Abstract
Posttraumatic stress disorder (PTSD) is associated with poor hippocampal function and disrupted pattern recognition. Cannabis use is highly prevalent in individuals with PTSD, yet the impact on these cognitive functions is poorly understood. Participants (n = 111) with a range of PTSD symptoms with and without regular cannabis use completed the mnemonic similarity task. We hypothesized that regular use would be associated with alterations in pattern separation ability in individuals with PTSD symptoms. High PTSD symptoms were associated with reduced pattern separation performance in minimal users. Regular users with high PTSD symptoms showed greater pattern separation, but reduced pattern separation with low PTSD symptoms. These results suggest that regular cannabis use may disrupt pattern separation and similar hippocampal-dependent processes, while it may improve pattern separation in individuals with high PTSD symptoms. These cross-sectional results require longitudinal follow-up studies to evaluate the causal effects of regular cannabis use on cognitive function in PTSD.
Subject terms: Psychology, Diseases, Signs and symptoms
Introduction
PTSD is often characterized as a disorder of ‘fear memory’ due to the chronic and persistent symptoms of intrusive trauma-related memories that disrupt functioning long-after the trauma occurred1,2. The impact of PTSD on memory, particularly the contextual modulation of fear memory, has become a focal point of investigation3–5. Contextual processing is using internal or external environmental cues to optimize the most appropriate behavioral response6–9. One integral component of contextual processing is pattern separation, which is the ability to distinguish between similar experiences and form distinct mnemonic representations10,11. By forming distinct memories and retrieving them accurately, pattern separation supports adaptive behavior in various contexts and enhances the ability to navigate and interact with the environment12,13. However, dysfunction in the ability to properly distinguish between similar but distinct cues might contribute to the persistence of traumatic memories and overgeneralization of fear responses to non-threatening stimuli that share similarities with stimuli associated with the traumatic event3,5,14,15.
Both preclinical and clinical studies have highlighted the role of bidirectional hippocampal-prefrontal cortex interactions in both retrieving past contextual associations and new contextual learning16–19. In particular, the hippocampus is implicated in mnemonic pattern separation10,13,20,21. PTSD is associated with structural and functional hippocampal dysfunction22–24, and has been implicated as both a pre-trauma risk factor for PTSD as well as a potential consequence of PTSD symptoms25–28. Collectively, these investigations indicate that contextual processes supported by the hippocampus, including pattern separation, are altered in individuals with PTSD29. Consistent with this hypothesis, compared to healthy control participants, treatment-seeking patients with PTSD demonstrate decreased performance in assessments of mnemonic pattern separation ability30.
In addition to PTSD, preclinical and clinical studies indicate that heavy cannabis use is also associated with memory impairment31–36. The hippocampus has a high concentration of cannabinoid receptors37–39, suggesting long-term use of cannabis may alter functions subserved by the hippocampus40. Results from numerous cross-sectional studies support an association between heavy cannabis use and reduced hippocampal volumes in both psychiatrically healthy individuals and those with various psychopathology including schizophrenia41–44. Some longitudinal studies suggest that long-term use of cannabis is associated with reductions in performance on numerous cognitive tasks and lower hippocampal volume compared to non-users45,46. However, some longitudinal investigations and meta-analyses examining both hippocampal volume and cognitive performance differences between cannabis users and non-users have shown mixed results47–54.
With the increase in the availability of cannabis, there is a great need to understand the impact of regular cannabis use on contextual learning mechanisms in individuals with PTSD. PTSD and problematic cannabis use are highly comorbid. Among a sample of returning US OEF/OIF Veterans diagnosed with Cannabis Use Disorder (CUD), more than 70% also had comorbid PTSD55. Results from the 2019-2020 National Health and Resilience in Veterans Study (NHRVS) showed that among Veterans with a current PTSD diagnosis, 28.9% self-reported cannabis use in the previous 6 months, compared to 11.9% of Veterans without PTSD56. Many individuals report using cannabis to manage symptoms, particularly sleep and anxiety57,58. It is unknown what daily/regular use of cannabis might do to hippocampal functions such as context-dependent pattern separation, a critical process governing contextual modulation of trauma memory and fear generalization3,5. Here we tested the hypothesis that regular cannabis use would be associated with significant disruptions in pattern separation memory processes in individuals with elevated PTSD symptoms. To test this hypothesis, we examined performance on the Mnemonic Similarity Task (MST), a widely used test reflective of hippocampal function59, in individuals reporting either minimal or regular cannabis use in a sample of trauma-exposed individuals with varying levels of PTSD symptoms.
Methods
Study population
Data for the current study were collected from two independent samples. For the first sample, participants (n = 90; Mage = 31.5, SD = 9.51; 34.44% female) with and without PTSD and with minimal or regular cannabis use were recruited to participate in a 2-day fear learning study (data not reported here). We supplemented this sample with a second cohort of participants (n = 33; Mage = 34.76, SD = 5.67; 100% male) from the Marine Resilience Study (MRS)60 who matched the same inclusion criteria as the first sample and who also completed the MST. Study protocols and procedures were approved by the Institutional Review Board at the University of California, San Diego, and complied with ethical standards involving human research participants. All participants provided written informed consent prior to participation.
Both cohorts were instructed to refrain from substance use before the testing day. Cannabis use was tracked by urine test in the first cohort with the minimal user group required to have a negative urine test at intake and both groups required to have a negative saliva test (Dräger Drug Test 5000, Houston TX) on the day of testing. Cannabis use assessment in the smaller MRS cohort was limited to self-report due to regulatory limitations. The combined study group consisted of 123 participants, all of whom were free from family or personal history of bipolar or psychotic disorders and antipsychotic medication use. Cannabis use group criteria were based on prior work61, with Regular Cannabis Users (n = 49 defined as using cannabis at least 3 times/week for >3 months or 2 times/week for > 6 months, and Minimal Users (n = 74) as abstaining from cannabis use for > 2 months. See Table 1 for details of cannabis use in the Regular User group.
Table 1.
