Abstract
The obligate intracellular bacterium Coxiella burnetii establishes an intracellular replicative niche termed the Coxiella-containing vacuole (CCV), which has been characterised as a bacterially modified phagolysosome. How C. burnetii withstands the acidic and degradative properties of this compartment is not well understood. We demonstrate that the key lysosomal protease cathepsin B is actively and selectively removed from C. burnetii-infected cells through a mechanism involving the Dot/Icm type IV-B secretion system effector CvpB. Overexpression of cathepsin B leads to defects in CCV biogenesis and bacterial replication, indicating that removal of this protein represents a strategy to reduce the hostility of the intracellular niche. In addition, we show that C. burnetii infection of mammalian cells induces the secretion of a wider cohort of lysosomal proteins, including cathepsin B, to the extracellular milieu via a mechanism dependent on retrograde traffic. This study reveals that C. burnetii is actively modulating the hydrolase cohort of its replicative niche to promote intracellular success and demonstrates that infection incites the secretory pathway to maintain lysosomal homoeostasis.
Subject terms: Cellular microbiology, Pathogens, Lysosomes
The zoonotic pathogen Coxiella burnetiiestablishes a unique intracellular niche within a lysosome-derived vacuole. Here Bird et al. undertook proteomic, cell biology and microbiology approaches to characterise this niche and the strategies employed by C. burnetii to maintain a balance between intracellular success and maintaining host cell homoeostasis.
Introduction
The mammalian lysosome is central to cellular homoeostasis, acting as a control centre for nutrient recycling and immune surveillance. Lysosomal degradation is primarily driven by >50 different hydrolases present in the lumen, and dysfunction of these enzymes is associated with a range of devastating disease pathologies including cancer, neurodegeneration and cardiovascular disease1,2. One major class of lysosomal proteases is the cathepsin family, which broadly serves to regulate protein degradation and turnover, thus maintaining cellular homoeostasis3. To regulate proteolysis, cathepsins are first synthesised in an immature zymogen form, containing an autoinhibitory pro-domain at their N- or C-terminus4. In the Golgi apparatus, immature cathepsins are tagged with mannose-6-phosphate, which directs their trafficking to the lysosome. Once inside this compartment, the low pH and activity of other proteases leads to cleavage of the pro-domain resulting in a mature, active cathepsin protein which participates in cellular catabolism. For obligate intracellular pathogens, avoiding lysosomal degradation is critical to a successful infection (reviewed in ref. 5). Following internalisation into a host cell, pathogens can interfere with maturation of the pathogen-containing phagosome or induce endomembrane damage to escape it, avoiding exposure to lysosomal content. The only known bacterial pathogen to successfully replicate inside a lysosome-derived compartment is the Gram-negative Coxiella burnetii. Exactly how C. burnetii withstands such a hostile intracellular environment is not fully understood.
C. burnetii is the causative agent of the global zoonosis Q fever, which can manifest in chronic and debilitating presentations such as endocarditis and chronic fatigue6. The primary disease reservoirs are ruminants such as cattle, sheep, and goats, and once shed to the environment, humans can become infected with C. burnetii through inhalation of aerosols containing even one bacterium7. The low infectious dose, aerosol transmission and high environmental resistance properties have led to C. burnetii being classified as a Category B potential bioterrorism agent by the US Centers for Disease Control and Prevention, and a Select Agent by the Federal Select Agent programme. Concerningly, analysis of C. burnetii seroprevalence in Australia indicates that up to 1 in 20 people have been exposed to this pathogen8.
Once internalised, C. burnetii establishes a spacious and fusogenic compartment termed the Coxiella-containing vacuole (CCV), which is decorated with both lysosomal and autophagosomal proteins9. From within this replicative niche, C. burnetii employs a type IV-B secretion system (T4SS) to translocate bacterial effector proteins across the bacterial and CCV membranes into the host cytosol. Disruption of the T4SS abolishes C. burnetii intracellular, but not axenic, replication, demonstrating the importance of this system for intracellular survival10,11. Early studies on the C. burnetii intracellular lifecycle report that the CCV contains an acidic pH and degradative enzymes such as acid phosphatase, leading to the theory that C. burnetii resides in a bacterially-modified phagolysosome12–15. Increasing lysosomal pH with chloroquine abolished C. burnetii replication, further supporting this notion16. However, the extent to which the CCV diverges from an endogenous phagolysosome is currently unclear, with reports indicating that the CCV pH (~5.2) is higher than native lysosomes (~4.8) and restoring CCV pH to 4.8 is toxic to C. burnetii. Further, induction of lysosome biogenesis was also found to be detrimental to intracellular replication17,18. However, it was also demonstrated that lysosomal hydrolase activity generates essential amino acids sensed by the C. burnetii regulatory two-component system PmrAB, promoting initial activation of the T4SS19. Interestingly, perturbation of the mannose-6-phosphate trafficking pathway, which directs hydrolases to lysosomes, did not affect C. burnetii viability or replication but did impact the morphology of CCVs20.
Given these reports, it is currently accepted that C. burnetii replicates in a proteolytically active lysosome-derived vacuole. However, the suite of lysosomal proteases present and active in the CCV has never been thoroughly examined. Here, we sought to investigate the abundance and activity of host lysosomal proteases of the cathepsin family during C. burnetii infection and to assess whether modulation of these proteases might impact bacterial success. Specifically, we hypothesised that C. burnetii might downregulate specific lysosomal proteases to create an environment that is permissive of bacterial replication. Using orthogonal experimental approaches, we demonstrate that the host lysosomal protease cathepsin B is removed from C. burnetii-infected cells in a T4SS-dependent manner and that overexpression of active cathepsin B is detrimental to C. burnetii. Furthermore, we show that this loss is dependent on a vacuolar environment established by the C. burnetii effector CvpB. Interestingly, we observed secretion of a suite of lysosomal proteins, including cathepsin B, into the extracellular milieu during infection via a mechanism that involves the default secretory pathway. Taken together, these data demonstrate that lysosomal proteolysis and protein trafficking are subverted during C. burnetii infection, significantly advancing our understanding of how this unique pathogen manipulates human lysosomal biology to cause disease.
Results
The lysosomal protease cathepsin B is actively removed from human cells during Coxiella burnetii infection
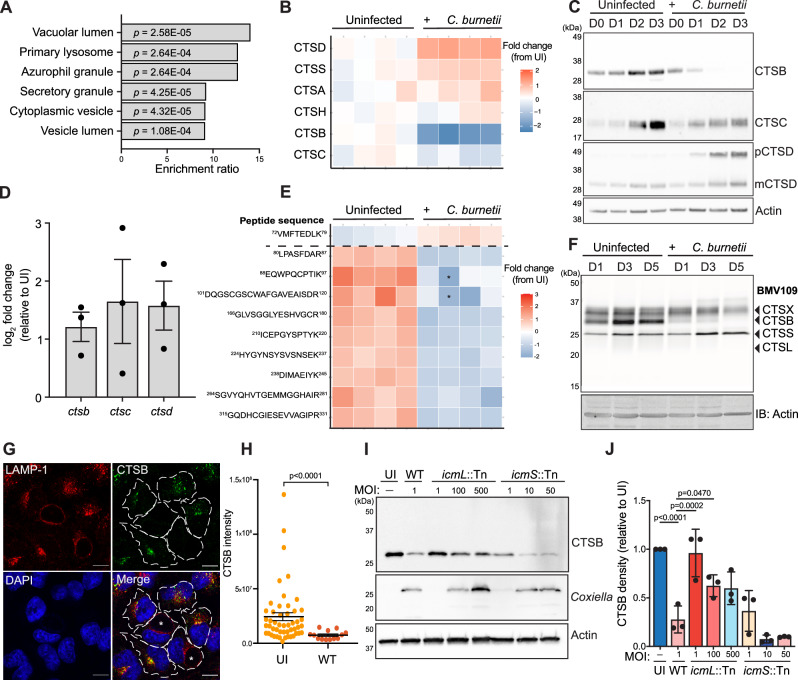
To globally assess host protease abundance during C. burnetii infection, we performed label-free mass spectrometry on lysates from uninfected and C. burnetii-infected THP-1 cells (Supplementary Fig 1A, B and Supplementary Data 1). Consistent with previous research21, we observed infection-dependent enrichment of proteins associated with lysosomes and vacuoles (Fig. 1A and Supplementary Table 1). Supporting this, examination of proteome Z-scores indicated an increase in the abundance of cathepsin proteases during infection, with the exceptions of cathepsin C (CTSC) which showed a small reduction, and cathepsin B (CTSB), which was dramatically decreased (Fig. 1B and Supplementary Fig 1C, D). This finding was supported by immunoblotting with an antibody to the heavy chain subunit of cathepsin B over 3 days of infection, which revealed that cathepsin B was undetectable by day 2 (Fig. 1C). This corresponded with an accumulation of immature (pro-) cathepsin D (pCTSD, Fig. 1C and Supplementary Fig 1E), aligning with reports that cathepsin B is involved in the proteolytic activation of cathepsin D22. Via immunoblotting, we also observed reduced abundance of cathepsin C at 3 days post-infection. However, unlike cathepsin B, this protein was not completely absent (CTSC, Fig. 1C) and the decrease in abundance was not significant as assessed by quantitative proteomics analysis (Supplementary Fig 1A and Supplementary Fig 1D), leading us to focus our investigation on cathepsin B. Analysis of transcript abundance using real-time quantitative PCR (RT-qPCR) confirmed that the changes in cathepsin expression during infection were not due to reduced transcription of these proteins (Fig. 1D). To increase coverage of cathepsin B peptides identified in our mass spectrometry experiments, we performed data-independent acquisition (DIA) mass spectrometry on uninfected and infected cell lysates23. Using this approach, we observed that the peptide 72VMFTEDLK79 from the pro-domain of cathepsin B increased in abundance during infection, in contrast to decreased abundance of peptides within the mature protein (Fig. 1E). In combination with the RT-qPCR data, this suggests that mature cathepsin B protein is being lost downstream of synthesis.
