Abstract
The way in which the extensive use of highly active antiretroviral therapy (HAART) has influenced the incidence of visceral leishmaniasis (VL) among human immunodeficiency type 1 (HIV-1)-infected patients is not yet understood. The present study assessed whether the incidence of symptomatic VL in HIV-infected patients has decreased since the introduction of HAART. Likewise, the role of other potential risk factors for VL was also analyzed. Therefore, 479 HIV-1-infected patients receiving antiretroviral treatment, according to the available drugs at each moment, were prospectively followed from April 1989 to June 2000 in two university hospitals in southern Spain. A bone marrow aspiration was performed when patients showed symptoms suggestive of kala-azar. A diagnosis of VL was made when Leishmania amastigotes were seen in Giemsa-stained samples or promastigotes were cultured in specific media. The median follow-up time was 1,380 [8 to 4,536] days. Twenty-one patients were diagnosed with symptomatic VL. The density of incidence of VL has decreased 64.8% as of January 1997, when HAART began to be used extensively in our area. The use of HAART was the main independent factor associated with VL; this therapy was a protective factor (adjusted hazard ratio [HR], 0.05; 95% confidence interval [CI], 0.02 to 0.15). CDC clinical category C at entry in the cohort (HR, 4.08; 95% CI, 1.46 to 11.35) and CD4+ cell counts below 300 cells/mm3 during the follow-up (HR, 3.96; 95% CI, 1.56 to 10.01) were also independently associated with kala-azar. A VL diagnosis prior to follow-up and low compliance with antiretroviral therapy were not independently associated with symptomatic VL, although statistical significance was almost reached (P = 0.1 and P = 0.08, respectively). In summary, the use of HAART has led to a fall in the incidence of symptomatic VL in HIV-infected patients. The main risk factor associated with kala-azar emergence in patients infected with HIV is deep immunosuppression.
Visceral leishmaniasis (VL) is a frequent disease among human immunodeficiency virus type 1 (HIV-1)-infected patients from the Mediterranean basin (12, 21). This disorder has been reported to be the fourth most common major AIDS-related disorder in southern Spain (8). Eleven percent of the patients attending an AIDS unit in our area harbor Leishmania infantum, almost half of them having subclinical VL (21). On the other hand, HIV and Leishmania coinfection is also an emerging disease in at least 30 countries worldwide, most of them in tropical regions (World Health Organization website [www.who.int/inf-fs/en/fact116]), but occasional cases have also been reported in Germany and the United States (2, 20).
In some cross-sectional and case report studies, an association between HIV-Leishmania coinfection and HIV advanced disease, intravenous drug use, and male gender has been found (12, 21). However, to date, the relationship between these potential risk factors and such coinfection has not been assessed in prospective cohort studies. The implementation of highly active antiretroviral therapy (HAART) has modified the incidence of most opportunistic diseases related with HIV infection (15, 19). HAART seems to prevent the development of overt kala-azar in patients with subclinical VL (7). Therefore, a decrease in the incidence of this disease would be expected after the introduction of HAART. However, some relapses and “de novo” overt VL cases in patients under HAART and well-suppressed HIV replication have been reported (14; J. L. Casado, R. Lopez-Vélez, and V. Piritado, 7th Conf. Retroviruses and Opportunistic Infections, abstr. 264, 2000). Because of this, prospective surveys assessing the incidence of VL in patients undergoing HAART are necessary.
This study was undertaken to assess whether the extensive use of HAART has decreased the incidence of symptomatic VL in HIV-infected patients. The impact of other potential risk factors for overt VL was also analyzed.
MATERIALS AND METHODS
Population and follow-up.
In April 1989, our tertiary-care AIDS units began a program aimed at studying the effect of antiretroviral therapy on the outcome of HIV infection. These units provide care to patients in southern Spain. Patients were included in this cohort when antiretroviral treatment was started. All individuals underwent clinical, hematological, biochemical, and immunological examinations at baseline, after 4 weeks, and every 12 weeks thereafter. Scheduled HIV load in plasma determinations were also performed at each visit as of December 1996. Up to June 2000, 515 HIV-infected patients had been enrolled in this cohort. We included in the present study all 479 patients who were seen at least twice.
Antiretroviral regimens.
