Abstract
Endocrine diseases are suspected contributors to lymphoid malignancies, but their precise association is unclear. This study aimed to investigate the causal relationship between various endocrine diseases—specifically type 2 diabetes, obesity, diabetic hypoglycemia, hyperlipidemia, and hyperthyroidism—and lymphoid malignancies, including lymphocytic leukemia and non-Hodgkin’s lymphoma with subtypes like diffuse large B-cell lymphoma and follicular lymphoma. Utilizing data from genome-wide association studies, a two-sample Mendelian randomization analysis was performed. The primary approach involved the inverse-variance weighted method, supplemented by other robust techniques such as the weighted median and MR-Egger regression to ensure reliability. The analysis indicated a significant causal connection between genetically predicted diabetic hypoglycemia and lymphocytic leukemia (Odds ratio = 1.0004, 95% Confidence interval = 1-1.0007, P = 0.03). Conversely, no associations were found for the other endocrine diseases with lymphoid malignancies (P > 0.05 for all). The findings suggest that while diabetic hypoglycemia may influence lymphocytic leukemia risk, further research is necessary to clarify the roles of other endocrine diseases in lymphoid malignancies, including cross-population validations and biological investigations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-99010-y.
Keywords: Endocrine diseases, Lymphoid malignancies, Mendelian randomization, Causal relationship, Genetic association, SNPs
Subject terms: Haematological cancer, Endocrinology
Introduction
Lymphoid malignancies are a broad and heterogeneous group of neoplasms, which can be divided into Hodgkin’s lymphoma and non-Hodgkin’s lymphoma (NHL)1,2. NHL accounts for 80–90% of malignant lymphomas. It principally displays lymph node involvement but can also spread to extranodal sites such as the spleen3,4. Diffuse Large B-Cell Lymphoma (DLBCL) represents almost 30% of all cases of NHL, and patients typically present with progressive lymphadenopathy, extranodal disease, or both5. Follicular lymphoma (FL) is the most common indolent lymphoma accounting for approximately 20-25% of all NHL. While it typically responds well to treatment, FL is frequently characterized by numerous relapses in the majority of patients. In the case of advanced-stage FL, it is currently deemed incurable6,7. Chronic lymphocytic leukemia (CLL), the most frequent type of leukemia in adults, is a lymphoproliferative disorder that is characterized by the expansion of monoclonal, mature CD5 + CD23 + B cells in the peripheral blood, secondary lymphoid tissues and bone marrow8. While the clinical manifestations of different lymphoma types may vary, enlarged lymph nodes, B-symptoms (such as fatigue, fever, night sweats, and weight loss), extranodal involvement, constitutional symptoms, localized symptoms, and disease progression with complications are common across the board9,10. However, despite advances in the understanding of the underlying mechanisms, the etiology of lymphoid malignancies remains incompletely understood, pointing to the need for further research into potential risk factors.
Endocrine diseases, which encompass a range of conditions affecting the endocrine glands and their hormonal secretions, including diabetes, obesity, thyroid dysfunction, and dyslipidemia. These diseases are also highly prevalent and have profound effects on the overall health of individuals. In recent years, the potential link between lymphoid malignancies and endocrine diseases has garnered significant interest. For instance, a previous study has implicated type 2 diabetes (T2D) in the incidence of NHL, as well as body mass index (BMI) and NHL incidence11. Furthermore, a meta-analysis found a moderate increase in the risk of NHL among patients with type 1 and 2 diabetes mellitus (DM), particularly in Asian populations, with the highest risk observed in patients within 1–2 years of DM diagnosis12. However, negative associations between type 1 diabetes and sarcoidosis and the risk of NHL have been observed13. Given the conflicting and inconclusive nature of the existing evidence, the causal relationship between endocrine diseases and lymphoid malignancies remains largely unknown. Further research is imperative to delineate the causal mechanisms, providing a solid theoretical basis for future studies and informing clinical practices.
Exploring the causal relationship between endocrine diseases and lymphoid malignancies is crucial for understanding the pathogenesis of these disorders and identifying potential targets for therapeutic intervention. Traditional observational studies, while informative, are often limited by the presence of confounding factors that can bias the observed associations. Mendelian randomization (MR), a genetic epidemiological approach, offers a promising alternative by utilizing germline genetic variants as proxies for modifiable exposures. This approach allows for the estimation of causal effects in the absence of confounding and reverse causation, providing more robust evidence of causation15.
In this study, we aimed to investigate the causal association between endocrine diseases and lymphoid malignancies using a two-sample MR analysis. By leveraging large-scale genome-wide association study (GWAS) datasets, we examined whether genetic variants associated with endocrine diseases were also predictive of lymphoid malignancies. This analysis aims to provide insights into the mechanisms underlying these associations, potentially informing prevention and management strategies.
Methods
Study design
As exhibited in Fig. 1, the selection of instrumental variables (IVs) from genetic variants in this MR analysis rigorously adhered to the three fundamental assumptions of MR:
Fig. 1.
Diagram of three stringent assumptions and core design of the present MR study.
The chosen IVs, namely the genetic variants, must demonstrate a strong association with the exposure.
These genetic variants should not be associated with any potential confounding factors.
The genetic variants can only affect the outcome indirectly through the exposure, excluding any direct or alternative pathways.
In this MR analysis, endocrine diseases including T2D, obesity, diabetic hypoglycemia (dHypo), hyperlipidemia (HPL) and hyperthyroidism (HTH) were considered as the exposures, with lymphoid malignancies encompassing Lymph node tumor, Lymphocytic Leukemia (LL) and NHL with its subtypes DLBCL and FL serving as the outcome. This study is based on publicly available GWAS data; therefore, no ethical approval was required.
Data source
Among the outcomes, DLBCL summary data, were derived from Wang et al., which investigated the causal relationship between genetically predicted telomere length (TL) and the risk of developing various hematologic diseases (HDs) using MR analysis16. In addition, the summary statistics of other lymphoid malignancies (Lymph node tumor, LL, NHL and FL) were obtained from the Integrative Epidemiology Unit (IEU) open GWAS project, an open database (https://gwas.mrcieu.ac.uk/). In regard to the exposures, the single-nucleotide polymorphisms (SNPs) associated with obesity and HPL were sourced from the FINNGen consortium, a comprehensive repository that has consolidated GWAS findings for numerous diseases, incorporating both genomic and electronic health record data from a cohort exceeding 100,000 Finnish individuals17. SNPs related to T2D, dHypo and HTH were obtained from IEU OpenGWAS (https://gwas.mrcieu.ac.uk/). The specific information is shown in the Table S1.