Fig. 1. Altered abundance and activity of lysosomal proteases during C. burnetii infection.
A, B THP-1 cells were uninfected (n = 4) or infected (n = 4) with C. burnetii (MOI 50) for 72 h prior to sample collection for mass spectrometry. A Gene ontology (GO) on significantly enriched proteins. GO analysis was performed using WebGestalt overrepresentation test and the cellular component functional database. P-values calculated using Fisher’s exact test with BH correction for multiple comparisons. B Heat map of LFQ intensities of protein abundance for all cathepsins identified. Each column represents one biological replicate. C Immunoblot showing cathepsin expression over 3 days in uninfected or C. burnetii-infected THP-1 lysates. D qRT-PCR analysis of transcript abundance for cathepsin B, C, and D in uninfected or infected THP-1 cells after 72 h of infection. Raw values were normalised against 18S rRNA transcript abundance and are presented as normalised log2 fold change from UI. Data reflect 3 biological replicates, error bars represent SEM. E Heatmap of cathepsin B peptides observed from DIA proteomic analysis of 72 h THP-1 infected cells. Peptides are ordered from N-terminus (top) to C-terminus (bottom), with horizontal dashed line indicating cleavage site at end of pro-domain. Imputed values denoted with an asterisk. F BMV109 labelling of active cysteine proteases at D1, 3, or 5 post infection. Gel is representative of 3 independent experiments. G Representative image of HeLa cells infected with C. burnetii (MOI 1) for 72 h and stained with antibodies against LAMP-1 and cathepsin B. Infected cells were identified by LAMP-1 staining and are denoted with an asterisk. H Quantification of imaging in G. Data points represent individual cells measured within one experiment (UI, n = 50, WT, n = 13). Error bars represent mean + SEM (I, J) Immunoblot of THP-1 cells infected with wild-type C. burnetii (WT), icmL::Tn or icmS::Tn mutants at the indicated MOI. Blot is representative of three independent experiments (quantified in J). Error bars reflect SD. CTSB cathepsin B, CTSC cathepsin C, pCTSD pro-cathepsin D, mCTSD mature cathepsin D; Scale bar = 10 μm. Source data are provided as a Source Data file. Mass spectrometry source data are available via ProteomeXchange with identifier PXD052888.
Having observed differential modulation of cathepsins during C. burnetii infection, we utilised a pan-reactive activity-based probe to measure cysteine cathepsin activity in live cells. BMV109 covalently binds to the active site of cathepsin X, B, S, and L, and emits fluorescence upon interaction with the respective target proteases, allowing visualisation of protease activity by in-gel fluorescence24,25. Live cell labelling of THP-1 cells with BMV109 confirmed that cathepsin B activity is lost during infection, with other cysteine cathepsins showing increased or unchanged activity (Fig. 1F). This was supported with immunoblots using antibodies against cathepsin L, X and S, confirming that these proteases show increased or unchanged expression during infection (Supplementary Fig 1F). At the single-cell level, immunofluorescence microscopy (IF) confirmed that cathepsin B was removed from wild-type-infected cells but not neighbouring uninfected cells (Fig. 1G, H).
C. burnetii infection is known to alter the endosomal compartment through occupation and modulation of lysosomes18,26. To determine whether the decreased cathepsin B levels were due to an altered endosomal compartment, we generated THP-1 cells stably overexpressing a fluorescently-tagged transcription factor EB (TFEB-GFP). As expected, TFEB overexpression led to increased expression of cathepsin B in uninfected cells (Supplementary Fig 1G, H), consistent with an increase in lysosomal biogenesis. However, infection of these cells with C. burnetii again led to cathepsin B removal, suggesting that decreased levels of proteolytically active cathepsin B during infection are not a consequence of the altered endosomal compartment.
We next aimed to elucidate whether cathepsin B removal was the result of C. burnetii T4SS activity. To achieve this, we used strains carrying transposon insertions in the icmL or icmS genes. The icmL::Tn mutant harbours a non-functional T4SS and is subsequently unable to translocate bacterial effector proteins to the host cytosol, leading to a complete abolishment of intracellular replication11. Similarly, the C. burnetii chaperone pair IcmSW has been shown to promote the translocation of a specific cohort of T4SS effectors, but disruption of IcmS also causes a partial replication defect27. We attempted to measure cathepsin B signal in cells infected with icmL::Tn or icmS::Tn via IF, but had limited success due to low bacterial loads and difficulty identifying infected cells (Supplementary Fig 2A, B). We compensated for this by infecting at multiplicities of infection (MOI) up to 500X greater than wild-type and examining cathepsin B abundance at the population level with immunoblotting. Infection with icmL::Tn at any MOI tested led to greater retention of cathepsin B than wild-type infection (Fig. 1I, J), indicating that T4SS activity is required for cathepsin B removal. Moreover, cathepsin B was lost during icmS::Tn infection at increasing MOI, signifying that translocation of the responsible effector(s) is IcmS-independent. Taken together, these data indicate that cathepsin B is removed during C. burnetii infection as a result of T4SS effector activity.
Overexpression of cathepsin B is detrimental to C. burnetii replication and CCV biogenesis
We hypothesised that C. burnetii may be actively removing cathepsin B to promote formation of a replicative niche that is less hostile than a classical host lysosome. To directly investigate the impact of cathepsin B expression on C. burnetii, we generated HeLa cell lines in which GFP-tagged wild-type cathepsin B (CTSB-GFP) or a catalytically inactive mutant (CTSBC108A-GFP) were overexpressed (Fig. 2A). Labelling of these cell lines with BMV109 confirmed that cathepsin B overexpression led to increased cathepsin B activity in CTSB-GFP cells, but not CTSBC108A-GFP cells (Fig. 2B). Immunoblotting of the same lysates demonstrated equivalence in cathepsin B expression in both overexpressing cell lines (Fig. 2B). Furthermore, infection of CTSB-overexpressing cell lines with C. burnetii led to a reduction, though not a complete absence, in cathepsin B (Fig. 2C). Interestingly, we observed that GFP was cleaved from the fusion protein in an infection-dependent manner (Fig. 2C, middle panel), however the relative abundance of the CTSB-GFP fusion protein (75 kDa) was unchanged during infection compared to uninfected cells.
Fig. 2. Overexpression of cathepsin B is detrimental to C. burnetii replication and CCV biogenesis.
A Schematic depiction of cathepsin B-GFP domains and processing events. Catalytic cysteine is indicated above with black arrowhead. SP signal peptide, Pro pro-domain (B) HeLa cells or HeLa cells expressing GFP only, cathepsin B-GFP (CTSB-GFP) or catalytically inactive cathepsin B-GFP (CTSBC108A-GFP) were labelled with BMV109 for 3 h prior to analysis of in-gel fluorescence (top) or immunoblotting for total cathepsin B (middle). Ponceau was used as a loading control (bottom). Pro pro-domain, SC single chain, HC heavy chain (C–F) HeLa, GFP, CTSB-GFP, or CTSBC108A-GFP cells were uninfected or infected with C. burnetii at a MOI of 5. After 72 h, cells were harvested for immunoblotting (C), quantitative PCR (D), or fixed for immunofluorescence microscopy (E, F). D To quantify bacterial intracellular replication, cells were harvested immediately following infection (D0), or at days 1, 3, 5, and 7 post-infection, genomic DNA was extracted, and C. burnetii genome equivalents quantified via qPCR with C. burnetii specific primers. Error bars represent standard deviation of six independent experiments that are represented by individual symbols. P values calculated by two-way ANOVA with Dunnett’s correction for multiple comparisons. E Representative images of those used for quantification in F. F Quantification of CCV area as determined by ImageJ analysis of confocal images. Top panel, data points reflect average CCV area for each replicate, n = 3. For each replicate, >100 CCVs were measured and quantified. Statistical significance was determined using one-way ANOVA and comparing each group to CTSB-GFP. Error bars represent SD. Bottom panel, representation of spread of CCV areas from one replicate. Black line denotes mean. Scale bar = 10 μm. Source data are provided as a Source Data file.
Quantification of C. burnetii intracellular replication over 5 days of infection revealed that replication was lower in CTSB-GFP cells than cells expressing GFP alone at day 3 (mean difference 122.9 ± 28.96, p = 0.006) and day 5 (457.1 ± 123.5, p = 0.03) post infection (Fig. 2D). Mutation of the cathepsin B active site (CTSBC108A-GFP) resulted in an intermediate phenotype but did not completely rescue the growth defect.
We also examined morphology of the CCVs in each cell line. IF revealed that CCVs were smaller in CTSB-GFP cells than HeLa parent cells or cells expressing GFP alone (mean difference 52.8 ± 10.87, p = 0.006 or 45.8 ± 10.87, p = 0.001, respectively, Fig. 2E, F). Cells expressing catalytically inactive cathepsin B (CTSBC108A-GFP) displayed an intermediate phenotype with respect to CCV area. Collectively, these data demonstrate that overexpression of cathepsin B is detrimental to C. burnetii replication and CCV biogenesis, indicating that active removal of this protein is an important element of C. burnetii intracellular success.
