Abstract
Muscle satellite cells (MSCs) are the most commonly used cells in cultured meat research and development. Enhancing MSC proliferation and differentiation while reducing cell culture costs is requisite to commercializing cultured meat. This study explored the effects of Glycyrrhiza uralensis crude water extract (GU-CWE) and licochalcone A and B (Lic A or B) on the proliferation and differentiation of chicken, bovine, and porcine MSCs. While GU-CWE and Lic A and B had negligible effects on bovine and porcine MSCs, GU-CWE significantly enhanced chicken MSC differentiation, and Lic A and B promoted both the proliferation and differentiation of chicken MSCs. Furthermore, GU-CWE was found to mitigate reactive oxygen species activity during chicken MSC differentiation and promote cell proliferation and adhesion in spheroid culture, thereby maintaining a spherical shape. Collectively, this study suggests that GU-CWE and Lic A and B can significantly reduce costs and safely increase the productivity of chicken MSCs in cultured meat production processes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-98386-1.
Keywords: Muscle satellite cells, Cultured meat, Glycyrrhiza uralensis, Proliferation, Differentiation
Subject terms: Cell biology, Stem cells
Introduction
Cultured meat, manufactured by culturing animal cells to mimic the texture and taste of traditional meat, is a promising future food technology. This innovation has garnered significant interest from both researchers and entrepreneurs, with the goal of commercializing it. Among the various cells explored for cultured meat production, muscle satellite cells (MSCs) are predominantly utilized owing to their efficacy in replicating skeletal muscle tissue structures1,2. Overcoming technical challenges, such as developing cost-effective and food-safe culture media for long-term cell cultivation, is crucial for cultured meat industrialization.
MSCs, which reside in skeletal muscles, express specific marker genes, including paired box 7 (Pax7)3. Physical stimuli, hormones, growth factors, and interactions with neighboring cells activate these cells, causing them to transform into myoblasts, which augment growth, proliferation, and differentiation. MSCs, well-known stem cells essential for maintaining and building skeletal muscle, undergo myogenesis, forming myotubes that are remarkably similar to myofibers, the fundamental functional units of skeletal muscle. These myotubes, which are multinucleated syncytial cells primarily comprising actin and myosin proteins in a sarcomere structure, are vital for replicating meat texture and flavor in cultured meat products. The myogenic regulatory factor (MRF) family, which includes myogenic factor 5 (Myf5), myoblast determination protein 1 (MYOD), myogenin (MYOG), and Myf6, coordinates myogenesis by sequentially regulating muscle-specific gene expression4–10. Myf5, MYOD, and Myf6 are myogenic determination factors that direct progenitor cells towards a muscle lineage, while MYOD, Myf6, and MYOG promote differentiation, with MYOG causing myoblasts to leave the cell cycle and fuse into multinucleated myofibers11.
Myostatin (MSTN), a well-known myokine produced and secreted by skeletal muscles, plays an important role in inhibiting muscle growth and maintaining muscle size. It binds to the activin type 2 receptor on muscle cells and circulates through the bloodstream, triggering a cascade of intracellular signals that enhance muscle protein breakdown by activating Suppressor of Mothers against Decapentaplegic proteins and regulating the Protein Kinase B/Forkhead Box O pathway, thereby negatively impacting muscle growth12–16. The inhibition of MSTN activity in MSCs to promote myogenesis has been investigated, thus potentially contributing to the long-term treatment of muscle-wasting diseases, such as sarcopenia and cachexia17–19. In the context of cultured meat production, targeting MSTN can also enhance the proliferation and differentiation of MSCs, which are pivotal for generating meat-like textures. Glycyrrhiza uralensis, commonly known as licorice, is an herb from the Leguminosae family that has long been used as a traditional remedy in Asia, including China and Korea. We have previously demonstrated that the G. uralensis crude water extract (GU-CWE) promotes mouse myoblast proliferation and differentiation20. Furthermore, licochalcones derived from G. uralensis and G. glabra physically bind to MSTN, downregulating MSTN expression as well as the muscle protein degradation genes Atrogin 1 and Muscle-specific RING finger protein 1, as demonstrated by in silico modeling21–23.
Our research extends these findings by exploring the effects of GU-CWE and licochalcone A and B (Lic A and B) on the proliferation and differentiation of MSCs from bovine, pig, and chicken. Interestingly, while these compounds exerted no significant effect on bovine and porcine MSCs, they were effective in enhancing the differentiation of chicken MSCs, indicating species-specific activity that may have important implications for the specialized production of cultured meat. This underscores the potential of natural compounds to differentiate MSC activity across diverse species, potentially leading to more efficient and targeted approaches in cultured meat technology.
