune system, emphasizing the need for in vivo models8–10. Therefore, more sophisticated tools, such as genetically engineered mouse models, are indispensable for comprehensively exploring T-cell malignancies.
HNRNPK, a DNA and RNA-binding protein, regulates numerous cellular processes through transcriptional, posttranscriptional, and translational mechanisms11,12. Notably, its oncogenic and tumor-suppressive functions are context-dependent. Both overexpression and abnormally low expression of HNRNPK can be pathogenic, likely due to the dysregulation of multiple cellular oncogenes or tumor suppressor genes13. In the context of hematological malignancies, it has been observed that a 9q21.32 deletion encompassing the HNRNPK gene occurs in acute myeloid leukemia patients, resulting in the loss of one copy of HNRNPK14,15. Interestingly, the recurrent deletion of HNRNPK has been reported in mycosis fungoides (MF) and other CTCL subtypes16,17. Therefore, there is a compelling need to establish a CD4 + T-cell-specific murine model of Hnrnpk that accurately represents the intact immune system and tumor microenvironment. Such a model would be pivotal in elucidating the intricate and multifaceted role of HNRNPK in CTCL, ultimately leading to a better understanding of the disease and developing more effective therapeutic interventions.
This work describes a novel Hnrnpk mouse model designed based on the CTCL gene sequencing results. Here, we primarily wanted to examine the basic impact of Hnrnpk-ko limited to CD4 + T cells in an inflamed skin site on subsequent chronic (allergic) inflammation at this site as suspected prelude to CTCL. In this mouse model, the immune homeostasis in peripheral blood remains relatively undisturbed under chronic antigen exposure, while T-cell infiltration is confined to the skin. The skin lesions predominantly exhibit lymphocytic infiltration characterized by CD3 + CD4 + cells, and the infiltration persists without regression, aligning with early-stage CTCL characteristics.
Result
Generation of Hnrnpk flox and Hnrnpk flox Cd4CreERT2 mice.
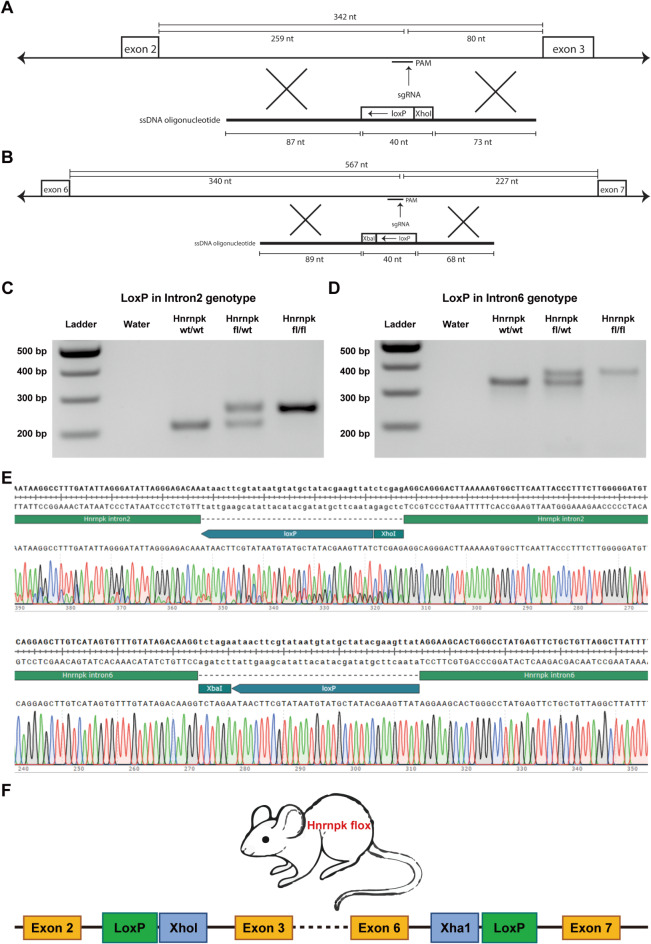
To generate conditional knockout Hnrnpk mice, we employed CRISPR/Cas9 RNPs and 200 bp single-stranded oligodeoxynucleotides (ssODN) to sequentially target intron 2 and in the resulting mouse intron 6 of the Hnrnpk gene, in murine oocytes on C57BL/J background We designed the 5’ ssODN (for intron 2) with a LoxP site, and introduced it into oocytes along with the CRISPR/Cas9 RNP complex.(Fig. 1A) Additionally, we designed the 3’ ssODN (for intron 6) with a LoxP site and 3’ HA, and introduced it into the resulting mouse line to target the LoxP site in intron 6. (Fig. 1B) (gRNA, ssODN listed in Table S1).
Fig. 1.
