Abstract
An outbreak of gastroenteritis affecting 158 of 219 (72%) guests and employees at a hotel is described. Food served at the hotel restaurant is believed to have been the source of the outbreak and to have been contaminated by sick employees working in the restaurant. A secondary attack rate of 22% was seen involving 43 persons in all. In stool specimens from seven of eight patients, Norwalk-like viruses (NLVs) were detected by electron microscopy. While NLV-specific PCR using primers JV12 and JV13 were negative, all specimens examined with primers NVp69 and NVp110 were positive. The failure of primers JV12 and JV13 was attributed to several mismatches in the JV12 primer. Genotyping and sequence analysis revealed that all samples had identical sequences and clustered with genogroup I, and the most closely related well-characterized genotype is Desert Shield. This is the first described food-borne outbreak associated with genogroup I virus in Sweden.
Since the discovery of the Norwalk virus in 1972 (26), Norwalk-like viruses (NLVs) have been recognized as the leading cause of acute nonbacterial gastroenteritis (6, 10, 15, 27, 36). The Norwalk virus was cloned and sequenced in the early 1990s and was found to belong to the human Caliciviridae (20, 23). On the basis of their sequences within the putative RNA-dependent RNA polymerase region and the capsid region, members of the genetically and antigenetically diverse NLVs can be divided into the two genogroups I and II (2, 13, 39). The genogroups can be further divided into various genotypes, but whether the various genogroups and genotypes differ in epidemiological, clinical, or other characteristics is presently unknown.
NLV-associated gastroenteritis is transmitted by the fecal-oral route and occurs both as sporadic community cases and as large outbreaks in, e.g., nursing homes, hospitals, schools, universities, vacation camps, cruise ships, hotels, and restaurants (10, 27). Many of the sporadic cases in the community are spread by person-to-person contacts, while outbreaks often are associated with the ingestion of food or water contaminated by the virus (4, 7, 12, 14, 25, 28).
Here we describe a food-borne outbreak of gastroenteritis associated with a genogroup I, Desert Shield-like calicivirus. Desert Shield virus was first isolated from U.S. military troops who had gastroenteritis while stationed in Saudi Arabia during operation Desert Shield in 1990 (17), and it was characterized a few years later (30). Transmission of the Desert Shield-like calicivirus has been suggested to occur by contaminated water as well as by person-to-person spread (11, 18, 33). We believed that our outbreak originated from an infected food handler.
MATERIALS AND METHODS
Description of outbreak and infection control measures.
On 2 and 3 May 2000, a major outbreak of gastroenteritis occurred at a hotel located near a university hospital in southern Sweden. The majority of the hotel guests were present or recently discharged patients from the hospital and their relatives. Not only guests living at the hotel but also medical staff working at the nearby university hospital ate at the hotel restaurant.
The outbreak began early on the morning of 2 May when the chef of the hotel restaurant started vomiting and had diarrhea while preparing breakfast and other meals for the guests. He left the hotel at 8 a.m. after working for only 1.5 h. The chef had met a friend with gastrointestinal symptoms the day before. On 3 May, five additional employees working in the hotel kitchen fell ill with gastroenteritis while preparing lunch for the hotel guests. Later that evening, infection control personnel at the university hospital were alerted when hotel guests who had eaten food prepared by the sick employees and served in the restaurant, but with no direct contact with the kitchen, started to fall ill with gastroenteritis. The food served at the restaurant on 2 and 3 May (breakfast buffet, ground beef, chicken, vegetarian paella, goulash, salad buffet, and desserts) was suspected to be contaminated and the source of the infection. The restaurant was closed, and guests at the hotel were not allowed to visit the hospital in order to limit the spread of the infection. On the morning of 4 May, when the magnitude of the outbreak was apparent, the hotel was closed and the hotel guests and employees were sent home. The hotel and the restaurant were not opened until 14 days later after a thorough disinfection of all contaminated settings. Medical staff working at the university hospital who had eaten at the restaurant were sent home immediately at the slightest signs of gastrointestinal symptoms and were not allowed to go back to work until 3 days after the symptoms had ceased to exist.
Epidemiological investigation and guests at the hotel.