Instrument selection
Initially, SNPs significantly associated to genome-wide endocrine diseases were filtered using a lenient threshold of P < 5 × 10⁻⁸. Among these, SNPs with a minor allele frequency (MAF) greater than 0.01 were further selected18. To eliminate linkage disequilibrium (LD) among SNPs, we applied a stringent criterion of R2 < 0.001 within a window size of 10,000kb19. If a chosen IV was absent from the outcome summary data, a proxy SNP with a high LD (R2 > 0.8) with that IV was identified and substituted20. The F-statistic for each SNP in the IVs was calculated to evaluate the strength of the IVs, aiming to exclude potential bias stemming from weak instrumental variables between the IVs and exposures. The F-statistic is calculated using the formula:
F = R2 × (N-2) / (1-R2), where R2 represents the proportion of exposure variation explained by the SNP in the IV, and the F-value should exceed 1021.
Statistical analysis
This analysis predominantly utilized the inverse variance weighted (IVW) method as the core analytical approach, evaluating the causal association between exposure and outcome risk by calculating the odds ratio (OR) alongside a 95% confidence interval (95% CI). IVW serves as the cornerstone for interpreting MR results, determining the weighted average of effect sizes by employing the inverse variance of each SNP as the weighting factor22. Additionally, the study’s findings underwent rigorous robustness testing employing methods such as MR-Egger, weighted median, and weighted mode. The MR-Egger method accounts for potential intercept terms, enabling precise causal effect estimates even in the presence of pleiotropy bias23. The weighted median method, on the other hand, assumes that half of the instrumental variables remain valid, supporting the analysis of the causal relationship between exposure and outcome24. All analyses in this study were performed using R version 4.0.5 and the “TwoSampleMR” package. Visual representations were achieved through scatter plots highlighting the effect relationship of IVs on exposure and outcome, funnel plots detecting potential biases, and forest plots displaying the effect estimates of SNPs and their consistency19.
Sensitivity analysis
Sensitivity analysis holds a pivotal position in detecting potential pleiotropy that could arise in MR studies. In this research, Cochran’s Q test was employed as a meticulous tool to assess the heterogeneity among IVs. When the resulting p-value exceeds 0.05, it signifies a low level of heterogeneity, implying that the variations in estimates among IVs are random and have a negligible impact on the IVW results22. Concurrently, acknowledging the significant role of genetic variation’s pleiotropy in estimating association effects, this study incorporated the MR-Egger regression method to scrutinize the presence of horizontal pleiotropy. If the intercept term of the MR-Egger regression tends towards zero or lacks statistical significance, it serves as an indicator of the absence of pleiotropy18. Furthermore, to enhance the accuracy of the analysis, this study harnessed the MR pleiotropy residual sum and outlier (MR-PRESSO) method to identify potential outliers, specifically SNPs with a p-value less than 0.05. After excluding these outliers, the causal association was re-estimated, thereby effectively correcting for horizontal pleiotropy21. Finally, the leave-one-out analysis was employed as a rigorous approach to validate the robustness and consistency of the obtained results21.
Results
Selection of instrumental variables
Eventually, 118, 8, 8, 15, and 13 independent SNPs were individually incorporated as IVs for T2D, obesity, dHypo, HPL, and HTH, respectively. In detail, the F-statistic values for T2D-related IVs ranged from 30 to 1578, averaging 76. Except for the MR analysis focusing on LL as the outcome, where two SNPs (rs6878122 and rs10087241) did not align with the summary data, all other SNPs matched successfully for each outcome. For obesity, F-statistic values for IVs spanned from 31 to 184, averaging 56. Notably, all SNPs aligned perfectly in the MR analysis with DLBCL as the outcome, but one SNP (rs4072287) did not match any information in the other types of lymphoid malignancies. The F-statistic values for dHypo IVs ranged from 39 to 447, averaging 140. Except for all SNPs aligning perfectly in the DLBCL MR analysis, two SNPs (rs9265531 and rs73410774) were absent from the summary statistics of Lymph node tumor and FL, while one SNP each (rs114058208 and rs9265531) was not present in LL and NHL, respectively. Concerning HPL-related SNPs, F-statistics ranged from 38 to 325, averaging 107. All SNPs aligned perfectly in the MR analyses for DLBCL and LL, but one SNP (rs151113958) was not available in the summary data for FL and Lymph node tumor. Lastly, the F-statistics of HTH-related IVs ranged from 30 to 195, averaging 64. Except for the MR analysis with DLBCL as the outcome, where one SNP (rs28375776) did not align with the summary data, all other SNPs matched successfully for each outcome. In summary, the F-statistics of all SNPs exceeded the conventional threshold of 10, indicating the adequacy of the IVs’ strength. A comprehensive list of the IVs and their corresponding information is provided in Table S2-S6.
