Abstract
The rates of cigarette use among American adults have dropped substantially throughout the last six decades, yet smoking remains the leading cause of preventable disease and death in the United States. It is crucial to identify the putative time-varying population-level factors of age, period, and cohort that influenced the decrease in smoking prevalence so we can maintain the downward trend. We used 49 years of data from the National Health and Nutrition Examination Survey (NHANES) and hierarchical age–period–cohort (HAPC) analysis to examine lifecycle, historical, and generational distribution of smoking among Americans aged 18–74 years old. The prevalence of smoking has declined tremendously from 1971 to 2020 because American adults over the age of ~ 27 had a lower probability of cigarette use, but the rates of decrease have been unequal among birth cohorts. We uncovered the putative temporal contributors to population-level decreases in the prevalence of current smoking among American adults over the last nearly fifty years. Policy-makers ought to prioritize tobacco control efforts that focus on young adults, and should address the cohort-specific challenges in order to maintain the downward trend in smoking prevalence and further reduce the number of preventable premature deaths due to cigarette use.
Keywords: Age, APC, Cigarette, Cohort, NHANES, Smoking
Subject terms: Diseases, Risk factors
Introduction
Cigarette smoking (hereinafter “smoking”) rates among American adults have dropped substantially since 1965 and reached an all-time low in 20211,2. The reduction of smoking prevalence is encouraging but smoking remains the leading cause of preventable disease and death in the U.S3. It is critical to find the putative temporal contributors to the population-level decreases in smoking prevalence so we can maintain the downward trend, adjust regulations, if need be, and attain the Healthy People 2030target of 6% current use of cigarettes among American adults4. The reported population-level trends in smoking, the country’s longest-running epidemic, might be a result of distinct, time-varying factors like age (association between age and smoking status), and/or period (differences in smoking prevalence due to changing sociocultural environments that shape smoking practices collectively among all individuals), and/or cohort(shifts in smoking status among groups of individuals that belong to different generations)1,5,6. More precisely, age effects are differences caused by the physiological, psychological, and behavioral changes across the lifespan5. Over the past six decades, for example, the largest decrease in smoking prevalence has been among 18–24-year-olds1,2. Period effects embody the impact of historical events that affect the entire population simultaneously regardless of age and birth cohort5. Some of the many tobacco-related events in American history, for example, include the 1964, 1988, and 2020 Surgeon General’s reports, the 2000 truth® campaign, the 2009 Family Smoking Prevention law, the 2018 Every Try Countscampaign, and the 2020 federal tobacco control strategic plan7–12. Cohort (i.e., generational) effects reflect the impact of macro-level contextual (e.g., environmental, social, cultural) pressures across the life course on a group of individuals born within a specified period (i.e., birth cohort)5. Published findings indicate that cohort differences in adult smoking may be associated with different social norms around cigarette use across cohorts13.
Regarding individual-level indicators, adult smoking rate is associated with a person’s age, sex, race/ethnicity, educational attainment, income, and marital status1,8,14–18. For example, between 1965 and 2019, the overall prevalence of adult smoking consistently was higher among men than women8,16,17. The patterns across racial/ethnic groups indicate that the smoking rates have continually been higher among non-Hispanic Black and non-Hispanic White compared to Hispanic adults, with non-Hispanic Whites consistently having the highest prevalence8,14,16–18. The overall smoking rate has dropped substantially through the years but annual prevalence remains the highest among those of low income or low educational attainment when compared to high-socioeconomic status (SES) same-aged peers1,2,16,18. Indeed, in 2014, the Surgeon General warned that socioeconomic deprivation contributes substantially to the observed disparities in the rates of smoking16. Lastly, married adults have the lowest prevalence of smoking but the relationship is complicated by the association between ‘detailed’ marital status (married, cohabitating, divorced, widowed, separated, single/never married) and race/ethnicity19.
In this paper we use data from the National Health and Nutrition Examination Survey (NHANES) and hierarchical (i.e., individuals are grouped within birth cohorts and time periods) age–period–cohort (HAPC) analysis to examine lifecycle (i.e., age), historical (i.e., period), and generational (i.e., cohort) distribution of smoking among American adults over the last five decades5. It is crucial to disentangle the age-specific smoking trends by birth cohort membership in order to ensure continued success of tobacco control policies in reducing smoking prevalence among all population groups. Indeed, although smoking remains the leading preventable cause of death, the Centers for Disease Control and Prevention (CDC) included tobacco control on its 1999 and 2011 lists of Ten Great Public Health Achievementsbecause of the unprecedented reduction in smoking rates after the transformative 1964 Surgeon General’s report3,7,20,21. Important for our current study, federal or state policies and/or initiatives may impact differently those smokers who initiated smoking before or after the implementation of the tobacco control measures22–25. There are multiple published reports about the prevalence of U.S. adult smoking and its variations over time but, to the best of our knowledge, we are the first to investigate smoking trends among Americans aged 18–74 years old using HAPC analysis1,2,5.
Methods
Data
NHANES is a cross-sectional dataset representative of the non-institutionalized United States population26. We used NHANES I (1971/74), NHANES II (1976/80), NHANES III (1988/94), and all 2-year “continuous” cycles (1999–2020). We restricted our sample to adults aged 18–74 years old27. Our final sample size is 45,964 after listwise deletion of missing values. We confirmed that there is no statistically significant difference between the full and our final sample size after exclusion due to missingness (t = −1.58; p= 0.11). We applied NHANES cycle-specific sampling weights to address differences in the unequal probabilities of selection and non-response26.
Measures
The outcome is a binary indicator representing current smoking status. We defined current smokers as those who reported smoking 100 or more cigarettes in their lifetime and are smoking every day or some days at the time of survey assessment26,27. We included covariates to represent sociodemographic characteristics shown to associate with smoking behavior. Operational definitions and descriptive information for all variables included in the models are available in Table 1.
Table 1.
