Abstract
Protein oxidation is a complex chemical process that pervades the entirety of the food domain. It is governed by two primary mechanisms: the direct oxidation by active entities and the indirect oxidation by secondary oxidation byproducts like lipid oxidation, influenced by many factors. The oxidation of proteins in livestock products readily occurs post-processing and storage through techniques such as freezing, cooking, ultrasonication, among others, leading to protein carbonylation and subsequent alterations in structure. Consequently, the purpose of this manuscript is to scrutinize the impacts of conventional processing and storage methodologies on protein oxidation in livestock products, delineating potential mechanisms, action sites, and influential factors implicated in this progression. Additionally, we delve into the ramifications of protein oxidation on the processing attributes of livestock products, while venturing into forthcoming trends and obstacles to set a groundwork for ensuring and regulating the caliber of these commodities.
Keywords: Livestock products, Storage, Processing, Protein oxidation, Quality
Highlights
-
•
Protein oxidation sites are mainly concentrated on amino acid side chain groups.
-
•
Protein oxidation and lipid oxidation occur simultaneously in livestock products.
-
•
Moderate protein oxidation improves the functional properties.
-
•
Indirect oxidation of protein is easy to occur in eggs and dairy products.
1. Introduction
As a vital component of the human diet, livestock products boast a wealth of proteins, fats, and other nutrients, furnishing ample energy and abundant nutrition for the human body. Nonetheless, within the processing and storage phases of livestock products, the generation of reactive oxygen species (ROS), reactive nitrogen species (RNS), and other dynamic entities can readily assail proteins, spurring protein oxidation reactions. These transformations alter protein structures to different extents, thereby influencing both the functional properties and the quality of associated food products (Feng et al., 2015; Li, Sun, et al., 2023; Zhu, Xing, et al., 2023).
Protein oxidation is a critical process in food science, akin in importance to the Maillard reaction and lipid oxidation (Hellwig, 2019). Like lipid oxidation, it proceeds through a free radical-mediated cascade with three stages: initiation, propagation, and termination (Wang, Cheng, et al., 2022). However, protein oxidation is more complex, as it can be directly triggered by reactive oxygen species (ROS) or indirectly induced by lipid oxidation products through reactions such as Michael addition and Schiff base formation (Huang, Sarkhel, et al., 2023; Soladoye et al., 2015; Zhu et al., 2024). This oxidation modifies proteins by fragmenting peptide backbones, altering amino acid side chains, and forming protein crosslinks, leading to changes in their structure and functional properties. These alterations ultimately affect the proteins' performance in food processing, underscoring the need to manage oxidation to preserve food quality (Baraibar et al., 2013).
Protein oxidation is a pervasive issue in the handling and preservation of livestock products, affecting meat, eggs, and milk. During processes like freezing, thawing, cooking, storage, and heating, pro-oxidants such as lipid oxidation by-products, enzymes, and metal ions are introduced, increasing the likelihood of oxidation. For example, freezing and thawing meat can degrade proteins and lipids, reducing tenderness (Utrera & Estévez, 2013; Xia et al., 2012). while prolonged heating can lead to protein aggregation and decreased digestibility (Bax et al., 2012; Yin et al., 2023; Zhang et al., 2020; Zhao et al., 2019). In eggs, oxidation contributes to texture changes, such as the “mudding” effect in salted egg yolks, and reduces emulsifying and foaming capabilities (Li et al., 2024; Xue, Liu, et al., 2023). In dairy, thermal sterilization can cause protein carbonylation and reduce solubility (Ma et al., 2019). Mechanical treatments like ultrasound and high pressure can also disrupt cell structures, exacerbating oxidation (Hong et al., 2024; Jiang, Xia, et al., 2024; Nie et al., 2021).
To mitigate these effects, researchers have proposed various strategies. Ultrasonic treatments can prevent protein aggregation (Zheng et al., 2024), while antioxidants can limit oxidation sites and structural changes (Jiang, Yang, et al., 2024; Lund et al., 2007; Soyer et al., 2010; Xie et al., 2020; Yeasmin Akter et al., 2015; Zhang, Gong, et al., 2023). Maintaining low temperatures, reducing oxygen exposure, shortening processing times, and careful mechanical interventions can also help (Lin et al., 2022; Liu et al., 2024; Zhang et al., 2024). Innovative approaches, such as using ozone brine curing for salted duck eggs, have shown promise in reducing curing time and salt content. These methods aim to preserve the functional and nutritional qualities of livestock products, ensuring their quality and usability in food processing (Wongnen et al., 2023b).
Research has shown that oxidative changes induced by reactive oxygen species (ROS) significantly affect protein structures and the functional properties of livestock products (Alavi et al., 2019; Feng et al., 2022; Kong et al., 2011; Li et al., 2019; Nawaz et al., 2022; Park & Xiong, 2007; Wang et al., 2018; Zhang, Gong, et al., 2023). While the causes and effects of protein oxidation in marine products are well understood, a systematic evaluation of how processing and storage methods influence protein oxidation in livestock goods is still lacking. A thorough investigation into these effects is crucial for advancing food science.
Understanding the relationship between protein oxidation and the functional properties of livestock products is essential for developing high-quality meat, eggs, and dairy products. This review explores the mechanisms, sites, and factors of protein oxidation, as well as common processing and storage methods and emerging technologies that influence oxidation. By highlighting these aspects, the review aims to encourage further research and innovation in this field, ultimately improving the quality and functionality of livestock products.
2. Protein oxidation
Protein oxidation pertains to alterations in the covalent bonds of proteins, directly or indirectly incited by potent agents. These modifications can be instigated outright by ROS or indirectly induced by ancillary by-products of oxidative stress reactions (Yang et al., 2023). The process of protein oxidation typically unfolds in three sequential phases: an inception stage (emanating from free radical generation), a propagation stage (involving free radical transfer and propagation), and a culmination stage (Domínguez et al., 2021). Upon the formation of a free radical upon a protein, it has the capacity to transition amid various sites, thereby catalyzing the oxidation of additional proteins within the system, ultimately resulting in protein aggregation, carbonylation, alterations in surface hydrophobicity, and disruptions to primary, secondary, and tertiary structures (Hellwig, 2020).
Throughout the course of food manipulation and preservation, proteins often find themselves exposed to oxidative environments. When food interfaces with molecular oxygen in gaseous form or dissolved within a liquid medium, the oxygen molecule itself exhibits diminished reactivity, yet can undergo a transformation into more reactive ROS. These potent agents can manifest as radical entities like the hydroxyl radical (HO⸱), superoxide radical (O2−), and hydroperoxide radical (HOO⸱), as well as non-radical forms such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) (Hellwig, 2020). The genesis of these active agents within food occurs through irradiation, photo-oxidation, enzymatic reactions, and the catalytic influence of transition metal ions (Schiano et al., 2019; Semagoto et al., 2014; Soladoye et al., 2015; Utrera & Estévez, 2013).
Furthermore, the study of protein oxidation can be conducted through in vitro models of induction, currently encompassing systems like the AAPH oxidation system and the Fenton oxidation system. Within these assessments, AAPH has the capability to generate peroxy radicals at 37 °C, thereby catalyzing protein oxidation (Cheng et al., 2021). Conversely, the Fenton oxidation system instigates protein oxidation by generating the potent HO⸱. Known as one of the most oxidative entities, HO⸱ readily initiates the oxidative modification of proteins (Zhang, Gong, et al., 2023). The oxidative resilience of meat, eggs, milk, and analogous foodstuffs is notably impacted by their constituent makeup, processing techniques, and storage conditions. Oxidative reactions within these comestibles can either be accelerated or impeded, with their oxidative stability contingent upon the extent, site, and form of oxidative modifications, alongside the dynamic equilibrium between antioxidants and pro-oxidant compounds (Bekhit et al., 2013).
