Abstract
Antifungal susceptibility testing may be an important aid in the treatment of patients with life-threatening yeast infections. In order to establish the suitability of different susceptibility test methods for fluconazole with yeasts, the Rosco tablet and the E-test were compared with the gold standard NCCLS broth macrodilution method for 106 yeast strains. These included 102 clinical isolates of Candida spp., including Candida glabrata (n = 30), Candida albicans (n = 20), Candida tropicalis (n = 13), Candida parapsilosis (n = 10), Candida krusei (n = 8), plus Cryptococcus neoformans (n = 3), Saccharomyces cerevisiae (n = 2), and 16 strains belonging to other Candida spp. Four American Type Culture Collection strains of Candida were included as quality controls. The NCCLS method was found to be too complex and labor-intensive for routine testing. The E-test is an accurate alternative, but experience in determining MICs and careful attention to procedural details are critically important. The Rosco tablet showed the best agreement with the NCCLS reference method, especially when newly established breakpoints of R ≤ 10 mm and S ≥ 21 mm were used.
During the last decade, the higher incidence of fungal infections in hospitalized patients has resulted in the use of systemic antifungal agents, especially fluconazole, which remains a first-line antifungal agent. Since fluconazole-resistant species have gained importance (14, 22, 23), and Candida albicans strains with decreased susceptibility to fluconazole have been described (14), it is important to use techniques which generate accurate and reproducible antifungal susceptibility test results.
Routine antifungal susceptibility testing is still not a recommended procedure (1), since most Candida species have a predictable susceptibility pattern and the reference test method, NCCLS broth macrodilution (9), is labor-intensive and therefore not readily applicable in routine laboratories with a high daily workload.
In the present study, the gold standard NCCLS broth macrodilution method (9) was compared with the Neo-Sensitabs method (Rosco, Taastrup, Denmark) (18) and the E-test (AB-Biodisk, Solna, Sweden) (12) for the determination of fluconazole susceptibility with different yeast species.
MATERIALS AND METHODS
Organisms.
A total of 102 clinical yeast isolates were included: 11 strains from the H. Hartziekenhuis Roeselare, 18 strains from the Academisch Ziekenhuis Brussel, 24 strains from the Algemeen Ziekenhuis St. Jan Brugge, and 49 strains from the Ghent University Hospital, Ghent, Belgium. The following species were studied: Candida glabrata (n = 30), C. albicans (n = 20), Candida tropicalis (n = 13), Candida parapsilosis (n = 10), Candida krusei (n = 8), Candida kefyr (n = 4), Candida guilliermondii (n = 4), Candida lusitaniae (n = 3), Candida norvegensis (n = 1), Candida lipolytica (n = 1), Candida humicola (n = 1), Candida pseudotropicalis (n = 1), Candida parakrusei (n = 1), Cryptococcus neoformans (n = 3), and Saccharomyces cerevisiae (n = 2). Two quality control strains (C. parapsilosis ATCC 22019 and C. krusei ATCC 6258) and two reference strains (C. albicans ATCC 90028 and C. parapsilosis ATCC 90018) were also included. All 106 isolates were identified to species level by internal transcribed spacer 2-PCR (20).
Inoculum preparation.
Prior to testing, each isolate was grown on Sabouraud agar (Becton Dickinson, BBL, Heidelberg, Germany) for 24 h at 35°C. Suspensions were prepared in 0.85% saline to achieve a 0.5 McFarland standard by spectrophotometric measurement.
NCCLS broth macrodilution.
Broth macrodilution testing was performed according to the NCCLS M27-A guidelines (10). Fluconazole (Pfizer, New York, N.Y.) was obtained as reagent-grade powder. Tubes containing twofold fluconazole dilutions, ranging from 0.25 to 256 mg/liter, in RPMI 1640 medium (Life Technologies, Gibco-BRL) and buffered with 0.164 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma, St. Louis, Mo.), were prepared in a single batch and stored frozen at −20°C until used. The macrobroth dilutions were incubated at 35°C and measured spectrophotometrically after 48 h (72 h for C. neoformans) to verify the presence or absence of growth. The turbidity was measured by diluting 0.2 ml of drug-free control growth with 0.8 ml of RPMI medium to produce an 80% inhibition standard (3).