Causal effects of endocrine diseases on lymphoid malignancies
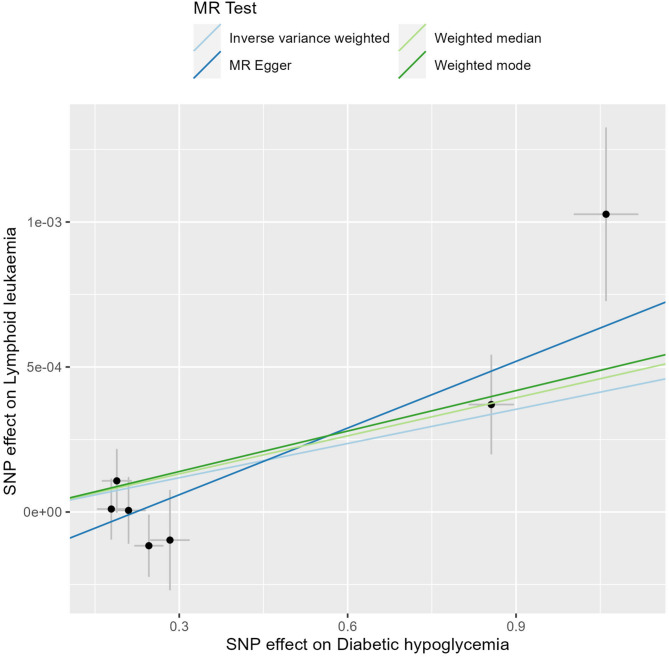
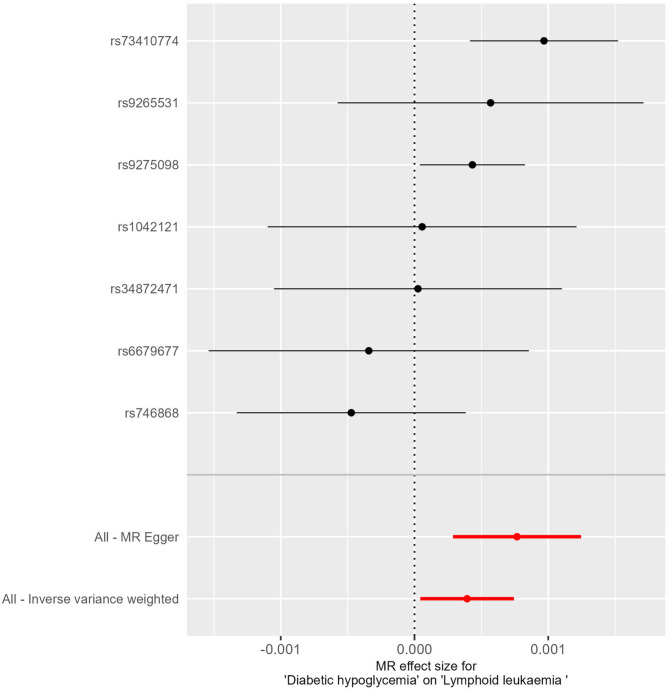
In this study, we employed the IVW method to rigorously assess the potential causal relationships between endocrine diseases and lymphoid malignancies, and the findings are systematically presented in Table 1. Notably, genetically predicted dHypo showed a significant causal association with LL (OR = 1.0004, 95%CI = 1-1.0007, P = 0.03). An OR value greater than 1 indicated that dHypo increases the risk of LL. Though the OR is small, it’s biologically significant in large genetic datasets, reflecting lifelong exposure. The MR-Egger and the Weighted median methods concurred with this finding (P < 0.05), while the Weighted mode did not exhibit causal association between dHypo and LL (P > 0.05). Furthermore, no causal association was observed between the remaining endocrine diseases and lymphoid malignancies (P > 0.05 for all). Figures 2 presents a scatter plot exhibiting the effect sizes of SNPs for dHypo and LL and the corresponding forest plots are illustrated in Figs. 3.
Table 1.
MR results of causal effects between endocrine diseases and lymphatic diseases risk.
| Outcome | Exposure | Methods | N. SNPs | OR (95% CI) | P |
|---|---|---|---|---|---|
| DLBCL | T2D | IVW | 114 | 1.01 (0.89–1.15) | 0.91 |
| MR Egger | 114 | 1.03 (0.75–1.4) | 0.87 | ||
| Weighted median | 114 | 0.97 (0.76–1.25) | 0.81 | ||
| Weighted mode | 114 | 0.98 (0.74–1.3) | 0.88 | ||
| Obesity | IVW | 8 | 1.08 (0.85–1.38) | 0.53 | |
| MR Egger | 8 | 1.16 (0.56–2.41) | 0.7 | ||
| Weighted median | 8 | 1.07 (0.78–1.48) | 0.67 | ||
| Weighted mode | 8 | 1.06 (0.74–1.52) | 0.77 | ||
| dHypo | IVW | 6 | 0.91 (0.76–1.07) | 0.25 | |
| MR Egger | 6 | 0.81 (0.62–1.07) | 0.21 | ||
| Weighted median | 6 | 0.87 (0.74–1.03) | 0.1 | ||
| Weighted mode | 6 | 0.87 (0.73–1.03) | 0.16 | ||
| HPL | IVW | 15 | 0.97 (0.86–1.08) | 0.57 | |
| MR Egger | 15 | 1.04 (0.79–1.37) | 0.77 | ||
| Weighted median | 15 | 0.92 (0.79–1.06) | 0.24 | ||
| Weighted mode | 15 | 0.9 (0.71–1.13) | 0.39 | ||
| HTH | IVW | 10 | 1.01 (0.83–1.23) | 0.93 | |
| MR Egger | 10 | 1.83 (1.07–3.13) | 0.06 | ||
| Weighted median | 10 | 1.11 (0.89–1.38) | 0.37 | ||
| Weighted mode | 10 | 1.16 (0.88–1.52) | 0.31 | ||
| LL | T2D | IVW | 113 | 1.0001 (0.9998–1.0004) | 0.64 |
| MR Egger | 113 | 1.0007 (0.9999–1.0014) | 0.08 | ||
| Weighted median | 113 | 1 (0.9994–1.0006) | 0.98 | ||
| Weighted mode | 113 | 1.0001 (0.9995–1.0007) | 0.79 | ||
| Obesity | IVW | 7 | 0.9999 (0.9993–1.0005) | 0.82 | |
| MR Egger | 7 | 0.9995 (0.9975–1.0015) | 0.62 | ||
| Weighted median | 7 | 0.9999 (0.9991–1.0006) | 0.69 | ||
| Weighted mode | 7 | 0.9998 (0.999–1.0007) | 0.71 | ||
| dHypo | IVW | 7 | 1.0004 (1-1.0007) | 0.03 | |
| MR Egger | 7 | 1.0008 (1.0003–1.0012) | 0.03 | ||
| Weighted median | 7 | 1.0004 (1.0001–1.0008) | 0.01 | ||
| Weighted mode | 7 | 1.0005 (1-1.0009) | 0.08 | ||
| HPL | IVW | 15 | 0.9999 (0.9997–1.0002) | 0.6 | |
| MR Egger | 15 | 0.9999 (0.9994–1.0004) | 0.77 | ||
| Weighted median | 15 | 0.9999 (0.9996–1.0002) | 0.5 | ||
| Weighted mode | 15 | 0.9999 (0.9995–1.0002) | 0.43 | ||
| HTH | IVW | 10 | 1.0002 (0.9998–1.0005) | 0.38 | |
| MR Egger | 10 | 1.0009 (0.9996–1.0022) | 0.23 | ||
| Weighted median | 10 | 1.0001 (0.9996–1.0006) | 0.7 | ||
| Weighted mode | 10 | 1 (0.9992–1.0007) | 0.92 | ||
| FL | T2D | IVW | 115 | 1.01 (0.82–1.24) | 0.92 |