Patients received antiretroviral therapy depending on drug availability at each moment and always according to international recommendations. Until 1993, zidovudine was the only antiretroviral drug commercially available. As of that date, both the patients who started the program and those taking monotherapy who showed clinical or immunological evidence of HIV disease progression were offered a dual combination of nucleoside reverse transcriptase inhibitors (NRTI) (i.e., zidovudine plus didanosine or zalcitabine). Lamivudine and stavudine were later included as a part of this regimen. Protease inhibitors were not extensively available in our hospitals until January 1997. Thereafter, all patients who received care in our units were offered HAART (i.e., a combination of two nonnucleoside reverse transcriptase inhibitors [NNRTI] and at least one protease inhibitor). As of 1999, HAART combinations including NNRTI (i.e., efavirenz or nevirapine) were also prescribed.
Self-reported adherence to antiretroviral therapy was measured as the proportion of pills taken out of the total number of pills prescribed. Patients receiving zidovudine were considered nonadherent, regardless of the data they reported, when we did not find an increase in the mean corpuscular volume that was equal to or greater than 10% of the baseline values.
None of the patients included in this study received specific primary or secondary antileishmanial prophylaxis. Pentamidine was not used as prophylaxis for Pneumocystis carinii pneumonia.
Diagnosis of symptomatic VL.
During the follow-up, a search for Leishmania spp. in a bone marrow aspirate was performed when patients showed unexplained fever, spleen enlargement, or a significant decline in leukocyte, platelet, or red blood cell count. When these symptoms coincided with a finding of Leishmania by Giemsa stain and/or Evans modified Tobie's medium culture, a diagnosis of symptomatic VL was made. A search for Leishmania spp. in samples other than bone marrow (e.g., liver or lymph node) was also performed when the responsible physician considered it appropriate. A more detailed description of the sample processing has been reported elsewhere (21).
Other laboratory methods.
HIV-1 RNA in plasma was measured by quantitative PCR (HIV Monitor test kit; Roche Molecular System; Hoffmann-La Roche, Basel, Switzerland) according to the manufacturer's instruction. The detection limit of this assay is 200 HIV-1 RNA copies/ml. CD4+ cell counts were measured by a conventional flow cytometry method.
Statistical study.
Continuous variables are expressed as median (range), and the categorical variables are expressed as number of cases (percent). The density of incidence of symptomatic VL was calculated as number of cases per one hundred patient-years. Patient-years for each individual patient were calculated as follows. A period of a year of follow-up was considered the unit; incomplete years were expressed as a fraction of the unit, obtained as the length of follow-up in this specific year measured in days divided by 365.
The relationship between symptomatic VL and the following potential risk factors was assessed: age, sex, intravenous drug use, previous episodes of symptomatic VL, Centers for Disease Control (CDC) clinical category C at entry in the cohort, evolution to CDC clinical category C during the follow-up, CD4+ cell counts at baseline, CD4+ cell count decline below 300 cells/mm3 during follow-up, time showing CD4+ cell counts below 300 cells/mm3, HAART use, time on HAART, low antiretroviral therapy compliance (i.e., adherence below 90% on at least two visits at any time during the follow-up), and time with poor adherence to the regimen.
Survival analysis was performed to study the relationship between VL emergence and these variables. In this study, the event was the development of the first episode of symptomatic VL during the follow-up. The study was censored on 30 June 2000. When a continuous variable included in this study was categorized, the median was selected as the cutoff value. Kaplan-Meier curves were used for all time-to-event analyses. The univariate study of the relationship between the abovementioned potential risk factors and the emergence of symptomatic VL was performed using the long rank test. We also used a Cox's proportional-hazards stepwise regression model to compute the risk hazard (95% confidence interval) and to determine the factors independently associated with the development of symptomatic VL. Cox's models for time-dependent and fixed variables were calculated, and the fittest one was chosen. All factors showing a univariate association level of ≤0.1 with symptomatic VL were entered into the multivariate analysis. All statistical procedures were carried out with the SPSS statistical package (Chicago, Ill.).
RESULTS
Characteristics of the population.
The main characteristics of the population are shown in Table 1. The population was followed for a median period of 1,380 days (range, 8 to 4,536 days). Ninety-five (20%) patients were lost to follow-up, and 83 (17%) patients died. Three hundred forty-seven (72%) patients received HAART at any time during the follow-up, and 220 of them (63%) had already taken antiretroviral drugs when HAART was started.