On 2 and 3 May, 144 guests and employees and 13 newborn babies were either living or working at the hotel. During the same days, 102 outside guests not living at the hotel but eating at the restaurant could be identified. These 246 persons were contacted and either asked to fill out a questionnaire or interviewed by telephone. They were asked whether they had eaten at the restaurant, about clinical symptoms, the start and duration of the symptoms, and whether secondary cases had occurred among their social contacts. Information was obtained from 219 of the 246 contacted guests and employees. In addition, information about the 13 newborn babies was given by their parents. The mean age of the 219 guests and employees (the 13 newborns not included) was 46, with a range of 7 months to 83 years. In addition to the 13 newborn babies, two guests were below 10 years of age (7 and 14 months old) and three were 80 years old or older (80, 82, and 83 years old). The 13 newborn babies were 1 to 5 days old.
Case definitions.
A patient with clinical symptoms was defined as a patient with one or more of the following symptoms: nausea, vomiting (one or more times in 24 h), diarrhea (more than two watery stools in 24 h), abdominal cramping, fever (≥38.0°C), headache, and/or muscle pains. A patient with gastrointestinal symptoms was defined as a patient with one or more of the following symptoms: nausea, vomiting, abdominal cramping, and/or diarrhea. A patient with gastroenteritis was defined as a patient with vomiting and/or diarrhea.
Environmental investigation.
Public health inspectors conducted an environmental assessment of the hotel restaurant and collected samples of foods served on 2 and 3 May (ground beef, chicken, vegetarian paella, goulash, salads, and desserts). The food samples were analyzed for pathogenic food-borne bacteria like Salmonella, Escherichia coli, Enterobacteriaceae, Staphylococcus, Bacillus cereus, and Clostridium perfringens and for yeast and mold species.
Laboratory investigation.
Bacterial cultures of stool samples from eight patients (seven hotel guests and one employee) were performed at the clinical microbiological laboratory at the University Hospital in Lund, Sweden. The stool samples were cultured for Salmonella, Shigella, Campylobacter, and Yersinia species.
EM.
Electron microscopy (EM) was performed essentially as previously described (15). Briefly, a 10% fecal suspension was prepared in phosphate-buffered saline, and a drop of the suspension was incubated for 1 min on a Formvar-carbon-coated grid and then stained with 2% tungstophosphoric acid. To increase the sensitivity, a second step was performed in which the fecal suspension was clarified at 20,000 × g for 30 min and the supernatant was pelleted directly on the grid at 150,000 × g for 10 min in a Beckman (Palo Alto, Calif.) Airfuge.
RNA extraction.
RNA was extracted from 100 μl of a 10% fecal suspension by use of the guanidinium thiocyanate-silica extraction method (5).
Detection of NLVs by RT-PCR.
Reverse transcriptase (RT)-PCR was performed with the primer pair JV12-JV13, which generates a 326-bp product (36, 37), and with primers designated NVp69 (upstream; 5′-GGC CTG CCA TCT GGA TTG CC-3′) and NVp110 (downstream; 5′-AC[A/T/G] AT[C/T] TCA TCA TCA TCA CCA TA-3′), which yielded a 151-bp fragment of the RNA polymerase region (nucleotides [nt] 4733 to 4884, corresponding to equivalent locations within the Norwalk sequence, M87661) (29).
Genotyping.
All PCR-positive samples were genotyped by reverse line blot hybridization essentially as described previously (38) with the modification that primer pair NVp110-NVp69, with primer NVp110 biotinylated, was used instead of primer pair JV12-JV13.
Sequencing.
The RT-PCR-positive samples were further characterized by sequence analysis using the NVp69 primer and the ABI Prism BigDye Terminator Cycle-Sequencing Ready Reaction kit on an ABI 310 automated sequencer. Sequences were edited using Seq Ed version 1.0.3 software and aligned using Se-al software. A 108-nt stretch of the RNA polymerase region was obtained and aligned against sequences obtained from the GenBank database. The dendrogram was produced by PAUP version 3.1.1 software.