Results
Effects of G. uralensis CWE on the proliferation and differentiation of chicken, bovine, and Porcine MSCs
An experiment was conducted to evaluate the effects of GU-CWE on the proliferation and differentiation of primary MSCs from cattle, pigs, and chickens used in cultured meat research (Fig. 1). While no significant changes in the proliferation of bovine and porcine MSCs were noted compared with that of the control, chicken MSCs exhibited a slight decrease in proliferation (Fig. 1A and Suppl Fig. 1A). Therefore, gene expression analysis was not conducted on bovine and porcine MSCs due to the absence of positive results in the MTS assay. Bovine and porcine MSCs displayed no significant variation in differentiation compared with the control. In contrast, chicken MSCs demonstrated a 28% increase in the creatine kinase activity, a marker of MSC differentiation, as well as an increase in myotube formation (Fig. 1B&C, and Suppl Fig. 1B&C). Interestingly, CWE treatment exclusively downregulated MSTN mRNA and protein expression in chicken MSCs compared with the control (Fig. 1D & E). These findings suggest that while GU-CWE exerts no significant effect on MSC proliferation, it does enhance chicken MSC differentiation and inhibit MSTN expression.
Fig. 1.
Chicken, bovine, and porcine MSC proliferation and differentiation and MSTN expression following CWE of G. uralensis treatment. Chicken, bovine, and porcine MSCs were cultured in growth media supplemented with or without CWE of G. uralensis for 4 days. (A) Cell proliferation according to MTS assay. When chicken, bovine, and porcine MSCs were at 100% confluency, growth media were switched to differentiation media (2% FBS) supplemented with or without CWE of G. uralensis and incubated for 2 days. (B) Chicken, bovine, and porcine MSC differentiation based on creatine kinase activity. (C) Chicken cell morphology using phalloidin staining (green: phalloidin, blue: nuclei). (D) & E) Chicken, bovine, and porcine MSTN mRNA expression according to real time RT-PCR and protein expression based on Western blotting. Control refers to non-treated cells. Results are presented as the mean ± SD (n > 3). * p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001.
Expression of differentiation markers in chicken MSCs treated with G. uralensis CWE and its effect under serum-free conditions
The effect of GU-CWE on the expression of differentiation marker genes during chicken MSC differentiation was evaluated. The expression levels of MYOD, MYOG, and myosin heavy chain (MYH) were significantly higher in the GU-CWE-treated group than in the control group, as evidenced by real-time RT-PCR, Western blot, and immunocytochemistry analyses (Fig. 2A-C). In addition, experiments similar to those shown in Fig. 2 were conducted without the addition of fetal bovine serum (FBS). This experiment excluded the commonly used 2% FBS in the MSC differentiation medium. In the absence of FBS, myotube formation was significantly reduced compared with that in the control with 2% FBS. However, GU-CWE treatment enhanced myotube formation. Similarly, creatine kinase activity as well as the expression of MYOG and MYH and the thrombospondin 1 (THBS1) gene, which reportedly involved in cell adhesion, were all increased (Fig. 3A-C). These findings indicate that the addition of GU-CWE during serum-free differentiation stages can maintain cell adhesion, potentially compensating for the detachment issues observed in serum-free conditions. Therefore, GU-CWE is a potentially viable additive for enhancing cell adhesion and differentiation in serum-free cultured meat production.
Fig. 2.
Myogenic mRNA and protein expression following CWE of G. uralensis treatment during chicken MSC differentiation. When chicken MSCs were at 100% confluency, growth media were switched to differentiation media (2% FBS) supplemented with or without CWE of G. uralensis and incubated for 2 days. A) & B) Myogenic (MYOD, MYOG, and MYH) mRNA expression according to real time RT-PCR and protein expression based on Western blotting. C) MYOD (green), MYOG (green), and MYH (green) protein expression according to immunocytochemistry. Control refers to non-treated cells. Results are presented as the mean ± SD (n > 3). * p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001.
Fig. 3.
Effect of CWE of G. uralensis on chicken MSC differentiation under serum-free conditions. When chicken MSCs were at 100% confluency, growth media were switched to differentiation media (2% FBS or serum free) supplemented with or without CWE of G. uralensis and incubated for 2 days. (A) Cell morphology using phalloidin staining (green: phalloidin, blue: nuclei). (B) MSC differentiation according to creatine kinase activity. (C) MYOD, MYH, and THBS1 protein expression based on Western blotting. 2% FBS-cultured cells were used as a control. Results are presented as the mean ± SD (n > 3). * p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001.
