Abstract
Housekeeping protein expression in tissue samples is often used to normalize immunoblotting data. However, utilizing common loading control proteins in pathological conditions without validation can be problematic. Here, we describe the alteration of commonly used loading control protein expressions in asthmatic lung samples and present a simpler and more reliable loading control for western blot analysis. Lung samples from control and asthmatic mice groups were used to assess the expression levels of three commonly used housekeeping proteins (beta-actin, alpha-tubulin, and glyceraldehyde-3-phosphate dehydrogenase). Ponceau S staining data was also obtained to assess the consistency of protein loading. Housekeeping protein expressions varied dramatically in asthmatic groups, ranging from 26 to 278 % compared to the control group (p < 0.05). On the other hand, no significant differences in Ponceau S staining were observed among the groups. There were no differences in protein extraction from lung samples among the groups, but significant differences were observed in bronchoalveolar lavage leukocyte populations (p < 0.05). Our data suggest that lung resident immune cells were dramatically altered in asthma groups, and protein expression of commonly used loading control proteins are unreliable for western blot analysis of asthmatic lungs. We recommend Ponceau S staining as a more reliable loading control for asthmatic lung samples to normalize western blot data.
Keywords: Western blot, Loading control, Asthma, Lung
Highlights
-
•
The expression of housekeeping proteins such as α-tubulin, β-actin, and GAPDH is altered in asthmatic mouse lung samples.
-
•
Ponceau S staining shows consistent protein levels across sexes and asthma models, making it a reliable control.
-
•
Asthma raises eosinophils in BAL fluid, possibly affecting the expression of common loading control proteins.
-
•
Our data has provided critical insight into the selection of appropriate loading controls in asthmatic mice research.
1. Introduction
Western blotting is a common laboratory technique for semiquantitative protein analysis that has been utilized over 40 years [1]. Since its first publicized use in 1979, immunoblotting has remained an invaluable tool for biological research and clinical diagnostics [2]. Several steps within the western blot protocol, including antibody selection, sample preparation, and normalization standards, may be subject to oversight, making the technique vulnerable to error [3]. In particular, the assumed stability and uniformity of the expression of common loading control proteins used is especially problematic because several studies have shown that housekeeping protein expression is not reliably consistent across experimental states [3,[5], [6], [7]]. For example, a study investigating housekeeping protein expression in the hearts of human diabetics found β-actin levels were reduced in the right atrium and left ventricle, while glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was reduced in only the right ventricle when considering age as a factor [4]. Similarly, the expressions of beta-actin and GAPDH decrease with age in human skeletal muscle [5]. Similarly, these housekeeping proteins have also been reported as unreliable loading controls in some preclinical experimental models such as myocardial infarction and hypoxic-ischemic brain injury [6,8]. This accumulating evidence suggests that it is critical to re-evaluate the validity of commonly used loading control protein expression in each experimental setting.
Preclinical studies play a vital role for identifying the mechanisms of many human diseases and the discovery of novel interventions. For preclinical asthma studies, the ovalbumin-induced experimental asthma model is the most frequently used model of experimental asthma because this method stimulates a Th2-driven immune response and produces airway hyperreactivity, conditions seen in human asthma [9]. In this model, female mice show increased ovalbumin-induced type 2 immune responses such as eosinophilia, IL-5, and IL-13 in the lungs compared to male mice [[9], [10], [11]]. These classic increased Th2 immune responses due to the experimental asthmatic insults were suppressed in ovariectomized mice [13]. Furthermore, castrated mice showed significantly higher sensitivity to ovalbumin-induced airway inflammation and performed like the female mice [[14], [15]]. Considering asthma is an inflammatory disorder that alters resident lung immune cell populations, the expression of commonly used housekeeping proteins such as alpha-tubulin, GAPDH, and beta-actin may be unreliable as loading controls. However, not many laboratories validate the loading control for immunoblotting assays used in asthmatic lung samples. Therefore, in this study, we analyzed the expression of three of the most commonly used housekeeping proteins for western blot (GAPDH, beta-actin, and alpha-tubulin) and compared them to total protein stain Ponceau S. We hypothesized that the expression of housekeeping proteins would be inconsistent across experimental asthmatic conditions and gonadectomy groups, and that total protein staining would be the most reliable method for normalization.