Cathepsin B is retained during infection with C. burnetii lacking the T4SS effector CvpB
In light of our findings that cathepsin B loss is dependent on a functional Dot/Icm T4SS, we aimed to determine which C. burnetii effector protein(s) might be responsible for loss of cathepsin B during infection. To achieve this, we infected HeLa cells with a library of C. burnetii mutants with transposons or deletions in 38 known T4SS effector-encoding genes (Supplementary Data 2). Given that this library is incomplete, we also ectopically expressed individual effector proteins (fused to an N-terminal 3xFLAG tag) in HeLa cells and used IF or western blotting (WB) to visualise cathepsin B (Supplementary Data 2). These approaches in combination allowed us to assess the impact of 134 unique C. burnetii proteins on cathepsin B abundance. Interestingly, we observed partial retention of cathepsin B during infection with a strain lacking the effector CvpB (also referred to as Cig2, encoded by gene CBU_0021) (Fig. 3A, B). This phenotype was able to be complemented, with cathepsin B lost after CvpB reintroduction (cvpB::Tn (pFLAG-cvpB)) (Fig. 3A, B). CvpB (Coxiella vacuolar protein B) is a highly conserved C. burnetii effector that localises to the CCV membrane during infection28,29 and has been partially characterised as having a key role in CCV biogenesis9,28,30 via modulation of phosphoinositide metabolism29. Furthermore, as with cathepsin B loss, translocation of this effector protein through the T4SS is independent of IcmS27. Disruption of CvpB leads to a unique multi-vacuole phenotype whereby small CCVs, which are still capable of bacterial replication, are unable to undergo homotypic fusion9. Analysis of cvpB::Tn-infected cells with IF revealed that cathepsin B puncta were observable in a portion of cells (Fig. 3C, white arrowheads, Fig. 3D). Quantitation of cathepsin B signal intensity revealed that infection with cvpB::Tn did not lead to a significant reduction in cathepsin B compared to uninfected cells, contrary to the phenotype observed during infection with wild-type or the complement (Fig. 3D). Proteomic analysis further confirmed increased abundance of cathepsin B during infection with cvpB::Tn compared to wild type (Fig. 3E and Supplementary Data 1). Taken together, these orthogonal experimental approaches indicate that cathepsin B is retained in cells infected with C. burnetii lacking the T4SS effector CvpB.
Fig. 3. Loss of cathepsin B during C. burnetii infection requires the T4SS effector CvpB.
A Immunoblot of THP-1 cells uninfected (UI) or infected with C. burnetii wild-type (WT), cvpB::Tn or cvpB::Tn(pFLAG-cvpB) at MOI 25 for 72 h. Blot is representative of 3 independent experiments, quantified in B. Error bars denote SD. C Representative image of HeLa cells infected with the above strains and stained with antibodies against cathepsin B and LAMP-1. Scale bar = 10 μm. Dashed lines denote cell periphery of uninfected cells, solid lines, infected cells. CCVs indicated with an asterisk. D Quantification of CTSB signal intensity in C. Individual data points are from two independent experiments, coloured in black or grey, respectively. WT n = 25, cvpB::Tn n = 32, cvpB::Tn(pFLAG-cvpB) n = 27. Statistical significance determined using Kruskal–Wallis test. Data are presented as mean (column height), error bars represent SD. E Cathepsin B label-free quantification (LFQ) values from THP-1 cells infected with WT, cvpB::Tn, or cvpB::Tn (pFLAG-cvpB) (n = 3, MOI 50) for 72 h. Statistical significance was calculated using one-way ANOVA with Tukey’s correction. Data are presented as mean (column height), error bars reflect SD. F BMV109 labelling (top) or immunoblot (bottom) on THP-1 cells infected with WT, cvpB::Tn or cvpB::Tn (pFLAG-cvpB) for 72 h (MOI 25). G Schematic depicting experimental design of data presented in H. H Analysis of bacterial intracellular replication in THP-1 cells infected with the indicated strains in the presence of DMSO or 10 μM Ca074Me. Data are presented as fold change relative to D0, with individual data points representing the mean of three biological replicates. Error bars denote SD. Statistical significance was examined using a paired t-test of each condition ± Ca074Me. I–K HeLa cells were transfected with pcDNA-3xFLAG-cvpB or pcDNA-3xFLAG for 18 h before immunofluorescence microscopy using antibodies against CTSB or FLAG. Scale bar = 10 μm. J Quantification of co-localisation in I. Area quantified is indicated in I by white line. K CTSB intensity in HeLa cells expressing 3xFLAG or 3xFLAG-CvpB. Data reflect the mean of three independent experiments (3xFLAG n = 70, 3xFLAG-CvpB n = 108). Statistical significance was examined using an unpaired t-test, error bars reflect SD. Source data are provided as a Source Data file. Mass spectrometry source data are available via ProteomeXchange with identifier PXD052954.
Independent groups have reported that C. burnetii lacking CvpB can still replicate to equivalent levels as wild-type bacteria in epithelial cells9,29. However, this is not the case in macrophages, where disruption of CvpB has a replication defect by 6 days post infection28,31. To comprehensively assess intracellular replication of the cvpB::Tn mutant in our laboratory, we conducted growth curves in both HeLa and THP-1 cells over a time course of infection, using different starting MOIs. In both cell types, cvpB::Tn showed delayed intracellular replication compared to wild-type (Supplementary Fig 3A, B). In THP-1 cells, equivalent levels were observed once replication reached a plateau. To assess the effect of bacterial load on cathepsin B, we therefore infected THP-1 cells with cvpB::Tn at MOIs of 25, 250 or 325. We observed a decrease in the amount of retained cathepsin B with increasing bacterial load, though it was not restored to wild-type levels (Supplementary Fig 3C, D), similar to what we observed for icmL::Tn (Fig. 1I).
To determine whether CvpB impacted the activity of other cathepsins, we utilised the BMV109 activity-based probe in the context of cvpB::Tn infection. No other cysteine proteases appeared to show altered activity when cvpB was disrupted (Fig. 3F), suggesting this phenotype is specific to cathepsin B. We also wanted to assess whether inhibition of cathepsin B activity would have any impact on C. burnetii replication. In this experiment, we chose to use a MOI of 0.1 so that we would observe a replication defect, which could theoretically be rescued by cathepsin B inhibition. Cells were treated with the specific inhibitor Ca074Me and intracellular replication was assessed over a time course. We observed that Ca074Me treatment did not alter replication dynamics of either wild-type or cvpB::Tn C. burnetii (Fig. 3G, H), indicating that the replication defect in C. burnetii cvpB::Tn is not a result of retained cathepsin B activity.
We next wanted to assess whether expression of CvpB alone was capable of modulating cathepsin B levels. When ectopically expressed, we observed partial co-localisation of 3xFLAG-CvpB with cathepsin B (Fig. 3I, J). However, there was no significant difference in overall cathepsin B abundance in the presence of 3xFLAG-CvpB compared to 3xFLAG alone (Fig. 3K). Co-immunoprecipitation experiments did not identify a direct physical interaction between CvpB and cathepsin B (Supplementary Fig 3E). Together, these data demonstrate that CvpB is necessary but not sufficient for cathepsin B removal during infection.
Though not biochemically characterised, CvpB has been shown to inhibit the phosphoinositide kinase PIKfyve, leading to enrichment of PI(3)P on the CCV membrane and contributing to the fusogenicity of the CCV29. To elucidate whether PIKfyve inhibition contributes to cathepsin B dynamics during infection, we utilised the PIKfyve inhibitor vacuolin-1. We hypothesised that if PIKfyve inhibition was responsible for cathepsin B loss, cvpB::Tn-infected cells would show reduced cathepsin B upon vacuolin-1 treatment. Consistent with the literature, vacuolin-1 induced lipidation of LC3-I to LC3-II in uninfected cells (32,33, Supplementary Fig 3F). However, we observed no change to cathepsin B abundance in cvpB::Tn-infected cells treated with vacuolin-1 (Supplementary Fig 3F, G). This indicates that the involvement of CvpB in cathepsin B loss is independent of PIKfyve inhibition.
To exclude the role of the proteasome in CvpB-mediated cathepsin B depletion, we treated cells with the proteasome inhibitor MG132. After a 6 h treatment window, we were able to observe proteasome inhibition, evidenced by an accumulation of ubiquitin in treated cells (Supplementary Fig 3H). However, proteasome inhibition did not have any impact on the abundance of cathepsin B (Supplementary Fig 3H, I), demonstrating that the proteasome is not responsible for cathepsin B loss during infection.
To date, the structure of CvpB has not been solved and low-confidence AlphaFold models limit the prediction of putative domains or binding regions. To identify the region(s) of CvpB that are required for cathepsin B loss, we generated a series of C. burnetii cvpB::Tn strains complemented with different N-terminal truncations, which all retain the C-terminal secretion signal required for translocation through the T4SS (Supplementary Fig 4A). None of these strains exhibited cathepsin B loss, suggesting that the N-terminus of CvpB is required for cathepsin B removal (Supplementary Fig 4B, C). However, we also observed that none of these truncations were able to complement the multi-vacuole phenotype (Supplementary Fig 4D, E), confirming that the first 306 amino acids of CvpB are required for CCV fusogenicity as previously reported29. Taken together, these data give rise to the hypothesis that cathepsin B retention in cells infected with both cvpB::Tn or strains complemented with CvpB lacking its N-terminus is a result of different CCV environments compared to wild-type infection, and not a direct interaction of CvpB with cathepsin B.