In order to investigate why primer pair JV12-JV13 failed to amplify the samples, a new primer was designed to cover the JV12 region. The BLAST search program was used to find the sequence in GenBank most closely related to the sequence obtained by NVp69. A new primer (5′-TGC TAT AGA GGA TGG TGG CCC TT-3′) was designed from this sequence and used in RT-PCR together with primer JV13, resulting in a 375-bp fragment. This fragment was sequenced with the JV13 primer, as described above, and a 320-nt stretch of the polymerase region was obtained.
Statistical analysis.
For statistical analysis, the nonparametric chi-square test and Fisher's exact test were utilized.
Nucleotide sequence accession number.
Sequence data have been submitted to GenBank and assigned accession number AF356599.
RESULTS
Incidence and clinical symptoms.
Information was obtained from 219 of the 246 (89%) contacted guests and employees; 214 responded to the questionnaire, and for an additional 5, information was obtained by interview. In all, 158 of the 219 (72%) guests and employees fell ill with clinical symptoms like nausea (89%), vomiting (77%), diarrhea (74%), abdominal cramping (61%), headache (39%), fever (30%), and myalgias (27%) (Table 1). There were no significant differences in attack rate, symptoms, or onset and duration of disease for the 78 guests living at the hotel and the 80 guests, of which 46 were hospital employees, only eating at the restaurant. A total of 156 patients had gastrointestinal symptoms (nausea, vomiting, gastrointestinal cramping, and/or diarrhea), and 147 had gastroenteritis (vomiting and/or diarrhea).
TABLE 1.
Clinical symptoms of patients falling ill from a food-borne calicivirus infection
| Symptom | No. of patients (percentage) |
|---|---|
| Clinical symptoms | 158 |
| Nausea | 140 (89) |
| Vomiting | 121 (77) |
| Diarrhea | 117 (74) |
| Abdominal cramping | 96 (61) |
| Headache | 61 (39) |
| Fever | 48 (30) |
| Myalgia | 43 (27) |
| Gastrointestinal symptomsa | 156 |
| Gastroenteritisb | 147 |
Defined as nausea, vomiting, diarrhea and/or gastrointestinal pains.
Defined as vomiting and/or diarrhea.
Patients with gastroenteritis.
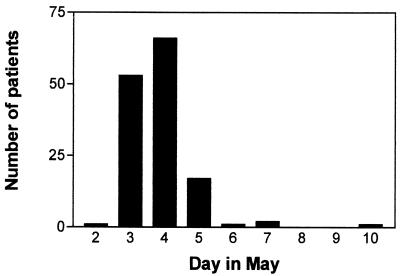
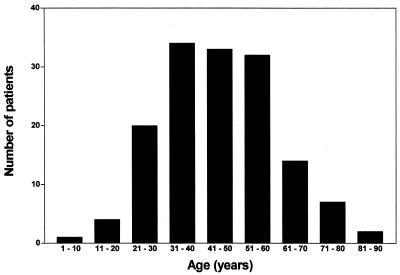
The first to fall ill with gastroenteritis was the chef of the restaurant on 2 May, followed the next day by five employees, all working in the restaurant kitchen. In all, 9 of 11 employees working in the kitchen had gastroenteritis on that and the following days. On 3 May, guests who had eaten at the restaurant, but with no direct contact with the kitchen, started to fall ill. The dates of onset of gastroenteritis for guests and employees were from 2 to 10 May, with a peak on 4 May (Fig. 1). Of the 147 patients with gastroenteritis, 98 (67%) were females and 49 (33%) were males. The mean and median ages were 46 and 45 years, respectively, with a range of 1 to 83 years. The age distribution can be seen in Fig. 2; one patient was below 10 years of age (14 months old), and two patients were >80 years old (82 and 83 years old).
FIG. 1.
Onset of symptoms for 147 patients with gastroenteritis from 2 to 10 May 2000.
FIG. 2.
Age distribution of 147 patients with gastroenteritis.
The duration of symptoms was known for 139 of the 147 patients. It was less than 24 h for 37 of the patients, 24 to 48 h for 61 patients, 48 to 72 h for 27 patients, and more than 72 h for 14 patients. One patient had to receive intravenous fluids to treat dehydration, but all patients recovered.