ROS activity and spheroid formation with G. uralensis CWE treatment
We investigated the effects of GU-CWE on reactive oxygen species (ROS) levels during chicken MSC differentiation. GU-CWE treatment significantly reduced ROS levels compared with the control, indicating that GU-CWE has an antioxidative effect (Fig. 4A). Additionally, GU-CWE treatment in a spheroid culture experiment elicited a more spherical morphology than the control, indicating an increase in cell adhesion. GU-CWE treatment also increased the expression of the MSC marker gene Pax7 and cell proliferation marker gene proliferating cell nuclear antigen (PCNA) (Fig. 4B). These findings confirm that GU-CWE treatment effectively inhibits ROS activity while also promoting MSC maintenance and proliferation in three-dimensional cultures, indicating that it harbors potential for muscle tissue formation.
Fig. 4.
ROS activity and spheroid culture following CWE of G. uralensis addition to chicken MSCs. When chicken MSCs were at 100% confluency, growth media were switched to differentiation media (2% FBS) supplemented with or without CWE of G. uralensis and incubated for 2 days. (A) ROS activity measurement. Chicken MSCs were cultured with proliferation media supplemented with or without CWE of G. uralensis for 2 days in a round-shaped well. After 2 days, round-shaped cells (spheroid) were collected, and RNA expression was analyzed. (B) Pax7 and PCNA mRNA expression according to real time RT-PCR. Control refers to non-treated cells. Results are presented as the mean ± SD (n > 3). * p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001.
Effects of lic A or B treatment on the proliferation and differentiation of chicken, bovine, and Porcine MSCs
Figure 5 illustrates the effects of Lic A and B on the proliferation and differentiation of MSCs from bovine, pigs, and chickens. Similar to the GU-CWE treatment experimental results, Lic A and B exerted no effect on the proliferation of bovine and porcine MSCs but significantly increased cell proliferation by 5% and 8%, respectively, in chicken MSCs (Fig. 5A-C, Suppl Fig. 2A&B). In addition, myotube formation was increased; and Lic A and B increased creatine kinase activity by 19% and 17%, respectively (Fig. 5D&E). Notably, both Lic A and B markedly decreased MSTN mRNA and protein expression in chicken MSCs, a phenomenon not observed in bovine and porcine MSCs (Fig. 5F&J, Suppl Fig. 3A).
Fig. 5.
Chicken, bovine, and porcine MSC proliferation and differentiation and MSTN expression following Lic A and B treatment. Chicken, bovine, and porcine MSCs were incubated in proliferation media supplemented with or without Lic A or B for 4 days. (A) Chicken, bovine, and porcine MSC proliferation according to MTS assay. (B) Chicken MSC morphology. (C) Cell number count based on direct cell counting. When chicken, bovine, and porcine MSCs were at 100% confluency, growth media were switched to differentiation media (2% FBS) supplemented with or without Lic A or B and incubated for 2 days (D) Chicken, bovine, and porcine MSC differentiation based on creatine kinase activity. (E) Chicken cell morphology using phalloidin staining (green: phalloidin, blue: nuclei). (F) MSTN mRNA expression according to real time RT-PCR. J) MSTN protein expression based on Western blotting. Control refers to non-treated cells. Results are presented as the mean ± SD (n > 3). * p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001.
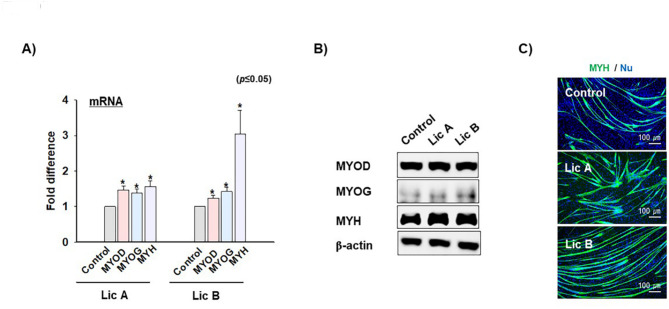
Furthermore, the effect of Lic A and B on the expression of MYOD, MYOG, and MYH on day 2 of chicken MSC differentiation was investigated. Both Licochalcones significantly increased the mRNA and protein expression levels of these marker genes compared with the control (Figs. 6A-C). However, the expression of MYH protein in bovine and porcine was not increased by Lic A and B (Suppl Fig. 3B). Altogether, Lic A and B promote chicken MSC differentiation and inhibit MSTN expression during the differentiation process, implying their potential utility in enhancing MSC differentiation in chickens.