Summary weighted statistics for all variables in the analysis among adults aged 18–74 years old, 1971–2020 NHANES [N = 45,964].
| Dependent Variable | Mean or % | SD | Min | Max | ||||
|---|---|---|---|---|---|---|---|---|
| Smoked at least 100 Cigarettes in Lifetime & Current Smoker | 27% | 0.44 | 0 | 1 | ||||
| Level-1 Variables | ||||||||
| Age | Respondent’s age at survey year | 46 | 15.88 | 18 | 74 | |||
| Centered around grand mean | ||||||||
| Sex | Respondent’s sex: 1 = women; 2 = men | 51% | 0.50 | 0 | 1 | |||
| Race/Ethnicity | Respondent’s race/ethnicity | |||||||
| Non-Hispanic White | 1 = white | 30% | 0.46 | 0 | 1 | |||
| Non-Hispanic Black | 2 = black | 11% | 0.32 | 0 | 1 | |||
| Other Race | 3 = other | 59% | 0.49 | 0 | 1 | |||
| Marital Status | Respondent’s marital status at survey administration: | |||||||
| Marital Status 1 | 1 = Married | 56% | 0.50 | 0 | 1 | |||
| Marital Status 2 | 2 = Widowed, Divorced, Separated | 25% | 0.43 | 0 | 1 | |||
| Marital Status 3 | 3 = Never Married | 19% | 0.39 | 0 | 1 | |||
| Income | Respondent’s parents’ household income in 2020 dollars [thousands] | $69,322 | $20,592 | $0 | $94,437 | |||
| Educational Attainment | Respondent’s highest level of education | |||||||
| Education 1 | 1 = < high school | 28% | 0.45 | 0 | 1 | |||
| Education 2 | 2 = high school degree | 27% | 0.44 | 0 | 1 | |||
| Education 3 | 3 = some college | 28% | 0.45 | 0 | 1 | |||
| Education 4 | 4 = college degree | 17% | 0.37 | 0 | 1 | |||
| Level-2 Variables | N | Min | Max | |||||
| Period | Survey Year | 13 | 1971 | 2020 | ||||
| Cohort | Five-Year birth cohort | 21 | 1899 | 2002 | ||||
Statistical analysis
We estimated age, period, and cohort effects using a recently developed HAPC modeling approach5,28. We used HLM 8.2 to specify a two-level mixed (fixed and random) effects model with this technique29. The possibility that respondents in the same survey year and/or cohort group have a similar probability of smoking simply because they share similar random period and/or cohort error components (i.e., social experiences) is addressed with this method. Respondents are nested within cells cross-classified in birth cohorts and survey years (i.e., two social contexts). Within period-cohort cells (Level-1) is a fixed effects quadratic age estimation along with other individual-level covariates within each period-by-cohort grouping. This portion of the model specifies how much of the change in smoking is due to variation in physiological changes across the lifespan and/or individual’s sociodemographic background, holding constant period and cohort effects. Between period-cohort cells (Level-2) are normally-distributed period and cohort random effects. This portion of the model specifies how much of the variation in smoking is due to changing social environments that influence the probability of smoking for all individuals concurrently (i.e., period effect) orchanging population composition attributable to collective event experience such as being born in the same year (i.e., cohort effect). We break exact linear dependency between the three explanatory variables (i.e., cohort = period – age) and deal with under-identification by grouping birth cohorts into frequently used 5-year intervals, and, after confirmation of a curvilinear relationship, treat smoking as a quadratic function of age30. We first estimated the bivariate association between age and smoking status (Model 1). Next, we jointly estimated the main effects of all Level-1 independent variables (Model 2). Finally, Model 3 is an additive model that includes a significant interaction effect between age and marital status.
Results
In Table 1, we display descriptive information and operational definitions for all variables included in the analysis. 27% (SD = 0.44) of respondents reported smoking 100 cigarettes in their lifetime and currently smoke every day or some days. We present fixed effects in the form of odds ratios along with random effect coefficient estimates of smoking from the multilevel models in Table 2. We observed a significant quadratic age effect in Model 1, net of random period and cohort effects. Each additional year of age increased the odds of smoking, but to a diminishing degree at older ages. The odds of smoking increased by 4% (OR = 1.04; CI = 1.01, 1.07), after adjustment for time period and birth cohort variation, with every year increase in age. This association weakened among older respondents (OR = 0.99; CI = 0.99, 1.01) across the life course with every passing year. We provide residual variance components at Level-2 in the lower portion of Table 2. Smoking varied significantly by time period and birth cohort, after controlling for the age effect.
Table 2.
Estimates from logit Cross-Classified random effects Age-Period-Cohort models of smoking.
| Fixed Effects | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | SE | CI | Odds Ratio | SE | CI | Odds Ratio | SE | CI | |
| Intercept, π0 | 0.19# | 0.96 | [0.03,1.19] | 0.02*** | 0.99 | [0.00,0.15] | 0.03*** | 0.99 | [0.00,0.19] |
| Age, π1 | 1.04* | 0.01 | [1.01,1.07] | 1.05** | 0.02 | [1.03,1.09] | 1.04*** | 0.02 | [1.01,1.07] |
| Age2, π1 | 0.99*** | 0.00 | [0.99,1.01] | 0.99*** | 0.00 | [0.99,1.01] | 0.99*** | 0.00 | [0.99,1.01] |
| Men, π2 | 1.40*** | 0.03 | [1.31,1.48] | 1.40*** | 0.03 | [1.32,1.49] | |||
| Race/Ethnicity [ref. = NH White] | |||||||||
| NH Black, π3 | 1.32*** | 0.06 | [1.18,1.47] | 1.32*** | 0.06 | [1.19,1.48] | |||
| Other Race, π4 | 2.58*** | 0.04 | [2.38,2.80] | 2.58*** | 0.04 | [2.38,2.79] | |||
| Marital Status [ref. = Marital Status 1: Married] | |||||||||