(A, B) The structure of designed single-stranded oligodeoxynucleotides (ssODN) for intron 2 and intron 6 of Hnrnpk. (C, D) Agarose gel electrophoresis image showing the PCR product of LoxP inserted in intron 2 and intron 6 of Hnrnpk. (Original gel is presented in Supplementary Fig. 1). (E) Sanger sequencing chromatogram of both modifications. (F) The structure of the Hnrnpk flox modified gene with two LoxP sites in Hnrnpk flox mouse.
We screened the offspring for the correct insertion of the LoxP sites on genomic DNA from ear-clip biopsies by PCR and Sanger Sequencing. (Fig. 1C, D and E) (Original gel is presented in Supplementary Fig. 1). (Primers listed in Table S2) To confirm correct integration loxP site in intron 6, TOPO-TA PCR product subcloning was performed. As shown in Fig. 1F, the Hnrnpk flox mice were inserted with two loxP sites flanking exon 3 to 5. We bred these conditional mice to homozygosity and the offspring are healthy.
-
2.
Hnrnpk knockout in CD4 + T cells confirmation.
To induce Hnrnpk knockout in CD4 + T cells, we crossed Cd4CreERT2 mice with Hnrnpk flox mice. Details of the cross-breeding scheme can be found in Fig. 2A. In Cd4CreERT2 mice, Cre recombinase is controlled by the Cd4 gene promoter and can be induced by tamoxifen. Homozygous Hnrnpk flox (Hnrnpk fl/fl) mice were crossed with the transgenic mice expressing Cre recombinase under the control of the Cd4 promoter (Cd4CreERT2 mice) to yield heterozygous littermates with the Cd4CreERT2 transgene. Offspring inherited both the targeted Hnrnpk fl/wt allele and the Cd4CreERT2 transgene.
Fig. 2.
(A) Breeding scheme used to create Hnrnpk flox/wt Cd4CreERT2 mouse. (B) Schematic diagram illustrating the Hnrnpk knockout using Tamoxifen in Hnrnpk flox/wt Cd4CreERT2 mouse. Tamoxifen was administered to mice for 5 continuous days via intraperitoneal injection (i.p.). On day 8, the spleen was collected, and CD4 + T cells were enriched. TAM: tamoxifen, i.p: intraperitoneal. (C) Representative flow cytometry plots showing CD3 + CD4 + T cells in splenocytes before and after CD4 + T cell enrichment. (D) Agarose gel electrophoresis image displaying the PCR product of recombinant Hnrnpk following Hnrnpk deletion (Hnrnpk KO) using three different sets of primers, and unrecombined Hnrnpk (Hnrnpk WT) also using three different sets of primers. (Original gel is presented in Supplementary Fig. 2).
In Hnrnpk flox Cd4CreERT2 mice, tamoxifen can selectively knock out Hnrnpk in CD4 + T cells. After five consecutive days of intraperitoneal tamoxifen injections in Hnrnpk fl/wt Cd4CreERT2 mice, splenocytes were collected and CD4 + T cells were enriched. (Fig. 2B) Flow cytometry analysis showed that CD4 + T cells accounted for approximately 94.1% after enrichment. (Fig. 2C). DNA was extracted from the enriched CD4 + T cells. Successful Hnrnpk deletion in CD4 + T cells of Hnrnpk flox Cd4CreERT2 mice treated with tamoxifen was confirmed through PCR analysis (primers listed in Table S3) showing the recombinant Hnrnpk (Hnrnpk KO). In contrast, the spleen CD4 + T cells enriched from mice not receiving tamoxifen injections showed no recombinant gene fragments following Hnrnpk deletion (Hnrnpk WT). (Fig. 2D) (Original gel is presented in Supplementary Fig. 2).
The flow cytometry results on the splenocytes from another 2 Hnrnpk fl/wt Cd4CreERT2 mice showed Hnrpnk protein level in CD4 + T cells was reduced after tamoxifen injection compared to two Hnrnpk fl/wt Cd4CreERT2 mice that did not receive tamoxifen (control group). (Supplementary Fig. 3)
-
3.
T-cell infiltration limited to cutaneous region and undisturbed immune homeostasis in peripheral blood in the conditional knockout mice with single-copy Hnrnpk deficiency in skin-resident CD4 + T cells.
In this experiment, Hnrnpk fl/wt Cd4CreERT2 mice were used to induce sustained chronic inflammation in the skin using OXA and topically applied tamoxifen to knock out Hnrnpk in CD4 + T cells within the skin. (Fig. 3A) One flank was treated and the collateral flank was left untreated as matched internal control. The specific application of reagents was implemented as previously described18,19. From these earlier experiments we know that without tamoxifen (no recombination at floxed sites) the chronic inflammation faded after discontinuation of the allergic challenges to a level observed in untreated flanks in the test animals.