Study Sample Demographics
| Minimal Cannabis Users (n = 69) | Regular Cannabis Users (n = 42) | |
|---|---|---|
| Age (SD)* | 34.16 (8.82) | 29.59 (7.94) |
| Sex | 26.09% women | 30.95% women |
| Race | ||
| Black/African American | 8.70% | 23.81% |
| American Indian/Alaskan Native | 0% | 2.38% |
| Asian | 14.49% | 4.76% |
| Native Hawaiian/Pacific Islander | 1.45% | 4.76% |
| White | 68.67% | 35.71% |
| Other/Not Reported | 8.70% | 28.57% |
| Ethnicity | ||
| Not Hispanic or Latino | 84.06% | 61.90% |
| Cuban | 0% | 0% |
| Mexican | 8.70% | 16.67% |
| Puerto Rican | 0% | 4.76% |
| South/Central American | 2.90% | 7.14% |
| Other Spanish Culture | 4.35% | 9.52% |
| PCL-5 Total Score (SD) | 26.7 (22.3) | 33.48 (21.93) |
| Re-experiencing cluster | 7.06 (6.10) | 8.67 (6.16) |
| Avoidance cluster | 2.97 (2.73) | 3.81 (2.70) |
| Negative Cognition & Mood cluster* | 7.54 (7.65) | 11.02 (8.07) |
| Hyperarousal cluster | 8.51 (7.19) | 9.88 (6.51) |
| BDI-II Total Score (SD)* | 16.71 (19.6) | 24.64 (18.78) |
| Cannabis Use (SD; range) | – | |
| Grams used/day | – | 1.18 (1.11; 0.015–5) |
| THC content | – | 31.2% (16%; 15–90%) |
| Days use/week | – | 4.86 (2.09; 1–7) |
| Years used at this rate | – | 4.28 (5.44; 1–31) |
Demographics and characteristics of sample used in the primary analyses. PCL-5 Posttraumatic Stress Disorder Checklist for the DSM-5. BDI-II Beck Depression Inventory, 2nd edition.
SD standard deviation.*p < 0.05.
From the combined study group, one outlier from the minimal cannabis user group was removed (>Q3 + 1.5*interquartile range), one individual was removed due to incomplete self-report cannabis use information, and one individual was removed due to missing self-reported PTSD symptoms. Additionally, 9 participants were excluded for having a negative lure discrimination index on the MST, which past work has suggested is indicative of a high response bias and inadequate encoding or retrieval of the initial target object62, leaving a total of 111 participants remaining for the final analysis (Minimal Users = 69; Regular Users = 42). The combined sample-size used in the current study provides 80% power to detect a medium-to-large effect size (Cohens f = 0.27) for main effects and interactions between two groups and one continuous variable (α<.05, F tests; G*Power version 3.1.9.6).
PTSD Checklist for DSM-5 (PCL-5)
The PTSD Checklist for DSM-5 was used to assay PTSD symptom severity. The PCL-5 is a 20-item self-report measure assessing past-month PTSD symptoms (Range: 0–80, >33 indicates probable PTSD diagnosis)63.
Beck Depression Inventory-II (BDI-II)
The BDI-II is a 21-item questionnaire measuring depressive symptoms over the past two weeks (Range: 0–63)64.
Customary Drinking and Drug Use Record (CDDR) – Cannabis Domain
Lifetime and 3-month cannabis use was measured using the Customary Drinking and Drug Use Record (CDDR), which assesses lifetime use, type, and route of cannabis products used, in addition to assessing abuse and dependence criteria, withdrawal effects, and substance-related difficulties65.
Mnemonic Similarity Task (MST)
Participants completed the mnemonic similarity task59,62,66. The task consisted of two phases, an incidental learning phase and a surprise recognition test. In the learning phase, participants viewed 192 images (2 s; 0.5 s inter-stimulus interval) on a computer screen. Participants had to respond to each image as an indoor or outdoor object. Immediately following the learning phase, participants completed the recognition test (192 trials). Participants had to respond to each item as “old”, “new”, or “similar.” One third of the trials consisted of images that appeared during the incidental learning phase (targets), one third of the images were new images not presented previously (foils), and one third of the images were similar but not identical to the images that appeared during the incidental learning phase (lures). The primary variable of interest was the lure discrimination index (LDI): [p(“similar” |lure) – p(“similar”|foil)], which we use as the measure of mnemonic pattern separation performance - the ability to successfully separate similar images from previously viewed images59,66,67.
Analytic approach
Because we measured MST performance from two different studies prior to combining the data for the current manuscript, we sought to reduce the between sample cohort variability. First, we Z-scored the MST LDI metric within the sample cohort. Second, we added a sample cohort as a covariate of non-interest. Primary hypothesis testing was conducted by computing a PTSD × Cannabis Use general linear model (GLM). PCL-5 total scores were added as a continuous predictor and Cannabis Use as a categorical variable on the MST LDI (Z-Scored). Analyses were performed in Jamovi (R Core Team, 2021; The Jamovi Project, 2022) using GAMLj: General Analyses for the Linear Model in Jamovi (Gallucci, 2019).
Results
Participant characteristics
Demographic and participant characteristics are reported in Table 1. When comparing regular vs. minimal users, regular cannabis users did not statistically differ on PCL-5 (p = 0.12) but had higher BDI-II total scores (p = 0.049; mean difference = 7.48, SEdifference = 3.76) and had higher PCL-5 Negative Cognition and Mood symptom cluster scores (p = 0.024; mean difference=3.49, SEdifference = 1.53). Self-reported PCL-5 and BDI-II were highly correlated (r = .80, p = 2.2 × 10-16). Regular cannabis users were younger than minimal cannabis users (p = 0.007; mean difference=4.49 years, SEdifference = 1.64). In the regular cannabis use group, participants reported using cannabis 4.86 days/week (SD = 2.09), 1.18 grams (SD = 1.11) per day, and using at this rate for 4.28 (SD = 5.44) years and cannabis use patterns did not relate to PTSD symptoms (grams per day: r = −0.04, p = 0.80, days/week: r = 0.04, p = 0.81, years of use at this rate: r = 0.12, p = 0.45) or to depression symptoms (rs < ± 0.02, ps > .90).
Cannabis Use Moderates the Effect of PTSD Symptoms on Mnemonic Pattern Separation
Results from the GLM indicated that the effects of PTSD symptom levels depended on Cannabis use (See Fig. 1, PTSD × Cannabis Use, F(1,106) = 12.38, p < 0.001, ; Main effect of PTSD F(1,106) = 0.002, p = 0.97, , Main effect of Cannabis use, F(1,106) = 0.26, p = 0.61, ). The interaction remained significant after controlling for age (p < 0.001, ) and depressive symptoms (p < 0.001, ). Simple effects analysis indicated that within the Minimal Cannabis Use group, increasing levels of PCL-5 severity was associated with lower mnemonic pattern separation performance, β = −0.341, t (106) = −2.91, p = 0.004. In contrast, in the Regular Cannabis Use group, increasing levels of PCL-5 severity was associated with greater mnemonic pattern separation performance, β = 0.334, t (106) = 2.20, p = 0.030. These patterns of results were not driven by adding the smaller MRS cohort, the results remained significant when only analyzing the larger subsample recruited for this study (n = 79; see Supplementary Table 1).
Fig. 1. Mnemonic pattern separation performance as a function of PTSD symptom severity in individuals who minimally use cannabis and in individuals with regular cannabis use.