| Marital Status 2: [Widowed, Divorced, Separated], π5 | 1.71*** | 0.03 | [1.59,1.85] | 1.35* | 0.13 | [1.03,1.77] | |||
| Marital Status 3: [Never Married], π6 | 1.23*** | 0.04 | [1.13,1.34] | 1.13* | 0.12 | [1.01,1.19] | |||
| Income, π7 | 0.99*** | 0.00 | [0.99,1.00] | 0.99*** | 0.00 | [0.99,1.00] | |||
| Educational Attainment [ref. = Education 4: College Degree] | |||||||||
| Education 1: [< High School], π8 | 5.55*** | 0.06 | [4.97,6.20] | 5.54*** | 0.06 | [4.96,6.19] | |||
| Education 2: [High School Degree], π9 | 3.97*** | 0.06 | [3.56,4.42] | 3.98*** | 0.06 | [3.57,4.42] | |||
| Education 3: [Some College], π10 | 2.55*** | 0.06 | [2.29,2.83] | 2.56*** | 0.06 | [2.30,2.85] | |||
| Age × Marital Status 2: [Widowed, Divorced, Separated] | 1.01* | 0.00 | [0.99,1.01] | ||||||
| Age x Marital Status 3: [Never Married] | 1.02*** | 0.00 | [1.01,1.02] | ||||||
| Random Effects | |||||||||
| Cohort | Coefficient | se | t Ratio | Coefficient | se | t Ratio | Coefficient | se | t Ratio |
| 1899 | 0.12 | 0.05 | 1.12 | 0.11 | 0.03 | 1.61 | 0.08 | 0.04 | 1.61 |
| 1904 | 0.09 | 0.06 | 1.19 | 0.10# | 0.06 | 1.69 | 0.10# | 0.06 | 1.75 |
| 1909 | 0.10 | 0.06 | 1.52 | 0.12* | 0.06 | 1.99 | 0.13* | 0.06 | 2.12 |
| 1914 | 0.19** | 0.06 | 1.64 | 0.20** | 0.06 | 3.13 | 0.21*** | 0.06 | 3.29 |
| 1919 | 0.19* | 0.07 | 2.85 | 0.20** | 0.07 | 2.75 | 0.21** | 0.07 | 2.89 |
| 1924 | 0.20* | 0.08 | 2.56 | 0.21** | 0.08 | 2.66 | 0.22** | 0.09 | 2.80 |
| 1929 | 0.22* | 0.09 | 2.43 | 0.23* | 0.10 | 2.63 | 0.24** | 0.10 | 2.75 |
| 1934 | 0.19* | 0.10 | 2.44 | 0.21* | 0.11 | 2.24 | 0.23* | 0.11 | 2.36 |
| 1939 | 0.23* | 0.10 | 1.96 | 0.25* | 0.13 | 2.42 | 0.27* | 0.12 | 2.54 |
| 1944 | 0.21* | 0.12 | 1.70 | 0.25* | 0.14 | 2.14 | 0.26* | 0.13 | 2.29 |
| 1949 | 0.24* | 0.13 | 1.88 | 0.27* | 0.15 | 2.18 | 0.29* | 0.14 | 2.31 |
| 1954 | 0.26* | 0.14 | 1.85 | 0.28* | 0.16 | 2.08 | 0.30* | 0.15 | 2.22 |
| 1959 | 0.28* | 0.15 | 1.87 | 0.30* | 0.17 | 2.06 | 0.32* | 0.16 | 2.19 |
| 1964 | 0.26 | 0.16 | 1.59 | 0.28* | 0.18 | 1.79 | 0.30* | 0.18 | 1.92 |
| 1969 | 0.25 | 0.17 | 1.46 | 0.29* | 0.19 | 1.72 | 0.31# | 0.18 | 1.84 |
| 1974 | 0.23 | 0.18 | 1.29 | 0.27* | 0.21 | 1.52 | 0.30 | 0.19 | 1.64 |
| 1979 | 0.26 | 0.19 | 1.37 | 0.30# | 0.21 | 1.56 | 0.32# | 0.20 | 1.67 |
| 1984 | 0.22 | 0.20 | 1.07 | 0.26 | 0.22 | 1.28 | 0.28 | 0.22 | 1.40 |
| 1989 | 0.21 | 0.12 | 0.97 | 0.25 | 0.21 | 1.16 | 0.27 | 0.21 | 1.28 |
| 1994 | 0.21 | 0.22 | 0.92 | 0.19 | 0.22 | 0.86 | 0.21 | 0.22 | 0.94 |
| 1999 | 0.12 | 0.24 | 0.51 | 0.04 | 0.24 | 0.19 | 0.05 | 0.24 | 0.20 |
| Period | |||||||||
| 1971 | −0.06** | 0.01 | −2.65 | −0.04** | 0.01 | −2.51 | 0.26* | 0.11 | 2.51 |
| 1976 | −0.08** | 0.04 | −3.37 | −0.06* | 0.02 | −2.87 | −0.06* | 0.09 | 2.18 |
| 1988 | −0.15** | 0.07 | −2.80 | −0.16** | 0.04 | −3.72 | −0.17** | 0.07 | 1.42 |
| 1999 | −0.21** | 0.07 | −2.79 | −0.32*** | 0.06 | −4.99 | −0.33*** | 0.05 | −1.30 |
| 2001 | −0.22* | 0.07 | −2.64 | −0.31** | 0.07 | −4.52 | −0.32*** | 0.04 | −1.20 |
| 2003 | −0.20* | 0.07 | −2.32 | −0.30** | 0.07 | −4.12 | −0.31** | 0.04 | −1.12 |
| 2005 | −0.22* | 0.08 | −2.43 | −0.32** | 0.07 | −4.07 | −0.33** | 0.03 | −1.61 |
| 2007 | −0.18# | 0.08 | −1.81 | −0.31** | 0.07 | −3.70 | −0.31** | 0.03 | −1.41 |
| 2009 | −0.20* | 0.09 | −1.97 | −0.32** | 0.08 | −3.70 | −0.33** | 0.03 | −2.21 |
| 2011 | −0.21* | 0.09 | −1.96 | −0.32** | 0.09 | −3.51 | −0.33** | 0.03 | −2.43 |
| 2013 | −0.18# | 0.10 | −1.58 | −0.30* | 0.10 | −3.10 | −0.31* | 0.02 | −1.70 |
| 2015 | −0.22# | 0.10 | −1.87 | −0.32* | 0.10 | −3.17 | −0.33** | 0.02 | −2.96 |
| 2017 | −0.28* | 0.11 | −2.43 | −0.26* | 0.11 | −2.37 | −0.27* | 0.11 | −2.51 |
| Variance Components | Variance | sd | p value | Variance | sd | p value | Variance | sd | p value |
| Cohort | 0.00547*** | 0.07398 | < 0.001 | 0.00668*** | 0.08173 | < 0.001 | 0.00687*** | 0.08286 | < 0.001 |
| Period | 0.00002** | 0.00000 | < 0.01 | 0.00001** | 0.00000 | < 0.01 | 0.00000** | 0.00000 | < 0.01 |
| Model Fit | 54258.76 | 50907.14 | 50878.76 | ||||||
Note: # p < 0.10, * p ≤ 0.05,** p ≤ 0.01, *** p ≤ 0.001.
Note: “SE” = Standard Error; “CI” = 95% Confidence Interval
In Fig. 1, we present the overall trends in smoking estimated from Model 1 in terms of predicted probabilities. We observed a clear curvilinear relationship between smoking and age (Fig. 1a). Specifically, there is an increase in the probability of smoking throughout early adulthood, with a persistent decline starting at ~ 27 years of age. In Fig. 1b, we display the estimated period effects in the form of predicted probabilities. We estimated smoking for each year at the mean age and averaged over all birth cohorts (intercept + period-specific random-effect coefficients). There is a sharp decline in smoking between 1971/74 and 1999/00, followed by a relative plateau until 2013/14 and another decline in 2015/16. We present in Fig. 1c the estimated cohort effects from Model 1 in terms of predicted probabilities. We computed smoking at the mean age and averaged over all periods [intercept + cohort-specific random effect coefficients]. The predicted probabilities of smoking were at a low point in the 1904 cohort, followed by a steady increase in the subsequent cohorts that peaked in the 1959 cohort, which was then followed by a downward trend among all cohorts until 1999, with a single exception, those born in 1979. It is important to note that these results are strongly confounded by age and, to a lesser extent, period effects, which makes it difficult to make any meaningful inferences based on this pattern.
Fig. 1.
Overall Age, Period, and Cohort Effects on Smoking: NHANES 1971–2020.