Fig. 6.
Myogenic mRNA and protein expression following Lic A or B treatment during chicken MSC differentiation. When chicken MSCs was at 100% confluency, growth media were switched to differentiation media (2% FBS) supplemented with or without Lic A or B and incubated for 2 days. A) & B) Myogenic (MYOD, MYOG, and MYH) mRNA expression according real time RT-PCR and protein expressions based on Western blotting. C) MYH (green) protein expression according to immunocytochemistry. Control refers to non-treated cells. Results are presented as the mean ± SD (n > 3). * p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.0001.
Discussion
The rapid population increase and corresponding surge in meat consumption are anticipated to lead to future environmental pollution and excessive livestock slaughtering issues. Cultured meat produced using scientific and hygienic cell culture processes emerges as a potential solution to these problems24–26. However, commercializing cultured meat necessitates overcoming several challenges, especially improving the efficiency of the costly cell culture media used in long-term cultivation processes essential for cultured meat production.
Various animal cells are employed in cultured meat production, with MSCs being particularly favored owing to their ability to replicate the texture and taste of meat1,27,28. Our research team has been focusing on isolating and culturing MSCs from skeletal muscle to examine the specific genes expressed during their proliferation and differentiation and determine how these genes assist these processes6,29,30. Recent advancements include the use of in silico techniques to identify substances that can bind specific target proteins within MSCs to enhance cell proliferation and differentiation23,31–33. Notably, natural compounds like quercetin, known for their anticancer, antioxidant, and anti-inflammatory properties, have been shown to promote the differentiation of MSCs derived from bovine, pig, and chicken, offering a cost-effective and food-safe option for cultured meat production processes33. Furthermore, compounds from G. uralensis, such as Lic A and B, bind to the well-known negative regulator of muscle growth, MSTN, and exhibit effects similar to those of GU-CWE23.
Therefore, this study endeavored to ascertain whether GU-CWE, Lic A, and Lic B, as in mice, promote the proliferation and differentiation of MSCs from bovine, pig, and chicken as well as whether these compounds are potentially useful as components of cell culture media in the production process of cultured meat. The study investigated the effects of GU-CWE on the proliferation and differentiation of bovine, porcine, and chicken MSCs and found that while GU-CWE did not significantly affect MSC proliferation across these species, it notably enhanced differentiation and suppressed the expression of the MSTN gene in chicken MSCs (Fig. 1& Suppl Fig. 1). To further explore the mechanism by which GU-CWE enhances chicken MSC differentiation, the gene expression of the transcription factors MYOD and MYOG, which are reportedly involved in the early and late stages of MSC differentiation, as well as MYH, a myotube structural protein formed during the later stages, was examined. The results revealed a significant increase in the expression of these genes following GU-CWE treatment (Fig. 2). Additionally, under serum-free medium conditions without the standard 2% FBS, GU-CWE treated media demonstrated enhanced differentiation as well as the increased expression of the MSC differentiation marker genes MYOG and MYH and cell adhesion gene THBS1, though at a lower level than that in media containing 2% FBS (Fig. 3). Cultured meat production methods must be sustainable, so they must avoid relying on serum or other animal-derived substances that are non-reproducible and in limited supply. As a result, switching to completely serum-free media, or reducing FBS in the culture medium, is critical for the mass production of biotechnological products, especially cultured meat34,35. Cell detachment, on the other hand, can occur in serum-free conditions, which may reduce the final cell differentiation rate36,37. Therefore, cultured meat production requires the establishment of culture conditions that can robustly differentiate satellite cells into mature muscle fibers without the use of animal components38. These results suggest that GU-CWE treatment promotes chicken MSC differentiation primarily by upregulating MYOD and MYOG gene expression while downregulating MSTN gene expression, resulting in the increased expression of MYH and THBS1.
Intriguingly, GU-CWE has previously been demonstrated to promote the proliferation and differentiation of mouse C2C12 myoblasts as well as enhanced skeletal muscle regeneration in vivo20,23. Notably, despite the close similarities between the mammalian MSCs from bovine and pig used in this study and the avian MSCs from chickens, GU-CWE’s effect was exclusively observed in chicken MSC proliferation. This suggests that GU-CWE’s mechanism of action may differ between species, indicating that further research is warranted to elucidate these differences and explore the potential species-specific pathways involved.