2. Methods
Asthma protocol: Sham and ovariectomy, and castration surgeries were conducted on 4-week-old C57BL/6J mice by the Jackson Laboratory. Animals were then shipped to WVSOM animal vivarium where housing conditions consisted of a 12H:12H dark-light cycle and a maintained temperature of 22° ± 2° celsius. Animals were provided food and water ad libitum. Once acclimatized, animals were randomly assigned to the following groups: 1) sham female vehicle control (VC), 2) ovariectomized (OVX) VC, 3) sham female asthma, 4) OVX asthma, 5) sham male VC, 6) castrated (CAST) VC, 7) sham male asthma, and 8) CAST asthma. For the experimental asthma procedure, ovalbumin sensitization and challenges were performed as described previously [16]. Mice were sensitized by i.p. injection on days 1 and 6 with ovalbumin (30 μg/mouse; Sigma-Aldrich) suspended in Imject alum (Thermo Fisher Scientific). Non-sensitized control (VC) animals only received the same volume of Imject alum without ovalbumin. On days 11–13, the allergen sensitized mice were exposed to challenges of aerosolized 5 % OVA in 0.9 % saline while the controls were exposed to 0.9 % saline only, for 20 min, twice a day with a minimum interval of at least 6 h using an ultrasonic nebulizer (De Vilbiss Healthcare). Animals were sacrificed within 24–48 h of the last challenge for tissue collection. All animal care and experimental procedures were performed as outlined in the Guide for the Care and Use of Laboratory Animals and were approved by the WVSOM Institutional Animal Care and Use Committee (IACUC # 2022-1).
Immunoblotting: Western blot was performed as described previously [17]. Briefly, approximately 100 mg lung tissues were pulverized in liquid nitrogen using a Spectrum™ Bessman Tissue Pulverizer (Fisher). Samples were then homogenized in RIPA lysis buffer supplemented with 1 mM DTT (Fisher), 1X protease inhibitor cocktail solution (Sigma), and 1X phosphatase inhibitor cocktail solution (Sigma). Samples were sonicated on ice, incubated for 15 min on ice, and centrifuged at 10,000 g at 4 °C for 10 min. The supernatant was removed, and protein concentrations were determined by BCA method using BSA as a standard (Pierce). Samples were diluted to 2 mg ml-1 in Laemmli sample buffer and boiled at 95 °C for 5 min. Equivalent amounts of protein were separated by SDS-PAGE 4–12 % gradient gels (Thermo Fisher Scientific), and then transferred onto nitrocellulose membranes (Thermo Fisher Scientific). Membranes were stained with Ponceau S solution (Thermo Fisher Scientific) for 5 min and washed 3 times in distilled H2O. Images were captured with a SynGene G:BOX Chemi XT 4 gel imaging system (Syngene). Membranes were then blocked with 5 % nonfat milk in TBST for 1 h at room temperature, washed 3 times in TBST, and incubated overnight at 4 °C with primary antibodies in 5 % BSA in TBST with 0.05 % NaN3 against 1) alpha tubulin (1:1000), 2) glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:2500, and 3) beta actin (1:2500). All antibodies were obtained from Cell Signaling Technology. After washing 3 times in TBST, membranes were incubated with anti-species secondary antibody conjugated with HRP (1:5000) in 5 % milk in TBST for 1 h at room temperature and then washed again 3 times with TBST. An enhanced chemiluminescence detection system (ECL Advance, Amersham Biosciences) was used to detect the antibodies, and images were captured with a SynGene G:BOX Chemi XT 4 image system (Syngene). Western blot images were then quantified by densitometric analysis using ImageJ (National Institutes of Health).