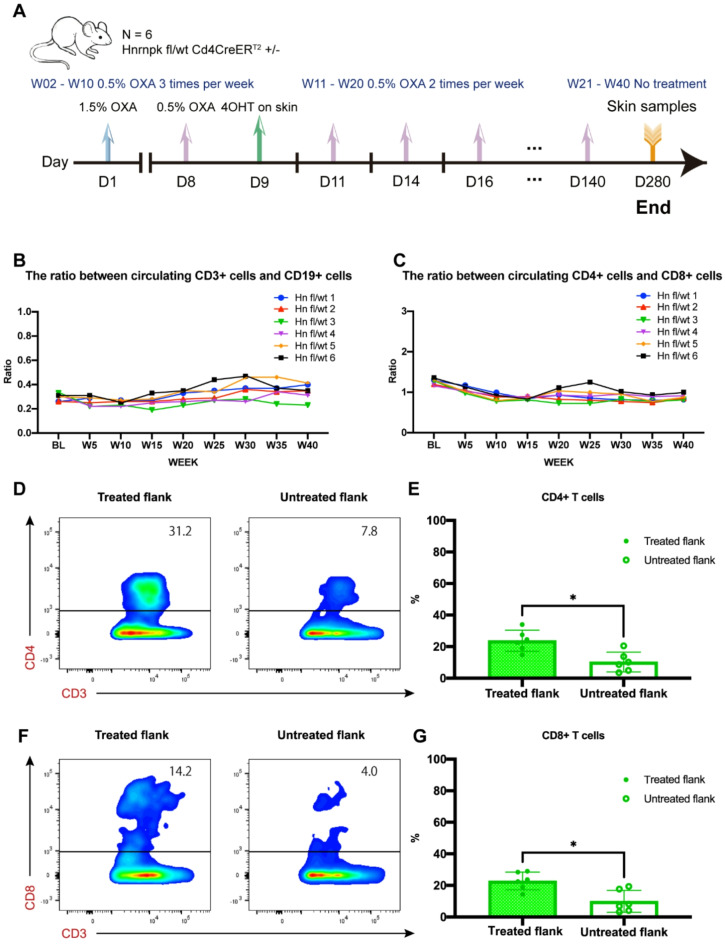
Fig. 3.
(A) Schematic diagram illustrating the experiment in Hnrnpk flox Cd4CreERT2 transgenic mice. D: day, W: week, OXA: oxazolone, 4OHT: 4-hydroxy-tamoxifen. Blood collection was performed 24 h after OXA application. Skin samples were collected at the end of the experiment. (B, C) Overview of the ratio between CD3 + cells and CD19 + cells, and between CD4 + cells and CD8 + cells in peripheral blood throughout the experiment. (D, E) Representative flow cytometry plots and quantitative analysis of CD3 + CD4 + T cells in treated and untreated flanks. P = 0.0159. (F, G) Representative flow cytometry plots and quantitative analysis of CD3 + CD8 + T cells in treated and untreated flanks. P = 0.0111. The data were presented as means ± SDs. Statistical analysis was performed using a pair t-test. Differences were considered statistically significant when P < 0.05 (*p < 0.05).
Flow cytometry analysis of peripheral blood during the 40-week experiment showed that repeated topical application of low-concentration OXA did not result in significant abnormalities in immune cell populations. The ratio of CD3 + cells to CD19 + cells in peripheral blood of all mice did not exhibit noticeable fluctuations (Fig. 3B). Similarly, the ratio of CD4 + cells to CD8 + cells in peripheral blood of all mice also did not show significant fluctuations. (Fig. 3C). The ratios of immune cell subpopulations remained relatively stable throughout the experiment.
Flow cytometry analysis showed successful cell extraction from mouse skin, and the proportion of CD4 + and CD8 + cells among CD3 + cells was determined. In the cells extracted from the skin, the percentage of CD4 + cells among CD3 + cells in the left flank (treated flank) was significantly higher compared to the percentage of CD4 + cells among CD3 + cells in the right flank (untreated flank) at a statistically significant level. (Fig. 3D,E). Similarly, the percentage of CD8 + cells among CD3 + cells in the left flank was significantly higher than that of CD8 + cells among CD3 + cells in the right flank. (Fig. 3F,G)
-
4.
Increased skin inflammation with dermal infiltrating lymphocytes, primarily characterized by CD3 + CD4 + cells in transgenic mice with single-copy Hnrnpk deficiency in skin-resident CD4 + cells.