During MSC differentiation, ROS generation act as an inhibitory factor against muscle differentiation39,40. G. uralensis is recognized for its antioxidative effects across diverse tissues41–43; moreover, we recently found GU-CWE treatment to reduce ROS levels in C2C12 cells44. Given the oxidative stress that cells experience during the proliferation process in cell culture, the antioxidative effects of GU-CWE during chicken MSC differentiation, as shown in Fig. 4A, indicate that GU-CWE possesses potential as an additive in cell culture media. Additionally, the spheroid culture experiments shown in Fig. 4B demonstrated that GU-CWE treatment not only promotes chicken MSC proliferation but also improves cellular adhesion, promoting the formation of spherical shapes. Spheroid culture carries the advantage of facilitating cell-to-cell signaling and mimicking the in vivo environment, which is critical for culturing cells in ways that reflect natural growth conditions45–48. The continuous cultivation of MSCs derived from skeletal muscle in the meat production process often leads to a decline in cell characteristics, proliferation, and differentiation efficiency. Considering this, future experiments are required to determine whether spheroid culture can maintain these qualities during the initial stages of chicken MSC cultivation, allowing for long-term cultivation. Carefully examining the reduction in necrosis of cells within the spheroid owing to nutrient and oxygen deficiency is imperative.
Consistent with the effects of GU-CWE, Lic A and B did not affect bovine and porcine MSCs proliferation and differentiation but specifically enhanced proliferation and differentiation rates as well as the expression of myogenic marker genes in chicken MSCs, while downregulating the expression of the MSTN gene (Figs. 5 and 6). The fact that these compounds bind to MSTN in in silico analyses suggests that treatments like GU-CWE can inhibit MSTN action, thereby enhancing myogenesis in chickens and offering insights into potential mechanisms for enhancing chicken MSC proliferation and differentiation.
Taken together, these findings suggest that GU-CWE and Lic A and B may serve as natural substances suitable for use in cultivated meat production involving chicken MSCs, potentially easing the high costs associated with culture media use in meat production. This opens avenues for further the exploration of natural substances that accelerate the industrialization of cultured meat.
Materials and methods
Animal experiment
This study was approved by the Institutional Animal Care and Use Committee of Yeungnam University (AEC2022-022). All procedures followed the ethical standards outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, as well as the ARRIVE guidelines.
Chicken, bovine and porcine MSCs isolation and culture
Leg muscles were used to isolate MSCs from chickens, cattle, and pigs. Briefly, muscles were minced and digested with 0.1% pronase (Roche, Mannheim, Germany) at 37 °C for 80 (Chicken) ~ 120 (bovine and porcine) minutes. After digestion, the supernatant was removed, and media [DMEM (Cytiva, Marlborough, USA) + 10% FBS (Fetal bovine serum, Cytiva) + 1% P.S (Penicillin and streptomycin, Thermo Fisher Scientific, Waltham, USA) was added, followed by pipetting. The supernatant was filtered with a 100 μm strainer and centrifuged. After centrifugation, the supernatant was removed, and growth medium [Ham’s F10 (Cytiva) + 10% FBS + 1% P.S + 5 ng/ml human FGF2 (fibroblast growth factor 2, Miltenyi, Auburn, USA)] was added to isolated MSCs, which were then cultured at 37 °C. To induce myogenic differentiation, MSCs were incubated in differentiation media [DMEM (Cytiva)] + 2% FBS or serum free + 1% P.S for 2 days.
CWE of G. uralensis and lic A/B treatment
GU-CWE and Lic (A or B) were added at concentrations of 100 µg/mL and 1 ng/mL, respectively, to evaluate their effects on MSC proliferation and differentiation.
MTS analysis
MSCs were cultured in growth media supplemented with GU-CWE and Lic A/ B for 4 days and subsequently incubated in growth media mixed with CellTiter 96™ One Solution Reagent for 2 h in a humidified 5% CO2 incubator at 37 ℃. The supernatant was collected and absorbance was measured at 490 nM using a VersaMax™ microplate reader (Synergy™ H1 Hybrid Multi-Mode Microplate Reader; BioTek, Winooski, VT, USA).
Cell counting
Chicken, bovine, and porcine MSCs were cultured in growth media containing Lic A or Lic B for 4 days to count the cells. The cells were then treated with trypsin-EDTA (Thermo Scientific) and counted using a hemocytometer.
Creatine kinase
MSCs were incubated with differentiation media supplemented with or without GU-CWE and Lic A/B for 4 days and subsequently washed with phosphate-buffered saline (PBS), collected, and centrifuged at 10,000×g for 15 min. Supernatants were analyzed using a creatine kinase assay kit (BioAssay Systems, CA, USA), and their absorbance was measured at 340 nm using a VersaMax™ microplate reader; thereafter, the following equation was applied.
 |
Real time RT-PCR
RNA extraction, cDNA synthesis, and real-time RT-PCR were conducted as previously described20. Supplementary Table S1 lists the primer sequences used.