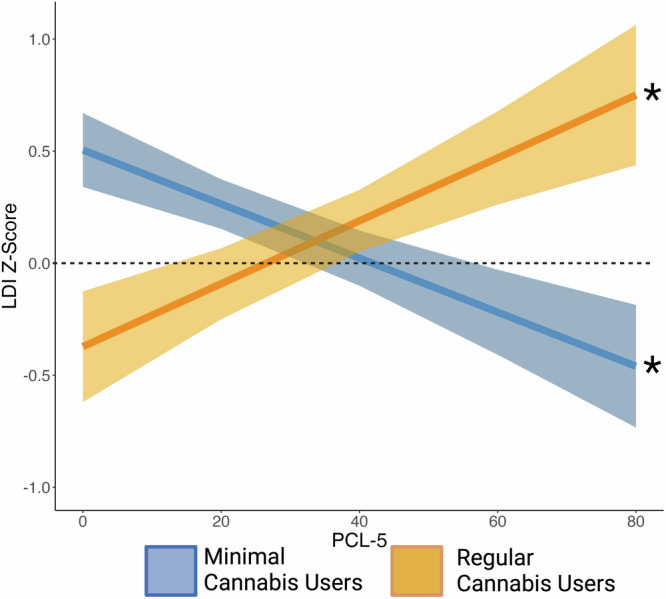
LDI Z-Score = Lure discrimination index, Z-scored; which measures mnemonic pattern separation performance. LDI was Z-scored within sample cohort (see Method). PCL-5 = PTSD Symptom Checklist for DSM-5. * Simple slope effects: p < .05. Ribbon reflects standard error of the mean. See online article for the color version of this figure. Figure was created using R (ggplot2) and BioRender.com.
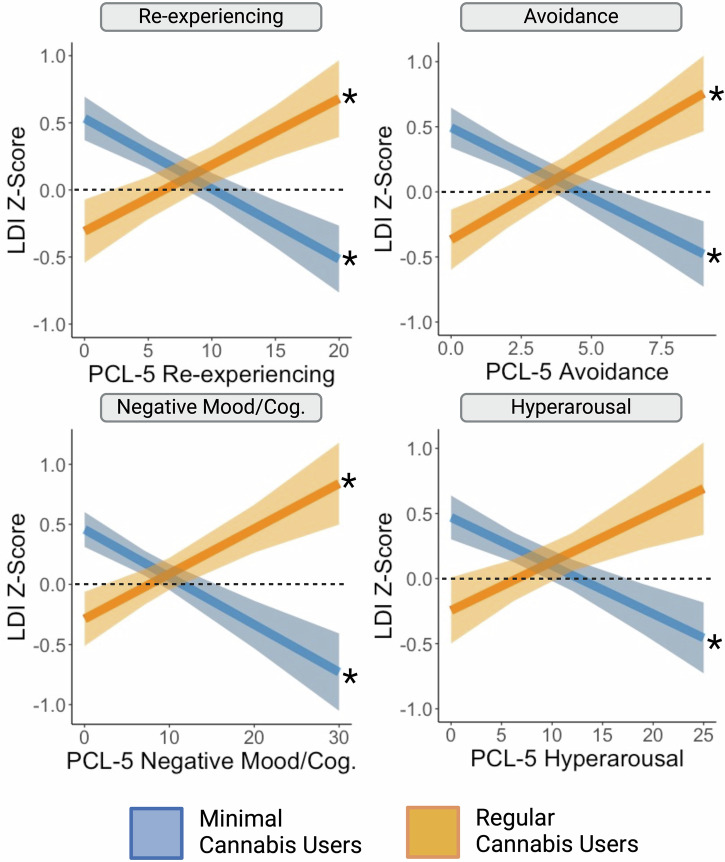
Cannabis Use Effects Across Different PTSD Symptom Types and Depression
Next, we further examined the symptom specificity of the findings. First, we computed separate models using each of the PCL-5 symptom subclusters. The effect of increasing mnemonic pattern separation performance with increasing symptom severity in the regular cannabis use group was significant for the re-experiencing, avoidance, and negative cognition and mood PCL-5 subclusters (ps < .05; Supplementary Tables 2 through 5; Fig. 2). For the minimal cannabis use group, the effect of decreasing pattern separation performance was significant in all four PCL-5 symptom subclusters (ps < .05). Second, when depression symptoms were added as a covariate, the improved pattern separation performance with elevated PTSD symptoms in the Regular Cannabis Use group was no longer significant, β=-0.003, t(104) = -0.01, p = .99; whereas the decreasing performance in the Minimal Cannabis Use group remained significant (p < .05).
Fig. 2. Mnemonic pattern separation performance as a function of PTSD sub-cluster symptom severity in individuals who minimally use cannabis and in individuals with regular cannabis use.
LDI Z-Score = Lure discrimination index, Z-scored; which measures mnemonic pattern separation performance. LDI was Z-scored within sample cohort (see Method). PCL-5 = PTSD Symptom Checklist for DSM-5. * Simple slope effects: p < .05. Ribbon reflects standard error of the mean. See online article for the color version of this figure. Figure was created using R (ggplot2) and BioRender.com.
Regular Cannabis Use Associated with Poor Pattern Separation Ability
To visualize the interaction between cannabis use and PTSD symptom severity on mnemonic pattern separation, we conducted a post-hoc analysis, comparing pattern separation ability between minimal and regular cannabis users across individuals with low (below 1 SD from the mean), mean (between -1 and +1 SD from the mean), and high (above 1 SD from the mean) PTSD symptoms. As shown in Fig. 3, at low PTSD symptoms (-1SD), pattern separation ability was significantly lower for regular cannabis users compared to minimal users (t = -2.69, p = .008). In contrast, at high PTSD symptoms (+ 1 SD), pattern separation ability was significantly higher for regular users than minimal users (t = 2.42, p = .017). At Mean levels of PCL, there were no group differences (t = -0.33, p = .74).
Fig. 3. Mnemonic pattern separation performance comparing Minimal versus Regular Cannabis Users as a function of low PCL (-1 SD), Mean PCL, and high PCL (+ 1 SD).

LDI Z-Score = Lure discrimination index Z-scored; which measures mnemonic pattern separation performance. Estimated marginal means are plotted for visualization purposes. LDI was Z-scored within sample cohort (see Method). PCL-5 = PTSD Symptom Checklist for DSM-5. *p < .05. Error bars reflect standard error. See online article for the color version of this figure. Figure was created using R (ggplot2) and BioRender.com.