In addition to isolation of intact cells from mouse skin, we made sections of paraffin embedded formalin fixed biopsies of mouse skin and performed histopathological analysis using haematoxylin and eosin (H&E) staining and immunohistochemistry (IHC). The H&E staining revealed a minor amount of epidermal cell swelling and lymphocyte infiltration in the dermis of the treated flanks. (Fig. 4A) No significant abnormalities were observed in the epidermis and dermis of the untreated flanks. Quantitative statistical analysis of the epidermal layers showed that the treated flanks exhibited thicker epidermis than the untreated flanks. (Fig. 4A)
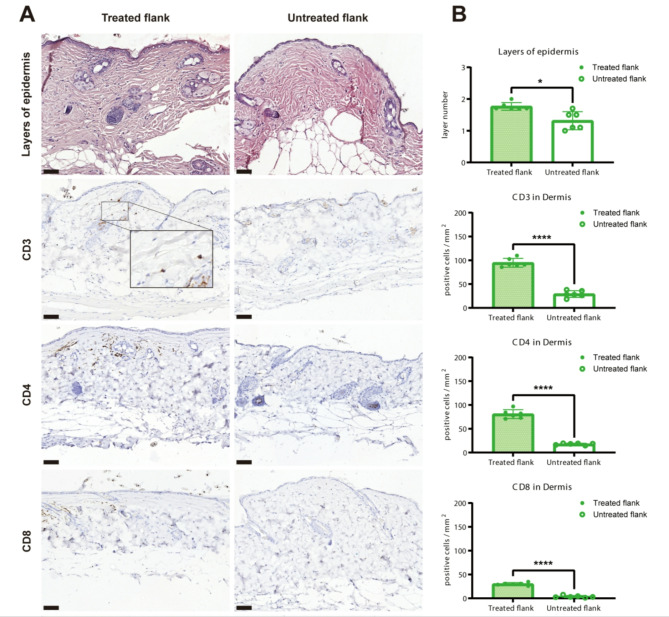
Fig. 4.
(A) Representative HE staining images and immunohistochemistry staining images of CD3, CD4 and CD8 in treated flanks and untreated flanks. (B) Quantitative analysis of the layer numbers of epidermis (P = 0.0312) and the quantitative analysis of CD3 (P = 0.000052), CD4 (P = 0.000019) and CD8 (P = 0.000002). The results were obtained by counting within five high-power fields (HPFs) at 20x magnification for each slide, with the mean values displayed in the figure. Scale bar = 50 μm. The data were presented as means ± SDs. A paired t-test or non-parametric test was used. A p-value of 0.05 or below was considered significant (*) and p < 0.0001 (****) was considered highly significant.
Furthermore, we conducted immunohistochemical staining on the mouse skin samples using T-cell (inflammation) markers CD3, CD4, and CD8. The staining results revealed scattered or focal distribution of CD3 + and CD4 + cells in the dermis of the treated flanks, and the presence of CD8 + cells, although with a scattered distribution. In contrast, the untreated flanks showed fewer CD3+, CD4+, and CD8 + lymphocyte infiltrations. (Fig. 4B) Quantitative analysis of CD3+, CD4+, and CD8 + infiltration in the dermis demonstrated a significant increase in the numbers of CD3+, CD4+, and CD8 + cells in the treated flanks compared to the untreated flanks. (Fig. 4B)
Discussion
We established a novel controlled knockout transgenic mouse model to study the function of one-copy loss of Hnrnpk in CD4 + T cells. HNRNPK has a dual role in tumor suppression and tumor promotion, and its expression may be upregulated or downregulated in different types of tumors. 20,13,21 Previous RNA sequencing studies have shown downregulation of HNRNPK expression in CD4 + T cells from patient skin lesions of mycosis fungoides (MF), the most common type of cutaneous T-cell lymphoma (CTCL)5,22 This abnormality may lead to HNRNPK dysfunction, affecting RNA processing and gene expression regulation23. Deletions in HNRNPK have also been identified in malignant cells from patients with Sézary syndrome (SS) and skin tumor samples from patients with MF17.
In further validating our Hnrnpk flox Cd4CreERT2 model, we conducted a comparison with the established Socs1 flox Cd4CreERT2 transgenic mouse model19. The Socs1 flox Cd4CreERT2 mice serve as targeting skin GEMMs (Genetically Engineered Mouse Models) in CTCL research. Specifically, the inflammatory responses observed following the deletion of Socs1 in CD4 + T cells suggest that Socs1 deletion may act as a potential initiator for CTCL18. This is similar to our approach in the Hnrnpk flox Cd4CreERT2 model, where the Socs1flox Cd4CreERT2 mice employ a targeted gene knockout strategy. This method has effectively simulated the very early characteristics of CTCL, underscoring the relevance and applicability of our technique in studying CTCL pathogenesis. The precision in gene manipulation and the resulting phenotypic manifestations observed in the Socs1 flox Cd4CreERT2 model lend credibility to the skin painting method and gene knockout technique used in our study.
Thanks to the reporter gene, deletion of the Socs1 gene in the CD4 + T cells of these hybrid mice can be detected via flow cytometry24. In the Hnrnpk flox Cd4CreERT2 mice, the knockout of Hnrnpk, which lacks a reporter gene, is verified by extracting DNA from enriched CD4 + T cells and employing PCR technology. These procedures have effectively confirmed the efficacy of the Cd4CreERT2 knockout tool.