TABLE 1.
Baseline characteristics of the population studied
| Parametera | Median (%) [range], n = 479 |
|---|---|
| Age (yrs) | 31 [12-75] |
| Male, no. (%) | 365 (76) |
| IDU, no. (%) | 293 (61) |
| Non-IDU, no. (%) | 186 (39) |
| CDC clinical category C, no. (%) | 111 (23) |
| Time in CDC clinical category C during follow-up (days) | 754 (1-2,775) |
| VL diagnosed before follow-up, no. (%) | 12 (2) |
| Baseline CD4+ cell count (cells/mm3) | 294 [0-1,516] |
| CD4+ cell count <300 cells/mm3 during follow-up, no. (%) | 257 (54) |
| Time with CD4+ count <300 cells/mm3 during follow-up (days) | 180 [0-3,060] |
| Compliance at any time <90%, no. (%) | 239 (50) |
| HAART at any moment, no. (%) | 347 (72) |
| No HAART, no. (%) | 132 (28) |
| Time on HAART during follow-up (days) | 830 [8-1,350] |
IDU, intravenous drug user.
Incidence of symptomatic VL.
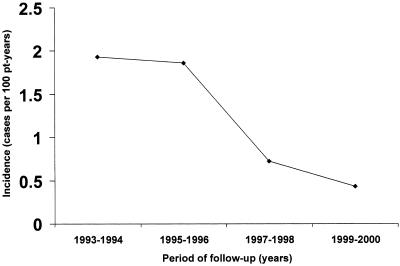
Two hundred thirty-four (48.8%) patients showed either unexplained fever, splenomegaly, or a significant decline in blood cell counts at some time during the follow-up. In 21 (4.4%) patients, at least one episode of overt VL was confirmed. Sixteen cases were observed between 1989 and 1996, whereas five cases of symptomatic VL occurred afterwards. Five of the cases that occurred before 1997 appeared in patients under monotherapy, and the remaining 11 cases were diagnosed in subjects who were on non-HAART combination therapy. The density of incidence of symptomatic VL during the period from 1989 to 1996 was 1.68 cases per 100 patient-years, whereas from 1997 to June 2000 it was 0.58 cases per 100 patient-years (Fig. 1). Thus, the incidence of symptomatic VL fell 64.8% from the first period to the second (Fig. 1). All five patients who experienced an episode of overt kala-azar as of 1997 showed uncontrolled HIV levels in blood (Table 2).
FIG. 1.
Incidence of symptomatic VL from 1993 to June 2000. pt, patient.
TABLE 2.
HIV RNA levels in plasma, pre-HAART and during HAART in patients who developed symptomatic VL while on HAART
| Patient no. | HIV RNA (lg copies/ml) | Pre-HAART therapya | Time on pre-HAART therapy (days) | HAART regimen | Time on HAART (days) |
|---|---|---|---|---|---|
| 1 | 4.7 | AZT+DDI+SQV | 375 | ||
| AZT+3TC+SQV+NVP | |||||
| 2 | 5.3 | AZT+3TC | 350 | DDI+D4T+IDV | 530 |
| 3 | 5.4 | AZT+DDC | 418 | AZT+3TC+IDV | 918 |
| 4 | 3.8 | AZT+DDC | 744 | DDI+D4T+IDV | 42 |
| 5 | 4.6 | D4T+DDI+SQV | |||
| D4T+DDI+IDV | 343 | ||||
| 3TC+NVP+NFV | |||||
| ABV+3TC+EFV+IDV+RTV |
Antiretroviral regimen received before HAART. Antiretroviral drug abbreviations: AZT (zidovudine), DDI (didanosine), DDC (zalcitabine), ABV (abacavir), D4T (stavudine), 3TC (lamivudine), NVP (nevirapine), EFV (efavirenz), SQV (saquinavir), IDV (indinavir), NFV (nelfinavir), or RTV (ritonavir).
Risk factors for symptomatic VL.
On the univariate analysis, HAART intake, CDC clinical category C at entry in the cohort (P = 0.0001) or during the follow-up (P = 0.0001), CD4+ cell counts below 300 cells/mm3 at baseline (P = 0.0001) or during the follow-up (P = 0.003), CD4+ cell counts of <300 cells/mm3 for longer than 180 days (P = 0.02), antiretroviral therapy compliance of <90%, and a history of VL (P = 0.0001) were associated with symptomatic VL (Table 3).