Bronchoalveolar lavage (BAL) differential leukocyte counts: BAL fluid samples were collected as described previously [18] with slight modifications. BAL fluids were collected by cannulating the trachea through a small incision with three lung lavages using 0.9 % sterile saline (1 ml saline/lavage). The BAL fluid samples were then centrifuged for 7 min at 400×g at 4°. The pellet was then resuspended in 200 μL of ACK lysis buffer (Thermo Fisher Scientific) and incubated for 10 min on ice to remove the erythrocytes. After the red blood cell lysis, the samples were centrifuged for 7 min at 400×g at 4 °C to collect the cell pellets. The cell pellets were then suspended in 0.5 ml 0.9 % sterile saline. The total number of cells in BAL fluids were counted by TC20 automated cell counter (Bio-Rad Laboratories). For the differential cell analysis, the cell suspension was further diluted by the addition of 0.5 mL cold sterile saline and aliquoted into each well of the cytofunnel. Slides were then placed in the Cytospin 3 (Shandon Scientific) and spun at room temperature for 5 min at 800 rpm. After completing the Cytospin procedure and air drying the slides for 24–36 h, cells were stained with Kwik-Diff staining solutions (Thermo Fisher Scientific) based on the manufacturer's protocol. Full slides were then digitally scanned and evaluated using a Leica Aperio Versa 8 system (Leica Biosystems). The different types of cells were counted under 40× magnification.
Data analysis: Results are presented as mean ± standard error of the mean (SEM). Regression analysis using GraphPad Prism was performed across the different amounts of protein, and a one-way analysis of variance (ANOVA) was performed for overall comparisons with the Bonferroni post hoc test.
3. Results
Appropriate sample load: To determine the sensitivity of protein band detection and the optimal loading amount of protein, gel electrophoresis, and immunoblotting analysis were performed using the half-log10 dose of protein (1, 3, 10 and 30 μg) with antibodies that recognize alpha-tubulin, GAPDH, and beta-actin (Fig. 1). In both male and female lungs from naive control C57BL/6 mice, the regression coefficients (R2) for Ponceau S data showed a very strong correlation (R2 = 0.965 and 0.956 for female and male mice, Fig. 1 A). Similarly, R2 values for alpha-tubulin (R2 = 0.685 and 0.684 for female and male mice, Fig. 1 B), GAPDH (R2 = 0.899 and 0.822 for female and male mice, Fig. 1 C), and beta-actin (R2 = 0.739 and 0.712 for female and male mice, Fig. 1 D) were strong or very strong. There were no differences across sex for all R2 values from each analyte (n = 4 each sex). Standard divisions of each protein load increased as the protein loading increased. For this reason, 20 μg of each sample was loaded in subsequent experiments to maximize both visibility and linearity.
Fig. 1.
Strength of the relationship between Ponceau S staining and western blot densitometry data with differing amounts of protein from control lung samples. Representative images and regression analyses of the relationship between densitometry results of total protein/specific proteins, and the different amounts of protein loadings (1–30 μg) for A) Ponceau S staining, B) alpha-tubulin, C) glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and D) beta-actin. Data are presented as fold changes relative to the 1 μg protein loading. (n = 4).
Uniform Ponceau S staining with asthmatic lung tissues, but not commonly used housekeeping proteins: To determine whether pathological conditions influence the expression of commonly used loading controls, western blot analyses were performed on asthmatic lung samples (Fig. 2). Consistent with immunoblotting experiments in Fig. 1, Ponceau S staining indicated uniform protein transfer to the membrane among control and experimental asthmatic groups (Fig. 2 A, ns, n = 8 each group).
Fig. 2.
Ponceau S staining is a reliable loading control in asthmatic lung samples.