In the Socs1 flox Cd4CreERT2 model, deletion of the Socs1 gene, combined with topical sensitizing stimuli, leads to autonomous skin inflammation exhibiting early MF characteristics. This closely mirrors the conditions observed in early-stage human CTCL25. Similarly, in our Hnrnpk flox Cd4CreERT2 mice, the targeted gene knockout resulted in a phenotype that accurately reflects early CTCL characteristics, particularly in early-stage MF. This similarity not only validates our methodological approach but also significantly enhances the potential of our model in revealing new insights into the molecular basis of CTCL. The ability to precisely knockout genes of interest, as demonstrated in both the Socs1 flox Cd4CreERT2 and our Hnrnpk flox Cd4CreERT2 models, is crucial for dissecting the complex interplay of genetic factors in the pathogenesis and progression of CTCL.
Our novel mouse model shows no significant disturbances in the immune cell population in peripheral blood, aligning with the localized nature of MF in CTCL and the challenge of distinguishing benign and malignant T cells in early-stage MF skin lesions4,8. In our new mouse model, skin inflammation persists even after the stimulus is removed, indicating a sustained chronic inflammatory response locally. However, there were no significant disturbances observed in the immune cell population in the peripheral blood. This pattern aligns with the early manifestations of mycosis fungoides (MF) in cutaneous T-cell lymphoma (CTCL), characterized by long-lasting and persistent inflammation limited to the skin2,26 Most MF patients also suffer from localized skin diseases, and distinguishing between benign and malignant T cells in early-stage MF lesions is challenging due to their shared T-cell characteristics and overlapping phenotypes27.
CTCL involves the coexistence of malignant CD4 + T cells, secreting cytokines, and normal tissue-resident memory T cells (TRM cells) that also contribute to the complex microenvironment through cytokine secretion28. CTCL cells, and CD4 + TRM cells, share a common tissue differentiation program likely influenced by the skin microenvironment29. In our mouse model, dermal infiltrating lymphocytes in Hnrnpk knockout mice primarily consisted of CD3 + CD4 + cells, mirroring the lymphocyte profile seen in human CTCL skin lesions. The increase in CD8 + T cells was less pronounced, suggesting an expansion of cytotoxic CD8 + T cells followed by gradual depletion.
Next to introducing the new mutant mouse model in this study, several limitations need to be addressed. The first limitation is the sample size of mice in long-term experiments. Additionally, further investigations are needed to validate the findings from sequencing studies in CTCL patients and explore the role of HNRNPK deletion in CTCL development and progression.
In conclusion, we established a novel genetically modified mouse model with conditional Hnrnpk knockout specifically in CD4 + T cells to investigate its role in CTCL pathogenesis. This model exhibits features consistent with CTCL, such as chronic skin inflammation, lymphocytic infiltration predominantly composed of CD3 + CD4 + cells. Our work provides insights into the mechanisms underlying CTCL progression and validates findings from sequencing studies in CTCL patients. Future mouse studies with larger sample sizes and longer follow-up are necessary to further enhance our understanding of HNRNPK involvement in CTCL.
Materials and methods
Ethical statement
All mouse experiments were supervised by the animal welfare committee (IvD) of the Leiden University Medical Center and approved by the national central committee of animal experiments (CCD) under the permit number AVD116002015271, in accordance with the Dutch Act on Animal Experimentation and EU Directive 2020/63/EU. The study is reported in accordance with the ARRIVE guidelines.
Animals
Hnrnpk flox (Hnrnpkem1Lumc; MGI:99894) mice where loxP sites were introduced in Hnrnpk intron 2 and intron 6 were generated at LUMC. Hnrnpk flox mice using CrisprCas9 RNP and 200 bp single-stranded oligodeoxynucleotides (ssODN). (all from IDT) Cd4CreERT2 mice (Tg (Cd4-cre/ERT2)11Gnri/J; MGI:5493114; JAX:022356) expressing CreERT2 recombinase under the control of the Cd4 promoter were purchased from The Jackson Laboratory (JAX). Hnrnpk flox mice were mated with Cd4CreERT2 mice to generate Hnrnpk flox Cd4CreERT2 mice. The offspring inherited both the targeted Hnrnpk flox allele and the Cd4CreERT2 transgenes.
Male Hnrnpk flox/wt Cd4CreERT2 + mice, 12–15 weeks old, were housed in a temperature-controlled room with a 12-hour light-dark cycle. Throughout the experiment, food and tap water were available ad libitum.
Tamoxifen and oxazolone application
Tamoxifen (TAM, Sigma-Aldrich, Netherlands) was dissolved in peanut oil at a concentration of 10 mg/ml, and 4-hydroxy-Tamoxifen (4OHT, Sigma-Aldrich, Netherlands) was dissolved in ethanol (20 mg/ml) and sonicated for 2 min. Tamoxifen was administered at a dose of 1 mg per mouse per day by intraperitoneal injection for 5 consecutive days. 4OHT was topically administered at a dose of 1 mg per mouse on the left shaved skin (2 cm × 3 cm). Oxazolone (4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one, Sigma-Aldrich, Netherlands) was dissolved in acetone. A freshly made solution of oxazolone was used for each experiment. The application schedule was as follows:
On the first day, 1.5% OXA (100 µl) was applied to the abdomen for induction. On the eighth day, 0.5% OXA (150 µl) was applied to the left flank. On the ninth day, the CRE-Lox system was activated with 4OHT to knock out Hnrnpk in CD4 + T cells. From week 2 to week 10, 0.5% OXA was applied three times per week to the left flank. From week 11 to week 20, 0.5% OXA was applied twice per week to the left flank. From week 21 to week 40, the mice were monitored for their overall condition, particularly their skin condition. The corresponding right flank was used as the control using the vehicle only.