2.1. Oxidation site
The locations susceptible to oxidation on proteins predominantly span the primary peptide chains and the side chains of amino acids, encompassing both aliphatic and aromatic amino acid side chains. While the reactivity towards ROS varies notably across different amino acids, all amino acids are potentially prone to assault by these active agents (Estévez et al., 2021). Notably, the side chain groups of amino acids stand out as primary targets for oxidative modification, culminating in alterations to protein carbonyl, sulfhydryl, and dityrosine levels, alongside the generation of protein free radicals (Xiong & Guo, 2020).
The formation of carbonyl derivatives stands as a pivotal indicator of protein oxidation. Specifically, the oxidation byproducts of lysine (Lys), threonine (Thr), arginine (Arg), and proline (Pro) result in α-aminoadiacyl semi-aldehyde, 2-amino-3-ketobutyric acid, glutamic semialdehyde, and 5-hydroxyproline, respectively, serving as primary precursors of carbonyl formation (Li et al., 2012). Noteworthily, within sulfur-containing amino acids, cysteine (Cys) and methionine (Met) exhibit heightened reactivity levels (Lund & Baron, 2010b). Cysteine readily undergoes oxidation to engender reversible disulfide bonds or irretrievable sulfoacid and its derivations, thereby influencing the protein's structure and functionality. On the other hand, methionine can transform into methionine sulfoxide, consequently altering protein conformation and stability. Consequently, the reduction in sulfhydryl group content has emerged as a widely employed significant metric of protein oxidation (Bao & Ertbjerg, 2019; Kehm et al., 2021).
Moreover, protein oxidation provokes specific oxidation products within tyrosine (Tyr), tryptophan (Trp), phenylalanine (Phe), and histidine (His) residues, such as dityrosine, kynurenine derivatives, m-tyrosine, and imidazoline derivatives (Fig. 1) (Ganjeh et al., 2024; Poojary & Lund, 2022). Multiple investigations have underscored that the elevation in carbonyl and dityrosine levels, coupled with the reduction in sulfhydryl levels, typify the standard characteristics of protein oxidation (Cheng et al., 2021; Wang et al., 2018; Yu et al., 2024).
Fig. 1.
The oxidation sites of protein.
2.2. Oxidation mechanism
2.2.1. Direct oxidation
The primary mechanism initiating protein oxidation stems from the assault of ROS on the main chain and amino acid side chain groups, triggering the oxidation process through the extraction of hydrogen atoms from proteins. Given the presence of an unpaired electron within the free radical, its reactivity is notably robust. It has the capability to engage with amino acids, peptides, and proteins through various avenues, encompassing hydrogen absorption, electron transfer (oxidation or reduction), addition, cleavage, rearrangement, dimerization, disproportionation, and substitution reactions (co-addition and elimination) (Davies, 2016; Zhang et al., 2022). These interactions directly instigate protein oxidation, resulting in the disruption of the main peptide chain and the oxidation of side chain groups, ultimately generating covalent crosslinks (Fig. 2).
Fig. 2.
Mechanism of protein oxidation (Domínguez et al., 2021; Huang, Sarkhel, et al., 2023; Domínguez et al., 2022; Soladoye et al., 2015; Zhu et al., 2009).
ROS (generated by pro-oxidants, oxidants, catalysts, metal ions, light, or enzymes) primarily seize hydrogen atoms within proteins through reactions like hydrogen capture, oxygen addition, and single electron reduction, yielding carbon-centered free radicals (C·). In the absence of oxygen, two C-centered free radicals amalgamate to fashion C—C cross-linked derivatives. Under aerobic circumstances, the C-centered radical undergoes a subsequent transformation into an alkyl peroxygen radical (COO·), prompting the extraction of hydrogen atoms from another protein molecule, thereby yielding an alkyl peroxide (COOH). Following this, COOH undergoes further reactions to engender an alkoxy radical (CO·) and its hydroxyl derivative (COH), catalyzed by a free peroxy radical (HO2·) or reduced iron (Fe2+). These COOH and alkyl radical derivatives possess the capacity to cleave polypeptide chains through α-amidation or diamide pathways, or establish protein covalent crosslinks via coupling reactions, ultimately culminating in protein carbonylation and fragmentation (Domínguez et al., 2021).
2.2.2. Indirect oxidation
Lipid oxidation byproducts and glycosylation byproducts may lead to the indirect oxidation of proteins, as elucidated by Luna et al. (Luna et al., 2021). Throughout the processing and storage of meat products, lipid oxidation ensues inevitably, giving rise to a plethora of intermediates including alkyl peroxy radicals, alkoxy radicals, active carbonyl compounds, and hydroperoxides. These free radicals possess the capability to directly impact proteins, provoking crosslinking and aggregation processes (Domínguez et al., 2021). Moreover, the secondary products stemming from lipid oxidation exhibit a heightened propensity to interact with proteins compared to their primary counterparts. Notably, compounds like 4-oxy-2-nonenal (4-ONE) and 4-hydroxy-2-nonenal (4-HNE) form enduring crosslinks with proteins, serving as significant indicators of oxidative stress (Huang, Sarkhel, et al., 2023). Malondialdehyde (MDA) stands as the prevailing active aldehyde among the secondary derivatives of lipid oxidation occurring throughout the processing and preservation of meat. Originating from various unsaturated fatty acids, MDA exhibits a robust capacity to instigate protein oxidation, as expounded by Chen et al. (Chen et al., 2023). The alteration induced by MDA on the spatial configuration of proteins through the oxidation of protein side chains and polypeptide framework elicits a profound impact on their functional attributes (Fig. 4a) (Estévez et al., 2021). In their study, Chen et al. (Chen et al., 2024) utilized MDA as a trigger to establish an oxidation system, alongside an in vitro digestion model to investigate the ramifications of protein oxidation on myofibril protein digestibility. Their findings unveiled a pattern wherein the augmentation of MDA concentration (0, 0.5, 1, 2, 5, 10 mmol/L) led to a suppression in the hydrolysis degree and digestibility of myofibril. Concurrently, there was a marked escalation in the levels of carbonyl groups, surface hydrophobicity, random coil formations, and MDA content. Conversely, a notable reduction was observed in the total sulfhydryl groups, α-helix formations, free amino groups, hydrolysis degree, and MDA binding quantity. These results underscore the proposition that the diminished digestibility of myofibril protein subsequent to freezing could potentially be linked to the protein oxidation pathway influenced by MDA—a byproduct of lipid oxidation.
Fig. 4.
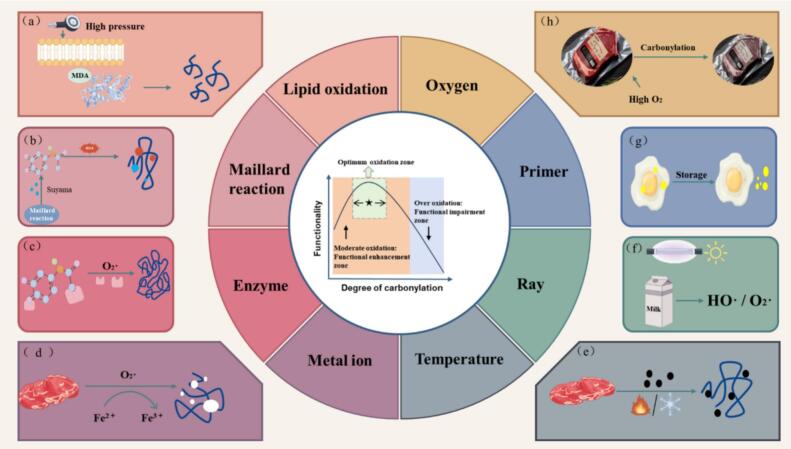
Factors affecting protein oxidation in livestock products (a: Fat oxidation products; b: Glycosylation products; c: Enzymes; d: Metal ions; e: Temperature; f: Irradiation; g: Endogenous substances; h: Packaging).