Twenty ug of total protein extract from the lungs of 1) sham female vehicle control (VC), 2) OVX VC, 3) sham female asthma, 4) OVX asthma, 5) sham male VC, 6) castrated (CAST) VC, 7) sham male asthma and 8) CAST asthma groups were analyzed by A) Ponceau S staining and immunoblotting for B) alpha-tubulin, C) glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and D) beta-actin. Data are presented as fold changes relative to each sham control croup. (n = 6∼8) Asterisks (∗, ∗∗, ∗∗∗∗) indicate p < 0.05, 0.01 and 0.001 by One-way ANOVA.
Compared to lungs from female control groups, alpha-tubulin expression was 77.9 ± 21.6 % and 66.1 ± 16.7 % higher in the female sham asthma and OVX asthma lungs (Fig. 2 B, p < 0.05, n = 7 each group), respectively. Although alpha-tubulin expression was 26.1 ± 19.5 % higher in male castrated asthmatic lungs than in male control lungs, this difference was not statistically significant due to the high data variability (p > 0.05, n = 7 each group). Similarly, the increase in alpha-tubulin expression observed in the male sham asthma mice group did not reach statistical significance (sham male VC: 1.00 ± 0.19 vs sham male asthma: 1.15 ± 0.17, A.U. p > 0.05).
Compared to lungs from female control groups, GAPDH expression was 78.1 ± 26.5 % and 68.1 ± 13.0 % higher in the female sham asthma and OVX asthma lungs (Fig. 2 C, p < 0.05, n = 7 each group), respectively. None of the male groups showed significant differences in GAPDH expression from male control lungs (Sham VC: 1.00 ± 0.12, Sham asthma: 1.31 ± 0.18, CAST asthma: 1.23 ± 0.25, A.U. p > 0.05).
Compared to lungs from female control groups, beta-actin expression was 257 ± 37.4 % and 278 ± 69.2 % higher in the female sham asthma and OVX asthma lungs (Fig. 2 D, p < 0.05, n = 7 each group), respectively. Although beta-actin expression in the lungs from male asthma and CAST asthma groups were higher than in male control lungs, there were no statistically significant differences (Sham VC: 1.00 ± 0.11, Sham asthma: 1.56 ± 0.29, CAST asthma: 1.69 ± 0.37, AU p > 0.05).
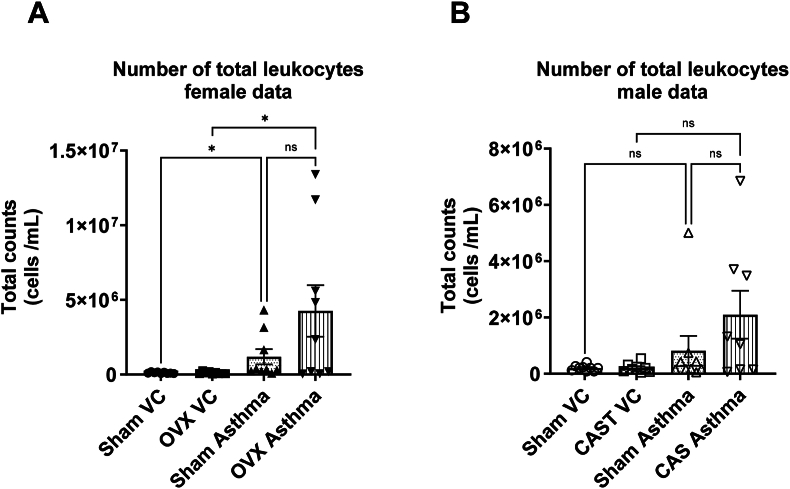
Altered eosinophil population with asthmatic lungs:Fig. 3 shows the total leukocyte counts from BAL fluid samples across different groups. Both the sham female and OVX sham groups had significantly higher leukocyte numbers compared to their respective controls (p < 0.05). There were no statistically significant differences between the sham female asthma and OVX asthma groups. Similarly, although there appeared to be trends of increased leukocyte numbers in the male and CAST asthma groups, these differences were not statistically significant in male BAL fluid. Further analysis of BAL cells indicated classic asthma-specific pathological changes in leukocyte populations in the asthmatic groups. Both male and female asthma groups exhibited severe eosinophilia compared to their respective sham control groups (male sham: 73.4 ± 3.0 %, CAST asthma: 76.1 ± 6.5 %, female sham: 67.4 ± 5.3 %, OVX asthma: 82.0 ± 4.6 %, Fig. 4, p < 0.05, n = 8–9 per group).