Peripheral blood samples were collected from mice via the tail vein weekly or biweekly, and flow cytometry was performed to analyze the immune cell populations in the peripheral blood. At the end of week 40, all mice were humanely euthanized by carbon dioxide inhalation and skin specimens were collected from both the left and right flanks of the mice for cell extraction and preparation of paraffin-embedded sections.
Cell isolation and DNA isolation
Intact skin cells were isolated by taking a 4 mm skin biopsy, which was then digested using the whole skin dissociation Kit (Miltenyi Biotec, NL) following the manufacturer’s instructions. CD4 + T splenocytes were isolated and enriched by processing the spleen using lysis buffer (from Hospital Pharmacy at LUMC) to obtain splenocytes. CD4 + T cells were enriched from the splenocyte suspension using The BD IMag™ Mouse CD4 + T Lymphocyte Enrichment Set (BD Biosciences, NL). DNA was isolated from ear-clips and the CD4 + T cells from splenocytes using DNeasy Blood & Tissue Kits (Qiagen, NL).
Genomic PCR was conducted to analyze the genotypes of mice using ear DNA and gene-specific primers (Table S1) for the Hnrnpk flox transgene and Hnrnpk wildtype gene. Genomic PCR was conducted on the DNA from enriched CD4 + splenoctyes to detect the recombinant Hnrnpk transgene and unrecombined Hnrnpk wildtype gene using the three different sets of gene-specific primers respectively (Table S2).
Flow cytometry
Samples from isolated skin cells, splenocytes before and after CD4 + T cell enrichment, and blood were used for flow cytometry. Blood samples were handled and stained as described previously18. Fluorescence-labelled antibodies, including Live/Dead marker (clone Zombie, BD, Netherlands), anti-mouse CD3 (clone 145-2C11, BD, Netherlands), anti-mouse CD19 (clone 1D3, Thermo Fisher Scientific, Netherlands), anti-mouse CD4 (clone RM4-5, Thermo Fisher Scientific, Netherlands), anti-mouse CD8 (clone 53 − 6.7, Biolegend, Netherlands), CD4 AlexaFluor647 (ThermoFisher, cat: 51-0042-82), HNRNPK (ThermoFisher, cat 11426-1-AP) and anti-Goat secondary AlexaFluor488 (ThermoFisher, cat A-11034), were used. Samples were processed in a BD Fortessa and Cytek Biosciences Aurora flow cytometer. The raw data were analyzed using FlowJo or Infinicyt software and median fluorescence intensity of Hnrnpk was analyzed.
Histological and immunohistochemical analyses
Skin samples were fixed with 4% paraformaldehyde, dehydrated, and paraffin-embedded. Section (4 μm-thick) were cut with a microtome (Leica 149MULTI0C1). Tissue sections were stained with hematoxylin and eosin. For immunohistochemistry analyses, tissue sections were deparaffinized, rehydrated, and blocked for endogenous peroxidase using 0.3% hydrogen peroxide and nonspecific antibody binding using a blocking buffer (SuperBlock, Thermo Fisher Scientific, The Netherlands). Antigen retrieval was performed using citric acid (pH 6.0) solution. The tissue sections were incubated with the following primary antibodies at 4 °C overnight: anti-mouse CD3 (1:200, D7A6E, Cell Signaling Technology, Netherlands), anti-mouse CD4 (1:100, D7D2Z, Cell Signaling Technology, Netherlands), anti-mouse CD8 (1:1600, 4SM15, eBioscience™, Netherlands). Then, sections were incubated with secondary antibodies at room temperature for 60 min. Sections were visualized with the Vectastain Elite Kit (Vector Labs, Netherlands) and diaminobenzidine (Dako Omnis, Agilent Dako, Netherlands). After counterstaining with hematoxylin, sections were mounted. The scanner (3DHISTECH, Pannoramic 250) was used for microscopic examination and image acquisition.
The layers of the epidermis were counted within 5 high-power fields (HPF) (20x magnification) of each slide, and the mean values were calculated for subsequent statistical analysis. The numbers of CD3+, CD4+, and CD8 + lymphocytes in the dermis were counted within 5 HPF (20x magnification) per case. The values were normalized to cells/mm2, and the mean numbers were assessed for further statistical analysis. The evaluations were conducted by two independent individuals who were blinded to sample information.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 8.0.1). A paired t-test was used to compare the treated flank and untreated flank in mice. A p-value of 0.05 or below was considered significant (*), p < 0.01 (**), p < 0.001 (***) and P < 0.0001 (****) was considered highly significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Roos Gaarkeuken and Yang Liu for technical assistance. We thank the Animal Facility of the Leiden University Medical Center (LUMC) for their excellent care of the animals and the Flow cytometry Core Facility of Leiden University Medical Center (LUMC) for their support.