C. burnetii infection promotes extracellular secretion of lysosomal proteins, including cathepsin B
We next aimed to determine the cellular pathway behind cathepsin B loss during infection and queried whether the absence of intracellular cathepsin B was correlated with increased secretion of the protein into the extracellular space. Hypersecretion of lysosomal proteases is known to occur in several disease states, including cancer and inflammatory diseases34. Immunoblotting on conditioned media collected from infected cells revealed increased extracellular abundance of the pro-forms of cathepsins B and D (Fig. 4A, B). Equivalent amounts of actin were present in the media collected from uninfected and infected cells, confirming that differences in extracellular cathepsin abundance were not a consequence of differential cell lysis. To globally assess whether increased secretion was specific to cathepsins, we performed label-free proteomics on conditioned media collected from C. burnetii-infected THP-1 (Fig. 4C, D, F) or HeLa (Fig. 4E, F, Supplementary Data 1) cells. Cathepsin B was not identified to be statistically significantly increased at the protein level in these experiments due to variability in abundance of peptides in the pro or mature domains. However, a single peptide was identified from the pro-domain of cathepsin B, and this peptide was significantly increased in abundance during infection (Fig. 4C). In both cell types, gene ontology analysis revealed enrichment of proteins associated with lysosomes and vacuoles (Fig. 4F and Supplementary Table 1). We identified 6 proteins that displayed a statistically significant increase in extracellular abundance during infection of both THP-1 and HeLa cells (Fig. 4H, I), 5 of which are annotated to have lysosomal subcellular localisation in UniProt. These 6 proteins included hydrolases (CTSD, GLB1, PLBD2, ASAH1) and membrane proteins/receptors (LAMP2, ANXA5). Overall, these findings indicate that C. burnetii infection leads to a non-specific increase in the secretion of lysosomal content, which includes immature lysosomal hydrolases such as cathepsin B.
Fig. 4. C. burnetii infection leads to the secretion of lysosomal content.
A Immunoblot on whole cell lysates (lysate) or conditioned media (media) from uninfected or C. burnetii-infected HeLa cells. Cells were infected for 48 h (MOI 100) before being changed to serum-free media for a further 16 h. Blot is representative of 3 independent experiments, quantified in B Error bars denote SD. C–I Conditioned media from uninfected (n = 4) or C. burnetii-infected (n = 4) THP-1 cells (C, D, G) or HeLa cells (E, F) were subject to label-free quantitative mass spectrometry. C Heatmap of MaxLFQ expression of peptides from cathepsin B, ordered from N-terminus (top) to C-terminus (bottom). Horizontal dashed line depicts end of pro-domain. Columns represent individual biological replicates. D, E Scatter plot of proteins enriched in the supernatant of C. burnetii-infected cells, where X-axis represents fold change (infected/uninfected) and Y-axis shows statistical significance as determined by a student’s t test. Proteins identified as located in the lysosome, lysosome lumen, or lysosome membrane (GO terms GO:0005764, GO:0043202, or GO:0005765, respectively) are coloured; all other proteins identified are depicted in grey. Horizontal dashed line denotes significance cut off (p < 0.05), vertical dashed line represents fold change > 1. Lysosomal proteins within these bounds are labelled with gene names. F, G GO analysis on significantly enriched proteins in D, E performed using WebGestalt overrepresentation test (Fisher’s exact test with BH correction for multiple comparisons) and the cellular component functional database. H Venn diagram depicting overlap of significantly enriched proteins in the supernatant from THP-1 and HeLa cells. I Proteins identified as significantly enriched in the supernatant of infected THP-1 and HeLa cells. Significance was determined using a student’s t test −log(p value) > 1.3 and log2 fold change > 1. Localisation is presented as designated in UniProt. Source data are provided as a Source Data file. Proteomics data have been deposited in ProteomeXchange with identifiers PXD052888 (THP-1) and PXD052890 (HeLa).
Secretion of cathepsin B is independent of CvpB and involves the default secretory pathway
Given that CvpB modulates cathepsin B dynamics during infection, we assessed whether CvpB was also mediating the secretion of lysosomal proteins. PIKfyve inhibition has been shown to induce protein secretion via secretory autophagy or exosome release35, so we examined the supernatant of cells infected with cvpB::Tn or cvpB::Tn (pFLAG-cvpB). Surprisingly, we saw no differences in the amount of cathepsin B in the extracellular media from wild-type and cvpB::Tn-infected cells (Fig. 5A, B). We deepened this analysis by performing proteomic analysis on supernatants and confirmed that the extracellular abundance of lysosomal proteins was not significantly altered when CvpB was disrupted, compared to WT or cvpB::Tn (pFLAG-cvpB) (Fig. 5C). This implies that while CvpB is involved in the intracellular loss of cathepsin B, this effector protein is not responsible for the global secretion of lysosomal content during infection.
Fig. 5. Secretion of lysosomal content is independent of CvpB and involves the default secretory pathway.
A, B Immunoblot on THP-1 lysates or media following infection with WT or cvpB::Tn. Blot is representative of 3 independent experiments, quantified in B. Statistical significance was determined by one-way ANOVA with Tukey’s correction. Error bars denote SD. C Scatter plot of proteins enriched in the supernatant of cvpB::Tn (n = 4) or cvpB::Tn(pFLAG-cvpB) (n = 3) as determined by proteomics analysis. X-axis represents fold change (relative to WT), Y-axis shows statistical significance as determined by Student’s t test. Proteins located in the lysosome, lysosome lumen, or lysosome membrane (GO terms GO:0005764, GO:0043202, or GO:0005765, respectively) are coloured; all other proteins identified are depicted in grey. Horizontal dashed line denotes significance (p < 0.05), vertical dashed line represents fold change >1. Lysosomal proteins within these bounds are labelled. D Immunoblot on THP-1 lysates or media following infection with WT or icmL::Tn at the indicated MOI. Blot is representative of 3 independent experiments, quantified in E. Mean is represented by column height, error bars represent SD. Statistical significance was determined using one-way ANOVA with Tukey’s correction. F qPCR determination of C. burnetii genome equivalents after 48 h of infection followed by 6 h treatment with DMSO (n = 4) or 1 μM brefeldin A (BFA) (n = 4). Statistical significance was examined using an unpaired t-test. Error bars denote SD. G, H THP-1 cells were treated with BFA as in F for 6 h before conditioned media and lysate fractions were collected for immunoblotting. Data reflect three biological replicates, error bars denote SD. I Schematic of experimental design for data presented in J. J THP-1 cells were uninfected (mock, n = 3) or infected with WT (n = 3) for 72 h before the secretomes were collected and applied to naïve cells for 24 h. After this time, treated cells were infected and harvested for qPCR analysis at D0, 1, and 3 post infection. Data points represent the mean of three biological replicates. Statistical significance was examined using an unpaired t-test at D3 post-infection. Error bars denote standard deviation. All source data are provided as a Source Data file. Mass spectrometry data have been deposited in ProteomeXchange with identifier PXD052954.
We then assessed whether lysosomal protein secretion was dependent on the C. burnetii Dot/Icm T4SS. We observed equivalent cathepsin B secretion during infection with WT and icmL::Tn C. burnetii (Fig. 5D, E), which increased with MOI. Interestingly, this was completely uncoupled from intracellular cathepsin B abundance, which was consistently absent in WT infected cells but retained in icmL::Tn infected cells. This supports a model in which the intracellular cathepsin B loss is a distinct process from the global lysosomal protein secretion observed during infection. Furthermore, these data indicate that lysosomal protein secretion is likely a host response to infection, rather than an active pathogenesis mechanism employed by C. burnetii.
To uncover the cellular mechanism behind this secretory phenotype, we assessed whether enrichment of lysosomal proteins in the supernatant of infected cells was reflective of lysosomal exocytosis. This process involves recruitment of lysosomes to the cell surface and fusion with the plasma membrane, leading to release of lysosomal content into the extracellular space36. To investigate whether this occurs during C. burnetii infection, we immunostained live, unpermeabilised cells with an antibody to the lumenal epitope of LAMP-137. During lysosomal exocytosis, this epitope is exposed on the cell surface and can be detected by confocal microscopy. As a positive control, cells were treated with the TRPML1 agonist ML-SA1, which resulted in a distinct LAMP-1 staining pattern at the cell periphery (Supplementary Fig 5A, top panel). This staining was not observed in untreated cells (Supplementary Fig 5A, middle panel) or in C. burnetii-infected cells (Supplementary Fig 5A, bottom panel). This suggests that the mechanism of lysosomal protein secretion during infection is distinct from lysosomal exocytosis.
It is now well established that disruption of the M6P trafficking pathway leads to hypersecretion of nascent lysosomal hydrolases38–41. Previous work found that C. burnetii could replicate in cells lacking GlcNAc1-phosphotransferase (GNPTAB−/−), which is responsible for adding an M6P to proteins destined for the lysosome20, suggesting this pathway is not required for intracellular replication. However, it remains unknown whether the M6P trafficking machinery is functional during C. burnetii infection. We saw no difference in the abundance or subcellular distribution of the cation-independent-M6P receptor (CI-M6PR) as assessed by confocal microscopy (Supplementary Fig 5B). This, combined with the observation that other lysosomal proteins such as cathepsin D are still delivered to CCVs9,14, suggest that M6P trafficking is functional during C. burnetii infection, and that secretion of lysosomal proteins is unlikely due to defects in the M6P machinery.
We then investigated whether we could pharmacologically inhibit the secretion of cathepsin B with brefeldin A (BFA), a classical inhibitor of the default secretory pathway from the Golgi apparatus to the plasma membrane. BFA inhibits vesicle formation between the ER and Golgi by inhibiting the small GTPase Arf1 and preventing COP-I coat recruitment42,43. Uninfected or C. burnetii-infected THP-1 cells were treated with 1 µM BFA for 6 h before both the media and cell fraction were collected for immunoblotting. BFA treatment had no significant effect on C. burnetii intracellular load as assessed by qPCR for determination of genome equivalents (Fig. 5F). As expected, BFA treatment led to accumulation of pro-cathepsin B in cell lysates, reflecting the zymogen form, which has not yet encountered a lysosomal environment for maturation. Furthermore, there was no change to the loss of cathepsin B in infected cells with the addition of BFA. Strikingly, BFA treatment completely abolished the secretion of pro-cathepsin B into the media. Taken together, this suggests that proteins are being secreted to the extracellular medium from the Golgi through the default secretory pathway.