| MR Egger | 115 | 1.23 (0.77–1.97) | 0.38 | ||
| Weighted median | 115 | 1.5 (1.09–2.06) | 0.01 | ||
| Weighted mode | 115 | 1.49 (0.96–2.31) | 0.08 | ||
| Obesity | IVW | 7 | 0.8 (0.53–1.21) | 0.29 | |
| MR Egger | 7 | 0.41 (0.1–1.61) | 0.26 | ||
| Weighted median | 7 | 0.73 (0.43–1.24) | 0.24 | ||
| Weighted mode | 7 | 0.71 (0.38–1.31) | 0.31 | ||
| dHypo | IVW | 6 | 1.13 (0.88–1.44) | 0.34 | |
| MR Egger | 6 | 0.81 (0.56–1.18) | 0.33 | ||
| Weighted median | 6 | 1.03 (0.8–1.33) | 0.81 | ||
| Weighted mode | 6 | 1 (0.77–1.3) | 0.99 | ||
| HPL | IVW | 14 | 1.1 (0.95–1.27) | 0.22 | |
| MR Egger | 14 | 1.09 (0.78–1.52) | 0.63 | ||
| Weighted median | 14 | 1 (0.81–1.23) | 0.99 | ||
| Weighted mode | 14 | 0.95 (0.72–1.27) | 0.75 | ||
| HTH | IVW | 11 | 0.95 (0.72–1.25) | 0.7 | |
| MR Egger | 11 | 0.83 (0.43–1.61) | 0.59 | ||
| Weighted median | 11 | 0.92 (0.69–1.23) | 0.56 | ||
| Weighted mode | 11 | 0.91 (0.67–1.25) | 0.58 | ||
| NHL | T2D | IVW | 115 | 1.01 (0.92–1.11) | 0.82 |
| MR Egger | 115 | 0.99 (0.8–1.22) | 0.91 | ||
| Weighted median | 115 | 0.99 (0.85–1.16) | 0.93 | ||
| Weighted mode | 115 | 0.96 (0.82–1.14) | 0.67 | ||
| Obesity | IVW | 7 | 0.98 (0.8–1.2) | 0.87 | |
| MR Egger | 7 | 0.68 (0.34–1.34) | 0.31 | ||
| Weighted median | 7 | 0.9 (0.72–1.13) | 0.37 | ||
| Weighted mode | 7 | 0.89 (0.71–1.11) | 0.33 | ||
| dHypo | IVW | 7 | 0.91 (0.82–1.02) | 0.12 | |
| MR Egger | 7 | 0.92 (0.75–1.14) | 0.49 | ||
| Weighted median | 7 | 0.92 (0.84–1.01) | 0.07 | ||
| Weighted mode | 7 | 0.93 (0.84–1.03) | 0.2 | ||
| HPL | IVW | 15 | 1.06 (1-1.13) | 0.06 | |
| MR Egger | 15 | 0.97 (0.84–1.12) | 0.7 | ||
| Weighted median | 15 | 1.03 (0.95–1.12) | 0.48 | ||
| Weighted mode | 15 | 1.01 (0.92–1.11) | 0.77 | ||
| HTH | IVW | 11 | 1.07 (0.81–1.4) | 0.64 | |
| MR Egger | 11 | 2.29 (0.99–5.3) | 0.09 | ||
| Weighted median | 11 | 0.97 (0.82–1.14) | 0.71 | ||
| Weighted mode | 11 | 1 (0.8–1.24) | 0.97 | ||
| Lymph node tumor | T2D | IVW | 115 | 0.92 (0.79–1.07) | 0.27 |
| MR Egger | 115 | 0.89 (0.63–1.24) | 0.49 | ||
| Weighted median | 115 | 0.89 (0.68–1.15) | 0.36 | ||
| Weighted mode | 115 | 0.92 (0.71–1.17) | 0.49 | ||
| Obesity | IVW | 7 | 1.02 (0.75–1.39) | 0.89 | |
| MR Egger | 7 | 1.27 (0.43–3.78) | 0.69 | ||
| Weighted median | 7 | 1.02 (0.72–1.46) | 0.91 | ||
| Weighted mode | 7 | 1.04 (0.7–1.55) | 0.84 | ||
| dHypo | IVW | 6 | 0.95 (0.82–1.09) | 0.44 | |
| MR Egger | 6 | 0.98 (0.77–1.26) | 0.9 | ||
| Weighted median | 6 | 0.95 (0.8–1.11) | 0.5 | ||
| Weighted mode | 6 | 0.95 (0.81–1.1) | 0.51 | ||
| HPL | IVW | 14 | 1.04 (0.95–1.15) | 0.4 | |
| MR Egger | 14 | 1.07 (0.86–1.33) | 0.56 | ||
| Weighted median | 14 | 1.08 (0.94–1.24) | 0.3 | ||
| Weighted mode | 14 | 1.09 (0.9–1.32) | 0.39 | ||
| HTH | IVW | 12 | 0.99 (0.87–1.13) | 0.91 | |
| MR Egger | 12 | 1.09 (0.8–1.5) | 0.59 | ||
| Weighted median | 12 | 1.05 (0.88–1.25) | 0.59 | ||
| Weighted mode | 12 | 1.05 (0.85–1.29) | 0.66 |
MR, mendelian randomization; N. SNPs, number of SNPs used in MR; IVW, inverse variance weighted; CI, confidence interval; OR, odds ratio; DLBCL, Diffuse Large B-Cell Lymphoma; LL, Lymphocytic Leukemia; FL, Follicular Lymphoma; NHL, Non-Hodgkin’s Lymphoma; T2D, type 2 diabetes; dHypo, diabetic hypoglycemia; HPL, hyperlipidemia; HTH, hyperthyroidism.
Fig. 2.
Scatter plots for analysis of causal effect of dHypo on LL.
Fig. 3.
Forest plots for analysis of causal effect of dHypo on LL.