Fig. 3.
Effects of asthma on total leukocyte cells in bronchoalveolar lavage (BAL) fluid. Bronchioalveolar lavage (BAL) fluid cell count measurements after ovalbumin challenges for (A) female and (B) male groups. Data are presented as means ± SEM. (n = 8∼9) Asterisks (∗) indicate p < 0.05 by One-way ANOVA.
Fig. 4.
Asthma increases the eosinophil population in bronchoalveolar lavage (BAL) fluid total leukocytes. A) & B) Differential counts for asthmatic groups showed extensive eosinophilia for female and male groups. C) & D) Representative images of Diff-quick staining of BAL fluid for sham female vehicle control (Sham F VC), ovariectomized vehicle (OVX VC), sham female asthma, OVX asthma, sham male VC, castrated (CAST) VC, sham male asthma and CAST asthma groups are shown. Differential counts for Asthmatic groups showed extensive eosinophilia. Scale bar = 50 μm, (n = 6 mice per group). Data are presented as means ± SEM. (n = 8∼9) Asterisks (∗∗∗∗) indicate p < 0.0001 by One-way ANOVA.
4. Discussion
The purpose of this study was to determine whether commonly used loading control proteins are expressed uniformly in asthmatic lung samples. Reliable loading controls are essential in western blotting because they allow for semi-quantitative analysis via normalization. Altered expressions of the housekeeping proteins used as loading controls may lead researchers to misinterpret results. In this study, we demonstrated that asthma conditions show increased expression of some of commonly used housekeeping proteins compared to control, and total protein staining confirmed by Ponceau S staining were not altered. Therefore, Ponceau S staining may offer a better alternative to loading control for the normalization of western blot analysis for asthmatic lung samples.
To our knowledge, this is the first study to systematically demonstrate that experimental asthma can alter alpha-tubulin, GAPHD, and beta-actin expression in the lungs of asthmatic mice (Fig. 2). These findings are in agreement with previous studies showing that experimental conditions can change housekeeping protein expression in different tissue samples [[3], [4], [5], [6]], highlighting the need for careful selection of loading controls for western blot analysis in each experimental setting. Although the exact mechanisms behind the asthma-associated increase in housekeeping proteins remain unclear, we observed a marked increase in BAL fluid leukocyte counts and eosinophilia in the asthmatic lungs (Fig. 4). The altered number of cells and leukocyte populations may partially explain the changes in commonly used housekeeping proteins in the lungs that we observed. However, the data from the present study do not establish a cause-and-effect relationship; further research is required to elucidate the mechanisms underlying the increased expression of these housekeeping proteins in the lungs of asthmatic mice.
Another important finding was that total protein staining, as confirmed by Ponceau S staining, was not altered (Fig. 2). This suggests that total protein staining may offer a better alternative for loading control in the normalization of western blot analysis for asthmatic lung samples. This finding is consistent with previous research showing that GAPDH and beta-actin are not reliable loading controls in vastus lateralis skeletal muscle from aged and untrained human subjects, whereas total protein staining provides a more reliable alternative for normalization [5]. Ponceau S offers several additional benefits, including affordability, near-immediate confirmation of equal loading before using additional resources (such as antibodies) for probing, and full reversibility without the need for a stripping buffer, which could otherwise affect the results [19,20]. Indeed, a study demonstrated that Ponceau S staining did not impact the sensitivity of western blotting [21]. Taken together, our data indicated total protein staining may offer a viable substitute to loading control protein for normalization of western blot analysis for asthmatic lung samples.