Lastly, we aimed to determine whether the secretion of lysosomal content was important for C. burnetii infection. To assess this, we treated naïve cells with the secretome collected from either uninfected cells (mock) or C. burnetii-infected cells (Fig. 5I) and then assessed the intracellular replication ability of C. burnetii. We saw no measurable difference in replication (Fig. 5J), indicating that the secretion of lysosomal content does not change susceptibility to infection in cell culture conditions.
Collectively, we propose that two distinct processes are being subverted during C. burnetii infection. Firstly, a cohort of lysosomal proteins is secreted into the extracellular medium directly from the Golgi via the default secretory pathway. This secretion is not specific to cathepsin B but rather is observed for several lysosomal proteins, which show unchanged intracellular abundance, including cathepsin D. This is likely a host response to infection rather than an active pathogenesis mechanism employed by C. burnetii. Secondly, the pool of mature cathepsin B, which is retained inside cells, is removed by C. burnetii as a result of the vacuolar environment established by the Dot/Icm effector CvpB (Fig. 6).
Fig. 6. C. burnetii subverts the host lysosome and trafficking pathways to facilitate intracellular success.
Proposed model for the response of mammalian cells to infection with WT or cvpB::Tn C. burnetii. Infection with both strains leads to secretion of a cohort of lysosomal proteins to the extracellular space through a mechanism that can be inhibited with BFA. Infection with WT, but not cvpB::Tn leads to removal of cathepsin B (CTSB) from the Coxiella-containing vacuole (CCV). Figure created in BioRender. Bird, L. (2025) https://BioRender.com/c7kaafo.
Discussion
While most intracellular pathogens have developed strategies to evade lysosomal degradation, Coxiella burnetii, the causative agent of Q fever, actively requires this environment for replication and virulence. Despite the established dogma that the C. burnetii replicative niche is proteolytically active, few studies have investigated the abundance or activity of lysosomal hydrolases present in the CCV14,44. We report that C. burnetii-infected cells exhibit loss of the lysosomal protease cathepsin B, a major workhorse of proteolysis in the lysosome (Fig. 1). Cathepsin B is a cysteine protease with broad substrate specificity, which is primarily involved in routine turnover of proteins in the lysosome. We hypothesise that C. burnetii removes cathepsin B to create a lysosomal environment that is less degradative and therefore more permissive of bacterial replication. Consistent with this, we observed that overexpression of cathepsin B had negative impacts on C. burnetii replication and CCV biogenesis (Fig. 2). During C. burnetii infection, the growing CCV is continuously damaged and repaired in an ESCRT-dependent manner45. Lysosome membrane permeabilization and release of cathepsin B into the cytosol can trigger activation of the NLRP3 inflammasome and subsequent pyroptotic cell death46. Removal of cathepsin B would therefore present an additional advantage in avoiding inflammatory cell death and clearance of infection.
We identified that cathepsin B is retained in cells infected with C. burnetii lacking the Dot/Icm effector CvpB, although still reduced compared to uninfected cells (Fig. 3). Although CvpB has been demonstrated to promote CCV biogenesis through inhibition of the phosphoinositide kinase PIKfyve29, we determined that the role of CvpB in cathepsin B loss was not due to PIKfyve inhibition (Supplementary Fig 3F). CvpB is a 93 kDa multi-domain protein which remains incompletely characterised. It is conceivable that the relationship between CvpB and cathepsin B is therefore due to a novel function of this effector. Expression of CvpB alone was not sufficient to alter cathepsin B levels, and pulldown experiments did not reveal a physical interaction between the two proteins, suggesting an indirect interaction. It is interesting to note that cvpB::Tn-infected cells show a distinct multi-vacuole phenotype as a result of PI(3)P depletion29,30. This prevents fusion of CCVs with autophagosomes and other CCVs. The extent to which the pH and degradative properties of cvpB::Tn CCVs differ from wild-type is not fully established, although initial reports demonstrate that they retain the ability to hydrolyse DQ-BSA and stain positive for cathepsin D9. We therefore hypothesise that retention of cathepsin B during cvpB::Tn infection is a result of an altered CCV environment. CCV biogenesis, facilitated by CvpB, creates an environment within which cathepsin B is lost, likely due to either instability and/or other Coxiella factors having the opportunity to target this lysosomal protein for degradation. A multi-vacuole phenotype has also been observed in the absence of autophagy-related proteins (e.g. ATG5, STX17)29,30,47. However, it has never been demonstrated that the CCVs formed in the absence of autophagy have the same properties as CCVs formed in the absence of CvpB. Future studies should investigate this question and test whether retention of cathepsin B is directly linked to defects in homotypic fusion. Importantly, CvpB mutants display attenuated virulence in both Galleria mellonella and SCID mice29,30,48. Whether this is linked to cathepsin B retention is worthy of further consideration.
Our finding that cathepsin B is absent from cells infected with C. burnetii has important implications for the C. burnetii research field. Initial reports that the CCV is a proteolytically active compartment utilised the cathepsin B Magic Red substrate as a proxy for general proteolytic activity in C. burnetii infected cells44. However, using the covalent chemical probe BMV109, we demonstrate a consistent loss of cathepsin B activity during C. burnetii infection, while the activity of other cathepsins remains relatively constant. As there is considerable overlap in cathepsin cleavage specificity, combined with promiscuous cleavage activities of cathepsins and other proteases, substrate-based probes such as Magic Red often fail to discern the nuances of individual proteases. Our results, therefore, suggest that more cautious interpretations of Magic Red assays in the context of C. burnetii infections are warranted.
Why C. burnetii infection promotes the loss of cathepsin B over other cathepsins remains unclear. We did not observe a decrease in other proteases of the cathepsin family except for a slight reduction, but not complete absence, in cathepsin C expression at 3 days post infection (Fig. 1C). It is important to note that a comprehensive profile of cathepsin B substrates remains elusive, however proteomic methods to identify carboxypeptidase substrates continue to improve and will likely provide an opportunity to elucidate the specific cathepsin B substrates that impact CCV biogenesis. This may aid in elucidating why cathepsin B is actively and selectively removed from C. burnetii-infected cells in contrast to other cathepsins. Interestingly, Qi et al. showed that genetic deletion of cathepsin B, but not cathepsin G or other non-lysosomal proteases, conferred enhanced intracellular resistance to the cytosolic bacterium Francisella novicida49. The authors demonstrated that cathepsin B deletion promotes lysosomal biogenesis and autophagy by governing the activities of transcription factor EB (TFEB) and the autophagy kinase ULK1. While C. burnetii infection is known to promote engagement of the autophagic pathway9,50, there is conflicting literature on whether TFEB is activated21,51 or inhibited52 during infection in a T4SS-dependent manner. It is likely that the true nature of TFEB signalling during infection is dynamic and influenced by multiple factors (as reviewed in ref. 5). Nonetheless, it is plausible that the reported increase in lysosomal biogenesis and autophagy observed during C. burnetii infection could be a downstream effect of the cathepsin B loss we observe in our study.
Our second major observation is that C. burnetii infection causes a suite of lysosomal proteins to be secreted from the Golgi apparatus to the extracellular space, which can be inhibited by Brefeldin A (BFA) and is independent of CvpB activity (Figs. 4 and 5). Although we observed enrichment of lysosomal proteins in the media, we did not detect LAMP-1 on the cell surface during infection, suggesting this was not reflective of lysosomal exocytosis. It was recently shown that β-coronaviruses incite lysosomal exocytosis for egress, and the authors determined that this pathway is insensitive to BFA53. In contrast, our findings show that lysosomal protein secretion could be inhibited by BFA, suggesting a distinct cellular pathway is engaged during C. burnetii infection.
We found that secretion of lysosomal proteins occurred even during infection with a C. burnetii Dot/Icm mutant (Fig. 5D, E) and was exacerbated with increasing bacterial load. We therefore propose that the secretion may be a compensatory mechanism by the host cell to maintain homoeostasis following infection. The occupation and modulation of host lysosomes by C. burnetii is known to globally alter the degradative capacity of the cell18,26, and reports have demonstrated that pathways such as secretory autophagy and exosome release can be incited as an alternative waste disposal pathway to maintain homoeostasis when lysosomal degradation is impaired54–56. Elucidating the mechanics of this pathway further will be important for a deeper understanding of the molecular mechanisms subverted during C. burnetii infection.
Redirection of lysosomal hydrolases is not unique to C. burnetii infection. Helicobacter pylori was recently shown to induce secretion of the lysosomal protease legumain to promote cleavage of extracellular matrix proteins and subsequent tumour invasion in a gastric cancer model57. Salmonella enterica Typhimurium encodes a T3SS effector, SifA, which reduces the hydrolytic capacity of lysosomes by interfering with retrograde trafficking of M6PR, causing secretion of hydrolases to the extracellular space58. Further research demonstrated that in addition to being secreted, cathepsins are trafficked to the nucleus during S. Typhimurium infection, where they contribute to non-canonical inflammasome activation and cell death59. While we demonstrate that treatment of naïve cells with the secretome from C. burnetii infected cells had no impact on intracellular replication of C. burnetii under the in vitro conditions tested (Fig. 5J), it should be investigated whether inflammatory pathways or the transcriptomic landscape of neighbouring cells is affected. Within cell culture conditions, these secreted proteases are in their zymogen form but in the context of infected tissue and inflammatory conditions, this may not be the case and thus it remains plausible that secretion of lysosomal enzymes influences the host response to infection.