TABLE 3.
Results of univariate and multivariate analysesa
| Parameter | No. of VL cases |
P
|
HR | 95% CI | |
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| Age >31 yr | 9 | 0.94 | |||
| Male gender | 18 | 0.34 | |||
| Intravenous drug use | 14 | 0.46 | |||
| Symptomatic VL before follow-up | 4 | 0.0001 | 0.1 | ||
| CD4+ cell <298 cells/mm3 at baseline | 15 | 0.0001 | 0.8 | ||
| CD4+ count <300 cells/mm3 during follow-up | 20 | 0.003 | 0.05 | 3.96 | 1.56-10.01 |
| CD4+ count <300 cells/mm3 for >180 days | 14 | 0.02 | 0.25 | ||
| CDC category C at baseline | 11 | 0.0001 | 0.007 | 4.08 | 1.46-11.35 |
| CDC category C during follow-up | 15 | 0.0001 | 0.8 | ||
| Time in CDC category C >754 days | 9 | 0.52 | |||
| Therapy compliance <90% | 17 | 0.03 | 0.08 | ||
| HAART | 5 | 0.0001 | 0.0001 | 0.05 | 0.02-0.15 |
| Time on HAART >830 days | 4 | 0.23 | |||
Hazard ratios and 95% confidence intervals (95% CI) are shown only for independent predictive variables in the stepwise multivariate analysis.
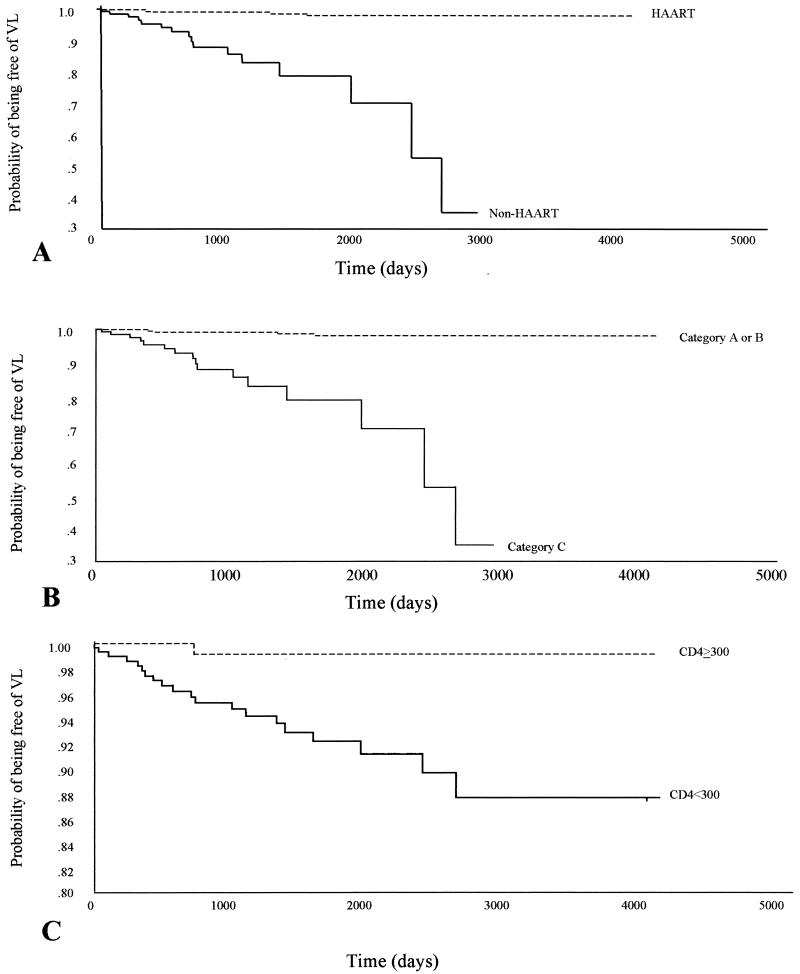
On the multivariate analysis, HAART intake was the most important independent factor associated with the emergence of symptomatic VL (Table 3). This therapy was identified as a protective factor (hazard ratio [HR] = 0.05; 95% confidence interval [CI], 0.02 to 0.15; P = 0.0001). CDC clinical category C at inclusion in the cohort (HR, 4.08 [95% CI, 1.46-11.35]; P = 0.007) and a CD4+ cell count below 300 cells/mm3 during the follow-up (HR, 3.96 [95% CI, 1.56 to 10.01]; P = 0.05) (Table 3) were also selected as risk factors independently associated with the emergence of VL on the multivariate analysis. The associations of poor antiretroviral treatment compliance and a history of VL with the development of symptomatic VL were close to the statistical significance level (P = 0.08 and 0.1, respectively). Kaplan-Meier curves for the factors independently associated with overt kala-azar are shown in Fig. 2.