Abbreviations
- 4OHT
4-Hydroxy-Tamoxifen
- ARRIVE
Animal research: Reporting of in vivo experiments
- CCD
Central committee on animal experiments
- CRISPR/Cas9
Clustered regularly interspaced short palindromic repeats/Cas9
- CTCL
Cutaneous T-cell lymphomas
- GEMMs
Genetically engineered mouse models
- HE
Hematoxylin and eosin
- HPF
High-power fields
- HNRNPK
Heterogeneous nuclear ribonucleoprotein K
- IHC
Immunohistochemistry
- IvD
Animal welfare committee
- LUMC
Leiden university medical center
- MF
Mycosis fungoides
- NHL
Non-Hodgkin’s lymphomas
- OXA
Oxazolone
- PCR
Polymerase chain reaction
- SOCS1
Suppressor of cytokine signaling 1
- ssODN
Single-stranded oligodeoxynucleotides
- TAM
Tamoxifen
Author contributions
Conceptualization: CPT and MV. Methodology: CPT, FRG, YL, MML, PH, FJB and MPV. Data curation: YL, MML, CB, JC, PH, FJB and MPV. Formal analysis: YL, MML, CB, JC, FJB, FRG and CPT. Writing - Original Draft Preparation: YL. Writing - Review and Editing: CPT, YL, MML, CB, JC, FRG, PH, FJB and MPV. All authors have read and agreed to the published version of the manuscript.
Data availability
The sequences generated are available in the EU Open Research Repository zenodo repository https://zenodo.org/records/15191320, and the original images used in this study are available in the supplementary files. All other data are available from the corresponding author upon reasonable request.
Declarations
Competing interest
The authors declare no competing interests.
Human and animal rights
Hnrnpk flox (Hnrnpkem1Lumc; MGI:99894) mice were generated at Leiden University Medical Center (LUMC). Cd4CreERT2 mice (Tg(Cd4-cre/ERT2)11Gnri/J; MGI:5493114; JAX:022356) were purchased from The Jackson Laboratory (JAX).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Willemze, R. Primary cutaneous lymphoma: the 2018 update of the WHO-EORTC classification. Presse Med.51, 104126. 10.1016/j.lpm.2022.104126 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Rein Willemze, L. C., Kempf, W., Swerdlow, S. H., Elaine, S. & Jaffe Emilio Berti, Fabio Facchetti, 5, and The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood (2018). [DOI] [PMC free article] [PubMed]
- 3.Tensen, C. P., Quint, K. D. & Vermeer, M. H. Genetic and epigenetic insights into cutaneous T-cell lymphoma. Blood139, 15–33. 10.1182/blood.2019004256 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Ren, J. et al. Integrated transcriptome and trajectory analysis of cutaneous T-cell lymphoma identifies putative precancer populations. Blood Adv.7, 445–457. 10.1182/bloodadvances.2022008168 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastidas Torres, A. N. et al. Genomic analysis reveals recurrent deletion of JAK-STAT signaling inhibitors HNRNPK and SOCS1 in mycosis fungoides. Genes Chromosomes Cancer. 57, 653–664. 10.1002/gcc.22679 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill, R. P. K. et al. Understanding cell lines, Patient-Derived xenograft and genetically engineered mouse models used to study cutaneous T-Cell lymphoma. Cells1110.3390/cells11040593 (2022). [DOI] [PMC free article] [PubMed]
- 7.Kalliara, E., Belfrage, E., Gullberg, U., Drott, K. & Ek, S. Spatially guided and single cell tools to map the microenvironment in cutaneous T-Cell lymphoma. Cancers (Basel). 15. 10.3390/cancers15082362 (2023). [DOI] [PMC free article] [PubMed]
- 8.Han, Z. et al. MicroRNA Regulation of T-cell exhaustion in cutaneous T cell lymphoma. J Invest Dermatol 142, 603–612 e607, (2022). 10.1016/j.jid.2021.08.447 [DOI] [PMC free article] [PubMed]
- 9.Trochopoulos, A. G. X. et al. Micellar curcumin substantially increases the antineoplastic activity of the alkylphosphocholine erufosine against TWIST1 positive cutaneous T cell lymphoma cell lines. Pharmaceutics 14, (2022). 10.3390/pharmaceutics14122688 [DOI] [PMC free article] [PubMed]