Western blot analysis
Protein (100 µg) was electrophoresed, transferred to PVDF membranes, and incubated with blocking reagent (3% skim milk or BSA) for 1 h at room temperature (RT). The membranes were incubated overnight with protein-specific primary antibodies (MYOD [1:500, Santa Cruz Biotechnology, CA, USA], MYOG [1:1,000, Abcam, MA, USA], MYH [1:250, Santa Cruz Biotechnology], MSTN [1:1,000, Cloud-Clone Corp, TX, USA], THBS1 [1:500, Cloud-Clone Corp], and β-actin [1:1,000, Santa Cruz Biotechnology]) in 1% skim milk or BSA in Tris-buffered saline at 4 °C. After 1 day, the membranes were washed and incubated for 2 h at RT with goat–mouse or rabbit horseradish peroxidase-conjugated antibodies (Santa Cruz Biotechnology). Subsequently, they were visualized using SuperSignal™ West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). The original blot image is provided in Suppl Fig. 4.
Immunocytochemistry
Immunocytochemistry was performed as previously described49. Fixed cells with 10% formaldehyde (Sigma Aldrich) were incubated in 0.2% Triton X-100 and subsequently incubated in 1% normal goat serum. The cells were incubated overnight with specific antibodies (MYOD, MYOG, or MYH [1:50]) at 4 °C, followed by Alexa Fluor 488 goat anti-mouse and rabbit secondary antibodies (1:100; Thermo Fisher Scientific) for 1 h at RT. The cells were stained with DAPI (Sigma Aldrich) and imaged using a Nikon fluorescence microscope (Nikon, Tokyo, Japan).
Phalloidin staining
Cells were washed with PBS, incubated with 10% formaldehyde for 10 min at RT, subsequently incubated with 0.2% Triton X-100, and stained with phalloidin (1:400; Invitrogen, CA, USA). The image was captured using a digital camera (Nikon, Japan).
ROS activity analysis
Chicken MSCs were incubated with differentiation media supplemented with or without GU-CWE for 2 days and subsequently incubated with 2’,7’-dichlorofluorescein (Sigma Aldrich) for 1 h at 37 ℃. After washing with PBS, fluorescence was measured using a VersaMax™ microplate reader (excitation 498 nm, emission 522 nm).
Spheroid culture
Chicken MSCs (50,000 cells) were cultured with growth media supplemented with or without GU-CWE for 2 days in spheroid microplates.
Statistical analysis
The T-test and Tukey’s Studentized Range test were used to assess the significance of differences in mean normalized gene expression and proliferation and differentiation rates. Statistical analysis was performed via one-way analysis of variance in SAS (version 9.0; SAS Institute, Cary, NC, USA), with GAPDH or β-actin serving as internal controls. P values < 0.05 were considered statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1A6A1A03044512) and a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2021R1A2C2004177). In addition, the study was supported by the Forestry (IPET) through High Value added Food Technology Development Program of the Korea Institute of Planning and Evaluation for Technology in Food and Agriculture funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (Grant nos. 321026-05).
Author contributions
E.J.L.: designed the work, performed the experiments, analyzed the data, wrote the manuscript. S.S.: performed the experiments, analyzed the data, wrote the manuscript. S.J.H.: contributed the materials. J.H.L.: performed the experiments. I.C.: designed the work, analyzed the data, wrote the manuscript.
Data availability
The data presented in this study are included in this article and the accompanying supplementary material.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally to this work.