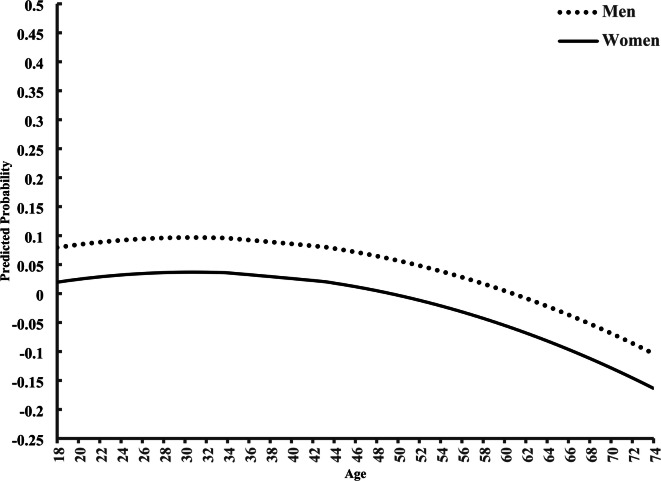
In Table 2, we display results from Model 2. Those with higher income (OR = 0.99; CI = 0.99, 1.00) have lower odds of smoking. Men (OR = 1.40; CI = 0.99, 1.00), relative to women, non-Hispanic Black (OR = 1.32; CI = 1.18, 1.47) and Other Race (OR = 2.58; CI = 2.38, 2.80), relative to non-Hispanic white, those who are widowed, divorced, and separated (OR = 1.71; CI = 1.59, 185) or those that never married (OR = 1.23; CI = 1.13, 1.34), relative to married, and respondents without a high school degree (OR = 5.55; CI = 497, 6.20) or those who graduated from high school (OR = 3.97; CI = 3.56, 4.42) or attended some college (OR = 2.55; CI = 2.29, 2.83), relative to college graduates, are more likely to smoke. Notably, when we consider Level-2 heterogeneity in period and cohort effects, Level-1 effects reported in prior published reports still hold. Also, as shown in the lower portion of Table 2, smoking varies significantly by cohort-specific (0.00668; p < 0.001), as well as period-specific (0.00001; p < 0.01), factors. A particularly significant and negative effect exists for respondents surveyed in 1999/00 (−0.32, p < 0.001), 2005/06, (−0.32, p < 0.01), 2009/10 (−0.32, p < 0.01), and 2011/12 (−0.32, p < 0.01), as evidenced by the estimated average effect coefficients for period. Still, significant positive estimated average cohort effect coefficients ‘push back’ against the period decline among those born in 1914/18 (0.20; p < 0.01), 1919/23 (0.20; p < 0.01), and 1924/28 (0.21, p < 0.01). We show in Model 2 that, after we adjust for all of the above conditions, the main age effect still is highly significant (OR = 1.05; CI = 1.03, 1.09). We also estimated predicted probabilities of smoking by sex from Model 2 (Fig. 2). The trajectories parallel one another from 18 to 74 years of age, but men, relative to women, are consistently more likely to smoke across the life course.
Fig. 2.
Predicted Age Variation in Sex on Smoking. Note: Model 2 includes all independent variables and is graphed by the reference categories.
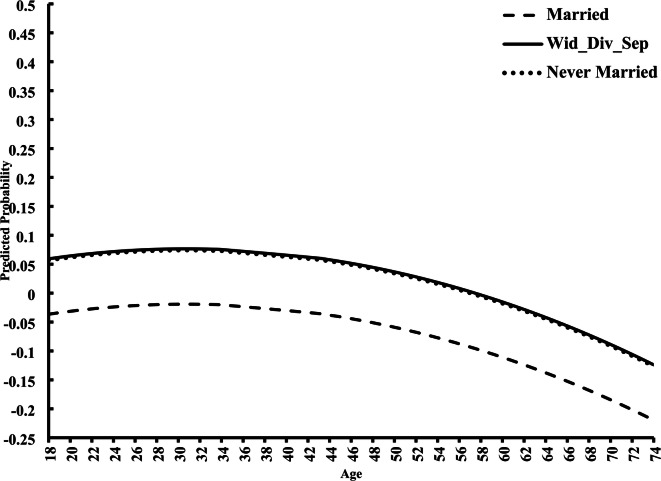
We found a significant interaction effect between age and marital status (Model 3). Individuals who are widowed, divorced, or separated, as well as those who never married, relative to those who are married, have an added 1% (OR = 1.01; CI = 0.99, 1.01) and 2% (OR = 1.02; CI = 1.01, 1.02) increase, respectively, in the odds of smoking with every year increase in age. We depict this variation graphically in the form of predicted probabilities (Fig. 3). Widowed, divorced, or separated, as well as never married, respondents begin with higher odds of smoking and maintain those higher odds throughout the aging process, compared to those smokers who are married.
Fig. 3.
Predicted Age Variation in Smoking by Marital Status. Note: Model 3 includes all independent variables and is graphed by the reference categories.
Discussion
Cigarette use is a crucial modifiable risk factor for premature mortality, and while U.S. adult smoking rates declined substantially throughout the last six decades, smoking still accounts for approximately 420,000 deaths annually1–3,31. In our present cross-sectional study, we used NHANES dataset and HAPC analysis to extend upon published research and found that the downward trend in U.S. adult smoking rates between 1971 and 2020 is driven mainly by negative period and age effects rather than cohort effect. In fact, if cohort effect also was negative, it would amplify the period and age effects to create an even steeper drop in rates of smoking. More specifically, we observed a decrease in smoking prevalence from 1971 to 2020 because American adults over the age of ~ 27 had a lower probability of cigarette use. The decline, however, has been unequal among birth cohorts (i.e., generations). As shown in Fig. 1a, the odds of smoking increase slightly among those between 18 and ~ 27 years of age, but this association weakens for smokers above the age of ~ 27. Indeed, the odds of being a current smoker decrease after the age of ~ 27, and the odds are comparable for those aged 18 or 38 years old. Notably, our current findings, using a newly developed statistical method, are in line with the results of previously published studies16,17. For example, the 2019 CDC data for American adult smokers indicate that the prevalence of current smoking peaks among 25–34-year-olds17. Also, in 2014, the Surgeon General reported that the prevalence peaks among those aged 26–44 years old16. We should note that the results of an APC analysis of smoking patterns among adults in England indicate that, after adjusting for the period and cohort effect, the odds of being a current smoker decrease after the age of ~ 2532. Given our current findings, and considering a large body of previously published results, young adulthood is a promising target for smoking initiation and/or cessation interventions to avoid the long-lasting sequelae of cigarette use3,33.