Exploratory analyses: role of sex on moderating effect of PTSD on pattern separation
Due to the importance of elucidating possible sex differences, we next conducted an exploratory analysis with sex added as a factor in our model; the PTSD x Cannabis Use interaction remained significant, F (1, 102) = 14.37, p < 0.001, . We also observed a PTSD x Cannabis Use x Sex interaction which approached significance, F (1, 102) = 3.87, p = 0.053, . Post-hoc simple effects analyses indicate that the PTSD x Cannabis interaction effect was stronger in women: Increasing PTSD severity in women was associated with higher pattern separation in regular cannabis users (p = 0.005) but lower pattern separation in minimal-cannabis users (p = 0.031). In men, increasing PTSD severity was associated with lower pattern separation in minimal-cannabis users (p = 0.048), but PTSD severity had no relationship with pattern separation in regular cannabis users (ps = 0.62; see Supplementary Fig. 1). However, due to the relatively small number of women in the sample (n = 31), these results should be taken cautiously.
Discussion
The aim of the current investigation was to test whether regular cannabis use would be associated with significant disruptions in hippocampal-dependent memory processes in individuals with elevated PTSD symptoms. We observed three primary results. First, we replicated the finding that individuals with elevated PTSD symptoms have impaired hippocampal-dependent function as measured by pattern separation ability30. Second, we found that the PTSD association with pattern separation performance is moderated by cannabis use. Individuals in the minimal cannabis use group showed the expected dysfunction in mnemonic pattern separation performance. In contrast, and unexpectedly, individuals in the regular cannabis use group exhibited better mnemonic separation ability with increasing levels of self-reported PTSD symptoms. Self-reported history of frequency, form, and %-tetrahydrocannabinol (THC) content was not associated with PTSD or depression symptoms, indicating that differences in patterns of use do not explain the paradoxical PTSD-related variation in pattern separation performance between these groups. Additionally, post-hoc analyses showed that individuals in the regular cannabis use group had significantly poorer pattern separation ability compared to the group with minimal cannabis use at lower levels of PTSD symptoms. Collectively, our findings highlight a complex relationship between PTSD symptoms, cannabis use, and hippocampus-dependent mnemonic pattern separation ability.
Regular cannabis use has been associated with various cognitive impairments34,49,68. Acute cannabis use in adolescence is associated with impairment in working memory and learning ability during adolescence31,33. Regular consumption of cannabis, particularly at high doses, has been linked to deficits in attention, memory, and executive functions in healthy individuals as well as a reduction in hippocampal volume41,43,69,70; although this negative impact is not always observed71,72. Some of these detrimental effects on cognitive performance and the hippocampus may be diminished after periods of abstinence or are less likely in users of cannabis products with higher cannabidiol (CBD) concentrations73–76. The effects of cannabis on cognition and brain structure likely depend on the age of use in addition to the amount, duration, frequency, and type (i.e., THC vs CBD ratio) of cannabis used77,78. Here, we show that regular cannabis use in those who are relatively asymptomatic from PTSD and depression symptoms have impaired mnemonic pattern separation ability. These findings are consistent with a double-blind, placebo-controlled within-subjects trial, which demonstrated that acute THC consumption immediately prior to the learning phase in a modified version of the MST impairs the encoding of perceptual details to discriminate between previous stimuli and semantically similar but different stimuli compared to placebo79.
Our finding of low levels of mnemonic pattern separation in minimal cannabis users with elevated PTSD are consistent with previous findings30 and with theoretical models that implicate impaired hippocampal functioning in the etiology and maintenance of PTSD3,5. PTSD symptom severity is associated with reduced hippocampal volume80,81, altered hippocampus activity and resting-state connectivity prospectively predict future PTSD symptoms following trauma82,83, and hippocampus structure and function are modified following treatment84,85. Hippocampal-dependent pattern separation impairment is a key mechanism underlying the overgeneralization of fear and contextual learning6,86,87. Overgeneralization of fear has been implicated in PTSD, wherein otherwise innocuous or “safe” stimuli are perceived as threatening, potentially leading to maladaptive avoidance behaviors which may strengthen the association of that stimuli with threat88. Our results are also consistent with prior imaging studies examining fear generalization in individuals with PTSD via overgeneralization gradients corresponding to the duration of altered fMRI BOLD signal change in regions employed in fear conditioning, including the hippocampus89–92.
The finding that regular cannabis use was associated with improved pattern separation ability in those reporting more severe PTSD symptoms was unexpected. One possible explanation for this observation is that the endocannabinoid system is altered by trauma exposure and in PTSD93. For example, meta-analytic evidence suggests that PTSD is associated with greater baseline anandamide (AEA) and 2-arachidonoglycerol (2-AG) endocannabinoid levels in serum and blunted release in response to stress94,95; while depression in some populations is associated with reduced serum levels of endocannabinoids96,97. Furthermore, positron emission tomography studies have reported increases in CB1R receptor availability in the brain in individuals with PTSD and depression patients relative to psychiatrically healthy individuals98–100. Moreover, animal studies consistently show that reduced endocannabinoid signaling at the CB1 receptor associated with emotional memory impairment can be normalized by increasing CB1 receptor signaling101. Thus, differences in baseline endocannabinoid levels and receptor availability suggest that patients with PTSD and depression symptoms may respond differently to cannabis use compared to non-symptomatic controls.
A second potential mechanism underlying the paradoxical PTSD-related improvement in pattern separation is the potential role of CBD in ameliorating cognitive impairment in neuropsychiatric disorders102,103. Experimental and meta-analytic evidence supports the hypothesis that CBD may reduce cognitive impairment associated with a range of different neuropsychiatric disease models104,105. For example, oral administration of CBD was associated with increased resting-state functional connectivity of the frontostriatal circuit106, improvement in working memory107, and enhancement of verbal memory108. A third possibility is dose-response effects of cannabis on cognition and memory. Rodent models suggest that hippocampal neurogenesis and the rescue of age-related deficits in memory is a result of biphasic dose-response to THC109. Consistent with the importance of dose in cannabis effects, recent work has shown regular and heavy cannabis use in healthy individuals is associated with impaired fear extinction110, but acute low dose THC appears to improve fear extinction and may help modulate amygdala reactivity in response to fear in those with PTSD111,112. In sum, existing work provides support for the notion that underlying differences in endocannabinoid signaling in PTSD may result in altered sensitivity or dose response shifts to CB1 agonists, resulting in differential responses to cannabis compared to psychiatrically healthy controls.
The paradoxical results observed in this study are in line with cannabis use associations in other neuropsychiatric disorders, suggesting transdiagnostic effects of cannabis on cognitive performance113,114. Regular and long-term cannabis use has been associated with improved, not impaired, cognitive performance in several neuropsychiatric disorders, including schizophrenia115, and bipolar disorder116. For example, individuals with Bipolar I disorder and comorbid Cannabis Use Disorder perform significantly better on several neurocognitive measures including attention, processing speed, and working memory117. Additionally, lifetime cannabis use is associated with better working memory and processing speed in individuals with Schizophrenia, even after controlling for the effect of antipsychotic medications118. Here, we report that pattern separation performance in regular cannabis users is also higher in individuals reporting elevated PTSD symptoms. We further observed that the positive association was not specific to PTSD symptoms by showing that this pattern was no longer significant after controlling for comorbid depressive symptoms. In our sample, PTSD and depression symptoms were highly collinear (r = 0.80), which is consistent with the high level of comorbidity between PTSD and depression119,120. Thus, we are unable to effectively separate PTSD from depression. However, our results support the hypothesis that the endocannabinoid system plays a role in cognition and highlight the need for future research to disentangle the distinct and overlapping contributions of various neuropsychiatric symptoms on the cognitive enhancements observed with regular cannabis use.