Disk diffusion.
Two milliliters of the 0.5 McFarland standard suspension was poured onto modified Shadomy agar (containing yeast nitrogen base, glucose, and asparagine) (Eδ-O Laboratories, Burnhouse, Scotland). A 15-μg fluconazole-containing tablet (Neo-Sensitabs; Rosco) was placed on the surface. The plates were incubated at 35°C, and zone diameters were read after 18 to 24 h (42 to 48 h for C. neoformans). The zones were measured to the diameter at which colonies of normal size occurred. The small and medium-sized colonies were considered nonresistant mutants, according to the manufacturer's guidelines.
E-test diffusion.
E-tests were carried out on RPMI-agar plates, prepared by adding sterile liquid RPMI 1640 (4.6%) to Bacto agar (1.5%) (Difco Laboratories, Detroit, Mich.) plates, which were stored at 4°C for a maximum of 1 week. The 0.5 McFarland inoculum was swabbed in three directions on the entire RPMI-agar plate, and the E-test strip (AB Biodisk, Solna, Sweden) was applied. The plates were incubated at 35°C, and MICs were read after 24 h (48 h for C. neoformans). The MIC was read at the intersection (at the point of approximately 80% growth inhibition) of the zone edge and the E-test strip. Illustrations for the interpretation of the results, as provided by the manufacturer, were consulted.
Breakpoints.
NCCLS breakpoints were used (9). Isolates were classified as susceptible if the MIC for the isolate was ≤8 mg/liter, susceptible-dose dependent if the MIC was ≥16 to ≤32 mg/liter, and resistant if the MIC was ≥64 mg/liter.
The Rosco criteria have recently changed, and only the more stringent diameters (R ≤ 16 mm and S ≥ 30 mm), formerly applicable only for systemic infections, are now advised for use.
Quality control.
Quality control was performed by testing four American Type Culture Collection (ATCC) Candida strains: C. parapsilosis ATCC 22019 and C. krusei ATCC 6258, used as quality control strains (11), were examined six times with all test methods; the two reference strains, C. albicans ATCC 90028 and C. parapsilosis ATCC 90018, were examined four or five times.
Interpretation of results.
Statistical analysis was done by linear regression, correlating NCCLS MICs and inhibition zone diameters, respectively, with E-test MICs. Results were also analyzed in terms of clinical categorization. The percentage of very major errors was calculated as the number of susceptible strains (according to the evaluated method) that were indicated to be resistant by the reference method, divided by the number of resistant strains according to the reference method. Analogously, the percentage of major errors was calculated as the number of resistant strains (according to the evaluated method) that were indicated to be susceptible by the reference method, divided by the number of susceptible strains according to the reference method. The percentage of minor errors was calculated as the number of susceptible strains according to the evaluated method (or the number of resistant strains according to the evaluated method) that were indicated to be susceptible-dose dependent by the reference method, divided by the total number of strains, and vice versa (10).
RESULTS
The MICs for the quality control strains, as determined by the NCCLS broth macrodilution and the E-test methods, were within the ranges established by Pfaller et al. (11). Diameters observed for the fluconazole Neo-Sensitabs diffusion method were within the ranges indicated by the manufacturer.
The comparison of fluconazole MICs for 102 strains analyzed by the NCCLS method and the E-test resulted in a correlation coefficient of 0.943. Table 1 shows the distribution of fluconazole broth macrodilution and E-test MICs.
TABLE 1.