Illustrated in Fig. 2 is the intricate process of indirect protein oxidation. Using lysine modification as a prime illustration, lipid oxidation byproducts infiltrate proteins by introducing carbonyl groups through the formation of Schiff bases or engaging in Michael addition reactions with amino groups on protein side chains. This biochemical interplay fosters protein carbonylation, thereby instigating crosslinking and aggregation phenomena (Akagawa, 2021; Lund & Baron, 2010a). Egg yolks are characterized by their elevated lipid content, and lipid oxidation can catalyze protein oxidation during the processing of egg-based products. Over the course of salted egg preservation, the yolks gradually assume a tender and pasty consistency, culminating in the development of an indistinct “mudding” appearance. Investigations indicate that throughout the storage duration of salted eggs, lipid oxidation propels the generation of an array of breakdown products such as aldehydes, ketones, and acids. These dynamic substances hold the potential to instigate or accelerate protein oxidation processes, resulting in the formation of dityrosine crosslinking compounds. This cascade leads to protein oxidation and consequential alterations in protein conformation—which serves as the primary driver behind the “mudding” transformation observed in salted egg yolks (Xue, Liu, et al., 2023).
Glycosylation marks the inaugural phase of the Maillard reaction, with non-enzymatic glycosylation notably capable of instigating protein oxidation. Under this process, reducing sugars undergo degradation to engender potent α-dicarbonyl entities (like glyoxal and methylglyoxal), which subsequently engage in nucleophilic interplays with basic amino acids within proteins (such as lysine and arginine). This interaction orchestrates protein carbonylation along the “Suyama” pathway (Fig. 4b). Moreover, during both the initial and advanced stages of glycosylation cascades, ROS are generated. These entities possess the capability to directly influence protein carbonylation or indirectly stimulate protein oxidation pathways (Estévez et al., 2021; Luna et al., 2021; Zhang et al., 2013).
As per the extant research findings (Domínguez et al., 2021; Hellwig, 2019; Huang, Sarkhel, et al., 2023; Nawaz et al., 2022), an intricate interplay emerges among protein oxidation, lipid oxidation, and the Maillard reaction (Fig. 3). Within livestock-derived products, protein oxidation and lipid oxidation often manifest concomitantly, facilitating the transfer of ROS between the two domains. The degradation of reducing sugars yields dynamic α-dicarbonyl entities that engage in reversible interactions with both lipids and proteins. Nevertheless, a comprehensive elucidation of the precise interaction dynamics among these three components, alongside the pathways of reactant introduction, remains constrained by the inadequacy of substantial empirical substantiation.
Fig. 3.
Relationship between protein oxidation, lipid oxidation, and the Maillard reaction (Domínguez et al., 2021; Hellwig, 2019; Huang, Sarkhel, et al., 2023; Nawaz et al., 2022).
The process of protein oxidation is intricate, influenced by a plethora of factors, making its alterations challenging to discern through scent cues, unlike the case with lipid oxidation. Currently, scholarly inquiries into the mechanisms governing protein oxidation exhibit a bias, warranting a more comprehensive exploration of both direct and indirect pathways. Furthermore, scant research exists on the comparability of protein oxidation mechanisms triggered by disparate agents (such as HO·, ROO·, Ray, etc.), and the distinctions in mechanisms between natural and induced oxidation remain underreported, underscoring the need for additional investigations.
2.3. Factors influencing protein oxidation in livestock products
Throughout the processing and preservation of meat, eggs, and dairy commodities, protein oxidation is subject to a diverse array of physical, chemical, and biological influences. These include enzymatic processes, metal ion catalysis, and irradiation, all of which have the potential to elicit oxidative alterations in proteins, consequently impacting their structural integrity and functional attributes.
2.3.1. Enzyme
Enzymatic reactions play a dualistic role in the realm of protein oxidation: they wield the ability to directly oxidize liberated amino acids while also exerting influence over the functional attributes of proteins by instigating the generation of active entities within food, thereby catalyzing protein oxidation and expediting reaction kinetics (Fig. 4c). Within the biosynthetic pathway, the enzymatic catalysis of amino acid oxidation stands as a commonplace occurrence. Notably, lysine oxidase assumes the role of catalyzing protein carbonylation, with its active locus exhibiting pronounced expression within mammalian connective tissue. This enzyme is capable of facilitating the oxidative deamination of select lysine and/or hydroxy-lysine residues ε-amino present in elastin and collagen, yielding α-amino fatty hemialdehyde (AAS). Highly reactive AAS residues further engage in reactions with lysine residues, fostering the genesis of both intermolecular and intramolecular covalent crosslinks (Akagawa, 2021).
In the realm of food-related enzymatic processes, there exists a roster of notable oxidases. Among these are glucose oxidase, which catalyzes the conversion of glucose into gluconic acid, liberating hydrogen peroxide in the process; Laccase, instrumental in generating phenolic free radicals, oxidizing phenolic compounds, and demonstrating potential for inducing protein crosslinking in controlled settings; Lipoxygenase, which oversees the oxidation of unsaturated lipids, culminating in the formation of hydroperoxides; and lactoperoxidase, a key participant in the generation of free radical peroxides (Lund & Baron, 2010b; Rubén Domínguez et al., 2022). Additionally, proteases, or proteolytic enzymes, are pivotal in the hydrolysis of proteins, instigating the liberation of peptides and thereby fostering protease-mediated protein oxidation (Sheng et al., 2024). Despite the prevalence of these pro-oxidase catalysts, the majority of organisms boast adept antioxidant mechanisms aimed at quelling reactive oxygen species. An ensemble of enzymes—comprising catalase, glutathione peroxidase, and superoxide dismutase—stands as the enzymatic bedrock of aerobic organisms' antioxidant defense systems (Zhang et al., 2022). It follows, therefore, that the extent of protein oxidation hinges on the delicate equilibrium between antioxidant enzymes and pro-oxidase entities.
2.3.2. Metal ion
Acting as a prevalent instigator of protein modifications, the process of single-electron reduction, orchestrated by metal ions, entails the binding of these ions to protein entities via coordination, chelation, and assorted interactions. This interaction, in turn, catalyzes the oxidation of amino acid side chains, yielding 1O2, whose conversion to H2O2 occurs either spontaneously or via alternative pathways. In their reduced molecular state, metal ions engage in binding with amino acids at designated metal-binding locales present on proteins and enzymes, forming chelates that subsequently react with H2O2, giving rise to highly reactive hydroxyl radicals (Fig. 4d) (Domínguez et al., 2021).
Within meat, intrinsic instigators or facilitators of oxidation are present, notably heme iron and transition metal ions. Utrera et al. (Utrera & Estévez, 2013) elucidated that the abundant heme iron content in beef patties markedly elevates the oxidation levels compared to pork and chicken counterparts. Moreover, investigations reveal that the heme iron concentration in pork's quadriceps and semimembranosus muscles surpasses that in the biceps, consequently reflecting higher oxidation levels (Utrera et al., 2012). Hence, when evaluating the impact of protein oxidation on meat quality, a comprehensive analysis should encompass intrinsic meat characteristics, including the livestock or poultry species, muscular regions, and their respective morphological attributes.
2.3.3. Irradiation
The utilization of irradiation technology proves efficacious in mitigating pathogenic microorganisms within food items, concurrently elongating their shelf life. Nonetheless, the generation of free radicals—comprising entities like HO⸱ and O2−—during irradiation may exacerbate the oxidation processes of proteins and lipids within meat and dairy products (Fig. 4f). Aromatic amino acids embedded in proteins' primary structures, encompassing tryptophan, tyrosine, phenylalanine, and histidine, act as principal targets for photooxidation (Zhang et al., 2013). Noteworthy, certain proteins harbor chromogenic prosthetic groups like riboflavin in milk and myoglobin in meat, endowing them with dual functionalities as both photosensitizers and substrates. Various irradiation methods such as Ultraviolet (UV), X-ray, γ-rays, and visible light, combined with sensitizers, have the capacity to instigate electron transfers and hydrogen extractions in proteins, engendering excited state free radicals that contribute to protein impairment and alterations in molecular properties.