FIG. 2.
Kaplan-Meier estimates of time to the development of symptomatic VL according to HAART intake (A), classification in CDC clinical category C at baseline (B), and CD4+ cell count during follow-up (C).
DISCUSSION
The results reported here demonstrate that the implementation of HAART has led to a sharp reduction in the incidence of symptomatic VL in HIV-infected patients in southern Spain. Likewise, this study shows that the main risk factor for symptomatic VL in patients infected with HIV is deep immunosuppression.
HAART has proven to reduce the frequency of most AIDS-related diseases (15, 19). This is the first study where the effect of HAART on the incidence of symptomatic VL has been quantified. In spite of the protective effect of HAART on overt VL emergence, de novo (14) and relapsing cases of symptomatic VL (Casado et al., abstract) have been reported in patients undergoing such therapy. In this work, all residual cases were observed in patients with uncontrolled HIV infection, due to the fact that antiretroviral therapy had either failed or not been started. Therefore, adequate control of viral replication and the subsequent immune reconstitution seem to prevent the development of symptomatic VL in HIV-infected patients.
The use of HAART could reduce the incidence of VL by two mechanisms. First, it may reduce the rate of evolution from latent Leishmania infantum infection to overt VL. Second, HAART might decrease the incidence of new infections by Leishmania spp. in HIV-infected patients. The first hypothesis is supported by investigations on subclinical VL (7). In addition to an increase in CD4 T-lymphocyte cell counts (13), HAART leads to an enhancement in the levels of Th1 cytokines (16). This fact could explain why patients on HAART are less prone to develop overt kala-azar, since an adequate Th1 response is associated with the control of Leishmania infections (6). To assess the second hypothesis, it would be necessary to perform studies using the leishmanin skin test and/or Leishmania serology. Thus, both symptomatic and asymptomatic cases of Leishmania infection could be analyzed. This matter is essential, since a high proportion of Leishmania infections in HIV-infected patients are subclinical (21). However, the sensitivity of these diagnostic tools is limited in HIV-infected patients due to the impaired cellular and humoral immune responses that these patients show (10, 22).
The protective effect of HAART on the development of symptomatic VL may even have been underestimated in this survey. In fact, 220 (63.4%) of 347 patients who received HAART during the follow-up and who were included in the statistical study in the HAART group had received NRTI previously. Thus, these drug-experienced patients could harbor viruses resistant to the NRTI used as a part of the HAART regimen (11). This fact could have led to a therapeutical failure and to a lesser protective effect of HAART.
Non-HAART regimens (monotherapy or combination therapy) do not seem to have significantly influenced the incidence of symptomatic VL. Monotherapy and combination therapies other than HAART were not associated with VL emergence in a statistical analysis restricted to the patients who received only these regimens (data not shown).
The main risk factor for symptomatic VL in HIV-infected patients included in this study was deep immunosuppression. All variables of those analyzed here, which indicate advanced HIV disease, were associated with the emergence of symptomatic VL on the univariate study. However, on the multivariate study, only CDC clinical category C at baseline and CD4+ cell counts below 300 cells/mm3 during the follow-up were selected as independent risk factors. This finding is probably due to the fact that all of the above mentioned variables are colineal.
Leishmania infection is a persistent disorder, although a clinical cure may be achieved using adequate treatment (1, 9). Because of this, a previous diagnosis of symptomatic VL was found to be associated with the development of kala-azar on the univariate analysis. However, when this variable was entered into a Cox's hazard model, it did not remain independently related with symptomatic VL. This fact could be due to the small number of patients with previous VL in this survey. Nevertheless, HAART could also prevent the relapse of symptomatic VL in HIV-infected patients who achieve satisfactory immune and virological responses. According to this, relapses in this study occurred only in patients who showed uncontrolled HIV replication.