The food served at the hotel restaurant was suspected to be contaminated and the source of the outbreak. For 211 of the 219 guests and employees, information about whether they had been eating at the restaurant on 2 and/or 3 May was available. In all, 200 of the 211 had been eating at the restaurant, and of these, 142 (71%) had fallen ill with gastroenteritis while 58 (29%) remained well. A significant correlation was seen between eating at the restaurant and falling ill with gastroenteritis (P < 0.001; Fisher's exact test), but no specific food could be associated with disease. Of the 58 guests who remained well despite eating at the restaurant, some had avoided the salads while others had eaten the same food as those who had fallen ill. Only one patient with gastroenteritis had not been eating at the restaurant. This patient worked as a cleaning person at the hotel.
For 61 of the patients with gastroenteritis who had been eating at the restaurant on either 2 or 3 May, an incubation period could be calculated. All of these patients fell ill within 2 days after eating at the restaurant; 2 patients fell ill on the day they ate at the restaurant, 44 patients fell ill the day after, and 15 patients fell ill 2 days after eating at the restaurant. This suggests that the infection was food borne until 6 May (Fig. 1) and thereafter was transmitted by person-to-person contact or from a contaminated environment.
Secondary gastrointestinal infections.
Of the 147 patients with gastroenteritis, 117 had social contacts with one or more persons, mostly family members, with no direct connection to the restaurant or hotel; 26 of these contacts resulted in secondary gastrointestinal infection (a secondary attack rate of 22%) affecting 43 persons in all. All secondarily infected persons were identified on clinical grounds alone, and no genotype identification was performed. The mean age of the secondarily infected persons was 36 years, with a range of 2 to 80 years. No case of gastroenteritis was observed among the 13 newborns at the hotel, although five parents had symptoms.
One probable secondary case was seen among patients at the university hospital. Two of the hotel guests with gastroenteritis had to be transferred to the cardiology department at the hospital, and one of them shared a wardroom with another patient who 2 days later had vomiting and diarrhea. Although no diagnostic tests were performed, this was judged to be a probable secondary case of calicivirus infection.
Environmental investigation.
No pathogenic bacteria, such as Salmonella, E. coli, Enterobacteriaceae, Staphylococcus, B. cereus, and C. perfringens, and no yeast or mold species could be detected from the collected food samples. The food was not examined for viruses.
Laboratory investigation.
Cultures of stool specimens from the eight tested patients did not yield any pathogenic bacteria, such as Salmonella, Shigella, Campylobacter, or Yersinia species. In stool specimens from seven of the eight patients, NLVs were identified by EM. Six of the seven positive specimens contained sufficient material to also be examined by an NLV-specific PCR utilizing primers JV12 and JV13 (37), and surprisingly, these primers failed to give a positive reaction. This led us to retest with a second set of NLV-specific primers designated NVp69 and NVp110, which gave a specific reaction in six of six specimens. Sequence analysis revealed that primer JV12 had six mismatches with the target sequence and primer JV13 had two mismatches, which may explain the failure with these primers.
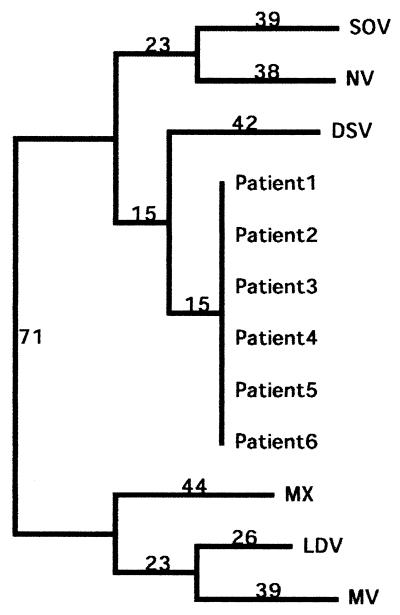
A modified reverse line blot hybridization assay (38) including primer pair NVp110-NVp69, with primer NVp110 biotinylated, was then used to genotype the six specimens as Desert Shield-like viruses. Sequence analysis of the six RT-PCR-positive samples showed that all of the samples had identical sequences (321 nt) and clustered with genogroup I and the most closely related Desert Shield virus with 82% identity on the nucleotide level (Fig. 3). The BLAST search showed that the strain in GenBank most closely related to the sequence obtained by NVp69 and JV13 was from a food-borne outbreak in Hokkaido, Japan, where oysters were the probable source of infection (accession number ABO19262). It has 98% identity on the nucleotide level.