We confirm previous reports and show that the prevalence of smoking among Americans aged 18–74 years old decreased from 1971 to 2020 and reached a historic low for this age group1,2. The smoking rate has fallen tremendously over the course of our study period, but we observed fluctuations across three distinct time intervals during these 49 years (Fig. 1b). Specifically, we see a substantial decrease in the probability of smoking from 1971/74 to 1999/00, as well as from 2013/14 to 2017/20, with a relative plateau between 1999/00 and 2013/14. Our findings have important implications for advancing the health of communities and populations. We show that the efficacy of past efforts aimed at reducing cigarette use among current smokers and discouraging cigarette consumption among non-smokers differed throughout these historical periods. Regarding the 1971/74 to 1999/00 period, our HAPC analysis indicates that the probability of smoking decreased substantially throughout these nearly 20 years (Fig. 1b), which is in line with past reports2,16,17. Clearly, several events coincided in the mid to late-20 th century that contributed to a dramatic shift in public attitudes about cigarette use and, as a result, decline in societal support for smoking. It is challenging to isolate the effects of a particular initiative, and we must consider the time lag between the public health program’s implementation and its effects. With that said, the reduction in rates of smoking resulted, in part, because of scientific evidence published in 1957 implicating cigarettes as a cause of lung cancer34 Moreover, the 1967 Fairness Doctrine required media to broadcast anti-smoking counter-advertisements, followed by the 1970 Public Health Cigarette Smoking Actbanning cigarette advertising on television and radio stations35. Furthermore, the 1988 Surgeon General’s report emphasized the scientific evidence about the tobacco plant’s addictive substance, nicotine12. These efforts, in addition to health warning labels on cigarette packs, as well as legislation restricting cigarette use in public places, contributed to negative public perceptions and made smoking a less acceptable social practice, which paralleled the changes in population-level smoking behavior22–25,35,36.
Concerning the 1999/00 to 2013/14 period, here we found minor intra-period fluctuations, but the probability of smoking did not change substantially during these 15 years (Fig. 1b). In terms of previously published reports, our findings for the 2003/04 to 2007/08 interval, for example, are consistent with the CDC estimates indicating that the proportion of current adult smokers did not change noticeably from 2004 to 200837,38. However, the CDC also reported a significant decrease in the prevalence of smoking from 1999 to 2004, as well as from 2008 to 201437–40. Thus, our current results only partially support the CDC report about the 1999/00 to 2013/14 trends in smoking prevalence37–40. Although it is beyond the scope of our analysis to explain precisely why we see this plateau, we offer some possible explanations. One consequence of the 1998 Master Settlement Agreement(MSA) was a drastic increase in the price of a pack of cigarettes41. While higher cigarette prices are generally associated with a decline in smoking prevalence, the decline is not uniform across demographic and socioeconomic subgroups15. Indeed, between 1998 and 2004, a per-pack cigarette price increase did not influence the rates of smoking among low-income individuals15. Given that impoverished Americans are more likely to smoke relative to their higher-income counterparts, the disproportionate effect of higher cigarette costs may, in part, have contributed to an overall slower rate of decrease in adult smoking prevalence during early to mid 2010 s15. Related, increases in cigarette taxes have been associated with only a slight decrease in smoking because, in part, more than 50% of adult U.S. current smokers between 2009 and 2010 practiced cigarette price-minimization strategies42. It is important to note that price sensitivity varies across age groups, and younger smokers are especially responsive to price increases16.
One of the Healthy People 2030 targets is to increase the national average tax on cigarettes from $2.14 in 2022 to $2.60, but tax increases alone are not the most effective strategy to rapidly decrease the overall rate of adult smoking because current cigarette smoking already is the lowest among the youngest adults43,44. Indeed, the U.S. did not meet the Healthy People 2010goal of 12% adult smoking prevalence until year 20211,45. Furthermore, in 2004 the CDC launched the Tobacco Cessation Quitlines, and two years later this service was available to smokers in all 50 states46. However, between 2011 and 2013, only a nominal percentage (between 1% and 2%) of smokers used the state-run quitlines, and almost 50% of smokers who enrolled in Quitline services between 2007 and 2008 completed only one cessation coaching session46,47. Notably, meta-analyses indicate that successful smoking interventions generally involve more than one coaching session48. Finally, the 2010 Affordable Care Act(ACA) included provisions that were designed to expand smoking cessation benefits and it established a fund that provided money to prevent and reduce cigarette use49. But, at the same time, the ACA allowed insurers to charge substantially higher premiums to cigarette users, which increased out-of-pocket costs for smokers, reduced insurance enrollment among smokers, and, ultimately, produced no difference in rates of smoking cessation between 2013 and 201449. Importantly, smokers living in states that limit or do not allow tobacco surcharges are more likely to have health insurance coverage, and insurance gain is associated with a higher likelihood of smoking cessation49. Also, even though several prescription-only, as well as over-the-counter, nicotine replacement products were available to smokers during this time period, not enough smokers used nicotine replacement therapy (NRT) when trying to quit and, consequently, the availability of NRT has not had a significant effect on population-level prevalence of smoking over the years50.
According to the CDC, current smoking among U.S. adults decreased between 2014 and 202039,51. Our present HAPC findings confirm these reports and indicate that the probability of smoking decreased significantly during this six-year time period (Fig. 1b). It is evident that a combination of synergistic population-level strategies helped accelerate this decline of cigarette use. Related, the time delay between the public health strategy’s enforcement and its effects makes it difficult to isolate the effects of a certain strategy. Indeed, the results of a campaign designed to prevent youth smoking initiation will become evident later than those of an initiative that targets adults, given that we are interested in the trends of current cigarette smoking prevalence among individuals aged 18 years and older11,25. Nonetheless, we offer some possible explanations. The 2014 FDA’s The Real Costcampaign was designed to prevent cigarette initiation, as well as to discourage escalation to persistent smoking, among 12–17-year-olds25. This campaign was associated with a nationwide reduction in smoking initiation among 11–19-year-olds from 2014 to 201652. Thus, the reduction in smoking uptake among American youths during this 2-year period contributed to the observed decline in adult current cigarette use between 2014 and 2020. Additionally, the Tobacco-21law that raised the minimum age for purchasing tobacco in the U.S. to 21 years is associated with lower odds of smoking among 12 th graders, as well as 18–20-year-olds53. Furthermore, since 2012, the CDC has implemented the Tips®campaign in order to motivate people to quit smoking24. By 2013, Tips®was associated with an estimated 220,000 sustained (6-month) quits among American adult cigarette smokers, and this number grew to an estimated 522,000 and 1,005,419 by 2015 and 2018, respectively54. Notably, Tips®is associated with lower probability of cigarette smoking relapse55. From 2018 to 2020, the FDA ran Every Try Counts™ campaign that targeted 25–54-year-olds in 35 U.S. counties who attempted to quit smoking but had previously been unsuccessful, and has prompted more than 15,000 people to sign up for text-messaging programs designed to help cigarette users quit11. We also must mention that use of electronic cigarettes (e-cigarettes) among American adults has increased significantly from 2014 to 2020, and e-cigarette use is associated with quitting combustible cigarette smoking56,57.