In the marketing realm of dairy products, the application of ultraviolet irradiation within refrigeration settings can incite the absorption of light energy by milk's photosensitizers, instigating the generation of singlet oxygen and sparking photooxidative reactions. This form of oxidation not only jeopardizes the vitamins present in milk but also diminishes its nutritional potency (Dalsgaard & Larsen, 2009; Schiano et al., 2019). Furthermore, as delineated by Rowe et al. (Rowe et al., 2004), the carbonyl levels in irradiated steak markedly surpassed those in non-irradiated samples, with myoglobin oxidation emerging as a pivotal factor in the discoloration progression of irradiated steak.
2.3.4. Other influencing factors
Beyond the aforementioned external influencers, internal factors also wield substantial influence over the protein oxidation trajectory. Notably, myoglobin and hemoglobin—oxidation-propelling proteins inherent in muscle tissue—stand as primary catalysts in the protein oxidation scheme (Wang et al., 2023). Throughout storage periods, the presence of riboflavin in egg whites experiences a pH elevation induced by hydrolysis, liberating riboflavin (functioning as a photosensitizer) that orchestrates protein oxidation via photosensitization mechanisms (Fig. 4g) (Li et al., 2024). Preceding their transformation into commercial entities, meats, eggs, and dairy products conventionally necessitate heating or preservation at low temperatures or freezing points. Adverse temperature settings can precipitate structural harm to muscle tissues, disrupt submicroscopic muscle architecture, and instigate the release of mitochondria, lysosomal enzymes, heme iron, and other oxidation-promoting agents, thereby expediting the pace of protein oxidation (Fig. 4e) (Pérez-Palacios, Ruiz, Martín, Barat, & Antequera, 2011).
The oxygen levels present constitute another pivotal element that impacts the course of protein oxidation, with elevated oxygen concentrations exacerbating protein carbonylation (Fig. 4h) (Li et al., 2022). Lund et al. (Lund et al., 2007) delved into the repercussions of enhanced air packaging (70 %O2/30 %CO2) and anaerobic packaging on protein oxidation within pork during a 14-day storage period at 4 °C. Their findings unveiled that the optimized air packaging within a high oxygen ambiance notably stimulated protein oxidation, culminating in reduced tenderness and succulence within the meat.
In conclusion, the exploration of protein oxidation, particularly the oxidation mechanisms within high-protein livestock products, has garnered considerable interest. Nonetheless, the intricacies of the protein oxidation process are intricate and subject to myriad influences, each factor demonstrating distinct operative mechanisms. Present literature remains inadequate in comprehensively investigating the interplay and correlations among these various factors. Consequently, delving deeper into the intricate mechanisms of protein oxidation induced by multiple factors holds immense significance for advancing our understanding in this domain.
3. Protein oxidation and its effect on functional properties during the processing and storage of meat products
Meat and its products are rich in protein, containing about 15–19 g per 100 g of fresh meat. During processing, mechanical actions disrupt cellular structures, weakening the antioxidant defense system. Endogenous pro-oxidants like myoglobin and iron ions, combined with external factors such as oxygen, light, fermentation, salting, storage, and heat, significantly increase the likelihood of protein oxidation (Wang et al., 2023). This interplay underscores the importance of controlling oxidation to maintain meat quality.
3.1. Water-holding capacity
The capacity of meat to retain water stands as a pivotal quality trait. Oxidation induces shifts in the protein's electrostatic charge, prompting partial unfolding and subsequent expansion of its globular structure. Unfolded protein configurations may aggregate post-oxidation via electrostatic interactions, consequently influencing their water retention capabilities (Bao & Ertbjerg, 2019). Within the realm of muscle texture attributes, myofibrillar proteins (MP) emerge as central players. Particularly, the myosin heavy chain (MHC) and actin regions are susceptible to oxidation, with methionine, cysteine, histidine, aspartate, and glutamate serving as prevalent sites for oxidation occurrences (Huang, Guan, et al., 2023).
The practice of frozen storage represents a prevalent approach to preserving meat; however, throughout the freezing, storing, and thawing phases, processes such as water infiltration removal, myosin deterioration, mechanical impairment, and MP crosslinking and aggregation can precipitate a decline in quality, notably affecting tenderness. Research indicates that alterations in meat quality during freezing primarily stem from lipid and protein oxidation processes (Cheng et al., 2023). Crucial elements influencing the degradation of frozen meat quality include the freeze-thaw temperature, thawing technique, and fluctuations in temperature levels. While conventional freezing temperatures (−18 °C) serve to thwart microbial proliferation and elongate product shelf life, a noteworthy portion of water, approximately 10 % to 20 %, remains in an unfrozen state. This phenomenon contributes to heightened concentrations of pro-oxidants within the cellular framework, including iron, hydrogen peroxide, and myoglobin, hastening the generation of ROS. Moreover, the presence of unfrozen water enveloping myofibrillar proteins fosters an environment rich in oxidation potential, further expediting protein oxidation processes (Utrera & Estévez, 2013). Consequently, the oxidation-induced structural degradation of frozen beef patties during cooking ensues, leading to the formation of lysine derivatives, diminished tryptophan fluorescence, and reduced myofibrillar protein retention (Manheem et al., 2023). Therefore, a prolonged duration of freezing may not entirely impede protein oxidation and could impact the sensory attributes of frozen meat products. In recent times, a novel freezing technique has garnered attention among researchers, involving the regulation of storage temperatures above the product's freezing point yet below 0 °C. This approach extends shelf life, curtails volatile salt nitrogen levels, and diminishes the total count of viable bacteria in yak meat by modulating protein and lipid oxidation processes (Fu et al., 2024). Furthermore, the incorporation of exogenous antioxidants, such as capsaicin, demonstrates efficacy in reducing oxidation sites, suppressing lipid and protein oxidation, and enhancing the textural characteristics and water retention properties of myofibrillar protein gels (Zhang et al., 2024).
The inexorable deterioration in quality of frozen meat during the thawing process predominantly arises from the development of extracellular ice crystals, lipid oxidation, protein oxidation, protein denaturation, and microbial proliferation. Irrespective of the thawing method employed, all methods contribute to the oxidation of lipids and proteins in pork, leading to heightened shear force in myofibrillar fibers and diminished tenderness. When juxtaposed with conventional temperature thawing at 20 °C, both refrigerator thawing at 4 °C and water thawing at 9 °C, microwave thawing yields an augmented inter-myofibrillar space, exacerbates the tearing of muscle fiber bundles, reduces enzyme activity, elevates cooking losses, increases carbonyl and thiobarbituric acid levels, and culminates in the most pronounced loss in tenderness (Xia et al., 2012). Nonetheless, appropriate ultrasonic intervention (with ultrasonic power levels below 300 W) administered during the freezing and thawing processes can impede protein aggregation, shield protein molecules from oxidation, alter the myofibrillar protein structure, diminish particle size, and amplify protein-water interactions. Consequently, this intervention boosts protein solubility and tenderness (Zhang et al., 2021; Zheng et al., 2024).