Overall, this study has established that lysosomal proteolysis and trafficking are actively modulated during C. burnetii infection, through both removal of mature cathepsin B from infected cells and secretion of a cohort of lysosomal hydrolases to extracellular space. Taken together, these findings contribute significantly to our understanding of how this unique intracellular bacterium subverts the host lysosome to cause disease.
Methods
Bacterial strains, plasmids and oligonucleotides
C. burnetii Nine Mile Phase II RSA439 were grown axenically in ACCM-2 liquid cultures at 37 °C with 5% CO2, and 2.5% O2 for 6–7 days60. For growth of mutants, media were supplemented with 350 μg/mL kanamycin or 3 μg/mL chloramphenicol as required. Strains used in this study are detailed in Supplementary Data 3. Escherichia coli pir2 or Stbl3 strains were cultivated in Luria-Bertani (LB) broth or LB agar plates supplemented with kanamycin (50 μg/mL), ampicillin (100 μg/mL), or chloramphenicol (12.5 μg/mL) as required. Plasmids used in this study are also detailed in Supplementary Data 3.
Custom oligonucleotides were ordered through Sigma Aldrich or Integrated DNA Technologies (IDT). A table of oligonucleotides used in this study can be found in Supplementary Data 3.
Cell culture
HeLa CCL2 cells (ATCC) were routinely passaged in Dulbecco’s Modified Eagle Media + GlutaMAX (DMEM) (Gibco, Thermo Fisher) supplemented with 10% heat-inactivated foetal calf serum (FCS). THP-1 human monocytic cells were maintained in Roswell Park Memorial Institute (RPMI) media with 10% FCS and differentiated with phorbol 12-myristate 13-acetate (PMA) at a concentration of 10−8 M for 72 h prior to infection. All cell lines were maintained at 37 °C with 5% CO2.
Generation of stable cell lines
HEK293T cells were transfected with lentiviral packaging plasmids alongside a lentiviral expression vector containing the protein of interest (see Supplementary Data 3). After 24 h, viral supernatant was collected and filtered through a 0.45 μm filter. Polybrene was added to a final concentration of 8 μg/ml before viral titres were added to HeLa cells in a 6-well plate and centrifuged for 1 h at 1583 × g at 32 °C. After 48 h, cells were incubated in media containing 5 μg/ml puromycin to select for successfully transduced cells. Expression of the desired protein was confirmed by WB.
C. burnetii infection of cultured cell lines
HeLa or differentiated THP-1 cells were infected with C. burnetii as described previously61. Briefly, C. burnetii genome equivalents from stationary phase cultures were quantified by RT-qPCR using primers specific for ompA. C. burnetii was added to cells at an MOI of 100, 50, 5 or 1, depending on the cell type and experimental design. Infected cells were centrifuged at 500 × g for 30 min or incubated at 37 °C with 5% CO2 for 4 h before washing once with phosphate-buffered saline (PBS) and once with respective cell culture media (DMEM or RPMI) supplemented with 5% FCS.
Antibodies and reagents
The following antibodies were used for WB or IF: anti-cathepsin B monoclonal (Cell Signalling Technology #31718, WB 1:2000, IF: 1:200), anti-cathepsin B polyclonal (R&D Systems, #AF965, WB 1:1000), anti-cathepsin C (Santa Cruz Biotechnology #sc-74590, WB 1:2000), anti-cathepsin D (Abcam #ab72915, WB 1:2000), anti-DotB (Edward Shaw Laboratory, WB 1:2000), anti-β-actin (Sigma Aldrich #A1978, WB 1:8000), anti-3xFLAG (Sigma, #F3165, IF 1:250) anti-LAMP-1 (Developmental Studies Hybridoma Bank #H4A3, IF 1:250), anti-Coxiella burnetii (Craig Roy Laboratory, IF 1:10,000), anti-LC3B (Novus, #NB100-2220, WB 1:2000), anti-CI-M6PR (Novus #NB300-514, IF 1:100), anti-Ubiquitin (Cell Signalling Technology #3936S, WB 1:2000).
Other reagents used in this study include brefeldin A (Invitrogen #00-4506-51), ML-SA1 (Sigma Aldrich #SML0627), Ca074Me (Sigma Aldrich #C5732)62, MG-132 (Sigma Aldrich #474790), and vacuolin-1 (Sigma Aldrich #673000). BMV109 was synthesised as described in refs. 24,25.
Western blotting
For analysis of protein expression in whole cell lysates, mammalian cells were infected with C. burnetii as above. At 3 dpi, cell lysates were harvested in 2X Laemmli sample buffer for SDS-PAGE. For examination of proteins in cell supernatants, media from cells were collected after 48 h of infection and centrifuged at 21,000 × g for 5 min to pellet cell debris. Supernatant was collected and centrifuged through Amicon® Ultra Centrifugal Filters with 10 kDa molecular weight cut-off (Merck). The concentrated supernatant was combined with 2X Laemmli sample buffer for SDS-PAGE. All samples were boiled at 95 °C prior to SDS-PAGE, then electrophoresed at 165 V for 45 min on an Any kDTM Mini-PROTEAN® TGX Stain-FreeTM protein gel (Bio-Rad), before being transferred to PVDF membranes and blocked for 1 h with Tris-buffered saline plus 0.1% Tween-20 (TBST) containing 5% skim milk. Primary and secondary antibodies (see above) were diluted in TBST containing 5% bovine-serum albumin (BSA). Chemiluminescence was detected using the Bio-Rad Clarity Western WCL kit using an Amersham 680 Imager (GE Healthcare) or Bio-Rad ChemiDoc MP Imager. Where indicated, BMV109 (1 μM) was added to cells 4 h prior to harvesting, and cathepsin activity was visualised by in-gel fluorescence by scanning with a Cy5 filter on a Typhoon 5 (GE Healthcare). All densitometry was performed using ImageLab software (BioRad). Intensity values were expressed as a percentage of the uninfected control for each experiment and then normalised against the respective expression of actin. Uncropped western blots are available in Source data.
qRT-PCR
Differentiated THP-1 cells were infected for 3 days with C. burnetii (see above). RNA extraction was performed using a phenol-chloroform method as previously described63. Briefly, cells were lysed in TriSURETM (Bioline) and incubated with chloroform to achieve phase separation. The RNA-containing layer was isolated and precipitated with isopropanol before being washed and quantified using a Nanodrop 2000 Spectrophotometer (ThermoFisher). Any contaminating genomic DNA was removed via rDNase I treatment, followed by addition of DNase inhibitor (Ambion, USA). RNA was reverse transcribed to cDNA using the iScriptTM cDNA synthesis kit (BioRad). RT-qPCR was performed using SYBR Green reaction mix (BioRad) and oligonucleotides listed in Supplementary Data 3. Real-time PCR was performed using a QuantStudioTM 7 Flex Real-Time PCR system (ThermoFisher). Transcript abundance for the RNA of interest was normalised against 18S rRNA abundance.
Immunofluorescence microscopy
HeLa cells were infected for 3 days with C. burnetii on 12 mm glass coverslips in 24-well plates. At 3 dpi, cells were fixed with 4% (w/v) paraformaldehyde for 15 min at room temperature. Fixed cells were permeabilised with 0.1% Triton X-100 in PBS at room temperature for 20 min before blocking in PBS containing 1% (w/v) bovine serum albumin and 0.01% Tween-20 for 1 h (blocking buffer). Stable cell lines expressing GFP were photo-bleached prior to immunostaining to quench endogenous GFP signal. Immunostaining was performed with antibodies diluted in blocking buffer. Prior to mounting onto glass slides, coverslips were incubated with PBS containing 4′, 6′-Diamidino-Z-phenylindole (DAPI, Life Technologies) at a dilution of 1:10,000. Images were acquired using a Zeiss LSM780 confocal microscope using a 63X oil objective lens (Zeiss, Germany). Image processing was performed in ImageJ.
Quantification of CTSB signal intensity
Quantification of CTSB in immunofluorescence images of transfected and infected cells was performed with CellProfiler64. Briefly, cells were manually outlined using FLAG (for transfected cells) or CTSB (for infected cells) staining, and background signal was subtracted using the mean intensity of a cell-free area. Nuclei were automatically identified from DAPI staining and masked, and CTSB puncta were identified using Otsu’s thresholding method. The integrated density of CTSB puncta was measured and combined per cell. Additionally, for infection experiments, CCVs were manually identified using DAPI staining and used to classify cells as either infected or uninfected.
LAMP-1 cell surface staining
To detect LAMP-1 on the plasma membrane, 3-day infected HeLa cells were washed thrice with warm PBS before being treated with 50 μM ML-SA1 in DMEM + 5% FCS for 5 min. After treatment, cells were transferred to a metal plate on ice to prevent re-endocytosis of LAMP-1. All subsequent procedures were performed at 4 °C. Live cells were stained with an antibody to the lumenal epitope of LAMP-1 (DSHB, 1:250) diluted in ice-cold PBS for 45 min on ice. Following this, cells were washed three times and then fixed with 4% PFA. Secondary antibody, DAPI staining, and image acquisition were performed as above.