FIG. 3.
Dendrogram based on a 321-nt stretch in the polymerase gene showing phylogenetic relationships between the identical samples from this outbreak and some prototype strains of calicivirus obtained from GenBank (accession numbers: SOV, Southhampton L07418; NV, Norwalk M87661; DSV, Desert Shield U04538; MX, Mexico U22498; LDV, Lordsdale X86557; MV, Melksham X81879). The numbers on the branches show the predicted nucleotide differences.
DISCUSSION
NLVs represent the major cause of acute nonbacterial gastroenteritis outbreaks among adults and the elderly (6, 10, 15, 27, 36). While the principal diagnostic method for detection of viruses in patients with gastroenteritis has been the examination of fecal specimens by EM, the introduction of novel molecular methods has made it possible to characterize different strains of NLVs. These viruses can now be divided into two genogroups, with genogroup I represented by the Norwalk virus and genogroup II represented by the Hawaii, Snow Mountain, and Lordsdale viruses (2, 8, 21, 31, 39). Genogroups I and II can be further subdivided into subgroups and genotypes as more extensive information becomes available (1, 3, 22, 32). In Sweden, genogroup II, Lordsdale-like viruses (8), has up to now been the dominant genotype (unpublished).
We describe a major outbreak of gastroenteritis associated with calicivirus genogroup I, at a hotel located at a university hospital in southern Sweden and involving 158 of 219 (72%) guests and employees. The diagnosis was based on the facts that caliciviruses were detected by EM and PCR and that sequencing of samples from six patients revealed the same sequence. The fact that the primer pair designated JV12-JV13 (37) failed to amplify the Desert Shield-like virus in this outbreak was unexpected, as these primers work very well for routine diagnostic work in Sweden, where most strains, however, belong to genogroup II. It should be mentioned that since we made this observation we have identified more Desert Shield-like viruses that fail to be recognized by JV12-JV13. The most reasonable explanation for the failure is mismatches in JV12.
The epidemiological and clinical symptoms of Desert Shield-like calicivirus are largely unknown (11, 17). It has been suggested that clinical symptoms occurring with this genotype are more closely associated with vomiting and not acute diarrhea alone (17). However, the clinical symptoms observed in general in this outbreak were consistent with those reported in previous NLV infections (14, 27). Vomiting and diarrhea were prominent symptoms in most affected individuals, but other symptoms, like headache, fever, and myalgias, were also seen. Calicivirus infections generally last 48 to 72 h and remit without sequelae (14, 27). Most of our patients recovered within 72 h, but for some the duration of the symptoms was rather extended.
Many guests living at the hotel were present or former patients at the university hospital, and it could be argued that their present or previous illness could have an influence on the attack rate, clinical symptoms, and/or duration of the calicivirus infection. However, this does not seem to be the case, as no significant differences in attack rate, clinical symptoms, or onset and duration of disease were seen for the guests living at the hotel and the previously healthy guests, many of whom were hospital employees who only ate at the restaurant.
Gastroenteritis was seen in patients from 1 to 83 years of age. This supports data showing that all ages can be affected and that the infection is not restricted to adults. It has recently been shown that human caliciviruses are common causative agents of gastroenteritis in children <2 years of age (19, 34); however, none of the 13 newborn babies at the hotel were affected, despite the fact that five parents had gastroenteritis. Whether this is due to their being protected from exposure to the virus or the fact that newborns were protected by maternal antibodies is not known.
The food served at the hotel restaurant was most probably contaminated by calicivirus and was the cause of the outbreak among the guests. This is supported by the fact that the outbreak started with the chef of the restaurant, followed by other employees working in the kitchen, before guests without any direct contact with the kitchen started to fall ill with gastroenteritis. It is also supported by the facts that a significant correlation was seen between eating at the restaurant and falling ill with gastroenteritis and that the epidemic curve of the outbreak, which is clustered around a peak value, points to a common event like exposure to a food-borne disease. For patients who had been eating at the restaurant on only 1 of the 2 days when the food was believed to be contaminated, an incubation period could be calculated. All of these patients fell ill within 2 days after eating at the restaurant, which is in agreement with the known incubation period for caliciviruses (27). This makes it probable that the infection after 5 May was no longer food borne but was transmitted either by person-to-person contact or from the environment.