Distribution of fluconazole MICs tested with the NCCLS macrobroth dilution and E-test methods
| Species (no. of strains) | Test | No. of strains inhibited at fluconazole concn (mg/liter):
|
No. of noninterpret- able results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.25 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ≥256 | |||
| C. glabrata (30) | NCCLS | 2 | 7 | 18 | 2 | 1 | ||||||||
| E-test | 1 | 7 | 20 | 2 | ||||||||||
| C. albicans (21) | NCCLS | 15 | 6 | |||||||||||
| E-test | 15 | 3 | 3 | |||||||||||
| C. tropicalis (13) | NCCLS | 1 | 5 | 2 | 1 | 4 | ||||||||
| E-test | 1 | 4 | 2 | 2 | 4 | |||||||||
| C. parapsilosis (12) | NCCLS | 1 | 6 | 3 | 1 | 1 | ||||||||
| E-test | 2 | 2 | 3 | 4 | 1 | |||||||||
| C. krusei (9) | NCCLS | 7 | 2 | |||||||||||
| E-test | 3 | 6 | ||||||||||||
| S. cerevisiae (2) | NCCLS | 1 | 1 | |||||||||||
| E-test | 1 | 1 | ||||||||||||
| Cryptococcus neoformans (3) | NCCLS | 1 | 1 | 1 | ||||||||||
| E-test | 1 | 1 | 1 | |||||||||||
| Other Candida. spp. (16) | NCCLS | 3 | 3 | 3 | 3 | 3 | 1 | |||||||
| E-test | 2 | 2 | 4 | 3 | 1 | 1 | 2 | 1 | ||||||
Four C. tropicalis strains did not give interpretable endpoints with either the NCCLS or the E-test because of substantial trailing. For 37% of the total number of strains tested, a double zone with growth of microcolonies throughout the ellipse zone was seen. This pattern was seen most often with C. albicans (18 of 21 strains [86%]) and C. glabrata (20 of 30 strains [67%]). Macrocolonies were seen with 5.6% (6 of 106) of the strains.
The E-test indicated lower mean MICs for C. albicans, C. parapsilosis, and C. tropicalis than did broth macrodilution, without changing the clinical interpretation according to the breakpoints used. However, one C. tropicalis strain with a MIC of >256 mg/liter by the reference method, appeared to be susceptible with the E-test (MIC = 0.5 mg/liter), resulting in a very major error. For the other Candida species tested, the E-test yielded lower mean MICs than the NCCLS method, but without a change in category. For C. glabrata, six of the nine susceptible strains were indicated to be susceptible-dose dependent, and one of the 20 susceptible-dose dependent strains was indicated to be resistant with the E-test. For C. krusei, four of the seven susceptible-dose dependent strains were indicated to be resistant with the E-test. One each of the two S. cerevisiae and three C. neoformans susceptible strains were indicated by the E-test method to be susceptible-dose dependent.
Thus, with the E-test, one very major error was observed (C. tropicalis, 25%), no major errors were found, and 23.5% minor errors were observed.
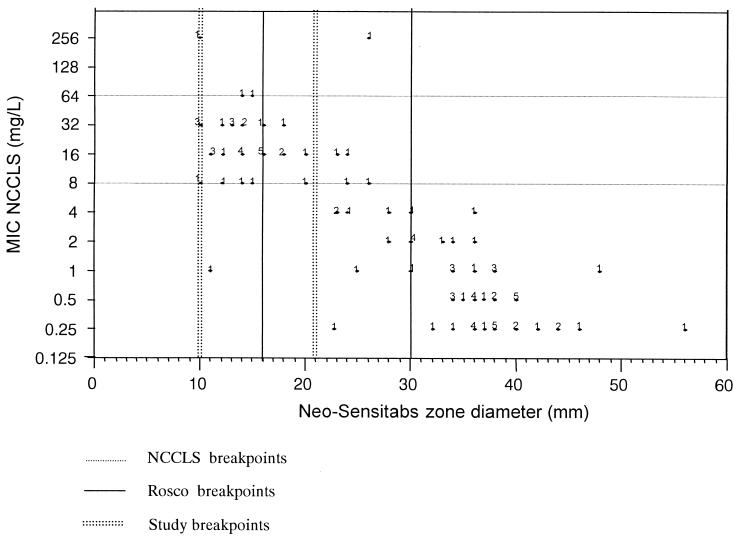
Figure 1 shows the correlation between the Neo-Sensitabs zone diameters and the NCCLS MICs. The correlation coefficient r was 0.873. No very major errors were seen, but major errors occurred with 14.1% of the strains tested. Using the breakpoints advised by the manufacturer, the Rosco tablet produced a minor error with the same C. tropicalis strain which produced a very major error with the E-test. In total, minor errors were observed with 29.4% of strains tested.