Cochran’s Q test revealed no heterogeneity for endocrine disease IVs in most lymphoid malignancy types, but considerable heterogeneity for HTH (Q = 77.53; P = 0) when considering NHL as the outcome (Table 2). The MR-Egger results indicated the presence of horizontal pleiotropy in the assessments exploring the associations between HTH and DLBCL, LL, as well as NHL (Table S7). Specifically, the following SNPs were excluded due to their potential to introduce bias or distort the analysis: rs1794280 from the HTH-DLBCL analysis, rs58722186 and rs1794280 from the HTH-LL analysis, and rs1794280 from the HTH-NHL analysis. These outliers were detected through a leave-one-out analysis (Figure S1) and were excluded to mitigate the impact of horizontal pleiotropy. As evident from Table S8, the MR-PRESSO outcomes revealed outliers in the assessments examining the associations between HTH and FL, HTH and DLBCL, HTH and NHL as well as dHypo and DLBCL, suggesting that outliers may potentially influence the causal associations. Guided by the MR-PRESSO results, certain SNPs were subsequently excluded from the respective analyses. After the removal of these outliers, the results showed a more consistent and reliable pattern, with no evidence of horizontal pleiotropy (Tables 2 and 3). In addition, the visualized results of corresponding analyzes after eliminating outliers and correcting for horizontal pleiotropy are exhibited in Figures S2.
Table 2.
The heterogeneity analysis by Cochran’s Q test and horizontal Pleiotropy analysis by MR-Egger regression.
| Outcome | Exposure | Heterogeneity (IVW) | Pleiotropy | |||
|---|---|---|---|---|---|---|
| Q | Q_pval | MR-Egger Intercept | P value | |||
| DLBCL | T2D | 103.58 | 0.73 | -0.0014 | 0.9 | |
| Obesity | 4.19 | 0.76 | -0.0117 | 0.85 | ||
| dHypo | 7.39 | 0.19 | 0.048 | 0.37 | ||
| HPL | 19.81 | 0.14 | -0.0248 | 0.56 | ||
| HTH | 12.81 | 0.17 | -0.126 | 0.05 | ||
| LL | T2D | 125.86 | 0.18 | 0 | 0.08 | |
| Obesity | 1.67 | 0.95 | 0.0001 | 0.65 | ||
| dHypo | 10.43 | 0.11 | -0.0002 | 0.11 | ||
| HPL | 9.39 | 0.81 | 0 | 0.94 | ||
| HTH | 7.34 | 0.6 | 0 | 0.31 | ||
| FL | T2D | 103.25 | 0.76 | -0.0161 | 0.35 | |
| Obesity | 4.7 | 0.58 | 0.1195 | 0.36 | ||
| dHypo | 6.78 | 0.24 | 0.142 | 0.1 | ||
| HPL | 11.43 | 0.57 | 0.0032 | 0.95 | ||
| HTH | 17.49 | 0.06 | 0.036 | 0.67 | ||
| NHL | T2D | 132.42 | 0.11 | 0.0018 | 0.82 | |
| Obesity | 8.01 | 0.24 | 0.0667 | 0.31 | ||
| dHypo | 12.36 | 0.05 | -0.0054 | 0.9 | ||
| HPL | 7.3 | 0.92 | 0.029 | 0.21 | ||
| HTH | 77.53 | 0 | -0.164 | 0.1 | ||
| Lymph node tumor | T2D | 135.56 | 0.08 | 0.0029 | 0.82 | |
| Obesity | 7.53 | 0.27 | -0.0388 | 0.7 | ||
| dHypo | 3.51 | 0.62 | -0.0161 | 0.73 | ||
| HPL | 11.61 | 0.56 | -0.0082 | 0.81 | ||
| HTH | 5.53 | 0.9 | -0.0258 | 0.52 | ||
IVW, inverse variance weighted; DLBCL, Diffuse Large B-Cell Lymphoma; LL, Lymphocytic Leukemia; FL, Follicular Lymphoma; NHL, Non-Hodgkin’s Lymphoma; T2D, type 2 diabetes; dHypo, diabetic hypoglycemia; HPL, hyperlipidemia; HTH, hyperthyroidism.
Table 3.
The Pleiotropy analysis by MR-PRESSO global test.
| Exposure | Outcome | Raw | Outlier corrected | Global P | Number of outliers | Distortion P | |||
|---|---|---|---|---|---|---|---|---|---|
| OR (CI%) | P | OR (CI%) | P | ||||||
| Type 2 diabetes | Diffuse large B-cell lymphoma | 1.01 (0.89–1.14) | 0.9 | NA | NA | 0.721 | NA | NA | |
| Obesity | 1.08 (0.9–1.31) | 0.44 | NA | NA | 0.788 | NA | NA | ||
| Diabetic hypoglycemia | 0.91 (0.76–1.07) | 0.3 | NA | NA | 0.31 | NA | NA | ||
| Hyperlipidemia | 0.97 (0.86–1.08) | 0.58 | NA | NA | 0.151 | NA | NA | ||
| Hyperthyroidism | 1.01 (0.83–1.23) | 0.93 | NA | NA | 0.18 | NA | NA | ||
| Type 2 diabetes | Lymphoid leukaemia |
1.0001 (0.9998–1.0004) |
0.64 | NA | NA | 0.174 | NA | NA | |
| Obesity |
0.9999 (0.9996–1.0003) |
0.69 | NA | NA | 0.96 | NA | NA | ||
| Diabetic hypoglycemia |
1.0004 (1-1.0007) |
0.07 | NA | NA | 0.134 | NA | NA | ||
| Hyperlipidemia |
0.9999 (0.9998–1.0001) |
0.53 | NA | NA | 0.843 | NA | NA | ||
| Hyperthyroidism | 1.0002 (0.9998–1.0005) | 0.36 | NA | NA | 0.589 | NA | NA | ||
| Type 2 diabetes | Follicular lymphoma | 1.01 (0.83–1.23) | 0.92 | NA | NA | 0.741 | NA | NA | |
| Obesity | 0.8 (0.55–1.15) | 0.27 | NA | NA | 0.628 | NA | NA | ||
| Diabetic hypoglycemia | 1.13 (0.88–1.44) | 0.38 | NA | NA | 0.338 | NA | NA | ||
| Hyperlipidemia | 1.1 (0.95–1.26) | 0.22 | NA | NA | 0.538 | NA | NA | ||
| Hyperthyroidism | 0.95 (0.72–1.25) | 0.71 | NA | NA | 0.09 | NA | NA | ||
| Type 2 diabetes | Non-Hodgkin’s lymphoma | 1.01 (0.92–1.11) | 0.82 | NA | NA | 0.113 | NA | NA | |
| Obesity | 0.98 (0.8–1.2) | 0.88 | NA | NA | 0.266 | NA | NA | ||
| Diabetic hypoglycemia | 0.91 (0.82–1.02) | 0.17 | NA | NA | 0.111 | NA | NA | ||
| Hyperlipidemia | 1.06 (1.01–1.11) | 0.02 | NA | NA | 0.932 | NA | NA | ||
| Hyperthyroidism | 1.07 (0.81–1.4) | 0.65 | NA | NA | < 0.001 | NA | NA | ||
| Type 2 diabetes | Lymph node neoplasm | 0.92 (0.79–1.07) | 0.27 | NA | NA | 0.086 | NA | NA | |
| Obesity | 1.02 (0.75–1.39) | 0.9 | NA | NA | 0.372 | NA | NA | ||
| Diabetic hypoglycemia | 0.95 (0.84–1.06) | 0.4 | NA | NA | 0.752 | NA | NA | ||
| Hyperlipidemia | 1.04 (0.95–1.14) | 0.38 | NA | NA | 0.497 | NA | NA | ||
| Hyperthyroidism | 0.99 (0.9–1.09) | 0.87 | NA | NA | 0.916 | NA | NA | ||
Discussion
The current study explored the potential causal associations between endocrine diseases and various types of lymphoid malignancies through two-sample MR study. The IVW method revealed a significant yet small causal relationship between genetically predicted dHypo and LL, indicating an increased risk of LL with dHypo. The marginal increase in risk suggests that the clinical impact of this association is likely to be limited. However, identifying even a small genetic predisposition to dHypo as a potential risk factor for LL highlights the importance of continued investigation into the interplay between metabolic disturbances and hematological malignancies, which could inform future preventive strategies or therapeutic targets.