We were not able to identify any specific food served at the restaurant as the main source of the infection. Guests in the restaurant who had eaten the same food either fell ill or remained well. The differences in outcome could be due to variations in infection dose or in immunity to this specific variant of calicivirus. Antibodies to NLVs are acquired in early childhood and can be detected in up to 90% of older children and adults (16, 35). Studies of the role of serum antibodies in mediating protection against NLVs, however, have yielded conflicting results. In adults, preexisting serum antibodies do not seem to be associated with protective immunity, but antibody levels become associated with protection after repetitive exposure (24).
Very few viruses are needed for infection, and therefore, transmission is possible by droplets and person-to-person contacts and from a contaminated environment, as well as from contaminated food. The only patient with gastroenteritis who did not eat at the restaurant worked as a cleaning person at the hotel and could have been infected from the environment when cleaning up after sick guests. It is known that caliciviruses can be persistent in the environment for a rather extended time and that they are relatively resistant to a variety of disinfectants (9).
Secondary transmission and a high secondary attack rate for family members and friends is a prominent feature of caliciviruses (27). We noted a secondary attack rate of 22% affecting mostly family members. Only one secondary case was seen at the university hospital, which may be attributed to the fact that guests at the hotel, as soon as an outbreak was suspected, were prohibited from visiting the hospital. Additionally, medical staff who had eaten at the restaurant and who worked at the hospital were sent home immediately at the slightest sign of gastrointestinal symptoms.
This is the first recorded outbreak of a Desert Shield-like virus in Sweden. Our data illustrate the limitations of using only one pair of PCR primers for diagnostic purposes and point to the importance of good food hygiene practices and the immediate exclusion of infected food handlers from work.
Acknowledgments
We thank Jan Vinje and Marion Koopmans for the reverse line blot hybridization kit and Margareta Thorhagen for excellent technical assistance.
This project (L. S.) received financial support from the EU: QLK1-CT-1999-00624 (Virus Safe Seafood) and QLK1-CT-1999-00594 (Foodborne Viruses in Europe).
REFERENCES
- 1.Ando, T., S. S. Monroe, J. R. Gentsh, Q. Jin, D. C. Lewis, and R. I. Glass. 1995. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and southern hybridization. J. Clin. Microbiol. 33:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando, T., M. N. Mulders, D. C. Lewis, M. K. Estes, S. S. Monroe, and R. I. Glass. 1994. Comparison of the polymerase region of small round structured virus strains previously classified in three antigenic types by solid-phase immune electron microscopy. Arch. Virol. 135:217-226. [DOI] [PubMed] [Google Scholar]
- 3.Ando, T., J. S. Noel, and R. L. Fankhauser. 2000. Genetic classification of “Norwalk-like viruses.” J. Infect. Dis. 181(Suppl. 2):S336-S348. [DOI] [PubMed] [Google Scholar]
- 4.Beller, M., A. Ellis, S. H. Lee, M. A. Drebot, S. A. Jenkerson, E. Funk, M. D. Sobsey, O. D. Simmons, S. S. Monroe, T. Ando, J. Noel, M. Petric, J. P. Middaugh, and J. S. Spika. 1997. Outbreak of viral gastroenteritis due to a contaminated well. International consequences. JAMA 278:563-568. [PubMed] [Google Scholar]