As displayed in Fig. 1c, using HAPC analysis, the effects of generation (i.e., cohort effect) look more variable than age and period effects. Specifically, adults born in the 1950 s, 1960 s, and 1970 s had the highest likelihood of smoking, while the three oldest (1899, 1904, 1909) birth cohorts, as well as the most recent (1999) birth cohort, showed the lowest probability of cigarette use. The lower probability of current smoking among the 1999 birth cohort, for example, may reflect reduced smoking initiation and increased smoking cessation rates. Crucially, neglecting cohort-related smoking patterns undoubtedly will lead to erroneous interpretation of trends, inappropriate conclusions about the efficacy of tobacco control policies, and inaccurate projections of cigarette consumption. Indeed, the findings of our HAPC analysis indicate that the adult smoking rates between 1971 and 2020 decreased due to the negative period effect (Fig. 1b), whereas the age effect on smoking is nonlinear, with peak rates observed in young adulthood (Fig. 1a), and the decline has been unequal, when we consider generational membership (Fig. 1c). These results provide indirect evidence that tobacco control policies over the decades resulted in an unequal, cohort-specific decline in the prevalence of smoking1. Hence, we cannot understate the significance of generational shifts in smoking behavior due to the social norms at a given point in time that signaled acceptability or disapproval of cigarette use, independent of, and beyond, the addictive properties of tobacco. We should note here that cohort-specific cigarette consumption varies greatly from state to state due to, in part, the differences in state-level tobacco control policies such as cigarette taxes and smoke-free air laws, as well as social norms and sociodemographic composition58.
In 2021, vis-à-vis sex differences, the overall prevalence of adult smoking was higher among men than women across all age groups1. Our present findings using HAPC analysis confirm these results and indicate that the smoking rate reductions for women and men parallel one another from 18 to 74 years of age, but men, relative to women, are consistently more likely to smoke across the life course (Fig. 2). It is evident that a combination of genetic, physiological, behavioral, socioeconomic, and sociocultural factors underlies each of the three phases of smoking behavior (i.e., initiation, intensity, cessation)59. Crucially, all of the above-mentioned factors interact with sex/gender to influence every stage of nicotine addiction (i.e., initiation, dependence, withdrawal, relapse)59. Specifically, women have greater craving during acute nicotine withdrawal and more difficulty quitting than men, but men experience greater nicotine-induced reinforcement/reward59. Moreover, in order to capture the complexity of sex/gender differences in nicotine addiction and population-level prevalence of smoking, we also must consider empirical evidence that crosses levels of analysis, from biological to social59. Indeed, hormones, personality traits, and social acceptance are just some of the many factors that influence differently smoking initiation and cessation among women versus men59. Thus, decision-makers and policy-makers must take into account sex/gender differences when designing population-based campaigns to combat this deadly epidemic59. For example, reducing the content of nicotine in cigarettes to very low levels would accelerate the rate of decline in smoking prevalence among all smokers concomitantly, but may prove to be more beneficial to women, relative to men60. Conversely, NRT (e.g., transdermal patch) increases the rate of quitting regardless of sex/gender, but may be more efficacious for men, compared with women60.
According to the 2021 CDC report, current cigarette use among U.S. adults varies by marital status, with those who are married having the lowest prevalence of smoking1. The findings of our current HAPC analysis confirm these estimates and indicate that married respondents begin with lower odds of smoking and maintain those lower odds throughout the aging process, compared to those smokers who are widowed, divorced, or separated, as well as those who never married (Fig. 3). It is well known that primary social relationships, such as marriage, influence the instigation, maintenance, cessation, and relapse of smoking behavior across the life course13. Indeed, a large body of literature has established that, in general, the marital relationship has a protective effect on smoking, whereas marital disruption (e.g., divorce or widowhood) is associated with more smoking13. Specifically, even smokers who are married are more likely to be former smokers than those who never-married, or are divorced or widowed13. Notably, cohabitating young adult smokers of both sexes in the United States are less likely than non-smokers to transition from cohabitation to marriage13. To inform evidence-based policymaking, since the early 1990 s, researchers have identified social support, social control, and social strain as some of the underlying mechanisms that constrain an individual’s smoking behavior13.
Although our findings are informative, we should note some potential limitations of this research. Here we used cross-sectional NHANES data, which restricts our ability to offer any causal explanations26. Nevertheless, using a repeated cross-sectional survey dataset that tracks multiple cohorts’ experiences over time enabled us to parse out the population-level mechanisms generating change in smoking28. Also, our outcome is a binary indicator representing current smoking status and, in the NHANES, these data are subjective (i.e., self-reported)26. Even though self-reports of smoking habits are subject to recall bias, as well as underreporting due to increasing stigma, studies over the years indicate that self-reported cigarette consumption is a valid measure for the assessment of trends in smoking prevalence61,62. Related, the inherent NHANES instrument limitations over a 49-year period may impact our estimates26. For example, Hispanic and non-Hispanic Asian adults are less likely to smoke than non-Hispanic White or non-Hispanic Black persons, but the early years of the NHANES do not have these data1,17,26. Likewise, the gene–environment interaction shapes individual variation in smoking behavior and nicotine dependence, but the publicly available NHANES does not have this information26. Furthermore, we did not include daily cigarette consumption (i.e., smoking intensity) among current smokers into the study. Similarly, we investigated combustible tobacco cigarette smoking trends among American adults and, due to NHANES data constraints, were unable to include e-cigarette use in our analyses, even though 4.5% of adults aged 18 and over were current e-cigarette users in 2021, and e-cigarette use is associated with quitting combustible cigarette smoking63,64. Additionally, all APC models have pros and cons65. Some contend, for example, that the HAPC model findings vary depending on the data structure and chosen constraints66–69. Accordingly, we performed several sensitivity analyses, and our findings remain the same when using 2-year cohort intervals, as well as alternate functional forms of age, indicating that these transformations make no substantive difference to the reported results70,71.
In our study we show that (1) current smoking rates among American adults over the last five decades have fallen because persons over the age of ~ 27 had a lower probability of cigarette use, and (2) the decline in prevalence of smoking from 1971 to 2020 has not been equal among birth cohorts. Our findings will be useful in the search for strategies that will make ‘tobacco endgame’ a reality in the United States72. To our first point, raising the minimum legal age of purchasing tobacco products to 21 years is an effective way to reduce cigarette consumption at the population level53,72. Also, future national tobacco control efforts must incorporate comprehensive evidence-based strategies that discourage smoking among young adults, namely significant increases in tobacco taxes73. Secondly, generational groups experience cigarettes differently when, as a birth cohort, they approach ages that are associated with a higher probability of smoking initiation16,17,33. Thus, policy-makers must develop and implement birth cohort-specific tobacco control strategies, and then conduct analyses to determine whether these policies resulted in a cohort-specific cigarette use disapproval and concurrent cohort-specific smoking rate decrease. Additionally, mass media campaigns designed to de-normalize smoking may be an efficacious public health strategy. For example, the American Legacy Foundation introduced the truth®national tobacco prevention counter-marketing campaign in 200011. Using HAPC analysis, we observed a step-wise decline in the prevalence of current smoking with each consecutive generation, starting and ending with the 1984 and the 1999 cohort, respectively (Fig. 1c). These findings may reflect reduced smoking initiation among individuals who were anywhere between 21 and 37 years of age in year 2020 (i.e., 1–16 years of age in year 2000), and indicate that truth®may have led to birth cohort-specific shifts in cigarette use11.