From a clinical perspective, isolating the path by which cannabis exerts potential negative and positive influences on cognition and what the implications are for clinical outcomes for PTSD will be important. Although we observed higher performance of hippocampal-dependent mnemonic pattern separation in regular cannabis users, this occurred only at higher levels of PTSD symptoms, suggesting that regular cannabis use is not improving self-reported psychiatric symptoms. Moreover, we conducted a cross-sectional study design, and therefore we cannot determine whether cannabis is a causal agent in producing improved cognition or modifies PTSD symptoms. Evidence is mixed regarding the beneficial impact of cannabis on PTSD. Some studies suggest a reduction in hyperarousal symptoms and significantly better PTSD symptom remission rates in regular users121. Whereas other studies show no long-term effect122, or a deleterious effect of cannabis on PTSD symptoms123. Yet, there remains a strong interest by patients and clinicians in the clinical application of cannabis to treat PTSD124,125. Despite a small number of individual studies indicating potential beneficial effects of cannabis in individuals with PTSD, meta-analyses highlight the lack of high-quality clinical trial results addressing the effects of cannabis on cognitive functioning and clinical outcomes in PTSD126. Currently, both the U.S. Department of Defense and U.S. Department of Veterans Affairs Clinical Practice Guidelines recommend against the use of cannabis in the treatment of PTSD due to the lack of evidence supporting efficacy127, underscoring the need to better understand the mechanisms by which cannabis may have salutary versus deleterious impacts on PTSD symptoms and cognition128.
Several limitations in the present study should be mentioned. First, we relied on self-report measures of cannabis use to define our groups, and urine test validation of THC was limited to the first cohort. Longitudinal investigations with experimental designs that validate %-THC/CBD concentrations, product label information, and quantification of cannabinoid metabolites in urine are clearly needed129–131. Second, our study included a small number of women, and therefore our exploratory finding of sex differences in pattern separation improvement within the regular cannabis use group should be interpreted cautiously. Evidence from animal and clinical studies suggests sex differences in the endocannabinoid system, including in PTSD populations100,132–134. Future research with larger samples of women and assaying reproductive hormone and cannabinoid levels is needed to clarify their combined impact on hippocampal-dependent mnemonic processes in PTSD135.
Collectively, our study contributes valuable insights into the intricate relationship between PTSD, cannabis use, and mnemonic pattern separation ability. The observed moderation effects of cannabis use on PTSD-associated cognitive dysfunction in cannabis users underscores the need for further research to elucidate the underlying mechanisms and potential therapeutic implications. Additionally, the differential impact of cannabis use in individuals with varying levels of PTSD symptoms highlights the complexity of these interactions, necessitating a comprehensive and nuanced approach to understanding the cognitive consequences of trauma and cannabis use.
Supplementary information
Acknowledgements
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Author contributions
J.R. served as lead for formal analysis, visualization, and writing–original draft. B.C. served as lead for data curation and supported data analysis, writing-review and editing. D.D., C.C., and G.C. supported data curation, data analysis, writing-editing. D.T.A. supported conceptualization, data analysis, writing- editing. D.G.B and V.B.R. served as lead for funding acquisition, and supported conceptualization, writing-original draft and editing, and supervision of investigation. D.M.S. supported supervision of data analysis, interpretation, and writing-original draft and editing. All authors contributed to writing, review, and editing of the manuscript.
Data availability
Data will be made available upon reasonable request and with appropriate data use agreement.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s44184-025-00126-w.
References
- 1.Brewin, C. R. The nature and significance of memory disturbance in posttraumatic stress disorder. Annu Rev. Clin. Psychol.7, 203–227 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Desmedt, A., Marighetto, A. & Piazza, P. V. Abnormal fear memory as a model for posttraumatic stress disorder. Biol. Psychiatry78, 290–297 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Acheson, D. T., Gresack, J. E. & Risbrough, V. B. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology62, 674–685 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunsmoor, J. E., Cisler, J. M., Fonzo, G. A., Creech, S. K. & Nemeroff, C.B. Laboratory models of post-traumatic stress disorder: The elusive bridge to translation. Neuron110, 1754–1776 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberzon, I. & Abelson, J. L. Context processing and the neurobiology of post-traumatic stress disorder. Neuron92, 14–30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asok, A., Kandel, E. R. & Rayman, J. B. The neurobiology of fear generalization. Front. Behav. Neurosci.12, 329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maren, S., Phan, K. L. & Liberzon, I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci.14, 417–428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glenn, D. E., Risbrough, V. B., Simmons, A. N., Acheson, D. T. & Stout, D.M. The future of contextual fear learning for PTSD research: a methodological review of neuroimaging studies. Curr. Top. Behav. Neurosci.38, 207–228 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Stout, D. M., Glenn, D. E., Acheson, D. T., Simmons, A. N. & Risbrough, V. B. Characterizing the neural circuitry associated with configural threat learning. Brain Res.1719, 225–234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yassa, M. A. & Stark, C. E. Pattern separation in the hippocampus. Trends Neurosci.34, 515–525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zotow, E., Bisby, J. A. & Burgess, N. Behavioral evidence for pattern separation in human episodic memory. Learn Mem.27, 301–309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayco-Gajic, N. A. & Silver, R. A. Re-evaluating circuit mechanisms underlying pattern separation. Neuron101, 584–602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leal, S. L. & Yassa, M. A. Integrating new findings and examining clinical applications of pattern separation. Nat. Neurosci.21, 163–173 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein, E. E. & McNally, R. J. Exploring behavioral pattern separation and risk for emotional disorders. J. Anxiety Disord.59, 27–33 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Lecei, A. & van Winkel, R. Hippocampal pattern separation of emotional information determining risk or resilience in individuals exposed to childhood trauma: Linking exposure to neurodevelopmental alterations and threat anticipation. Neurosci. Biobehav Rev.108, 160–170 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Maurer, A. P. & Nadel, L. The continuity of context: a role for the hippocampus. Trends Cogn. Sci.25, 187–199 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang, F. C. & Liang, K. C. Interactions of the dorsal hippocampus, medial prefrontal cortex and nucleus accumbens in formation of fear memory: difference in inhibitory avoidance learning and contextual fear conditioning. Neurobiol. Learn Mem.112, 186–194 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Zelikowsky, M., Hersman, S., Chawla, M. K., Barnes, C. A. & Fanselow, M. S. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J. Neurosci.34, 8462–8466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Voogd, L. D. et al. The role of hippocampal spatial representations in contextualization and generalization of fear. Neuroimage206, 116308 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Kassab, R. & Alexandre, F. Pattern separation in the hippocampus: distinct circuits under different conditions. Brain Struct. Funct.223, 2785–2808 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Sahay, A., Wilson, D. A. & Hen, R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron70, 582–588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Casale, A. et al. Grey matter volume reductions of the left Hippocampus and Amygdala in PTSD: A coordinate-based meta-analysis of magnetic resonance imaging studies. Neuropsychobiology81, 257–264 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Kunimatsu, A., Yasaka, K., Akai, H., Kunimatsu, N. & Abe, O. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imaging52, 380–396 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Lambert, H. K. & McLaughlin, K. A. Impaired hippocampus-dependent associative learning as a mechanism underlying PTSD: A meta-analysis. Neurosci. Biobehav. Rev.107, 729–749 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbertson, M. W. et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci.5, 1242–1247 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logue, M. W. et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite enigma-pgc study: subcortical volumetry results from posttraumatic stress disordr Consortia. Biol. Psychiatry83, 244–253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitman, R. K. et al. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann. N. Y Acad. Sci.1071, 242–254 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie, H. et al. Relationship of hippocampal volumes and posttraumatic stress disorder symptoms over early posttrauma periods. Biol. Psychiatry Cogn. Neurosci. Neuroimaging3, 968–975 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy-Gigi, E., Sudai, E. & Bar, M. Context as a barrier: Impaired contextual processing increases the tendency to develop PTSD symptoms across repeated exposure to trauma. J. Anxiety Disord.100, 102765 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Bernstein, E. E., Brühl, A., Kley, H., Heinrichs, N. & McNally, R. J. Mnemonic discrimination in treatment-seeking adults with and without PTSD. Behav. Res Ther.131, 103650 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Schwartz, R. H., Gruenewald, P. J., Klitzner, M. & Fedio, P. Short-term memory impairment in cannabis-dependent adolescents. Am. J. Dis. Child143, 1214–1219 (1989). [DOI] [PubMed] [Google Scholar]
- 32.Millsaps, C. L., Azrin, R. L. & Mittenberg, W. Neuropsychological effects of chronic cannabis use on the memory and intelligence of adolescents. J. Child Adolesc. Subst. Abus.3, 47–55 (1994). [Google Scholar]
- 33.Solowij, N. et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology216, 131–144 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Blest-Hopley, G., O’Neill, A., Wilson, R., Giampietro, V. & Bhattacharyya, S. Disrupted parahippocampal and midbrain function underlie slower verbal learning in adolescent-onset regular cannabis use. Psychopharmacology238, 1315–1331 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen, H. T. & Mackie, K. Adolescent Δ(9)-Tetrahydrocannabinol exposure selectively impairs working memory but not several other mPFC-mediated behaviors. Front Psychiatry11, 576214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prini, P. et al. Neurobiological mechanisms underlying cannabis-induced memory impairment. Eur. Neuropsychopharmacol.36, 181–190 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Zou, S. & Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci.19. 10.3390/ijms19030833 (2018). [DOI] [PMC free article] [PubMed]
- 38.Davies, S. N., Pertwee, R. G. & Riedel, G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology42, 993–1007 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Laaris, N., Good, C. H. & Lupica, C. R. Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology59, 121–127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier, M. H. et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA109, E2657–E2664 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yücel, M. et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch. Gen. Psychiatry65, 694–701 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Lorenzetti, V., Solowij, N. & Yücel, M. The role of cannabinoids in neuroanatomic alterations in Cannabis users. Biol. Psychiatry79, e17–e31 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Lorenzetti, V. et al. Gross morphological brain changes with chronic, heavy cannabis use. Br. J. Psychiatry206, 77–78 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Solowij, N. et al. Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophr. Res143, 179–184 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Meier, M. H. et al. Long-term cannabis use and cognitive reserves and hippocampal volume in midlife. Am. J. Psychiatry179, 362–374 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, Y., Zuo, C., Wang, W., Xu, Q. & Hao, L. Reduction in hippocampal volumes subsequent to heavy cannabis use: a 3-year longitudinal study. Psychiatry Res295, 113588 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Garimella, A., Rajguru, S., Singla, U. K. & Alluri, V. Marijuana and the hippocampus: A longitudinal study on the effects of marijuana on hippocampal subfields. Prog. Neuropsychopharmacol. Biol. Psychiatry101, 109897 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Kroon, E., Kuhns, L. & Cousijn, J. The short-term and long-term effects of cannabis on cognition: recent advances in the field. Curr. Opin. Psychol.38, 49–55 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Lovell, M. E., Akhurst, J., Padgett, C., Garry, M. I. & Matthews, A. Cognitive outcomes associated with long-term, regular, recreational cannabis use in adults: A meta-analysis. Exp. Clin. Psychopharmacol.28, 471–494 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Lorenzetti, V., Chye, Y., Silva, P., Solowij, N. & Roberts, C. A. Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. Eur. Arch. Psychiatry Clin. Neurosci.269, 59–71 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Lorenzetti, V. et al. Brain anatomical alterations in young cannabis users: is it all hype? a meta-analysis of structural neuroimaging studies. Cannabis Cannabinoid Res8, 184–196 (2023). [DOI] [PubMed] [Google Scholar]
- 52.McKetin, R., Parasu, P., Cherbuin, N., Eramudugolla, R. & Anstey, K. J. A longitudinal examination of the relationship between cannabis use and cognitive function in mid-life adults. Drug Alcohol Depend.169, 134–140 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Tait, R. J., Mackinnon, A. & Christensen, H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction106, 2195–2203 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Scott, J. C. et al. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry75, 585–595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bryan, J. L., Hogan, J., Lindsay, J. A. & Ecker, A. H. Cannabis use disorder and post-traumatic stress disorder: The prevalence of comorbidity in veterans of recent conflicts. J. Subst. Abus. Treat.122, 108254 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Hill, M. L., Loflin, M., Nichter, B., Norman, S. B. & Pietrzak, R. H. Prevalence of cannabis use, disorder, and medical card possession in U.S. military veterans: Results from the 2019-2020 National Health and Resilience in Veterans Study. Addict. Behav.120, 106963 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Bonn-Miller, M. O., Babson, K. A. & Vandrey, R. Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend.136, 162–165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metrik, J. et al. The mediating roles of coping, sleep, and anxiety motives in cannabis use and problems among returning veterans with PTSD and MDD. Psychol. Addict. Behav.30, 743–754 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stark, S. M., Kirwan, C. B. & Stark, C. E. L. Mnemonic similarity task: a tool for assessing hippocampal integrity. Trends Cogn. Sci.23, 938–951 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker, D. G. et al. Predictors of risk and resilience for posttraumatic stress disorder among ground combat Marines: methods of the Marine Resiliency Study. Prev. Chronic Dis.9, E97 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cousijn, J. et al. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage59, 3845–3851 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Kirwan, C. B. & Stark, C. E. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem.14, 625–633 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bovin, M. J. et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol. Assess.28, 1379–1391 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Beck, A. T., Steer, R. A. & Brown, G. Beck Depression Inventory–II (BDI-II). APA PsychTests (1996).