- 10.Patil, K. et al. Molecular pathogenesis of cutaneous T cell lymphoma: Role of chemokines, cytokines, and dysregulated signaling pathways. Semin Cancer Biol.86, 382–399. 10.1016/j.semcancer.2021.12.003 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Barboro, P., Ferrari, N. & Balbi, C. Emerging roles of heterogeneous nuclear ribonucleoprotein K (hnRNP K) in cancer progression. Cancer Lett.352, 152–159. 10.1016/j.canlet.2014.06.019 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Mucha, B. et al. Tumor suppressor mediated ubiquitylation of HnRNPK is a barrier to oncogenic translation. Nat. Commun.13, 6614. 10.1038/s41467-022-34402-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallardo, M. et al. Aberrant HnRNP K expression: all roads lead to cancer. Cell. Cycle. 15, 1552–1557. 10.1080/15384101.2016.1164372 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naarmann-de Vries, I. S. et al. Characterization of acute myeloid leukemia with del(9q) - Impact of the genes in the minimally deleted region. Leuk. Res.76, 15–23. 10.1016/j.leukres.2018.11.007 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Dayyani, F. et al. Loss of TLE1 and TLE4 from the del(9q) commonly deleted region in AML cooperates with AML1-ETO to affect myeloid cell proliferation and survival. Blood111, 4338–4347. 10.1182/blood-2007-07-103291 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallardo, M. et al. HnRNP K is a haploinsufficient tumor suppressor that regulates proliferation and differentiation programs in hematologic malignancies. Cancer Cell.28, 486–499. 10.1016/j.ccell.2015.09.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park, J., Daniels, J., Wartewig, T., Ringbloom, K. G., Martinez-Escala, M. E., Choi,S., Thomas, J. J., Doukas, P. G., Yang, J., Snowden, C., Law, C., Lee, Y., Lee, K.,Zhang, Y., Conran, C., Tegtmeyer, K., Mo, S. H., Pease, D. R., Jothishankar, B., Kwok, P. Choi, J. Integrated genomic analyses of cutaneous T-cell lymphomas reveal the molecular bases for disease heterogeneity. Blood, 10.1182/blood.2020009655 (2021). [DOI] [PMC free article] [PubMed]
- 18.Luo, Y., de Haan, V. M., Kinderman, S., de Gruijl, P. & van Hall, F. R. Socs1-knockout in skin-resident CD4(+) T cells in a protracted contact-allergic reaction results in an autonomous skin inflammation with features of early-stage mycosis fungoides. Biochem. Biophys. Rep.35, 101535. 10.1016/j.bbrep.2023.101535 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo, Y. et al. In vivo modelling of cutaneous T-cell lymphoma: The role of SOCS1. Front. Oncol.12, 1031052. 10.3389/fonc.2022.1031052 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, Z., He, Q. H., Liu, J., Xue, L. & Fox, W. The emerging roles of HnRNPK. J. Cell. Physiol.235, 1995–2008. 10.1002/jcp.29186 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Chen, Y., Xiao, Z. Y., Chen, Z., Li, S. & Zou, Y. Role of heterogeneous nuclear ribonucleoprotein K in tumor development. J. Cell. Biochem.120, 14296–14305. 10.1002/jcb.28867 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Fan, X. et al. Cytoplasmic HnRNPK interacts with GSK3beta and is essential for the osteoclast differentiation. Sci. Rep.5, 17732. 10.1038/srep17732 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu, Y. et al. New insights into the interplay between Non-Coding RNAs and RNA-Binding protein HnRNPK in regulating cellular functions. Cells810.3390/cells8010062 (2019). [DOI] [PMC free article] [PubMed]
- 24.Aghajani, K., Keerthivasan, S., Yu, Y. & Gounari, F. Generation of CD4CreER(T(2)) Transgenic mice to study development of peripheral CD4-T-cells. Genesis50, 908–913. 10.1002/dvg.22052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krejsgaard, T. et al. Malignant inflammation in cutaneous T-cell lymphoma-a hostile takeover. Semin Immunopathol.39, 269–282. 10.1007/s00281-016-0594-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieyra-Garcia, P. et al. Benign T cells drive clinical skin inflammation in cutaneous T cell lymphoma. JCI Insight. 410.1172/jci.insight.124233 (2019). [DOI] [PMC free article] [PubMed]
- 27.Roediger, B. & Schlapbach, C. T cells in the skin: lymphoma and inflammatory skin disease. J. Allergy Clin. Immunol.149, 1172–1184. 10.1016/j.jaci.2022.02.015 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Stolearenco, V. et al. Cellular interactions and inflammation in the pathogenesis of cutaneous T-Cell lymphoma. Front. Cell. Dev. Biol.8, 851. 10.3389/fcell.2020.00851 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertschi, N. L., Bazzini, C. & Schlapbach, C. The concept of pathogenic TH2 cells: collegium internationale allergologicum update 2021. Int. Arch. Allergy Immunol.182, 365–380. 10.1159/000515144 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences generated are available in the EU Open Research Repository zenodo repository https://zenodo.org/records/15191320, and the original images used in this study are available in the supplementary files. All other data are available from the corresponding author upon reasonable request.