Although it is beyond the scope of the present study, asthma research has shown notable differences in prevalence, severity, and outcomes between males and females, with females experiencing more severe asthma [12]. Our data indicate that female asthmatic animals generally showed larger increases in alpha-tubulin, GAPDH, and beta-actin expression compared to male asthmatic groups. Similarly, OVX asthmatic mice appeared to exhibit more severe eosinophilia. We speculate that female sex hormones, such as estrogen and progesterone, may contribute to these observed sex differences. However, the current study was not designed to investigate the role of sex hormones in asthma. Further studies will be needed to determine why female asthmatic animals appear to develop a more severe form of the disease.
5. Conclusion
Our data suggests that commonly used loading control protein expressions are altered in asthmatic mouse lungs, and these altered expressions were associated with an altered BAL fluid immune cell population. Ponceau S staining was found to be reliable in asthmatic lung samples and between sexes, with comparable sensitivity and a higher linear correlation than the housekeeping proteins studied. For this reason, we recommend Ponceau S staining as a reliable loading control for western blot analysis in asthmatic lung samples. Furthermore, protein loads of 20 μg are recommended due to band visibility. Researchers should take care that the loading control is appropriate to the model in order to best normalize data.
CRediT authorship contribution statement
Abigail R. Patterson: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Alexandra P. Crawford: Writing – review & editing, Formal analysis, Data curation. Amanda S. Hatcher: Formal analysis, Data curation. Dovenia S. Ponnoth: Supervision, Resources, Methodology. Shinichi Asano: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Data reported in this manuscript were supported by the West Virginia IDeA Network for Biomedical Research Excellence (P20GM103434). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2025.102018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Moritz C.P. 40 years Western blotting: a scientific birthday toast. J. Proteonomics. 2020;212 doi: 10.1016/j.jprot.2019.103575. [DOI] [PubMed] [Google Scholar]
- 2.Eaton S.L., Roche S.L., Llavero Hurtado M., Oldknow K.J., Farquharson C., Gillingwater T.H., Wishart T.M. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh R., Gilda J.E., Gomes A.V. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev. Proteomics. 2014;11:549–560. doi: 10.1586/14789450.2014.939635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang M., Yan J., Wu A., Zhao W., Qin J., Pogwizd S.M., Wu X., Yuan S., Ai X. Alterations of housekeeping proteins in human aged and diseased hearts. Pflügers Archiv. 2021;473:351–362. doi: 10.1007/s00424-021-02538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigelsø A., Dybboe R., Hansen C.N., Dela F., Helge J.W., Guadalupe Grau A. GAPDH and β-actin protein decreases with aging, making Stain-Free technology a superior loading control in Western blotting of human skeletal muscle. J. Appl. Physiol. Bethesda Md. 2015;118:386–394. doi: 10.1152/japplphysiol.00840.2014. 1985. [DOI] [PubMed] [Google Scholar]
- 6.Nie X., Li C., Hu S., Xue F., Kang Y.J., Zhang W. An appropriate loading control for western blot analysis in animal models of myocardial ischemic infarction. Biochem. Biophys. Rep. 2017;12:108–113. doi: 10.1016/j.bbrep.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson R.E., Carroll H.P., Harris A., Maher E.R., Selby P.J., Banks R.E. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics. 2005;5:566–571. doi: 10.1002/pmic.200400941. [DOI] [PubMed] [Google Scholar]