Li et al. (Li et al., 2021) unearthed that stress-induced protein oxidation and denaturation escalated as storage time elongated. Stress triggered the liberation of Fe2+, instigating site-targeted metal catalyzed oxidation of lysine, arginine, proline, and threonine residues within mutton samples. This process culminated in the creation of carbonyl derivatives, profoundly impacting water retention and texture, the magnitude of which hinged on the intensity of pressure. Subsequent analyses disclosed that a pressure of 200 MPa dismantled the tertiary and quaternary protein structures, laying bare internal hydrophobic and sulfhydryl groups, augmenting the surface hydrophobicity of myosin and actomyosin. Additionally, sulfhydryl groups underwent oxidation to form disulfide bonds, thereby enhancing the gel properties of myofibrillar proteins (Liu, Xu, et al., 2021; Zhang et al., 2017). However, escalating pressure levels led to the oxidative aggregation of proteins, rupture of the intricate network structure, and diminished water retention (Xue et al., 2018). Zhu et al. (Zhu, Zhang, et al., 2023) corroborated through an AAPH oxidative stress experiment the existence of a dose-dependent correlation between oxidation and myofibrillar proteins. Moderate oxidation (at 3 mmol/L AAPH) induced covalent bond aggregation of myosin, fostering protein-protein interactions and enhancing water retention. On the contrary, excessive oxidation (at 5 and 10 mmol/L AAPH) resulted in the partial degradation of MHC subtypes, diminished storage modulus, irregular microstructure, and significantly reduced textural properties.
3.2. Digestibility
Thermal treatments, encompassing methods such as cooking, microwave cooking, frying, and baking, exert widespread utility in both domestic kitchens and industrial food processing realms to elevate the quality, digestibility, and safety standards of meat products. Nonetheless, the application of high-temperature heat instigates the generation of ROS, liberation of heme, and deactivation of antioxidant enzymes, thereby hastening the oxidation of lipids and proteins in meat products (Soladoye et al., 2015). Research indicates that the intensity of protein oxidation amplifies with escalating heating temperatures and prolonged heating durations, as evidenced by several studies (Bax et al., 2012; Yin et al., 2023; Zhang et al., 2020). During heat treatment at 70 °C, amino acid residues undergo oxidation and the protein structure unfolds, creating additional active sites for digestive enzymes. This process enhances the digestibility of actomyosin. Nevertheless, as the heat escalates to 100 °C, proteins undergo extensive cross-linked polymerization. Excessive oxidation impedes protein aggregation and hydrolysis sites, suppresses the liberation of peptides, and ultimately diminishes the digestibility of pork (Zhao et al., 2019).
Salting, a time-honored preservation technique, extends the longevity of products by diminishing water activity and impeding microbial proliferation. Inclusion of sodium chloride can compromise cellular membrane integrity and stimulate the liberation of free iron from iron-binding compounds. Simultaneously, salt partly restrains antioxidant enzyme functionality and heightens oxidative pressure within muscle tissues. Furthermore, sodium chloride collaboratively fosters protein aggregation through oxidation. An optimal concentration of NaCl (0.1 mol/L) diminishes protein binding ability, expedites myoglobin breakdown by pepsin, and augments the digestion and assimilation of thermally treated beef myofibrillar proteins. Conversely, elevated NaCl concentrations (0.6 mol/L) dampen antioxidant enzyme function, fostering the conversion of ROS into RNS. This metabolic shift subsequently instigates and exacerbates oxidative strain in cured meat products (Du et al., 2018; Liu et al., 2011; Wang, Yu, et al., 2022).
3.3. Emulsification characteristic
The emulsion liquid system primarily arises from the dispersion of the oil phase within the aqueous phase, with the oil phase manifesting as diminutive spherical droplets. Playing a pivotal role in meat, myofibrillar protein's capacity for emulsification significantly influences water retention, oil retention, flavor, and the shelf life of meat products.
Emulsions crafted from natural myofibrillar proteins are prone to disrupting the delicate oil-water equilibrium, leading to flocculation, precipitation, and phase division. Consequently, technologies such as ultrasonication, high-pressure homogenization, magnetic field manipulation, and others are harnessed to refine the emulsifying performance of myofibrillar protein. Employing magnetic field treatment, for instance, can prompt alterations in myofibrillar protein conformation, unravel the protein architecture, and expose additional active sites. By doing so, the process hinders protein oxidation and bolsters its emulsification capabilities (Jiang, Xia, et al., 2024). Research indicates that exposure to magnetic fields of 3, 6, and 9mT heightens the surface hydrophobicity of myofibrillar proteins, unfurls secondary structures, reveals more active regions, amplifies protein-lipid interactions, and fosters the formation of a more resilient interfacial layer, thereby enhancing the emulsification efficacy of myofibrillar protein. Furthermore, the application of magnetic field treatment modifies the charge properties of myofibrillar proteins on the surface of oil droplets. This adjustment elevates the electrostatic repulsion between droplets, curbing the likelihood of flocculation. Conversely, exposure to a high-intensity magnetic field (12mT) can render the interfacial layer fragile, prompting droplet coalescence and the formation of larger droplets, ultimately diminishing the emulsifying prowess of myofibrillar protein (Jiang, Yang, et al., 2024).
High-intensity ultrasound (HIU) has the capacity to trigger protein denaturation and fortify the stability of myofibrillar protein emulsions. Liu et al. (Liu, Zhang, et al., 2021) affirmed that subjecting pork myofibrillar protein to 450 W ultrasonic treatment fostered protein dispersion, heightened protein solubility, and augmented the interplay and bonding among interfacial proteins, thereby enhancing emulsion stability. Nonetheless, excessive HIU intensity (520 W) can perturb the hydrophobic interactions of myofibrillar protein, induce protein aggregation, diminish interfacial adsorption, and detrimentally impact emulsion stability (Hong et al., 2024). In the realm of meat product processing, a judicious dose (2 mol/L) of NaCl can elevate the carbonyl content of myofibrillar protein, enhancing emulsion flocculation and aggregation. Conversely, a high NaCl concentration (6 mol/L) may instigate extensive protein aggregation and the formation of numerous pores in emulsion gels. The surplus of oxidation byproducts impedes the establishment of covalent bonds between adsorbed proteins on lipid droplet surfaces and continuous phase proteins, disrupts the three-dimensional gel matrix, and diminishes the emulsifying prowess of myofibrillar protein (Feng et al., 2017).
3.4. Other characteristics
Myoglobin (Mb) serves as a natural pigment protein essential in defining the hue of meat, with its quantity and redox state dictating the meat's color palette. Comprising globin and heme—a compound housing four pyrrole rings alongside iron ions—myoglobin may exhibit iron atoms in a reduced or oxidized form. The color of meat is primarily influenced by heme, a pivotal determinant in this aspect. Throughout the processing and preservation of meat items, variables such as temperature, pH levels, light exposure, and processing techniques can compromise Mb stability, trigger heme porphyrin ring cleavage (Jiang, Xia, et al., 2024), and instigate the creation of highly reactive intermediates that incite protein oxidation, thereby instigating alterations in protein functionality and color profiles (Manzoor et al., 2023). The oxidation process of myoglobin metamorphoses crimson oxygenated myoglobin into brown ferrimyoglobin through the interaction of lipid oxidation byproducts (such as aldehydes and ketones) with proteins, catalyzed by ions (Zamuz et al., 2018). Furthermore, Zhu et al. (Zhu, Xing, et al., 2023) delineated that low-frequency alternating magnetic field thawing (LF-MFT) can overhaul the myofibrillar protein architecture, markedly expedite protein oxidation, and escalate dityrosine concentration. Pork thawed under LF-MFT at an intensity of 4mT displayed minimal alterations in color and myoglobin content, underscoring how moderate protein oxidation could enhance meat coloration (Sheng et al., 2024).