Taken together, we uncovered the putative time-varying population-level factors of age, period, and cohort that influenced the decrease in the prevalence of current smoking among American adults over the last nearly fifty years. Policy-makers ought to prioritize tobacco control national efforts that focus on young adults, and should address the cohort-specific challenges in order to maintain the downward trend in smoking prevalence and further reduce the number of preventable premature deaths due to cigarette use.
Acknowledgements
We thank Chris Bader, Ed Day, Pete Simi, Karen Snedker, and Robert Wagmiller for their comments on an earlier version of the manuscript.
Author contributions
AWK: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization; DK: Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Project administration, Resources, Supervision, Visualization. All authors read and approved the final manuscript.
Funding
This research did not receive any grant funding from agencies in the public, commercial, or not-for-profit sectors.
Data availability
The dataset analyzed during the current study are available at https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cornelius, M. E. et al. Tobacco product use among adults – United States, 2021. MMWR Morb Mortal. Wkly. Rep.72, 475–483 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovino, G. A. et al. Surveillance for selected Tobacco-Use Behaviors – United States, 1900–1994. Centers for disease control and prevention. CDC surveillance summaries, 1994. MMWR Morb Mortal. Wkly. Rep.43 (SS-3), 1–50 (1994). [PubMed] [Google Scholar]
- 3.Le, T. T. T., Mendez, D. & Warner, K. E. New estimates of smoking-attributable mortality in the U.S. From 2020 through 2035. Am. J. Prev. Med.66 (5), 877–882 (2024). [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2030. Smoking. Reduce current cigarette smoking in adults — TU-02.
- 5.Yang, Y. & Land, K. C. Age—period—cohort analysis of repeated cross-section surveys. Fixed or random effects? Sociol. Methods Res.36 (3), 297–326 (2008). [Google Scholar]
- 6.Ryder, N. B. The cohort as a concept in the study of social change. Am. Social Rev.30, 843–861 (1965). [PubMed] [Google Scholar]
- 7.U.S. Public Health Service. Smoking and Health. Report of the Advisory Committee To the Surgeon General of the Public Health Service (U.S. Department of Health, Education, and Welfare, Public Health Service, Centers for Disease Control, 1964). (PHS publication no. 1103). [Google Scholar]
- 8.U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, (2020).
- 9.United States. Congress. Public Law 111–31, Family Smoking Prevention and Tobacco Control Act (U.S. Government Printing Office, 2009). [Google Scholar]
- 10.Healton, C. Who’s afraid of the truth? Am. J. Public. Health. 91 (4), 554–558 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. Every Try Counts Campaign. https://www.fda.gov/tobacco-products/public-health-education-campaigns/every-try-counts-campaign
- 12.U.S. Center for Health Promotion and Education. The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General (Office on Smoking and Health, 1988). [Google Scholar]
- 13.Christakis, N. A. & Fowler, J. H. The collective dynamics of smoking in a large social network. N Engl. J. Med.358 (21), 2249–2258 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrazola, R. A. et al. US cigarette smoking disparities by race and Ethnicity — Keep going and going! Prev. Chronic Dis.20, 220375 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett, B. E. et al. Socioeconomic differences in cigarette smoking among sociodemographic groups. Prev. Chronic Dis.16, 180553 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014). [Google Scholar]
- 17.National Center for Health Statistics, Health, U. & States 2020–2021. Table SmokSex. Current cigarette smoking among adults aged 18 and over, by sex, race, and age: united States, selected years 1965–2019. Hyattsville, MD. https://www.cdc.gov/nchs/data/hus/2020-2021/smoksex.pdf
- 18.Nguyen-Grozavu, F. T. et al. Widening disparities in cigarette smoking by race/ethnicity across education level in the united States. Prev. Med.139, 106220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsey, M. W. Jr et al. Association between marital status and cigarette smoking: variation by race and ethnicity. Prev. Med.119, 48–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). Ten great public health achievements – United States, 1900–1999. MMWR Morb Mortal. Wkly. Rep.48 (12), 241–243 (1999). [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). Ten great public health achievements – United States, 2001–2010. MMWR Morb Mortal. Wkly. Rep.60 (19), 619–623 (2011). [PubMed] [Google Scholar]
- 22.Congress of the United States. Congressional budget office. Raising the Excise Tax on Cigarettes: Effects on Health and the Federal Budget. Pub. No. 4036. (2012). [Google Scholar]
- 23.Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. State Tobacco Activities Tracking and Evaluation (STATE) System. STATE System Smokefree Indoor Air Fact Sheet. https://www.cdc.gov/statesystem/factsheets/sfia/SmokeFreeIndoorAir.html
- 24.Centers for Disease Control and Prevention (CDC). National Center for Chronic Disease Prevention and Health Promotion. Tips From Former Smokers. https://www.cdc.gov/tobacco/campaign/tips/index.html
- 25.U.S. Food and Drug Administration. The Real Cost Cigarette Prevention Campaign. https://www.fda.gov/tobacco-products/public-health-education-campaigns/real-cost-campaign
- 26.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data1971 (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2020). https://wwwn.cdc.gov/nchs/nhanes/default.aspx [Google Scholar]
- 27.Meza, R. et al. Trends in US adult smoking prevalence, 2011 to 2022. JAMA Health Forum. 4 (12), e234213 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, Y. & Land, K. C. A mixed models approach to the age–period–cohort analysis of repeated cross-section surveys, with an application to data on trends in verbal test scores. Sociol. Methodol.36 (1), 75–97 (2006). [Google Scholar]
- 29.Raudenbush, S. W. et al. HLM 8.2: Hierarchical Linear and Nonlinear Modeling (Scientific Software International, 2021). [Google Scholar]
- 30.Thomas, R. K. Population composition. In Concepts, Methods and Practical Applications in Applied Demography (Springer, 2018). [Google Scholar]
- 31.Inoue-Choi, M. et al. Dose-response association of low-intensity and nondaily smoking with mortality in the united States. JAMA Netw. Open.3 (6), e206436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breton, M. O. et al. Understanding long-term trends in smoking in England, 1972–2019: and age–period–cohort approach. Addiction117 (5), 1392–1403 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Barrington-Trimis, J. L. et al. Trends in the age of cigarette smoking initiation among young adults in the US from 2002 to 2018. JAMA Netw. Open.3 (10), e2019022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bing, R. J. et al. Report of Study Group on Smoking and Health. March 6; Legacy Tobacco Documents Library, UCSF: (1957). https://www.industrydocuments.ucsf.edu/tobacco/docs/#id=fphw0123
- 35.Federal Trade Commission. Report To Congress Pursuant To the Federal Cigarette Labeling and Advertising Act (United States Federal Trade Commission, 1967). [Google Scholar]
- 36.Cummings, K. M. & Proctor, R. N. The changing public image of smoking in the united States: 1964–2014. Cancer Epidemiol. Biomarkers Prev.23 (1), 32–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults — United States, 2004. MMWR Morb Mortal. Wkly. Rep.54 (44), 1121–1124 (2005). [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC). Current cigarette smoking among adults and trends in smoking cessation — United States, 2008. MMWR Morb Mortal. Wkly. Rep.58 (44), 1227–1232 (2009). [PubMed] [Google Scholar]
- 39.Jamal, A. et al. Current cigarette smoking among adults — United States, 2005–2014. MMWR Morb Mortal. Wkly. Rep.64 (44), 1233–1240 (2015). [DOI] [PubMed] [Google Scholar]
- 40., C. D. C. & Centers for Disease Control and Prevention. Cigarette smoking among adults — United States, 1999. MMWR Morb Mortal. Wkly. Rep.50 (40), 869–873 (2001). [PubMed]
- 41.Ruel, E. et al. After the master settlement agreement: trends in the American tobacco retail environment from 1999 to 2002. Health Promot Pract.5 (3 Suppl), 99S–110S (2004). [DOI] [PubMed] [Google Scholar]
- 42.Xu, X. et al. Cigarette price-minimization strategies by U.S. Smokers. Am. J. Prev. Med.44 (5), 472–476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2030. Smoking. Increase the national average tax on cigarettes — TU-21.