FIG. 1.
Scatter diagram of NCCLS MICs for fluconazole and zone diameters obtained with 15-μg fluconazole disks.
Lowering the disk diffusion breakpoints to ≤10 mm for resistance (R) and ≥21 mm for susceptibility (S) apparently resulted in a better correspondence between the disk diffusion and broth macrodilution methods (Fig. 1). There was a major error with only 1.4% of strains, and fewer minor errors (15.7%) were observed (Table 2). The C. tropicalis strain which was resistant by the NCCLS method but susceptible with the E-test was also indicated to be susceptible with the Neo-Sensitabs using the study criteria.
TABLE 2.
Errors with the E-test and disk diffusion (two-diameter interpretation criteria) compared to the NCCLS broth macrodilution methoda
| Error type | No. (%) of strains
|
||
|---|---|---|---|
| E-test | Rosco break- points (R ≤ 16 mm, S ≥ 30 mm) | Suggested break- points (R ≤ 10 mm, S ≥ 21 mm) | |
| Very major error | 1 (25) | 0 (0) | 1 (25) |
| Major error | 0 (0) | 10 (14.1) | 1 (1.4) |
| Minor error | 24 (23.5) | 30 (29.4) | 16 (15.7) |
According to the NCCLS broth macrodilution method, 69 strains were susceptible, 29 strains were susceptible-dose dependent, and 4 strains were resistant. Suggested breakpoints were determined, using data from Fig. 1.
DISCUSSION
Since yeast infections are increasing and more resistant strains are observed (15, 16), a comparison of the currently available routine methods for fluconazole susceptibility testing with the gold standard method was undertaken.
The spectrophotometric reading performed in the NCCLS method provided an objective result. The subjective interpretation of minimal turbidity, as well as the trailing effect (seen particularly with C. albicans), was circumvented largely by producing an 80% inhibition standard (5, 13). However, interpretation of the precise meaning of “a prominent decrease in turbidity from the control” remains problematic (17). The MICs obtained corresponded with published data from the literature (2, 12, 18).
Several studies have shown that the E-test is an accurate method for MIC determination with numerous bacteria, including fastidious microorganisms and also Candida spp. (6, 7, 19). To minimize the problem of trailing endpoints due to partial inhibition by fluconazole, the E-test was performed on RPMI-agar with 2% glucose and the point of intersection was determined at 80% growth inhibition, as recommended previously (12). In addition, retesting the microcolonies from the inner growth zone showed that a greater degree of resistance for these isolates could be excluded. The distinction between these microcolonies and true macrocolonies is not always straightforward and requires experience.
The correlation of the E-test with NCCLS MICs was acceptable. However, to the best of our knowledge, no r values for fluconazole susceptibility testing of yeasts have been published. The MICs obtained were comparable to those published in other studies (6, 19). Only one C. tropicalis strain was resistant according to the NCCLS reference method but susceptible with the E-test (a very major error), but since only four resistant strains were available for testing, this caused a high very major error ratio (25%). To obtain a more realistic estimate of the very major error ratio, a larger number of fluconazole-resistant C. tropicalis strains should be tested. Minor errors were due to a shift towards higher susceptibilities with the E-test. Overall, lower mean MICs were observed with the E-test, and this is in agreement with previous work (21).
Four of the 13 C. tropicalis strains gave noninterpretable results with the NCCLS broth macrodilution method and the E-test because of substantial trailing and could not be included in the data set. These species-dependent difficulties have also been seen in other studies (3). To prevent variability in endpoint reading, plates were always scored by the same person. However, in a routine laboratory, trailing might constitute an important source of endpoint variability (4).
The methodology will have to be adapted for testing of S. cerevisiae var. boulardii strains, since the two isolates in this study could not be grown on RPMI macrobroth or RPMI-agar for the E-test, although these isolates grew on Sabouraud agar.