Throughout the refrigeration course, the concentrations of flavor constituents undergo fluctuations corresponding to the duration of freezing. The carbonyl content within beef exhibits a linear escalation during its stint in frozen storage. Research has illustrated that the distinct flavor elements encompassing alcohols, aldehydes, and phenols emerge through the oxidative processes involving lipids and proteins (Al-Dalali et al., 2022). Furthermore, Dominguez et al. (Domínguez et al., 2014) delved into the ramifications of four distinct cooking methods—baking, grilling, microwaving, and frying—on cooking loss, oxidation pathways, and volatilization attributes of foal meat. Their findings unveiled a notable impact of heat application on cooking loss (p < 0.01), with microwaving exhibiting a heightened cooking loss at 32.5 %, contrasting with the lower rates observed in barbecuing (22.5 %) and frying (23.8 %). Across all methods, there was an elevation in total volatile compound content post-cooking compared to raw meat. Grilled steak particularly showcased elevated levels of volatile compounds, suggesting a concomitant rise in volatile compound generation with escalating cooking temperatures, potentially due to thermal instigation of porphyrin ring oxidative degradation, liberation of heme iron, and intensification of protein oxidation (Domínguez et al., 2014; Hellwig, 2020), thereby augmenting aldehyde and straight-chain alkane content while dampening ester and ketone levels. Thus, oxidation emerges as a pivotal factor in configuring the hallmark flavors characteristic of meat.
Various processing and storage methods, such as freezing, thawing, cooking, and marinating, can induce oxidation in meat products, affecting their quality. Thawing techniques, in particular, lead to the oxidation of pork lipids and proteins, reducing tenderness. Extended freezing increases pro-oxidants like Fe2+, H2O2, and Mb, accelerating ROS formation. Mechanical processes (e.g., pressure, ultrasound) release Fe2+, triggering metal-catalyzed oxidation at specific amino acid residues, impacting water-holding capacity and emulsification. Protein oxidation influences meat functionality in a dose-dependent manner, highlighting the need to control oxidation levels to improve product quality (See Fig. 5.).
Fig. 5.
Effect of protein oxidation on functional properties during processing and storage of livestock products (Feng et al., 2022; Kong et al., 2011; Li et al., 2024; Liu et al., 2011; Liu, Hou, et al., 2023; Sheng et al., 2024; Wongnen et al., 2023b; Zhang et al., 2020; Zhang, Gong, et al., 2023; Zhang, Li, et al., 2023; Zhu, Zhang, et al., 2023).
4. Protein oxidation and its effect on functional properties during the processing and storage of egg products
Eggs and their derivatives are rich sources of high-quality livestock protein, with a single egg providing 3 %–4 % of an adult's energy needs and 11 % of their protein requirements (Bhat et al., 2021). Poultry egg proteins are divided into two main categories: egg white proteins and egg yolk proteins. These proteins are not only highly nutritious but also exhibit excellent functional properties, such as foaming, gelation, and emulsification, making them indispensable in the culinary world. However, improper handling during processing and storage can degrade their nutritional value and impair their functional benefits, highlighting the importance of careful management to preserve their quality.
4.1. Gel property
In the livestock and poultry tissues, protein oxidation exists, and the degree of oxidation varies depending on the species and age of the livestock and poultry (Zhang et al., 2022). The aging process of hens is associated with a decrease in the Haugh units (HU) of eggs. As the HU of eggs decreases, the spatial structure of egg white protein aggregates to form insoluble aggregates, the negative charge on the protein surface decreases significantly, the electrostatic repulsion and stability of egg white protein solution decrease, causing the aggregation of egg white protein during heating. As the age of the laying hens increases, the gel strength and water-holding capacity of egg white protein decrease with the decrease in HU. It is worth noting that the content of free thiol groups in egg white protein with lower HU values decreases. The loss of thiol groups is a sign of protein oxidation; therefore, protein oxidation is an important reason for the differences in the thermal gelation of egg white proteins of different qualities during the aging process of laying hens (Li, Mi, et al., 2023).
The process of protein oxidation bears significance during the storage of eggs as well. Within the confinement of storing fresh duck eggs, the pH level ascends, triggering the liberation of riboflavin from the egg white. Acting as an inherent photosensitizer, riboflavin orchestrates protein oxidation via photosensitizing reactions, inclusive of singlet oxygen emission (Li et al., 2024). Over the duration of storage, the protein carbonyl group content escalates while the sulfhydryl group content dwindles, signifying the oxidation of egg yolk proteins during this period. Post a 6-month storage span, the reconstituted yolk powder unveils a lax gel configuration (Tian et al., 2023). Moderate protein oxidation can enhance the gel characteristics of proteins; however, excessive oxidation yields adverse effects. Bao et al. (Bao et al., 2017) observed that upon ovalbumin modification by AAPH, controlled oxidation (0-1 mmol/L) fosters carbonyl formation within ovalbumin, unveils hydrophobic group exposure, reinforces hydrophobic interactions among molecules, and augments gel potency. Conversely, heightened oxidation levels (1-5 mmol/L) impede disulfide bond formation, lessen surface hydrophobicity, spur protein breakdown into polypeptides, thereby engendering a lax gel network structure, along with reduced gel robustness and water retention capacity of ovalbumin.
The molecular architecture of poultry egg protein undergoes transformation under the influence of heat, salt, alkali, and mechanical action, culminating in gel formation through thermal induction. Abundant in lipids and proteins, egg yolks are predisposed to both direct and indirect oxidation. Research indicates that by meticulously blending egg white and egg yolk, subsequently subjecting them to reheating (100 °C, 30 min), the elasticity and texture of egg yolk gel can be enhanced. Agitation disrupts the protein framework, facilitating the even dispersion of lipids and proteins within the gel structure, thereby amplifying gel suppleness. Moreover, the amalgamation of egg yolk lipoproteins with oxygen molecules during thorough mixing may provoke oxidation, consequently influencing its functional attributes. Hence, stirring prior to heating stands as a means to refine the gel characteristics of egg yolk proteins (Liu, Wang, et al., 2023).
Sodium chloride exerts an influence on the gel characteristics of egg proteins by modulating the interactions between protein molecules. A milieu of heightened ionic strength augments the protein's susceptibility to free radicals and pro-oxidative elements. Wongnen et al. (Wongnen et al., 2023b) treated low-salt salted duck eggs with ozone brine and observed that ozone facilitated protein oxidation, hastened protein modification aggregation, impeded salt infiltration into the eggs, diminished the salt content of salted eggs, and enhanced the gel attributes of these preserved eggs. Notably, the presence of protein carbonyl groups post-boiling in salted egg yolk surpasses that in salted egg white, potentially linked to the instigation of protein oxidation by lipid oxidation within the yolk (Wongnen et al., 2023a). Furthermore, protein oxidation transpires throughout the preservation of egg derivatives. Xue et al. (Xue, Liu, et al., 2023) additionally substantiated that protein oxidation stood as the primary cause behind the mudding of salted egg yolk consistency.
Alkali treatment stands as an exclusive culinary technique, and preserved eggs represent its quintessential manifestation. Crafted from fresh poultry eggs under the collective impact of NaOH, NaCl, and CuSO4, preserved eggs exemplify a distinctive delicacy. Throughout the curing journey, the elevated pH level catalyzes a sequential metamorphosis of egg white, transitioning from liquefaction to solidification and eventual discoloration, culminating in the creation of a dark-brown, translucent gel with a distinctively flavorful profile (Ai & Jiang, 2021). To avert undue alkali-induced damage to the egg white gel, a judicious incorporation of metallic compounds becomes imperative to regulate the infiltration of lye. This strategic addition elevates the levels of inorganic elements, such as sodium, iron, magnesium, and copper in the egg white, towering above those found in fresh egg white (Zhao et al., 2014). Through their interaction with proteins, metal ions induce structural alterations, fostering protein aggregation via intermolecular forces. Research outcomes reveal that the firmness of the egg white gel undergoes an initial rise followed by attenuation with mounting NaOH concentrations. Subdued levels of metal ions aid in constructing a robust microstructure, heightening the gel's textural finesse. Conversely, heightened metal ion concentration amplifies electrostatic repulsion amidst egg white protein molecules, impeding protein amalgamation and consequently diminishing gel rigidity (Xue, Han, et al., 2023). Noteworthy in the evolution of preserved eggs, metal ions potentially act as catalysts or accelerants in protein oxidation. Nonetheless, conclusive evidence delineating the precise mechanism of protein oxidation during the pickling of preserved eggs remains elusive, underscoring the necessity for further exploration to illuminate this intricate process.