- 65.Brown, S. A. et al. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J. Stud. Alcohol59, 427–438 (1998). [DOI] [PubMed] [Google Scholar]
- 66.Stark, S. M., Yassa, M. A., Lacy, J. W. & Stark, C. E. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia51, 2442–2449 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yassa, M. A. et al. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus21, 968–979 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broyd, S. J., van Hell, H. H., Beale, C., Yücel, M. & Solowij, N. Acute and chronic effects of cannabinoids on human cognition-a systematic review. Biol. Psychiatry79, 557–567 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Paul, S. & Bhattacharyya, S. Cannabis use-related working memory deficit mediated by lower left hippocampal volume. Addict. Biol.26, e12984 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Schacht, J. P., Hutchison, K. E. & Filbey, F. M. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology37, 2368–2376 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koenders, L. et al. Longitudinal study of hippocampal volumes in heavy cannabis users. J. Psychopharmacol.31, 1027–1034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meier, M. H. et al. Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction113, 257–265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bourque, J. & Potvin, S. Cannabis and cognitive functioning: from acute to residual effects, from randomized controlled trials to prospective designs. Front. Psychiatry12, 596601 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demirakca, T. et al. Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend.114, 242–245 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Schuster, R. M. et al. One Month of cannabis abstinence in adolescents and young adults is associated with improved memory. J. Clin. Psychiatry79. 10.4088/JCP.17m11977 (2018). [DOI] [PMC free article] [PubMed]
- 76.Yücel, M. et al. Hippocampal harms, protection and recovery following regular cannabis use. Transl. Psychiatry6, e710 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Figueiredo, P. R., Tolomeo, S., Steele, J. D. & Baldacchino, A. Neurocognitive consequences of chronic cannabis use: a systematic review and meta-analysis. Neurosci. Biobehav Rev.108, 358–369 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Sagar, K. A. et al. An observational, longitudinal study of cognition in medical cannabis patients over the course of 12 months of treatment: preliminary results. J. Int Neuropsychol. Soc.27, 648–660 (2021). [DOI] [PubMed] [Google Scholar]
- 79.Doss, M. K., Weafer, J., Gallo, D. A. & de Wit, H. Δ(9)-Tetrahydrocannabinol during encoding impairs perceptual details yet spares context effects on episodic memory. Biol. Psychiatry Cogn. Neurosci. Neuroimaging5, 110–118 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith, B. M., Thomasson, M., Yang, Y. C., Sibert, C. & Stocco, A. When fear shrinks the brain: a computational model of the effects of posttraumatic stress on hippocampal volume. Top. Cogn. Sci.13, 499–514 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Gosnell, S. N. et al. Hippocampal Volume in Psychiatric Diagnoses: Should Psychiatry BiomarkerResearch Account for Comorbidities? Chronic. Stress4, 2470547020906799 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fitzgerald, J. M. et al. Hippocampal resting-state functional connectivity forecasts individual posttraumatic stress disorder symptoms: a data-driven approach. Biol. Psychiatry Cogn. Neurosci. Neuroimaging7, 139–149 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Rooij, S. J. H. et al. The role of the hippocampus in predicting future posttraumatic stress disorder symptoms in recently traumatized civilians. Biol. Psychiatry84, 106–115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butler, O. et al. Hippocampal gray matter increases following multimodal psychological treatment for combat-related post-traumatic stress disorder. Brain Behav.8, e00956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu, X. et al. Exposure-based therapy changes amygdala and hippocampus resting-state functional connectivity in patients with posttraumatic stress disorder. Depress Anxiety35, 974–984 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Besnard, A. & Sahay, A. Adult Hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology41, 24–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dunsmoor, J. E. & Paz, R. Fear generalization and anxiety: behavioral and neural mechanisms. Biol. Psychiatry78, 336–343 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Lange, I. et al. Behavioral pattern separation and its link to the neural mechanisms of fear generalization. Soc. Cogn. Affect Neurosci.12, 1720–1729 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berg, H. et al. Salience and central executive networks track overgeneralization of conditioned-fear in post-traumatic stress disorder. Psychol. Med.51, 2610–2619 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaczkurkin, A. N. et al. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatry174, 125–134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morey, R. A., et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl. Psychiatry5, e700 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morey, R. A., Haswell, C. C., Stjepanović, D., Dunsmoor, J. E. & LaBar, K. S. Neural correlates of conceptual-level fear generalization in posttraumatic stress disorder. Neuropsychopharmacology45, 1380–1389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bassir Nia, A., Bender, R. & Harpaz-Rotem, I. Endocannabinoid system alterations in posttraumatic stress disorder: a review of developmental and accumulative effects of trauma. Chronic Stress3. 10.1177/2470547019864096 (2019). [DOI] [PMC free article] [PubMed]
- 94.Hill, M. N. et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology38, 2952–2961 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gowatch, L. C. et al. Endocannabinoids and stress-related neurospsychiatric disorders: a systematic review and meta-analysis of basal concentrations and response to acute psychosocial stress. Cannabis Cannabinoid Res9, 1217–1234 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hill, M. N., Miller, G. E., Carrier, E. J., Gorzalka, B. B. & Hillard, C. J. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology34, 1257–1262 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mazurka, R. et al. Endocannabinoid concentrations in major depression: effects of childhood maltreatment and relation to hippocampal volume. Transl. Psychiatry14, 431 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi, K. et al. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J. Psychiatr. Res46, 882–889 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Hungund, B. L. et al. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol. Psychiatry9, 184–190 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Neumeister, A. et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol. Psychiatry18, 1034–1040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee, T. T., Hill, M. N. & Lee, F. S. Developmental regulation of fear learning and anxiety behavior by endocannabinoids. Genes Brain Behav.15, 108–124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osborne, A. L., Solowij, N. & Weston-Green, K. A systematic review of the effect of cannabidiol on cognitive function: Relevance to schizophrenia. Neurosci. Biobehav Rev.72, 310–324 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Ortiz, R., Rueda, S. & Di Ciano, P. Use of cannabidiol (CBD) for the treatment of cognitive impairment in psychiatric and neurological illness: A narrative review. Exp. Clin. Psychopharmacol.31, 978–988 (2023). [DOI] [PubMed] [Google Scholar]