Lymphocytic leukemia (LL) is a hematological malignancy that arises from the clonal transformation of either B- or T-lymphoid progenitor cells. It is distinguished by the uncontrolled proliferation and accumulation of these cells within the bone marrow, leading to a suppression of normal hematopoiesis and the clinical manifestations of anemia, thrombocytopenia, and neutropenia25. Furthermore, these abnormal cells can infiltrate into extracranial tissues such as the meninges, gonads, thymus, liver, spleen, or lymph nodes, causing further complications26. It is noteworthy that LL is most common in children, with Acute Lymphoblastic Leukemia (ALL) being more prevalent at this age stage. On the other hand, CLL tends to occur more frequently in older adults27. Several factors have been associated with an increased risk of developing LL, including physical factors such as ionizing radiation, chemical factors such as benzene and its derivatives, as well as some biological and viral factors28,29. In addition, genetic factors also play a role in the pathogenesis of LL. A previous MR study focusing on the relationship between TL and the risk of 11 hematological diseases has revealed a positive association between genetically predicted longer TL and an increased risk of developing ALL16. On the other hand, dHypo is a condition that occurs when someone with diabetes has an insufficient amount of glucose in their blood, resulting from increased insulin levels or decreased counter regulation in patients with diabetes30. Insulin is a naturally occurring protein hormone produced by the body, and also a widely prescribed medication for diabetic patients to help control blood glucose levels and delay the onset of complications like retinopathy, nephropathy, and neuropathy31. However, overly aggressive blood glucose control can increase the risk of hypoglycemia32. A previous study has demonstrated that dHypo might disrupt brain function, increase cardiovascular risk and mortality, and is associated with cardiac arrhythmias, including sudden death32. Regarding the relationship between dHypo and LL, most previous studies have focused on children with ALL, who have an increased probability of developing hypoglycemia during medication therapy33,34. In addition, previous studies have demonstrated the relationship between endocrine diseases and LL, in which LL was considered exposure. Xu et al. found that LL may increase the risk of diabetes, suggesting the need for diabetes prevention among leukemia survivors35. Another review summarized the risk factors and prevalence of metabolic syndrome among pediatric ALL and hematopoietic stem cell transplantation (HSCT) survivors, emphasizing the need for early and continuous screening to identify those at risk and implement preventive measures36.
This is the first study to establish a relationship between LL and dHypo. We found that dHypo may increase the risk of LL, and the potential mechanism may be related to impaired immune function or cellular stress. Specifically, plasma glucose is the main energy source for immune system cells such as lymphocytes and macrophages. When diabetic hypoglycemia occurs, the energy supply of these cells may be affected, leading to impairment of their functions37. Further, the normal function of the immune system is crucial for identifying and eliminating abnormal cells like leukemia cells. When immune function is weakened, leukemia cells may be more likely to escape from immune surveillance, increasing the risk of developing lymphocytic leukemia38. On the other hand, hypoglycemic state may lead to a series of metabolic abnormalities in the body, such as enhanced gluconeogenesis and increased fatty acid decomposition. These metabolic abnormalities may further cause disorders in the internal environment of cells and therefore affect the normal functions of cells39,40. Long-term cellular stress may lead to cell damage and gene mutation, increasing the risk of malignant transformation of cells. In the case of lymphocytic leukemia, this malignant transformation may manifest as abnormal proliferation and differentiation of leukemia cells41,42. Although it is generally considered that hypoglycemia is not a serious condition, the proposed mechanisms linking dHypo to LL progression are intriguing but remain speculative at this stage. Given the acute and transient nature of hypoglycemia, it is important to approach these potential links with caution and further studies are needed to elucidate whether and how dHypo might influence immune function and subsequently affect LL progression.
The existing clinical evidence regarding the relationship between endocrine diseases and lymphoid malignancies is inconclusive and often contradictory. For instance, Wang et al. observed a moderate elevation in the risk of NHL among patients suffering from T1D and T2D, particularly pronounced in Asian populations12. Conversely, negative associations between type 1 diabetes and sarcoidosis and the risk of NHL have been observed13. Similarly, Chao et al. found that T2D, which involved altered immune function and chronic inflammation, was positively associated with the risk of NHL. Furthermore, Leukemia and Hodgkin lymphoma survivors showed the highest risks for any endocrine disease and hypothyroidism, respectively43. But the authors also admitted that evidence from case-control studies remained inconclusive due to methodological limitations and further prospective studies were needed to confirm this association44. However, no causal association was found between T2D and NHL, or T2D and DLBCL in this study. There are several reasons that might explain this contradiction, such as lifestyle, environmental factors, and drug therapy. T2D patients are often accompanied by unhealthy lifestyles, such as lack of exercise and unhealthy dietary habits. These factors may be related to the increased risk of lymphoid malignancies31. Environmental factors such as pollution and occupational exposure may also affect the risks of T2D and lymphoid malignancies simultaneously45. Additionally, long-term use of certain medications (such as insulin, hypoglycemic agents, etc.) by diabetic patients may indirectly affect the risk of lymphoid malignancies46. Also, some medications may be associated with the risk of lymphoid malignancies by altering immune system functions or affecting cell proliferation47.