4.2. Foaming properties
The characteristics of foaming properties not only foaming power but also foam stability, with the foaming properties of egg white protein intricately influenced by protein oxidation. Zhang et al. (Zhang, Gong, et al., 2023) have demonstrated that a measured level of protein oxidation (5 mmol/L H2O2) can enhanced the EWP interfacial, rheological and foaming properties by forming more disordered and flexible structures, with more hydrophobic interactions and cleavage sites. Correspondingly, findings by Duan et al. (Duan et al., 2018) suggest that oxidation treatment could modify molecular arrangement and interaction of egg white proteinvia changing their dityrosine formation, sulfhydryl-disulfide interchange, surface hydrophobicity and secondary structures (Table 1). A judicious level of oxidation treatment (0.2 mmol/L AAPH) is shown to enhance the foaming efficacy of egg white protein. Conversely, when subjected to an overabundance of oxidation treatment (25 mmol/L AAPH), a substantial accumulation of egg white protein is observed, thereby diminishing its foam stability at this juncture of oxidative progression, oxidation treatment might be a useful approach to modify foaming ability of egg white protein.
Table 1.
Protein oxidation markers in livestock products.
| Materials | Oxidation system | Oxidation marker |
References | ||
|---|---|---|---|---|---|
| Carbonyl | Sulfhydryl | Dityrosine | |||
| Beef | AAPH | ↑ | ↓ | – | (Yin et al., 2023) |
| Duck | AAPH | ↑ | – | – | (Zhu, Zhang, et al., 2023) |
| Myofibrillar | AAPH | ↑ | ↓ | – | (Yu et al., 2024) |
| Pork | Fenton | ↑ | ↓ | – | (Li et al., 2012) |
| Egg white powder | Fenton | ↑ | ↓ | ↑ | (Wang et al., 2018) |
| Egg white powder | Fenton | – | ↓ | – | (Alavi et al., 2019) |
| Egg | AAPH | ↑ | ↓ | (Cheng et al., 2021) | |
| Egg white | AAPH | – | ↑ and then ↓ | ↑ | (Duan et al., 2018) |
| Ovalbumin | Fenton | ↑ | ↓ | – | (Li et al., 2019) |
| albumin | AAPH | ↑ | ↓ | – | (Bao et al., 2017) |
| Egg white | AAPH | – | ↑ and then ↓ | ↑ | (Zhang, Gong, et al., 2023) |
| Whey protein (WPI) | AAPH | ↑ | ↓ | ↑ | (Feng et al., 2015) |
| Whey protein (WPI) | Fenton | ↑ and then ↓ | – | – | (Berton-Carabin, Schröder, Rovalino-Cordova, Schroën, & Sagis, 2016) |
| Whey protein Concentrate (WPC) | Fenton | ↑ | ↓ | – | (Feng et al., 2022) |
| Whey protein (WPI) | Fenton | – | ↓ | ↑ | (Kong et al., 2011) |
“-” indicates that the system is not queried.
Furthermore, the evolution of egg white protein's foamability throughout storage appears intricately linked to protein oxidation and the yield of ROS. Investigations have revealed a marked elevation in the levels of carbonyl and tyrosine within egg white over storage durations, concomitant with a decline in free sulfhydryl group content, a phenomenon likely associated with the ongoing oxidation process (Li et al., 2024). Notably, it is worth underscoring that measured protein oxidation not only enhances the foamability of egg white protein but also manifests superior functional attributes under specific pH regimes. For instance, at a pH level of 11, both the foamability and foam stability of egg white protein exhibit substantial enhancement, a transformation potentially linked to oxidative aggregation occurrences (Alavi et al., 2019). These revelatory insights furnish a pivotal theoretical foundation for the utilization of egg white protein in the realm of food processing.
4.3. Emulsifying properties
Emulsification stands as a pivotal functional facet of egg protein. Within the egg yolk, approximately 33 % comprises lipids and 16 % consists of proteins, predominantly complex proteins, notably lipoproteins. These lipoproteins encompass yolk high phosphorus protein, low-density lipoprotein, high-density lipoprotein, among others, with their distinctive composition dictating their unique emulsifying characteristics. For the long-term preservation of egg yolks, spray drying represents a prevalent technique. Nonetheless, the elevated temperatures inherent in the spray drying process can instigate lipid chain oxidation reactions, fostering the generation of an abundance of lipid free radicals and active oxidation byproducts. This cascade of events culminates in the oxidative transformation of protein structures and the carbonylation of the lipoproteins themselves (Van der Plancken et al., 2005). The process of spray drying encourages the aggregation of oxidized proteins, leading to the formation of a more viscoelastic film, thus markedly augmenting the emulsifying capacity of egg white protein. Consequently, spray-dried egg yolks exhibit a heightened propensity to yield stable emulsions compared to fresh yolks (Rannou et al., 2015). Moreover, studies have elucidated that ultrasonic treatment represents another avenue for enhancing the emulsification prowess of egg yolks, with its efficacy closely intertwined with the intensity of ultrasonic power. Moderate ultrasonic treatment at 225 W effectively bolstered the emulsification efficacy and stability of egg yolks, attributed to the enhancement of surface hydrophobicity and the reinforcement of the hydrophilic-lipophilic balance within low-density lipoprotein components of egg yolks. This enhancement resulted in escalated adsorption rates and heightened interface stability. However, excessive ultrasonic treatment may disrupt the emulsion structure, thereby diminishing emulsion stability (Xie et al., 2020) (See Table 2).
Table 2.
Effects of measure on protein oxidation and its consequences.