Label-free quantitative mass spectrometry
Sample preparation
HeLa or THP-1 cells were seeded in 10 cm dishes at a density of 1.1 × 106 or 1.1 × 107 cells per dish, respectively. Cells were infected with C. burnetii as above, with n = 4 biological replicates for each condition. After 48 h of infection, culture media were removed and replaced with fresh media containing no FCS (serum-free), and cells were incubated in this medium for 18 h. Conditioned media were collected and centrifuged at 3000 × g for 10 min to pellet any cell debris. The supernatant was then concentrated through Amicon® Ultra Centrifugal Filters with a 10 kDa molecular weight cut off (Merck). The concentrated supernatant was combined with a lysis buffer containing 10% sodium dodecyl sulphate (SDS), 100 mM Tris-HCl pH 8). Samples were boiled at 95 °C on a thermomixer with shaking at 2000 rpm to shear DNA before the protein content was quantified using a BCA assay (Pierce). One hundred micrograms protein was used for mass spectrometry sample preparation. Briefly, samples were reduced with dithiothreitol (DTT) to a final concentration of 10 mM at 95 °C for 10 min, then alkylated with 40 mM iodoacetamide for 30 min at room temperature in the dark. The reaction was quenched by the addition of another 10 mM DTT, then samples were acidified with 12% phosphoric acid. Samples were combined with a binding/wash buffer containing 100 mM Tris pH 8, 90% methanol and loaded onto S-trap mini columns (ProtiFi, USA). Columns were washed 4x with binding/wash buffer and then on-column digestion was performed using 1 μg trypsin per 33 μg protein diluted in 50 mM Tris-HCl pH 8. Digestion was performed overnight at 37 °C in a humified container. The following day, samples were eluted via sequential centrifugation using 50 mM Tris-HCl pH, followed by 0.2% formic acid, then 50% acetonitrile. Each spin was 4000 × g and the flow-through from each was collected. Eluate was vacuum dried before being resuspended in mass spectrometry running buffer (0.1% trifluoreoacetic acid, 2% acetonitrile) and subjected to clean up on C18 stage tips65,66.
Reverse phase liquid chromatography–mass spectrometry
C18 enriched proteome samples were re-suspended in mass spectrometry running buffer and separated using a two-column chromatography setup composed of a PepMap100 C18 20-mm by 75-mm trap (Thermo Fisher Scientific) and a PepMap C18 500-mm by 75-mm analytical column (Thermo Fisher Scientific) using a Dionex Ultimate 3000 UPLC (Thermo Fisher Scientific). Samples were concentrated onto the trap column at 5 μl/min for 6 min with Buffer A (0.1% formic acid, 2% DMSO) and then infused into an Orbitrap Lumos™, Orbitrap EclipseTM or Q-Exactive (Thermo Fisher Scientific) at 300 nl/min via the analytical columns. Peptides were separated by altering the buffer composition from 3% Buffer B (0.1% formic acid, 77.9% acetonitrile, 2% DMSO) to 23% B over 89 min, then from 23% B to 40% B over 10 min and then from 40% B to 80% B over 5 min. The composition was held at 80% B for 5 min before being returned to 3% B for 10 min. The mass spectrometer was operated in a data-dependent mode automatically switching between the acquisition of a single Orbitrap MS scan (300–2000 m/z, maximal injection time of 50 ms, an Automated Gain Control (AGC) set to a maximum of 400k and a resolution of 60k) and 3 s of Orbitrap MS/MS HCD scans of precursors (NCE 35%, a maximal injection time of 80 ms, a AGC of 125k and a resolution of 30k).
Data independent acquisition-mass spectrometry
Purified peptide samples were re-suspended in Buffer A* and separated using a trap and elute setup with a Pepmap nano-trap column (C18, 100 Å, 75 µm × 2 cm) and 5.5 cm high throughput uPAC Neo analytical column (Thermo Scientific) on a Vanquish Neo UHPLC (Thermo Scientific) coupled to an Orbitrap Astral mass spectrometer (Thermo Scientific). Peptides were separated using a gradient composed of 0.1% v/v formic acid (solvent A) and 80% acetonitrile with 0.1% v/v formic acid (solvent B). A flow rate of 750 nl/min was used with a gradient of (i) 0–0.3 min 3–6% B, (ii) 0.3–23 min, 6–23.5% B (iii) 23–26.7 min, 23.5–40% B (iv) 26.7–28.7 min 40–50% B and (v) 28.7–28.8 min, 50–99% B. Prior to sample loading, the analytical column was washed at 2 μl/min (combine flow and pressure control) for 14 column volumes, then the trap column was washed (combine flow and pressure control) with 2 zebra wash cycles at 80% acetonitrile. For DIA experiments, MS1 scans covering the range 380–980 m/z were acquired in the orbitrap at a resolution of 120,000 at m/z 200 between with an AGC target was 500% with a maximum IT of 5 ms in profile mode. MS2 scans were carried out in the Astral analyser with isolation window of 2 m/z, normalised HCD collision energy of 27, normalised AGC target of 500% and maximum injection time of 3 ms.
Data analysis
Raw data files were searched in FragPipe (v.18.0)67,68 against Homo sapiens (UniProt accession: UP000005640, downloaded 2023, 82518 entries) and Coxiella burnetii RSA439 NMII (UniProt accession: UP000002671, downloaded 2023, 1812 entries) reference proteomes. FragPipe parameters were set to default unless otherwise specified. Label-free quantification (LFQ) was undertaken allowing for the following modifications: cysteine carbamidomethylation as a fixed modification (+57.0215 Da) and methionine oxidation (+15.9949 Da) and N-terminal acetylation (+42.0106 Da) as variable modifications. Enzyme cleavage specificity was set to ‘stricttrypsin’, cleavage at K or R, allowing a maximum of 2 missed cleavages. Protein and peptide FDR were calculated using Philosopher with default settings. DIA datasets were searched using DIA library-free analysis within DIA-NN (version 1.8.1)69. Data files were searched against the Homo sapiens and Coxiella burnetii RSA439 NMII proteomes (see above for UniProt identifiers). Fixed and variable modifications, and protease specificity were defined as outlined above. For all datasets, the resulting data were further analysed using Perseus (v.1.6.0.7)70. A log2 transformation was applied and data were filtered to contain valid values in a minimum of three out of four biological replicates in at least one group (i.e. uninfected/infected). Imputation was performed to replace missing values based off a downshifted normal distribution (σ-width = 0.3, σ-downshift = −2.3). Statistical comparison between groups was performed using a student’s t test with a multiple hypothesis correction undertaken using a permutation-based FDR. Downstream data visualisation was performed using the ggplot2 package in R (v.4.2.1).
Statistical analysis
Calculation of statistically significant differences between groups was performed in GraphPad Prism 9 (GraphPad Inc., USA). Where required, a one-way or two-way analysis of variance (ANOVA) with Tukey’s post-hoc test was performed with p < 0.05 as a significance threshold, assuming equal standard variance between groups.
For mass spectrometry data, log2 fold change in protein abundance between groups was calculated using a two-sided t-test. To adjust for multiple hypothesis testing, a Benjamini-Hochberg correction was performed using a false discovery rate (FDR) of 5%. Enrichment analysis was undertaken using WebGestalt71,72 on significantly enriched proteins (−log(p value) > 1.3 and log2 fold change >1) using the gene ontology cellular component functional database and the H. sapiens protein-coding genome as background.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description Of Additional Supplementary File
Source data
Acknowledgements
We wish to thank the Biological Optical Microscopy Platform (BOMP) and the Mass Spectrometry and Proteomics Facility (MSPF) at the University of Melbourne for access to and maintenance of key resources. L.E.B., A.R.Z., and B.X. are supported by RTP scholarships from the Australian Government. L.E.E.M. was funded by a grant from the Australian National Health and Medical Research Council (GNT2011119). Research in H.J.N.’s laboratory was funded by the Australian National Health and Medical Research Council (GNT2010841).
Author contributions
L.E.B.: Conceptualisation, experimental investigation, data analysis and visualisation, writing—first draft, review and editing. B.X., A.D.H., A.R.Z., P.N., and D.R.T.: Experimental investigation. N.E.S. Experimental investigation—mass spectrometry and data analysis. L.E.E.M., and H.J.N.: Conceptualisation, experimental investigation, review and editing, supervision, funding acquisition.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE73 partner repository with the dataset identifiers PXD052888, PXD052890 and PXD052954. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Laura E. Edgington-Mitchell, Hayley J. Newton.
Contributor Information
Laura E. Edgington-Mitchell, Email: laura.edgingtonmitchell@unimelb.edu.au
Hayley J. Newton, Email: hayley.newton@monash.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-59283-3.