In addition, the contradictory results may also be attributed to the limitations of this study. Firstly, although the F-statistics of all SNPs exceeded the conventional threshold of 10, indicating sufficient strength of the IVs, the relatively low F-statistics in some cases, such as those associated with obesity and hyperthyroidism, may still raise concerns about the power of the MR analysis. This could potentially limit the ability to detect weak causal effects. Secondly, the study was based on genetic associations and SNPs, which may not fully capture the complexity of the relationships between endocrine diseases and lymphatic malignancies. Environmental and lifestyle factors, as well as gene-environment interactions, play crucial roles in disease development and progression, but were not comprehensively addressed in this study. Thirdly, the identification of most dHypo-associated SNPs primarily originated from studies involving populations with diabetes, but the GWAS study did not clearly distinguish whether the included cases were mainly T1DM or T2DM. Consequently, this restricts the broader applicability of the findings. Future studies incorporating larger and more diverse datasets, would help validate and expand upon these findings.
Furthermore, future research could focus on the following directions of effort. Firstly, this study primarily examined the existence of a unidirectional causal relationship between endocrine diseases and lymphoid malignancies. However, bidirectional MR studies hold greater potential in uncovering reciprocal associations and offering a more nuanced understanding of the intricate interplay between these two conditions. Secondly, while the study found a significant causal association between genetically predicted dHypo and LL, the effect size was small (OR = 1.0004), indicating a modest increase in risk. Future studies should aim to replicate this finding in larger and more diverse populations to confirm the robustness of this association. Additionally, further investigation into the biological mechanisms underlying this link could provide targets for intervention and prevention. Thirdly, the current study utilized MR to infer causal relationships based on genetic associations. However, these findings should be interpreted with caution and complemented by other types of evidence, such as experimental studies and epidemiological data. Future research should aim to integrate genetic, epidemiological, and experimental data to provide a more comprehensive understanding of the causal pathways between endocrine diseases and lymphoid malignancies.
In conclusion, while our MR analysis validated the causal relationship between dHypo and LL, the causal relationships with other endocrine-lymphatic pairings remain unclear. Future research should prioritize bidirectional MR, replicate findings in diverse populations, probe biological mechanisms, and integrate genetic, epidemiological, and experimental evidence. Such a comprehensive approach will be instrumental in delineating the causal pathways between endocrine diseases and lymphoid malignancies, ultimately informing more targeted prevention and treatment strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
Conception and design: Chenze Zhao and Ni Zhu_Collection and assembly of data: Yu Zhang, Lili Qian and Qing GuoData analysis and interpretation: Chenze Zhao and Ni ZhuManuscript writing: All authorsFinal approval of manuscript: All authors.
Funding
Zhejiang Provincial Natural Science Foundation of China [Grant No. LTGY23H290001]. Zhejiang Provincial Natural Science Foundation of China [Grant No. LQ20H020012]. Zhejiang Provincial Natural Science Foundation of China [Grant No. LTGY23H270004].
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ware, A. D., Davis, K. & Xian, R. R. Molecular pathology of mature lymphoid malignancies. Surg. Pathol. Clin.14, 529–547. 10.1016/j.path.2021.06.001 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Singh, R. et al. Non-Hodgkin’s lymphoma: A review. J. Family Med. Prim. Care. 9, 1834–1840. 10.4103/jfmpc.jfmpc_1037_19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan, Z. A. et al. The diagnostic dilemma of Splenic Non-Hodgkin’s lymphoma and Splenic abscess: A narrative review. Cureus14, e31944. 10.7759/cureus.31944 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu, S., Xu, M., Zhong, L., Tong, X. & Qian, S. Recent advances in nanobiotechnology for the treatment of Non-Hodgkin’s lymphoma. Mini Rev. Med. Chem.24, 895–907. 10.2174/1389557523666230915103121 (2024). [DOI] [PubMed] [Google Scholar]
- 5.Sehn, L. H., Salles, G., Diffuse Large, B-C. & Lymphoma N Engl. J. Med.384, 842–858, doi:10.1056/NEJMra2027612 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linton, K. M. et al. Personalised therapy in follicular lymphoma - is the dial turning? Hematol. Oncol.10.1002/hon.3205 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon, M. J., Smith, M. R. & Nastoupil, L. J. Follicular lymphoma: The long and winding road leading to your cure? Blood Rev.57, 100992. 10.1016/j.blre.2022.100992 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Bosch, F. & Dalla-Favera, R. Chronic lymphocytic leukaemia: From genetics to treatment. Nat. Rev. Clin. Oncol.16, 684–701. 10.1038/s41571-019-0239-8 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Klausen, U., Jørgensen, N. G. D., Grauslund, J. H., Holmström, M. O. & Andersen, M. H. Cancer immune therapy for lymphoid malignancies: Recent advances. Semin. Immunopathol.41, 111–124. 10.1007/s00281-018-0696-7 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Stevens, W. B. C., Netea, M. G., Kater, A. P. & van der Velden, W. J. F. M. ‘Trained immunity: consequences for lymphoid malignancies. Haematologica101, 1460–1468. 10.3324/haematol.2016.149252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maskarinec, G. et al. Association of obesity and type 2 diabetes with Non-Hodgkin lymphoma: the multiethnic cohort. Cancer Epidemiol. Biomarkers Prev.32, 1348–1355. 10.1158/1055-9965.Epi-23-0565 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, Y. et al. Association between type 1 and type 2 diabetes and risk of non-Hodgkin’s lymphoma: A meta-analysis of cohort studies. Diabetes Metab.46, 8–19. 10.1016/j.diabet.2019.04.006 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Shi, X., Wallach, J. D., Ma, X. & Rogne, T. Autoimmune diseases and risk of Non-Hodgkin lymphoma: A Mendelian randomisation study. Cancer Med.13, e70327. 10.1002/cam4.70327 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drozd-Sokolowska, J. et al. Type 2 diabetes mellitus compromises the survival of diffuse large B-cell lymphoma patients treated with (R)-CHOP – the PLRG report. Sci. Rep.10.1038/s41598-020-60565-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birney, E. Mendelian randomization. Cold Spring Harbor Perspect. Med.10.1101/cshperspect.a041302 (2021). [Google Scholar]