- 8.Goasdoue K., Awabdy D., Bjorkman S.T., Miller S. Standard loading controls are not reliable for Western blot quantification across brain development or in pathological conditions. Electrophoresis. 2016;37:630–634. doi: 10.1002/elps.201500385. [DOI] [PubMed] [Google Scholar]
- 9.Kips J.C., Anderson G.P., Fredberg J.J., Herz U., Inman M.D., Jordana M., Kemeny D.M., Lötvall J., Pauwels R.A., Plopper C.G., Schmidt D., Sterk P.J., Van Oosterhout A.J.M., Vargaftig B.B., Chung K.F. Murine models of asthma. Eur. Respir. J. 2003;22:374–382. doi: 10.1183/09031936.03.00026403. [DOI] [PubMed] [Google Scholar]
- 10.Takeda M., Tanabe M., Ito W., Ueki S., Konnno Y., Chihara M., Itoga M., Kobayashi Y., Moritoki Y., Kayaba H., Chihara J. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirol. Carlton Vic. 2013;18:797–806. doi: 10.1111/resp.12078. [DOI] [PubMed] [Google Scholar]
- 11.Warren K.J., Sweeter J.M., Pavlik J.A., Nelson A.J., Devasure J.M., Dickinson J.D., Sisson J.H., Wyatt T.A., Poole J.A. Sex differences in activation of lung-related type 2 innate lymphoid cells in experimental asthma. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2017;118:233–234. doi: 10.1016/j.anai.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blacquière M.J., Hylkema M.N., Postma D.S., Geerlings M., Timens W., Melgert B.N. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int. Arch. Allergy Immunol. 2010;153:173–181. doi: 10.1159/000312635. [DOI] [PubMed] [Google Scholar]
- 13.Riffo-Vasquez Y., Ligeiro de Oliveira A.P., Page C.P., Spina D., Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2007;37:459–470. doi: 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- 14.Yu C.-K., Liu Y.-H., Chen C.-L. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinae-sensitized mice. J. Microbiol. Immunol. Infect. 2002;35:199–202. http://www.ncbi.nlm.nih.gov/pubmed/12380796 [PubMed] [Google Scholar]
- 15.Hayashi T., Adachi Y., Hasegawa K., Morimoto M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand. J. Immunol. 2003;57:562–567. doi: 10.1046/j.1365-3083.2003.01269.x. [DOI] [PubMed] [Google Scholar]
- 16.Nadeem A., Ponnoth D.S., Ansari H.R., Batchelor T.P., Dey R.D., Ledent C., Mustafa S.J. A2A adenosine receptor deficiency leads to impaired tracheal relaxation via NADPH oxidase pathway in allergic mice. J. Pharmacol. Exp. Therapeut. 2009;330:99–108. doi: 10.1124/jpet.109.151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asano S., Rice K.M., Kakarla S., Katta A., Desai D.H., Walker E.M., Wehner P., Blough E.R. Aging influences multiple indices of oxidative stress in the heart of the Fischer 344/NNia × Brown Norway/BiNia rat. Redox Rep. 2007;12:167–180. doi: 10.1179/135100007X200254. [DOI] [PubMed] [Google Scholar]
- 18.Patel M., Narke D., Kurade M., Frey K.M., Rajalingam S., Siddiquee A., Mustafa S.J., Ledent C., Ponnoth D.S. Limonene-induced activation of A2A adenosine receptors reduces airway inflammation and reactivity in a mouse model of asthma. Purinergic Signal. 2020;16:415–426. doi: 10.1007/s11302-020-09697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moritz C.P. Tubulin or not tubulin: heading toward total protein staining as loading control in western blots. Proteomics. 2017;17 doi: 10.1002/pmic.201600189. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Calvo I., Ocón B., Martínez-Moya P., Suárez M.D., Zarzuelo A., Martínez-Augustin O., de Medina F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 21.Sander H., Wallace S., Plouse R., Tiwari S., Gomes A.V. Ponceau S waste: Ponceau S staining for total protein normalization. Anal. Biochem. 2019;575:44–53. doi: 10.1016/j.ab.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.