- 5.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caul, E. O. 1996. Viral gastroenteritis: small round structured viruses, caliciviruses and astroviruses. Part I. The clinical and diagnostic perspective. J. Clin. Pathol. 49:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniles, N. A., D. A. Bergmire-Sweat, K. J. Schwab, K. A. Hendricks, S. Reddy, S. M. Rowe, R. L. Frankhauser, S. S. Monroe, R. L. Atmar, R. I. Glass, and P. Mead. 2000. A foodborne outbreak of gastroenteritis associated with Norwalk-like viruses: first molecular traceback to deli sandwiches contaminated during preparation. J. Infect. Dis. 181: 1467-1470. [DOI] [PubMed] [Google Scholar]
- 8.Dingle, K. E., P. R. Lambden, E. O. Caud, and I. N. Clarke. 1995. Human enteric Caliciviridae: the complete genome sequence and expression of virus-like particles from a genetic group II small round structured virus. J. Gen. Virol. 76:2349-2355. [DOI] [PubMed] [Google Scholar]
- 9.Doultree, J. C., J. D. Druce, C. J. Birch, D. S. Bowden, and J. A. Marshall. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 41:51-57. [DOI] [PubMed] [Google Scholar]
- 10.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 178:1571-1578. [DOI] [PubMed] [Google Scholar]
- 11.Gray, J. J., J. Green, C. Cunliffe, C. Gallimore, J. V. Lee, K. Neal, and D. W. Brown. 1997. Mixed genogroup SRSV infections among a party of canoeists exposed to contaminated recreational water. J. Med. Virol. 52:425-429. [PubMed] [Google Scholar]
- 12.Green, K. Y. 1997. The role of human caliciviruses in epidemic gastroenteritis. Arch. Virol. Suppl. 13:153-165. [DOI] [PubMed] [Google Scholar]
- 13.Green, S. M., K. E. Dingle, P. R. Lambden, E. O. Caul, C. R. Ashley, and I. N. Clarke. 1994. Human enteric Caliciviridae: a new prevalent small round-structured virus group defined by RNA-dependent RNA polymerase and capsid diversity. J. Gen. Virol. 75:1883-1888. [DOI] [PubMed] [Google Scholar]
- 14.Hedberg, C. W., and M. T. Osterholm. 1993. Outbreaks of food-borne and waterborne viral gastroenteritis. Clin. Microbiol. Rev. 6:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedlund, K. O., E. Rubilar-Abreu, and L. Svensson. 2000. Epidemiology of calicivirus infections in Sweden, 1994-1998. J. Infect. Dis. 181(Suppl. 2):S275-S280. [DOI] [PubMed] [Google Scholar]
- 16.Hinkula, J., J. M. Ball, S. Lofgren, M. K. Estes, and L. Svensson. 1995. Antibody prevalence and immunoglobulin IgG subclass pattern to Norwalk virus in Sweden. J. Med. Virol. 47:52-57. [DOI] [PubMed] [Google Scholar]
- 17.Hyams, K. C., A. L. Bourgeois, B. R. Merrell, P. Rozmajzl, J. Escamilla, S. A. Thornton, G. M. Wasserman, A. Burke, P. Echeverria, K. Y. Green, A. Z. Kapikian, and J. N. Woody. 1991. Diarrheal disease during Operation Desert Shield. N. Engl. J. Med. 325:1423-1428. [DOI] [PubMed] [Google Scholar]
- 18.Hyams, K. C., J. D. Malone, A. Z. Kapikian, M. K. Estes, X. Jiang, A. L. Bourgeois, S. Paparello, R. E. Hawkins, and K. Y. Green. 1993. Norwalk virus infection among Desert Storm troops. J. Infect. Dis. 167:986-987. [DOI] [PubMed] [Google Scholar]
- 19.Inouye, S., K. Yamashita, S. Yamadera, M. Yoshikawa, N. Kato, and N. Okabe. 2000. Surveillance of viral gastroenteritis in Japan: pediatric cases and outbreak incidents. J. Infect. Dis. 181(Suppl 2):S270-S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, X., D. Y. Graham, K. Wang, and M. K. Estes. 1990. Norwalk virus genome cloning and characterization. Science 250:1580-1583. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, X., D. O. Matson, G. M. Ruiz-Palacios, J. Hu, J. Treanor, and L. K. Pickering. 1995. Expression, self-assembly, and antigenicity of a Snow Montain agent-like calicivirus capsid protein. J. Clin. Microbiol. 33:1452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang, X., J. Wang, and M. K. Estes. 1995. Characterization of SRSVs using RT-PCR and a new antigen ELISA. Arch. Virol. 140:363-374. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51-61. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, P. C., J. J. Mathewson, H. L. DuPont, and H. B. Greenberg. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J. Infect. Dis. 161:18-21. [DOI] [PubMed] [Google Scholar]