For disk diffusion-based susceptibility testing carried out with Rosco tablets on modified Shadomy agar, the zone edge was taken where the colonies reached a normal size, and faint growth in the inhibition zone was disregarded.
Using the Rosco breakpoints (R ≤ 16 mm and S ≥ 30 mm), 14.1% major errors occurred. Using the lower breakpoints (R ≤ 10 mm and S ≥ 21 mm), deduced from the comparative study described in this paper (Fig. 1), fewer major errors (1.4%) were observed. Minor errors consisted of a shift towards lower susceptibilities with both sets of criteria. In general, lower mean diameter values were observed with the disk diffusion method.
The original breakpoints to be used, as proposed by the manufacturer of fluconazole tablets (Rosco), were based on different studies consisting of MIC determinations for isolates from patients treated with fluconazole daily (100 or >100 mg), on area under the curve/MIC determinations, and on clinical outcome. The present study used regression analysis to determine diameter breakpoints and did not take into account the pharmacokinetic aspects. The lower inhibition diameter breakpoints proposed here (R ≤ 10 mm and S ≥ 21 mm) are comparable to those established previously (R ≤ 12 mm and S ≥ 20 mm [8] and R ≤ 9 mm and S ≥ 20 mm [M. Vandevenne, I. Vandenbossche, G. Verschraegen, and L. Van Nimmen, Abstr. Natl. Symp. Belgische Vereniging Klinische Biologie-Société Belge de Biologie Clinique, abstr. 10, 2000]). Use of the diameter values proposed by Rosco caused too many major errors. By lowering those values, major error rates became more acceptable and fewer minor errors were seen. The Rosco tablet, used in combination with the diameter values proposed in this paper, appears to be an accurate alternative for the more expensive E-test.
In summary, the performance of susceptibility tests according to the NCCLS reference method on a large scale is technically difficult. The E-test is a very acceptable alternative. The disk diffusion method with the diameter values proposed by Rosco produced too many major errors. By lowering these values to R ≤ 10 mm and S ≥ 21 mm, acceptable major error rates and fewer minor errors were obtained, making the Rosco tablet a reasonable alternative for the more expensive E-test. More resistant strains, especially C. tropicalis, should be tested to put the percentage of very major errors into perspective.
As a final caution it should be emphasized that whatever technique is used, experience in determining MICs and the interpretation of inhibition zone diameters, together with careful attention to procedure details, is critically important when performing fluconazole susceptibility testing for yeasts.
Acknowledgments
We thank Ignace Surmont (H. Hartziekenhuis Roeselare), Sabine Lauwers (Academisch Ziekenhuis Brussel), and Herman Van Landuyt (Algemeen Ziekenhuis St. Jan Brugge) for providing us with 53 of the clinical isolates used.