- 104.Batalla, A., Bos, J., Postma, A. & Bossong, M. G. The impact of cannabidiol on human brain function: a systematic review. Front Pharm.11, 618184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bomfim, A. J. L. et al. Effects of the acute and chronic administration of cannabidiol on cognition in humans and animals: a systematic review. Cannabis Cannabinoid Res8, 955–973 (2023). [DOI] [PubMed] [Google Scholar]
- 106.Grimm, O. et al. Probing the endocannabinoid system in healthy volunteers: Cannabidiol alters fronto-striatal resting-state connectivity. Eur. Neuropsychopharmacol.28, 841–849 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Lees, R. et al. Effect of four-week cannabidiol treatment on cognitive function: secondary outcomes from a randomised clinical trial for the treatment of cannabis use disorder. Psychopharmacology240, 337–346 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hotz, J., Fehlmann, B., Papassotiropoulos, A., de Quervain, D. J. & Schicktanz, N. S. Cannabidiol enhances verbal episodic memory in healthy young participants: A randomized clinical trial. J. Psychiatr. Res.143, 327–333 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Calabrese, E. J. & Rubio-Casillas, A. Biphasic effects of THC in memory and cognition. Eur. J. Clin. Invest48, e12920 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Papini, S. et al. Chronic cannabis use is associated with impaired fear extinction in humans. J. Abnorm Psychol.126, 117–124 (2017). [DOI] [PubMed] [Google Scholar]
- 111.Rabinak, C. A. et al. Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: a preliminary study. Psychopharmacology237, 1813–1826 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zabik, N. L., Rabinak, C. A., Peters, C. A. & Iadipaolo, A. Cannabinoid modulation of corticolimbic activation during extinction learning and fear renewal in adults with posttraumatic stress disorder. Neurobiol. Learn Mem.201, 107758 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gallego-Landin, I., García-Baos, A., Castro-Zavala, A. & Valverde, O. Reviewing the role of the Endocannabinoid system in the pathophysiology of depression. Front Pharm.12, 762738 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ibarra-Lecue, I. et al. The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochem Pharm.157, 97–107 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Kayir, H., Ruffolo, J., McCunn, P. & Khokhar, J. Y. The relationship between cannabis, cognition, and schizophrenia: it’s complicated. Curr. Top. Behav. Neurosci.63, 437–461 (2023). [DOI] [PubMed] [Google Scholar]
- 116.Selloni, A., Bhatia, G., Ranganathan, M. & De Aquino, J. P. Multimodal correlates of cannabis use among U.S. veterans with bipolar disorder: an integrated study of clinical, cognitive, and functional outcomes. J. Dual Diagn.18, 81–91 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Braga, R. J., Burdick, K. E., Derosse, P. & Malhotra, A. K. Cognitive and clinical outcomes associated with cannabis use in patients with bipolar I disorder. Psychiatry Res200, 242–245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Menendez-Miranda, I. et al. History of lifetime cannabis use is associated with better cognition and worse real-world functioning in schizophrenia spectrum disorders. Eur. Addict. Res.25, 111–118 (2019). [DOI] [PubMed] [Google Scholar]
- 119.Post, L. M., Feeny, N. C., Zoellner, L. A. & Connell, A. M. Post-traumatic stress disorder and depression co-occurrence: Structural relations among disorder constructs and trait and symptom dimensions. Psychol. Psychother.89, 418–434 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Elhai, J. D. et al. Testing whether posttraumatic stress disorder and major depressive disorder are similar or unique constructs. J. Anxiety Disord.25, 404–410 (2011). [DOI] [PubMed] [Google Scholar]
- 121.Bonn-Miller, M. O. et al. The long-term, prospective, therapeutic impact of cannabis on post-traumatic stress disorder. Cannabis Cannabinoid Res.7, 214–223 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonn-Miller, M. O. et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS One16, e0246990 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilkinson, S. T., Stefanovics, E. & Rosenheck, R. A. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J. Clin. Psychiatry76, 1174–1180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abizaid, A., Merali, Z. & Anisman, H. Cannabis: A potential efficacious intervention for PTSD or simply snake oil?. J. Psychiatry Neurosci.44, 75–78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yarnell, S. The use of medicinal Marijuana for posttraumatic stress disorder: a review of the current literature. Prim. Care Companion CNS Disord.17,10.4088/PCC.15r01786 (2015). [DOI] [PMC free article] [PubMed]
- 126.Orsolini, L. et al. Use of Medicinal Cannabis and Synthetic Cannabinoids in Post-Traumatic Stress Disorder (PTSD): A Systematic Review. Medicina55,10.3390/medicina55090525 (2019). [DOI] [PMC free article] [PubMed]
- 127.Schnurr, P. P. et al. The management of posttraumatic stress disorder and acute stress disorder: Synopsis of the 2023 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann. Intern. Med.177, 363–374 (2024). [DOI] [PubMed] [Google Scholar]
- 128.Hill, M. L. et al. Cannabis use and trauma-focused treatment for co-occurring posttraumatic stress disorder and substance use disorders: A meta-analysis of individual patient data. J. Anxiety Disord.102, 102827 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Larsen, S. F., Johnson, A. J., Larimer, M. E., Dager, S. R. & Kleinhans, N. M. Self-report methodology for quantifying standardized cannabis consumption in milligrams delta-9-tetrahydrocannabinol. Am. J. Drug Alcohol Abus.49, 723–732 (2023). [DOI] [PubMed] [Google Scholar]
- 130.Lorenzetti, V. et al. The International Cannabis Toolkit (iCannToolkit): a multidisciplinary expert consensus on minimum standards for measuring cannabis use. Addiction117, 1510–1517 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Watkins, S. L., Karliner-Li, P., Lee, Y. O., Koester, K. A. & Ling, P. M. A mixed-methods study to inform the clarity and accuracy of cannabis-use and cannabis-tobacco co-use survey measures. Drug Alcohol Depend.224, 108697 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hill, M. N., Karacabeyli, E. S. & Gorzalka, B. B. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology32, 350–357 (2007). [DOI] [PubMed] [Google Scholar]
- 133.Ney, L. J., Matthews, A., Bruno, R. & Felmingham, K. L. Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neurosci. Biobehav Rev.94, 302–320 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Xing, G. et al. Cannabinoid receptor expression and phosphorylation are differentially regulated between male and female cerebellum and brain stem after repeated stress: implication for PTSD and drug abuse. Neurosci. Lett.502, 5–9 (2011). [DOI] [PubMed] [Google Scholar]
- 135.Santoro, A. et al. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. Int. J. Mol. Sci. 22. 10.3390/ijms22020972 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request and with appropriate data use agreement.