- 25.Kapikian, A. Z. 1996. Overview of viral gastroenteritis. Arch. Virol. Suppl. 12:7-19. [DOI] [PubMed] [Google Scholar]
- 26.Kapikian, A. Z., R. G. Wyatt, R. Dolin, T. S. Thornhill, A. R. Kalica, and R. M. Chanock. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan, J. E., G. W. Gary, R. C. Baron, N. Singh, L. B. Schonberger, R. Feldman, and H. B. Greenberg. 1982. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann. Intern. Med. 96:756-761. [DOI] [PubMed] [Google Scholar]
- 28.Kohn, M. A., T. A. Farley, T. Ando, M. Curtis, S. A. Wilson, Q. Jin, S. S. Monroe, R. C. Baron, L. M. McFarland, and R. I. Glass. 1995. An outbreak of Norwalk virus gastroenteritis associated with eating raw oysters: implications for maintaining safe oyster beds. JAMA 273:466-471. [DOI] [PubMed] [Google Scholar]
- 29.Le Guyader, F., M. K. Estes, M. E. Hardy, F. H. Neill, J. Green, D. W. Brown, and R. L. Atmar. 1996. Evaluation of a degenerate primer for the PCR detection of human caliciviruses. Arch. Virol. 141:2225-2235. [DOI] [PubMed] [Google Scholar]
- 30.Lew, J. F., A. Z. Kapikian, X. Jiang, M. K. Estes, and K. Y. Green. 1994. Molecular characterization and expression of the capsid protein of a Norwalk-like virus recovered from a Desert Shield troop with gastroenteritis. Virology 200:319-325. [DOI] [PubMed] [Google Scholar]
- 31.Lew, J. F., A. Z. Kapikian, J. Vadesuso, and K. Y. Green. 1994. Molecular characterization of Hawaii virus and other Norwalk-like viruses: evidence for genetic polymorphism among human caliciviruses. J. Infect. Dis. 170:535-542. [DOI] [PubMed] [Google Scholar]
- 32.Noel, J. S., T. Audo, J. P. Leite, K. Y. Green, K. F. Dingle, M. K. Estes, Y. Seto, S. S. Monroe, and R. I. Glass. 1997. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J. Med. Virol. 53:372-383. [DOI] [PubMed] [Google Scholar]
- 33.Ohyama, T., S. Yoshizumi, H. Sawada, Y. Uchiyama, Y. Katoh, N. Hamaoka, and E. Utagawa. 1999. Detection and nucleotide sequence analysis of human caliciviruses (HuCVs) from samples in non-bacterial gastroenteritis outbreaks in Hokkaido, Japan. Microbiol. Immunol. 43:543-550. [DOI] [PubMed] [Google Scholar]
- 34.Pang, X. L., S. Honma, S. Nakata, and T. Vesikari. 2000. Human caliciviruses in acute gastroenteritis of young children in the community. J. Infect. Dis. 181(Suppl 2):S288-S294. [DOI] [PubMed] [Google Scholar]
- 35.Sakuma, Y., S. Chiba, R. Kogasaka, H. Terashima, S. Nakamura, K. Horino, and T. Nakao. 1981. Prevalence of antibody to human calicivirus in general population of northern Japan. J. Med. Virol. 7:221-225. [DOI] [PubMed] [Google Scholar]
- 36.Vinje, J., S. A. Altena, and M. P. Koopmans. 1997. The incidence and genetic variability of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J. Infect. Dis. 176:1374-1378. [DOI] [PubMed] [Google Scholar]
- 37.Vinje, J., and M. P. Koopmans. 1996. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J. Infect. Dis. 174:610-615. [DOI] [PubMed] [Google Scholar]
- 38.Vinje, J., and M. P. Koopmans. 2000. Simultaneous detection and genotyping of“Norwalk-like viruses” by oligonucleotide array in a reverse line blot hybridization format. J. Clin. Microbiol. 38:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, J., X. Jiang, H. P. Madore, J. Gray, U. Desselberg, T. Ando, Y. Seto, I. Oishi, J. F. Lew, K. Y. Green, and M. K. Estes. 1994. Sequence diversity of small, round-structured viruses in the Norwalk virus group. J. Virol. 68:5982-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]