The sharing of needles has been reported to be a transmission route for Leishmania infection (3). Consequently, an association between intravenous drug abuse and VL has been found in previous studies (21). However, this relationship was statistically weak (21). Thus, a survey like this that analyzes a small portion of L. infantum infection cases, those with overt symptoms, could not be potent enough to prove such a relationship. Therefore, studies in which the leishmanin skin test and/or Leishmania serology is performed in HIV-seronegative people are more reliable to analyze this issue.
A low adherence to antiretroviral therapy is related to inadequate control of HIV replication, which leads to the emergence of mutant HIV strains (17) and, subsequently, to treatment failure (18). Although in this study an independent association between low antiretroviral treatment compliance and emergence of symptomatic VL has not been found, the level of statistical significance was almost reached. The failure to detect a clear association could be explained by several reasons. First, compliance with the antiretroviral treatment was self-reported by patients; therefore, estimations of adherence were not completely accurate (4). Besides, the compliance with different therapy regimens used during the follow-up (i.e., monotherapy, dual combination, and HAART) was not considered separately. Probably, a low adherence to monotherapy or combined therapy does not lead to the same virological and immunological consequences as a low compliance with HAART. In any case, the fact that low antiretroviral treatment adherence was associated with symptomatic VL on the univariate study could indicate that adherence is a risk factor for the development of symptomatic VL.
The ability of HAART to reduce the incidence of VL is yet another reason to develop programs to make this therapy accessible and to improve adherence. In countries where HIV-Leishmania coinfection is endemic, this approach may have a major impact, since VL causes morbidity and mortality by itself and enhances the effects of HIV infection (5, 23) in HIV-seropositive patients.
Acknowledgments
We thank Ana Fernández-Palacín (Unidad de Investigación, Hospital Universitario de Valme, Seville, Spain) for statistical assistance.
REFERENCES
- 1.Aebisher, T. 1994. Recurrent cutaneous leishmaniasis: a role for persistent parasites. Parasitol. Today 10:25-28. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht, H., I. Sobottka, C. Emminger, H. Jablonowski, G. Just, A. Stoehr, T. Kubin, B. Salzberger, T. Lutz, and J. van Lunzen. 1996. Visceral leishmaniasis emerging as an important opportunistic infection in HIV-infected persons living in areas nonendemic for Leishmania donovani. Arch. Pathol. Lab. Med. 120:189-198. [PubMed] [Google Scholar]
- 3.Amela, C., D. López-Gay, J. C. Alberdi, and J. Castilla. 1996. Injecting drug use as risk factor for visceral leishmaniasis in AIDS patients. Eur. J. Epidemiol. 12:91-96. [DOI] [PubMed] [Google Scholar]
- 4.Bansberg, D., F. Hecht, E. Charlebois, A. Zolopa, M. Holodniy, L. Sheiner, J. Bamberger, M. Chesney, and A. Moss. 2000. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 14:357-366. [DOI] [PubMed] [Google Scholar]
- 5.Bernier, R., B. Barbeau, M. J. Trembley, and M. Olivier. 1998. The lipophosphoglycan of Leishmania donovani up-regulates HIV-1 transcription in T-cells through the nuclear factor κB elements. J. Immunol. 160:2881-2888. [PubMed] [Google Scholar]
- 6.Carvalho, E. M., A. Barral, D. Pedral-Sampaio, M. Barral-Netto, R. Badaro, H. Rocha, and W. D. Johnson. 1992. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J. Infect. Dis. 165:535-540. [DOI] [PubMed] [Google Scholar]
- 7.de la Rosa, R, J. A. Pineda, J. Delgado, J. Macías, F. Morillas, J. Martin-Sánchez, M. Leal, A. Sánchez-Quijano, and E. Lissen. 2001. Influence of highly active antiretroviral therapy on the outcome of subclinical visceral leishmaniasis in HIV-infected patients. Clin. Infect. Dis. 32:633-635. [DOI] [PubMed] [Google Scholar]