- 44.Pierce, J. P. et al. Declines in cigarette smoking among US adolescents and young adults: indications of independence from e-cigarette vaping surge. Tob. Control. 10.1136/tc-2022-057907 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics. Healthy People 2010. October 31; (2019). https://wonder.cdc.gov/DATA2010/
- 46.Increases in. Quitline calls and smoking cessation website visitors during a National tobacco education campaign — March 19–June 10, 2012. MMWR Morb Mortal. Wkly. Rep.61 (34), 667–670 (2012). [PubMed] [Google Scholar]
- 47.Glover-Kudon, R. M. gates EF. The role of quitlines in tobacco cessation: an introduction. Am J Prev Med. ;60(3 Suppl 2):S99–S102. (2021). [DOI] [PubMed]
- 48.Matkin, W., Ordonez-Mena, J. M. & Hartmann-Boyce, J. Telephone counseling for smoking cessation. Cochrane Database Syst. Rev.5 (5), CD002850 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friedman, A. S., Schpero, W. L. & Busch, S. H. Evidence suggests that the ACA’s tobacco surcharges reduced insurance take-up and did not increase smoking cessation. Health Aff (Millwood). 35 (7), 1176–1183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cummings, K. M. & Hyland, A. Impact of nicotine replacement therapy on smoking behavior. Annu. Rev. Public. Health. 26, 583–599 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Cornelius, M. E. et al. Tobacco product use among adults — United States, 2020. MMWR Morb Mortal. Wkly. Rep.71, 397–405 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farrelly, M. C. et al. Association between the real cost media campaign and smoking initiation among youths — United States, 2014–2016. MMWR Morb Mortal. Wkly. Rep.66, 47–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agaku, I. T. et al. A rapid evaluation of the US federal tobacco 21 (T21) law and lessons from statewide T21 policies: findings from population-level surveys. Prev. Chronic Dis.19, 210430 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy-Hoefer, R. et al. Association between the tips from former smokers campaign and smoking cessation among adults, united States, 2012–2018. Prev. Chronic Dis.17, E97 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis, K. et al. The impact of the Tips from former Smokers(®) campaign on reducing cigarette smoking relapse. J. Smok. Cessat.2022, 3435462 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali, F. R. M. et al. E-cigarette unit sales, by product and flavor type — United States, 2014–2020. MMWR Morb Mortal. Wkly. Rep.69, 1313–1318 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartmann-Boyce, J. et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev.11, CD010216 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holford, T. R. et al. Smoking histories by state in the U.S. Am. J. Prev. Med.64 (4 Suppl 1), S42–S52 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sieminska, A. & Jassem, E. The many faces of tobacco use among women. Med. Sci. Monit.20, 153–162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theodoulou, A. et al. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev.6 (6), CD013308 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatziandreu, E. J. et al. The reliability of self-reported cigarette consumption in the united States. Am. J. Public. Health. 79 (8), 1020–1023 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liber, A. C. & Warner, K. E. Has underreporting of cigarette consumption changed over time? Estimates derived from US National health surveillance systems between 1965 and 2015. Am. J. Epidemiol.187 (1), 113–119 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Kramarow, E. A. & Elgaddal, N. Current Electronic Cigarette Use among Adults Aged 18 and Over: United States, 2021. NCHS Data Brief, No 475 (National Center for Health Statistics, 2023). [PubMed] [Google Scholar]
- 64.Lindson, N. et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev.1, CD010216 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keyes, K. M., Rutherford, C. & Smith, G. S. Alcohol-induced death in the USA from 1999 to 2020: a comparison of age–period–cohort methods. Curr. Epidemiol. Rep.9, 161–174 (2022). [Google Scholar]
- 66.O’Brien, R. M. Mixed models, linear dependency, and identification in age–period–cohort models. Stat. Med.36 (16), 2590–2600 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Bell, A. & Jones, K. Should age–period–cohort analyst accept innovation without scrutiny? A response to Reither, masters, Yang, powers, Zheng and land. Soc. Sci. Med.128, 331–333 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Bell, A. & Jones, K. The hierarchical age–period–cohort model: why does it find the results that it finds? Qual. Quant.52, 783–799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo, L. & Hodges, J. S. Constraints in random effects age–period–cohort models. Sociol. Methodol.50 (1), 276–317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reither, E. N. et al. Should age–period–cohort studies return to the methodologies of the 1970s? Soc. Sci. Med.128, 356–365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reither, E. N. et al. Clarifying hierarchical age–period–cohort models: a rejoinder to bell and Jones. Soc. Sci. Med.145, 125–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim, S. C. J. et al. US tobacco 21 is paving the way for a tobacco endgame. Tob. Use Insights. 14, 1179173X211050396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feirman, S. P. et al. Computational models used to assess US tobacco control policies. Nicotine Tob. Res.19 (11), 1257–1267 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed during the current study are available at https://wwwn.cdc.gov/nchs/nhanes/default.aspx.