REFERENCES
- 1.Bille, A. L., and S. D. Brown. 1996. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J. Clin. Microbiol. 34:2154-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canton, E., J. Peman, A. Carrillo-Muñoz, A. Orero, P. Ubeda, A. Viudes, and M. Gobernado. 1999. Fluconazole susceptibilities of bloodstream Candida sp. isolates as determined by National Committee for Clinical Laboratory Standards method M27-A and two other methods. J. Clin. Microbiol. 37:2197-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo, A. L., F. Barchiesi, D. A. McGough, and M. G. Rinaldi. 1995. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J. Clin. Microbiol. 33:535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drouhet, E., B. Dupont, L. Improvisi, M. A. Viviani, and A. M. Tortorano. 1986. Disc agar diffusion and microplate automatized techniques for in vitro evaluation of antifungal agents on yeasts and sporulated pathogenic fungi, p. 31-49. In K. Iwata and H. Vanden Bossche (ed.), In vitro and in vivo evaluation of antifungal agents. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 5.Espinel-Ingroff, A. 1994. Etest for antifungal susceptibility testing of yeasts. Diagn. Microbiol. Infect. Dis. 19:217-220. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., L. Steele-Moore, and J. N. Galgiani. 1994. Evaluation of two growth control dilutions for the determination of 90% (MIC-90%) and 80% (MIC-80%) inhibition fluconazole MIC endpoints. Diagn. Microbiol. Infect. Dis. 20:81-86. [DOI] [PubMed] [Google Scholar]
- 7.Favel, A., C. Chastin, A. L. Thomet, P. Regli, A. Michel-Nguyen, and A. Penaud. 2000. Evaluation of the E test for antifungal susceptibility testing of Candida glabrata. Eur. J. Clin. Microbiol. Infect. Dis. 19:146-148. [DOI] [PubMed] [Google Scholar]
- 8.Goutaland, C., and M. A. Piens. 1996. Méthodes de détermination de la sensibilité de souches de Candida au fluconazole: mise au point d'une microméthode et corrélation à deux autres techniques. Pathol. Biol. 44:724-728. [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 10.National Committee for Clinical Laboratory Standards. 1998. Development of in vitro susceptibility testing criteria and quality control parameters. Tentative guideline M23-T3. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 11.Pfaller, M. A., M. Bale, B. Buschelman, M. Lancaster, A. Espinel-Ingroff, J. H. Rex, M. G. Rinaldi, C. R. Cooper, and M. R. McGinnis. 1995. Quality control guidelines for National Committee for Clinical Laboratory Standards recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J. Clin. Microbiol. 33:1104-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., S. A. Messer, A. Karlsson, and A. Bolmström. 1998. Evaluation of the Etest method for determining fluconazole susceptibilities of 402 clinical yeast isolates by using three different agar media. J. Clin. Microbiol. 36:2586-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller, M. A., S. A. Messer, and S. Coffmann. 1995. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D0870. J. Clin. Microbiol. 33:1094-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redding, S., J. Smith, G. Farinacci, M. Rinaldi, A. Fothergill, J. Rhine-Chalberg, and M. Pfaller. 1994. Development of resistance to fluconazole by Candida albicans during treatment of oropharyngeal candidiasis in AIDS: documentation by in vitro susceptibility testing and DNA subtype analyses. Clin. Infect. Dis. 18:240-242. [DOI] [PubMed] [Google Scholar]
- 15.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, O. P. Dib, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1996. Detection and significance of fluconazole resistance in oropharyngeal candidiasis in human immunodeficiency virus-infected patients. J. Infect. Dis. 174:821-827. [DOI] [PubMed] [Google Scholar]
- 16.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex, J. H., M. A. Pfaller, M. G. Rinaldi, A. Polak, and J. N. Galgiani. 1993. Antifungal susceptibility testing. Clin. Microbiol. Rev. 6:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandven, P. 1999. Detection of fluconazole-resistant Candida strains by a disk diffusion screening test. J. Clin. Microbiol. 37:3856-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sewell, D. L., M. A. Pfaller, and A. L. Barry. 1994. Comparison of broth macrodilution, broth microdilution, and ETest antifungal susceptibility tests for fluconazole. J. Clin. Microbiol. 32:2099-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turenne, C. Y., S. E. Sanche, D. J. Hoban, J. A. Karlowsky, and A. M. Kabani. 1999. Rapid identification of fungi, using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 37:1846-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Eldere, J., L. Joosten, A. Verhaeghe, and I. Surmont. 1996. Fluconazole and amphotericin B antifungal susceptibility testing by National Committee for Clinical Laboratory Standards broth macrodilution method compared with E-test and semiautomated broth microdilution test. J. Clin. Microbiol. 34:842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warnock, D. W., J. Burke, N. J. Cope, E. M. Johnson, N. A. von Fraunhofer, and E. W. Williams. 1988. Fluconazole resistance in Candida glabrata. Lancet ii:1310. [DOI] [PubMed] [Google Scholar]
- 23.Wingard, J. R., W. G. Merz, M. G. Merz, M. G. Rinaldi, T. R. Johnson, J. E. Karp, and R. Saral. 1991. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 325:1274-1277. [DOI] [PubMed] [Google Scholar]