- 8.Delgado, J., J. A. Pineda, J. Gallardo, M. Leal, J. Macías, A. Sánchez-Quijano, and E. Lissen. 1998. Influence of medical intervention on the presentation of AIDS defined by clinical episodes. Med. Clin. (Barcelona) 110:125-127. [PubMed] [Google Scholar]
- 9.De Rosell, R. A., R. de Jesús de Durán, O. Rosell, and A. M. Rodríguez. 1992. Is leishmaniasis ever cured? Trans. R. Soc. Trop. Med. Hyg. 86:251-253. [DOI] [PubMed] [Google Scholar]
- 10.Gallardo, J. A., J. A. Pineda, J. Macias, R. Torronteras, and E. Lissen. 1996. Specificity of a commercial indirect immunofluorescence technique in the diagnosis of visceral leishmaniasis in HIV-1 infected patients. Trans. R. Soc. Trop. Med. Hyg. 90:383.. [DOI] [PubMed] [Google Scholar]
- 11.Gómez-Cano, M., A. Rubio, T. Puig, M. Pérez-Olmeda, L. Ruiz, V. Soriano, J. A. Pineda, L. Zamora, N. Xaus, B. Clotet, and M. Leal. 1998. Prevalence of genotypic resistance to nucleoside analogues in antiretroviral-naive and atiretroviral-experienced HIV-infected patients in Spain. AIDS 12:1015-1020. [PubMed] [Google Scholar]
- 12.Gradoni, L., A. Scalone, M. Gramiccia, and M. Troiani. 1996. Epidemiological of surveillance of leishmaniasis in HIV-1 infected individuals in Italy. AIDS 10:785-791. [DOI] [PubMed] [Google Scholar]
- 13.Hammer, S. M., K. E. Squieres, M. D. Hugues, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Erron, J. E. Feienberg, H. H. Baltour, L. R. Deyton, J. A. Chodakewitz, and M. A. Fischl. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4+ cells counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 14.Jiménez-Expósito, M. J., C. Alonso-Villaverde, P. Sardá, and L. Masana. 1999. Visceral leishmaniasis in HIV-infected patients with non-detectable HIV-1 viral load after highly active antiretroviral therapy. AIDS 13:152-153. [PubMed] [Google Scholar]
- 15.Kaplan, J. E., D. Hanson, M. S. Dworkin, T. Frederick, J. Bertolli, M. L. Lindegren, S. Holmberg, and J. L. Jones. 2000. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. Suppl. 1:S5-S14. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann, G. R., J. Zaunders, and D. A. Cooper. 1999. Immune reconstitution in HIV- infected subjects treated with a potent antiretroviral therapy. Sex. Transm. Infect. 75:218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta, S., R. Moore, and N. Graham. 1997. Potential factors affecting adherence with HIV therapy. AIDS 11:1665-1670. [DOI] [PubMed] [Google Scholar]
- 18.Moatti, J., M. Carrieri, B. Spire, J. Gastaut, J. Cassuto, J. Moreau, et al. 2000. Adherence to HAART in French HIV-infected injecting drug users: the contribution of buprenorphine drug maintenance treatment. AIDS 14:151-155. [DOI] [PubMed] [Google Scholar]
- 19.Palella, F. J., K. Delaney, A. Moorman, M. Loveless, J. Fuhrer, G. Satten, D. Aschman, and S. Holmberg. 1998. Declining morbidity and mortality among patients with advanced immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 20.Parkas, V., J. Godwin, and H. Murray. 1997. Kala-azar comes to New York. Arch. Intern. Med. 157:921-923. [PubMed] [Google Scholar]
- 21.Pineda, J. A., J. A. Gallardo, J. Macías, J. Delgado, C. Regordán, F. Morillas, F. Relimpio, J. Martin-Sánchez, A. Sánchez-Quijano, M. Leal, and E. Lissen. 1998. Prevalence of and factors associated with visceral leishmaniasis in human immunodeficiency virus type 1-infected patients in southern Spain. J. Clin. Microbiol. 36:2419-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sears, S. D., R. Fox, R. Brookmeyer, R. Leavitt, and B. F. Polk. 1987. Delayed hipersensitivity skin testing and anergy in a population of gay men. Clin. Immunol. Immunopathol. 45:177-183. [DOI] [PubMed] [Google Scholar]
- 23.Wolday, D., N. Berhe, H. Akuffo, and S. Britton. 1999. Leishmania-HIV interaction: immunopathogenic mechanisms. Parasitol. Today 15:182-187. [DOI] [PubMed] [Google Scholar]