- 16.Wang, Y. et al. Genetically predicted telomere length and the risk of 11 hematological diseases: a Mendelian randomization study. Aging (Albany NY). 16, 4270–4281 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature613, 508–518. 10.1038/s41586-022-05473-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal Pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet.50, 693–698. 10.1038/s41588-018-0099-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization. Jama10.1001/jama.2021.18236 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Brion, M. J. A., Shakhbazov, K. & Visscher, P. M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol.42, 1497–1501. 10.1093/ije/dyt179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep.4, 330–345. 10.1007/s40471-017-0128-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowden, J. & Holmes, M. V. Meta-analysis and Mendelian randomization: A review. Res. Synthesis Methods. 10, 486–496. 10.1002/jrsm.1346 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuber, V., Colijn, J. M., Klaver, C. & Burgess, S. Selecting likely causal risk factors from high-throughput experiments using multivariable Mendelian randomization. Nat. Commun.10.1038/s41467-019-13870-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent Estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol.40, 304–314. 10.1002/gepi.21965 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luca, D. C. Update on lymphoblastic leukemia/lymphoma. Clin. Lab. Med.41, 405–416. 10.1016/j.cll.2021.04.003 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Malard, F. & Mohty, M. Acute lymphoblastic leukaemia. Lancet395, 1146–1162 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Inaba, H. & Mullighan, C. G. Pediatric acute lymphoblastic leukemia. Haematologica105, 2524–2539. 10.3324/haematol.2020.247031 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom, M., Maciaszek, J. L., Clark, M. E., Pui, C. H. & Nichols, K. E. Recent advances in genetic predisposition to pediatric acute lymphoblastic leukemia. Expert Rev. Hematol.13, 55–70. 10.1080/17474086.2020.1685866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceppi, F., Cazzaniga, G., Colombini, A., Biondi, A. & Conter, V. Risk factors for relapse in childhood acute lymphoblastic leukemia: prediction and prevention. Expert Rev. Hematol.8, 57–70. 10.1586/17474086.2015.978281 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Ortiz, M. R. Hypoglycemia in diabetes. Nurs. Clin. North Am.52, 565–574. 10.1016/j.cnur.2017.07.006 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Tinajero, M. G. & Malik, V. S. An update on the epidemiology of type 2 diabetes. Endocrinol. Metab. Clin. North Am.50, 337–355. 10.1016/j.ecl.2021.05.013 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Amiel, S. A. et al. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol.7, 385–396. 10.1016/s2213-8587(18)30315-2 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Visavachaipan, N., Aledo, A., Franklin, B. H. & Brar, P. C. Continuous glucose monitoring: A valuable monitoring tool for management of hypoglycemia during chemotherapy for acute lymphoblastic leukemia. Diabetes. Technol. Ther.15, 97–100. 10.1089/dia.2012.0181 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Trelinska, J. et al. Hypoglycemia and glycemic variability among children with acute lymphoblastic leukemia during maintenance therapy. Leuk. Lymphoma. 52, 1704–1710. 10.3109/10428194.2011.580024 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Xu, R., Zheng, T., Ouyang, C., Ding, X. & Ge, C. Causal associations between site-specific cancer and diabetes risk: A two-sample Mendelian randomization study. Front. Endocrinol.10.3389/fendo.2023.1110523 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bielorai, B. & Pinhas-Hamiel, O. Type 2 diabetes mellitus, the metabolic syndrome, and its components in adult survivors of acute lymphoblastic leukemia and hematopoietic stem cell transplantations. Curr. Diab. Rep.10.1007/s11892-018-0998-0 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Iqbal, A. et al. Effect of hypoglycemia on inflammatory responses and the response to Low-Dose endotoxemia in humans. J. Clin. Endocrinol. Metabolism. 104, 1187–1199. 10.1210/jc.2018-01168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arruga, F. et al. Immune response dysfunction in chronic lymphocytic leukemia: dissecting molecular mechanisms and microenvironmental conditions. Int. J. Mol. Sci.10.3390/ijms21051825 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yong, J., Johnson, J. D., Arvan, P., Han, J. & Kaufman, R. J. Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nat. Reviews Endocrinol.17, 455–467. 10.1038/s41574-021-00510-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Packer, M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc. Diabetol.10.1186/s12933-020-01041-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masle-Farquhar, E. et al. STAT3 gain-of-function mutations connect leukemia with autoimmune disease by pathological NKG2Dhi CD8 + T cell dysregulation and accumulation. Immunity55, 2386–2404e2388. 10.1016/j.immuni.2022.11.001 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Andersen, B. L. et al. Cells, cytokines, chemokines, and cancer stress: A biobehavioral study of patients with chronic lymphocytic leukemia. Cancer124, 3240–3248. 10.1002/cncr.31538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrahão, R. et al. Late endocrine diseases in survivors of adolescent and young adult cancer in California: a population-based study. Br. J. Cancer. 130, 1166–1175. 10.1038/s41416-024-02594-x (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chao, C. & Page, J. H. Type 2 diabetes mellitus and risk of Non-Hodgkin lymphoma: A systematic review and Meta-Analysis. Am. J. Epidemiol.168, 471–480. 10.1093/aje/kwn160 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Rebelo, A., Oliveira, J. & Sousa, C. Severe mercaptopurine-induced hypoglycemia in acute lymphoblastic leukemia. Pediatr. Hematol. Oncol.37, 245–247. 10.1080/08880018.2020.1713940 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Xie, X. et al. Benefits and risks of drug combination therapy for diabetes mellitus and its complications: a comprehensive review. Front. Endocrinol. (Lausanne). 14, 1301093. 10.3389/fendo.2023.1301093 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang, J. L. et al. Post-transplantation cyclophosphamide, tacrolimus and Low-Dose ATG as GVHD prophylaxis for allogeneic peripheral stem cell transplantation for adult patients with lymphoid malignancies: A single arm phase II study. Front. Med. (Lausanne). 8, 630160. 10.3389/fmed.2021.630160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.