References
- 1.Cocchiaro, P. et al. The multifaceted role of the lysosomal protease cathepsins in kidney disease. Front. Cell Dev. Biol.5, 114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang, X. & Robbins, J. Proteasomal and lysosomal protein degradation and heart disease. J. Mol. Cell. Cardiol.71, 16–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller, S., Dennemärker, J. & Reinheckel, T. Specific functions of lysosomal proteases in endocytic and autophagic pathways. Biochim. Et. Biophys. Acta Proteins Proteom.1824, 34–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turk, V. et al. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Et. Biophys. Acta Proteins Proteom.1824, 68–88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird, L. E., Edgington-Mitchell, L. E. & Newton, H. J. Eat, prey, love: pathogen-mediated subversion of lysosomal biology. Curr. Opin. Immun.83, 102344 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Eldin, C. et al. From Q Fever to Coxiella burnetii Infection: a Paradigm Change. Clin. Microbiol. Rev.30, 115–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tissot-Dupont, H., Amadei, M. A., Nezri, M. & Raoult, D. Wind in November, Q fever in December. Emerg. Infect. Dis.10, 1264–1269 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gidding, H. F. et al. Q fever seroprevalence in Australia suggests one in twenty people have been exposed. Epidemiol. Infect.148, e18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton, H. J. et al. A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog.10, e1004286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beare, P. A. et al. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. mBio2, e00175–00111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey, K. L., Newton, H. J., Lührmann, A. & Roy, C. R. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog.7, e1002056 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akporiaye, E. T., Rowatt, J. D., Aragon, A. A. & Baca, O. G. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect. Immun.40, 1155–1162 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton, P. R., Stueckemann, J., Welsh, R. M. & Paretsky, D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect. Immun.21, 556–566 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinzen, R. A., Scidmore, M. A., Rockey, D. D. & Hackstadt, T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun.64, 796–809 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurin, M., Benoliel, A. M., Bongrand, P. & Raoult, D. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun.60, 5013–5016 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackstadt, T. & Williams, J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. USA78, 3240–3244 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulye, M., Samanta, D., Winfree, S., Heinzen, R. A. & Gilk, S. D. Elevated cholesterol in the Coxiella burnetii intracellular niche is bacteriolytic. mBio810.1128/mBio.02313-16 (2017). [DOI] [PMC free article] [PubMed]
- 18.Samanta, D., Clemente, T. M., Schuler, B. E. & Gilk, S. D. Coxiella burnetii Type 4B Secretion System-dependent manipulation of endolysosomal maturation is required for bacterial growth. PLoS Pathog.15, e1007855 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newton, P. et al. Lysosomal degradation products induce Coxiella burnetii virulence. Proc. Natl. Acad. Sci. USA117, 6801–6810 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, H. E., Hoyt, F. H. & Heinzen, R. A. Replication of Coxiella burnetii in a lysosome-like vacuole does not require lysosomal hydrolases. Infect. Immun.8710.1128/iai.00493-19 (2019). [DOI] [PMC free article] [PubMed]
- 21.Padmanabhan, B. et al. Biogenesis of the spacious Coxiella-containing vacuole depends on host transcription factors TFEB and TFE3. Infect. Immun.88, e00534–00519 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurent-Matha, V., Derocq, D., Prébois, C., Katunuma, N. & Liaudet-Coopman, E. Processing of human cathepsin D is independent of its catalytic function and auto-activation: involvement of cathepsins L and B. J. Biochem.139, 363–371 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doerr, A. DIA mass spectrometry. Nat. Methods12, 35–35 (2015). [Google Scholar]
- 24.Edgington-Mitchell, L. E., Bogyo, M. & Verdoes, M. in Activity-Based Proteomics: Methods and Protocols (eds Overkleeft, H. S. & Florea, B. I.) 145–159 (Springer New York, 2017).
- 25.Verdoes, M. et al. Improved quenched fluorescent probe for imaging of cysteine cathepsin activity. J. Am. Chem. Soc.135, 14726–14730 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson, C. L. & Heinzen, R. A. High-content imaging reveals expansion of the endosomal compartment during coxiella burnetii parasitophorous vacuole maturation. Front. Cell Infect. Microbiol.7, 48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson, C. L., Beare, P. A. & Heinzen, R. A. Dependency of Coxiella burnetii type 4B secretion on the chaperone IcmS. J. Bacteriol.20110.1128/jb.00431-19 (2019). [DOI] [PMC free article] [PubMed]
- 28.Larson, C. L. et al. Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication. Infect. Immun.83, 661–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez, E. et al. Coxiella burnetii effector CvpB modulates phosphoinositide metabolism for optimal vacuole development. Proc. Natl. Acad. Sci. USA113, E3260–E3269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler, L. J. et al. Effector protein Cig2 decreases host tolerance of infection by directing constitutive fusion of autophagosomes with the Coxiella-containing vacuole. mBio7, 10.1128/mbio.01127-16 (2016). [DOI] [PMC free article] [PubMed]
- 31.Steiner, S. & Roy, C. R. CRISPR-Cas9-based approaches for genetic analysis and epistatic interaction studies in Coxiella burnetii. mSphere9, e0052324 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, Y. et al. Vacuolin-1 potently and reversibly inhibits autophagosome-lysosome fusion by activating RAB5A. Autophagy10, 1895–1905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano, O. et al. Vacuolin-1 inhibits autophagy by impairing lysosomal maturation via PIKfyve inhibition. FEBS Lett.590, 1576–1585 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Yan, D., Wang, H. W., Bowman, R. L. & Joyce, J. A. STAT3 and STAT6 signaling pathways synergize to promote cathepsin secretion from macrophages via IRE1α activation. Cell Rep.16, 2914–2927 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hessvik, N. P. et al. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell Mol. Life Sci.73, 4717–4737 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buratta, S. et al. Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic and endo-lysosomal systems go extracellular. Int. J. Mol. Sci.21, 2576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews, N. W. in Lysosomes: Methods and Protocols (eds Öllinger, K. & Appelqvist, H.) 205–211 (Springer New York, 2017).
- 38.Marschner, K., Kollmann, K., Schweizer, M., Braulke, T. & Pohl, S. A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science333, 87–90 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Pechincha, C. et al. Lysosomal enzyme trafficking factor LYSET enables nutritional usage of extracellular proteins. Science378, eabn5637 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Richards, C. M. et al. The human disease gene LYSET is essential for lysosomal enzyme transport and viral infection. Science378, eabn5648 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, W. et al. GCAF(TMEM251) regulates lysosome biogenesis by activating the mannose-6-phosphate pathway. Nat. Comm.13, 5351 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donaldson, J. G., Finazzi, D. & Klausner, R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature360, 350–352 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Helms, J. B. & Rothman, J. E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature360, 352–354 (1992). [DOI] [PubMed] [Google Scholar]
- 44.Howe, D., Shannon, J. G., Winfree, S., Dorward, D. W. & Heinzen, R. A. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect. Immun.78, 3465–3474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radulovic, M. et al. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J.3710.15252/embj.201899753 (2018). [DOI] [PMC free article] [PubMed]
- 46.Jin, C. & Flavell, R. A. Molecular mechanism of NLRP3 inflammasome activation. J. Clin. Immunol.30, 628–631 (2010). [DOI] [PubMed] [Google Scholar]
- 47.McDonough, J. A. et al. Host pathways important for Coxiella burnetii infection revealed by genome-wide RNA interference screening. mBio4, e00606–e00612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Schaik, E. J., Case, E. D., Martinez, E., Bonazzi, M. & Samuel, J. E. The SCID mouse model for identifying virulence determinants in Coxiella burnetii. Front. Cell Infect. Microbiol.7, 25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi, X. et al. Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection. J. Exp. Med.213, 2081–2097 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romano, P. S., Gutierrez, M. G., Berón, W., Rabinovitch, M. & Colombo, M. I. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol.9, 891–909 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Larson, C. L., Sandoz, K. M., Cockrell, D. C. & Heinzen, R. A. Noncanonical inhibition of mTORC1 by Coxiella burnetii promotes replication within a phagolysosome-like vacuole. mBio10, 10.1128/mbio.02816-18 (2019). [DOI] [PMC free article] [PubMed]
- 52.Killips, B., Heaton, E. J. B., Augusto, L., Omsland, A. & Gilk, S. D. Coxiella burnetii inhibits nuclear translocation of TFEB, the master transcription factor for lysosomal biogenesis. J. Bacteriol.206, e0015024 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh, S. et al. β-coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell183, 1520–1535.e1514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eguchi, T. et al. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc. Natl. Acad. Sci. USA115, E9115–E9124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shelke, G. V., Williamson, C. D., Jarnik, M. & Bonifacino, J. S. Inhibition of endolysosome fusion increases exosome secretion. J. Cell. Biol.222. 10.1083/jcb.202209084 (2023) [DOI] [PMC free article] [PubMed]
- 56.Solvik, T. A. et al. Secretory autophagy maintains proteostasis upon lysosome inhibition. J. Cell Biol.221. 10.1083/jcb.202110151 (2022). [DOI] [PMC free article] [PubMed]
- 57.Kovalyova, Y. et al. An infection-induced oxidation site regulates legumain processing and tumor growth. Nat. Chem. Biol.18, 698–705 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGourty, K. et al. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science338, 963–967 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selkrig, J. et al. Spatiotemporal proteomics uncovers cathepsin-dependent macrophage cell death during Salmonella infection. Nat. Microbiol.5, 1119–1133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Omsland, A. et al. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl. Environ. Microbiol.77, 3720–3725 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau, N., Thomas, D. R., Lee, Y. W., Knodler, L. A. & Newton, H. J. Perturbation of ATG16L1 function impairs the biogenesis of Salmonella and Coxiella replication vacuoles. Mol. Microbiol.117, 235–251 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edgington-Mitchell, L. E. et al. Cysteine cathepsin activity suppresses osteoclastogenesis of myeloid-derived suppressor cells in breast cancer. Oncotarget6, 27008–27022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chomczynski, P. & Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem.162, 156–159 (1987). [DOI] [PubMed] [Google Scholar]
- 64.Carpenter, A. E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol.7, R100 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rappsilber, J., Ishihama, Y. & Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem.75, 663–670 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc.2, 1896–1906 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Kong, A. T., Leprevost, F. V., Avtonomov, D. M., Mellacheruvu, D. & Nesvizhskii, A. I. MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry–based proteomics. Nat. Methods14, 513–520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu, F., Haynes, S. E. & Nesvizhskii, A. I. IonQuant enables accurate and sensitive label-free quantification with FDR-controlled match-between-runs. Mol. Cell. Proteom.2010.1016/j.mcpro.2021.100077 (2021). [DOI] [PMC free article] [PubMed]
- 69.Demichev, V., Messner, C. B., Vernardis, S. I., Lilley, K. S. & Ralser, M. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods17, 41–44 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods13, 731–740 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Elizarraras, J. M. et al. WebGestalt 2024: faster gene set analysis and new support for metabolomics and multi-omics. Nucleic Acids Res.10.1093/nar/gkae456 (2024). [DOI] [PMC free article] [PubMed]
- 72.Zhang, B., Kirov, S. & Snoddy, J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res.33, W741–W748 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res.50, D543–D552 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description Of Additional Supplementary File
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE73 partner repository with the dataset identifiers PXD052888, PXD052890 and PXD052954. Source data are provided with this paper.