References
- 1.Shaikh, S. et al. Cell types used for cultured meat production and the importance of myokines. Foods1010.3390/foods10102318 (2021). [DOI] [PMC free article] [PubMed]
- 2.Guan, X., Zhou, J., Du, G. & Chen, J. Bioprocessing technology of muscle stem cells: implications for cultured meat. Trends Biotechnol.40, 721–734. 10.1016/j.tibtech.2021.11.004 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Seale, P. et al. Pax7 is required for the specification of myogenic satellite cells. Cell102, 777–786. 10.1016/s0092-8674(00)00066-0 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Bentzinger, C. F., von Maltzahn, J. & Rudnicki, M. A. Extrinsic regulation of satellite cell specification. Stem Cell. Res. Ther.110.1186/scrt27 (2010). [DOI] [PMC free article] [PubMed]
- 5.Brack, A. S. & Rando, T. A. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell. Stem Cell.10, 504–514. 10.1016/j.stem.2012.04.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, E. J. et al. Depot-specific gene expression profiles during differentiation and transdifferentiation of bovine muscle satellite cells, and differentiation of preadipocytes. Genomics100, 195–202. 10.1016/j.ygeno.2012.06.005 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Jan, A. T., Lee, E. J., Ahmad, S. & Choi, I. Meeting the meat: delineating the molecular machinery of muscle development. J. Anim. Sci. Technol.58, 18. 10.1186/s40781-016-0100-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryson-Richardson, R. J. & Currie, P. D. The genetics of vertebrate myogenesis. Nat. Rev. Genet.9, 632–646. 10.1038/nrg2369 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Fang, J. et al. Skeletal muscle regeneration via the chemical induction and expansion of myogenic stem cells in situ or in vitro. Nat. Biomed. Eng.5, 864–879. 10.1038/s41551-021-00696-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henze, H. et al. Denervation alters the secretome of myofibers and thereby affects muscle stem cell lineage progression and functionality. NPJ Regen Med.910.1038/s41536-024-00353-3 (2024). [DOI] [PMC free article] [PubMed]
- 11.Zammit, P. S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell. Dev. Biol.72, 19–32. 10.1016/j.semcdb.2017.11.011 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Relizani, K. et al. Blockade of actriib signaling triggers muscle fatigability and metabolic myopathy. Mol. Ther.22, 1423–1433. 10.1038/mt.2014.90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorgens, S. W., Eckardt, K., Jensen, J., Drevon, C. A. & Eckel, J. Exercise and regulation of adipokine and myokine production. Prog Mol. Biol. Transl Sci.135, 313–336. 10.1016/bs.pmbts.2015.07.002 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Lee, J. H. & Jun, H. S. Role of myokines in regulating skeletal muscle mass and function. Front. Physiol.10, 42. 10.3389/fphys.2019.00042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy, A. & Kumar, A. Supraphysiological activation of TAK1 promotes skeletal muscle growth and mitigates neurogenic atrophy. Nat. Commun.13, 2201. 10.1038/s41467-022-29752-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abati, E., Manini, A., Comi, G. P. & Corti, S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell. Mol. Life Sci.79, 374. 10.1007/s00018-022-04408-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White, T. A. & LeBrasseur, N. K. Myostatin and sarcopenia: opportunities and challenges - a mini-review. Gerontology60, 289–293. 10.1159/000356740 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Baig, M. H. et al. Myostatin and its regulation: A comprehensive review of myostatin inhibiting strategies. Front. Physiol.13, 876078. 10.3389/fphys.2022.876078 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson, A. M. et al. Inhibiting myostatin signaling partially mitigates structural and functional adaptations to hindlimb suspension in mice. NPJ Microgravity. 9, 2. 10.1038/s41526-022-00233-4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, E. J. et al. Isolation and characterization of compounds from Glycyrrhiza uralensis as therapeutic agents for the muscle disorders. Int. J. Mol. Sci.2210.3390/ijms22020876 (2021). [DOI] [PMC free article] [PubMed]
- 21.Maria Pia, G. D., Sara, F., Mario, F. & Lorenza, S. Biological effects of licochalcones. Mini Rev. Med. Chem.19, 647–656. 10.2174/1389557518666180601095420 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Shaikh, S., Lee, E. J., Ahmad, K. & Choi, I. Therapeutic potential and action mechanisms of licochalcone B: a mini review. Front. Mol. Biosci.11, 1440132. 10.3389/fmolb.2024.1440132 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad, K. et al. Licochalcone A and B enhance muscle proliferation and differentiation by regulating myostatin. Phytomedicine125, 155350. 10.1016/j.phymed.2024.155350 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Deliza, R., Rodríguez, B. & Reinoso-Carvalho, F. Lucchese-Cheung, T. Cultured meat: a review on accepting challenges and upcoming possibilities. Curr. Opin. Food Sci.52, 101050 (2023). [Google Scholar]
- 25.Ibrahim, N. E. S. Making cultured meat tasty. Nat. Reviews Bioeng.2, 198–198 (2024). [Google Scholar]
- 26.Martins, B. et al. Advances and challenges in cell biology for cultured meat. Annu. Rev. Anim. Biosci.12, 345–368. 10.1146/annurev-animal-021022-055132 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Kang, D. H. et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated Bioprinting. Nat. Commun.12, 5059. 10.1038/s41467-021-25236-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joo, S. T. et al. A comparative study on the taste characteristics of satellite cell cultured meat derived from chicken and cattle muscles. Food Sci. Anim. Resour.42, 175–185. 10.5851/kosfa.2021.e72 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, E. et al. Effect of sex steroid hormones on bovine myogenic satellite cell proliferation, differentiation and lipid accumulation in myotube. Asian-Australasian J. Anim. Sci.23, 649–658 (2010). [Google Scholar]
- 30.Lee, E. J. et al. Fibromodulin: a master regulator of myostatin controlling progression of satellite cells through a myogenic program. FASEB J.30, 2708–2719. 10.1096/fj.201500133R (2016). [DOI] [PubMed] [Google Scholar]
- 31.Alonso-Martin, S. et al. Gene expression profiling of muscle stem cells identifies novel regulators of postnatal myogenesis. Front. Cell. Dev. Biol.4, 58. 10.3389/fcell.2016.00058 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baig, M. H. et al. Methylglyoxal and advanced glycation end products: insight of the regulatory machinery affecting the myogenic program and of its modulation by natural compounds. Sci. Rep.7, 5916. 10.1038/s41598-017-06067-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad, S. S. et al. Targeting myostatin using Quercetin as a media supplement to improve myogenesis for cultured meat production: an in Silico and in vitro study. Curr. Res. Food Sci.8, 100678. 10.1016/j.crfs.2024.100678 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saini, A. et al. Asymmetric cellular responses in primary human myoblasts using Sera of different origin and specification. PLoS One. 13, e0192384. 10.1371/journal.pone.0192384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolkmann, A. M., Post, M. J., Rutjens, M. A. M., van Essen, A. L. M. & Moutsatsou, P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology72, 111–120. 10.1007/s10616-019-00361-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayman, E. G., Pierschbacher, M. D., Suzuki, S. & Ruoslahti, E. Vitronectin–a major cell attachment-promoting protein in fetal bovine serum. Exp. Cell. Res.160, 245–258. 10.1016/0014-4827(85)90173-9 (1985). [DOI] [PubMed] [Google Scholar]
- 37.Stout, A. J. et al. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol.5, 466. 10.1038/s42003-022-03423-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messmer, T. et al. A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nat. Food. 3, 74–85. 10.1038/s43016-021-00419-1 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Zhang, Q. et al. Reactive oxygen species generated from skeletal muscles are required for gecko tail regeneration. Sci. Rep.6, 20752. 10.1038/srep20752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lian, D., Chen, M. M., Wu, H., Deng, S. & Hu, X. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxid. (Basel). 1110.3390/antiox11040755 (2022). [DOI] [PMC free article] [PubMed]
- 41.Kim, H. J., Seo, J. Y., Suh, H. J., Lim, S. S. & Kim, J. S. Antioxidant activities of licorice-derived prenylflavonoids. Nutr. Res. Pract.6, 491–498. 10.4162/nrp.2012.6.6.491 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou, H. et al. Immunomodulatory and antioxidant effects of Glycyrrhiza uralensis polysaccharide in Lohmann brown chickens. Front. Vet. Sci.9, 959449. 10.3389/fvets.2022.959449 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui, X., Lou, L., Zhang, Y. & Yan, B. Study of the distribution of Glycyrrhiza uralensis production areas as well as the factors affecting yield and quality. Sci. Rep.13, 5160. 10.1038/s41598-023-31946-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fatima Qadri, A. et al. Effect of Glycyrrhiza uralensis crude water extract on the expression of nitric oxide synthase 2 gene during myogenesis. Heliyon10, e34747. 10.1016/j.heliyon.2024.e34747 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutherland, R. M., McCredie, J. A. & Inch, W. R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst.46, 113–120 (1971). [PubMed] [Google Scholar]
- 46.Johnson, N., Filler, A. C., Sethi, A., Smith, L. R. & Leach, J. K. Skeletal muscle spheroids as Building blocks for engineered muscle tissue. ACS Biomater. Sci. Eng.10, 497–506. 10.1021/acsbiomaterials.3c01078 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalmao-Fernandez, A. et al. Development of three-dimensional primary human myospheres as culture model of skeletal muscle cells for metabolic studies. Front. Bioeng. Biotechnol.11, 1130693. 10.3389/fbioe.2023.1130693 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin, G. Z. Enhanced growth and myogenic differentiation of spheroid-derived C2C12 cells. Biosci. Biotechnol. Biochem.85, 1227–1234. 10.1093/bbb/zbab018 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Lee, E. J. et al. Transthyretin maintains muscle homeostasis through the novel shuttle pathway of thyroid hormones during myoblast differentiation. Cells810.3390/cells8121565 (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are included in this article and the accompanying supplementary material.