| Material | Measure | Functional characteristics | Consequence | References |
|---|---|---|---|---|
| Mutton | Storage under high pressure conditions (200 MPa/500 MPa) at 4 °C for 1, 3, or 7 days. | Water holding capacity | An application of 200 MPa pressure can enhance the textural attributes of lamb, while a pressure of 500 MPa exacerbates the color and water retention properties of the meat. Stress-induced protein oxidation escalates with prolonged storage duration, consequently resulting in diminished water retention capacity in lamb. | (Li et al., 2021) |
| Duck | Marinated (infused with 1.5 g/kg of ginger, 0.5 g/kg of fennel, and 0.5 g/kg of cinnamon) at 4 °C for 24 h. | Digestibility | Throughout the curing process, oxidation and aggregation of sarcoplasmic proteins result in diminished nutritional quality. | (Du et al., 2018) |
| Beef | Frozen storage (−18 °C, 20 weeks) | – | Protein cross-linking and Schiff base formation, accompanied by texture degradation, diminished tryptophan fluorescence, and the creation of lysine derivatives. Beef is more prone to oxidation owing to the pro-oxidative influence of heme. | (Utrera & Estévez, 2013) |
| Pork | Ultrasonic assisted vacuum tumbling (0 W, 100 W, 300 W, 60 min, 120 min) | Solubility | The configuration and physicochemical attributes of myofibrillar protein underwent alteration with the rise in carbonyl content.. | (Zhang et al., 2021) |
| horseflesh | Low-frequency magnetic field (0, 3, 6, 12mT treatment 1 h) | – | High intensity magnetic field (> 12mT) can significantly inhibit hydroxyl radical induced heme iron oxidation. | (Jiang, Xia, et al., 2024) |
| Pork | Packaging (70 %O2, 30 %CO2) | Water holding capacity | In high‑oxygen packaging environments, the sulfhydryl group content diminished, leading to protein oxidation and a reduction in the succulence of the meat. | (Lund et al., 2007) |
| Pork | Packaging (oxygen concentration 40 %, 60 %, 80 %) | Water holding capacity | A diminished oxygen concentration of 40 % notably enhances the hue and delicacy of the meat, concurrently escalating the degree of protein oxidation with augmented oxygen levels. | (Li et al., 2022) |
| Chicken | Frozen storage (−7 °C, −12 °C, −18 °C) | – | Protein oxidation manifested across three discrete temperatures. With heightened temperatures, the magnitude of protein oxidation increased significantly, notably surpassing in chicken leg compared to chicken breast. | (Soyer et al., 2010) |
| Foal meat | Bake/fry/microwave/fry | Special flavor | Barbecue and frying have little effect on oxidation, and moderate oxidation is beneficial to the formation of flavor | (Domínguez et al., 2014) |
| Pork | Heating (60 °C, 100 °C, 140 °C, 30 min) | Digestibility | Protein oxidation caused by heating decreased the pepsin digestibility of pork | (Bax et al., 2012) |
| Beef | Frozen storage (0, 2, 4, 6 months at −18 °C) | Special flavor | The content of carbonyl group increases linearly during freezing storage. | (Al-Dalali et al., 2022) |
| Pork | NaCl(0, 0.2, 0.6 M) | Emulsibility | High concentration of NaCl (6 M) reduced the emulsification of myofibrillar protein emulsion gel. | (Feng et al., 2017) |
| Egg white powder | Storage (0, 1, 3, 6 months at 37 °C) | Gel property | Storage oxidizes protein in egg white powder and affects its gelatinicity after rehydration. | (Tian et al., 2023) |
| Egg white | Pressure (100-700 MPa) | Gel property | High-pressure-induced EWP gels are softer and more elastic than heat-induced gels. | (Van der Plancken et al., 2005) |
| Egg white | Storage (1, 7, 14, 21, 28d at 40 °C) | Foaming characteristic, Emulsibility | The levels of carbonyl groups and dityrosine rose, while the amount of sulfhydryl groups declined. Moderate oxidation bolstered the foaming and emulsifying capacities of egg white protein, although excessive oxidation proved detrimental. | (Li et al., 2024) |
| Whey Protein (WPI) | Extrusion temperature (50, 75, 100 °C) | Digestibility | After extrusion treatment, the increase/decrease decreased, and extrusion at 50 °C had the best digestibility. | (Jiang et al., 2023) |
| Lactoprotein | Fluorescence irradiation (400-600 nm) | Hydrolyzability | Upon exposure to fluorescence irradiation, oxidation occurred in tryptophan, histidine, methionine, and tyrosine, leading to an elevation in the carbonyl group content. | (Dalsgaard & Larsen, 2009) |
| Camel milk | Dry heat (80 °C, 5, 10, 24 h) | Solubility | Drying and heating treatment decreased the sulfhydryl content of whey protein, increased its solubility and improved emulsification activity and stability. | (Ma et al., 2019) |
| Milk | Light, temperature (70 °C, 0, 48, 96, 144 h) | Digestibility | The presence of unsaturated fatty acids promotes protein oxidation and affects digestibility. | (Obando et al., 2015) |
| β- lactoglobulin | Ultraviolet radiation(302 nm, 1.1 ± 0.5 mW cm−2-1.9 ± 0.1 mW cm−2) | Heat stability | The content of carbonyl group increases, and the thermal stability of β-lactoglobulin decreases. | (Fitzner et al., 2023) |
| Whey Protein (WPI) | Ultra-high magnetic field (5, 10, 15, 20mT) | Foaming characteristic | The sulfhydryl content of the protein diminished, resulting in a more structured protein framework, aggregation, a notable increase in particle size, and substantial enhancements in antioxidant activity, foaming attributes, stability, as well as water/oil characteristics. | (Liu, Hou, et al., 2023) |
| Whey Protein (WPI) | Ozonation(30–480 min, at a temperature of 20 °C and relative humidity of 70 %) | Foaming characteristic | After ozone treatment, the free sulfhydryl group was reduced, the protein structure was changed, and the foamability and foam stability were improved. | (Segat et al., 2014) |
| Milk | Exposure to ultraviolet radiation (6 W, 302 nm UV lamp) under storage conditions of 25 or 40 °C and at relative humidities of 11 %, 33 %, or 75 % | Solubility | Uv-induced milk protein decreased and changed color after photooxidation. | (Semagoto et al., 2014) |
“-” indicates that the system is not queried.
External factors play a pivotal role in shaping the emulsifying attributes of egg white protein. As eggs are stored, the dynamics of egg white emulsification undergo a transformation characterized by an initial rise followed by a decline, a phenomenon intricately linked to protein oxidation. In the initial phase of storage, the onslaught of free radicals augments protein flexibility, exposes non-polar groups, and amplifies oil-water interface adsorption, culminating in peak emulsification efficacy around the 7-day mark. However, beyond this threshold, oxidation induces protein aggregation, diminishes flexibility, and consequently lowers emulsification outcomes (Li et al., 2024). Ovalbumin, boasting the highest protein content and free sulfhydryl group concentration in egg white (Pathania et al., 2019), exhibits a significantly altered emulsification performance in response to oxidative influences. Li et al. (Li et al., 2019) revealed that hydroxyl radical-induced oxidation augments the carbonyl content of egg albumin while reducing the sulfhydryl content, thereby reshaping the surface chemical groups and structure of egg albumin and consequently impacting its interfacial traits. Optimal oxidation in the range of 0–2 h can elevate egg albumin emulsification rates by 1.2–1.8 times, whereas excessive oxidation beyond 3 h may impede emulsification efficacy.
4.4. Digestive properties
Oxidation heightens the susceptibility of egg white protein to pepsin while concurrently unraveling its spatial structure, exposing additional sites for enzymatic breakdown, thereby enhancing its digestibility (Zhang, Gong, et al., 2023). Extensive literature corroborates that heat treatment substantially enhances the digestibility of egg protein by augmenting enzyme diffusion rates and bolstering the sensitivity of hydrolysis sites (Farjami et al., 2021). In a study by Nolasco et al. (Nolasco et al., 2021) the impact of various cooking methods on egg protein digestibility was assessed through in vitro digestion experiments. The findings revealed that eggs boiled in vigorously bubbling water for 10 min exhibited superior digestibility compared to eggs fried at 191 °C for 90 s. Moreover, it is noted that alkali-induced protein gels demonstrate enhanced digestibility compared to heat-induced counterparts. This phenomenon arises from the comparatively dense structure of heat-induced egg white gels, as opposed to the orderly and porous microstructure observed in preserved egg gels, rendering the latter more amenable to digestive enzymes. Research indicates that after a 14-day pickling process at 25 °C involving 4 %NaOH, 3 %NaCl, and 0.3 %CuSO4, preserved eggs undergo hydrolysis of egg white proteins into smaller peptides and amino acids, thus facilitating digestion (Guo et al., 2019). Moreover, Deng et al. (Deng et al., 2020) observed that the addition of varying concentrations of KCl, CaCl2, ZnCl2, and FeCl3 can enhance the digestibility of egg white gels, with digestibility increasing in tandem with metal ion concentration. Lower concentrations of metal ions promote the formation of a structured microarchitecture in egg white gels. Conversely, elevated concentrations of metal ions can disrupt the structure, rendering it more susceptible to enzymatic breakdown.
The utilization of autoclaving technology is prevalent in the production of eggs and egg derivatives. Its ability to disrupt and alter protein structures can heighten enzyme accessibility, thereby enhancing protein digestibility (Bhat et al., 2021). Employing precise pressure-heat treatments (PHT) in the preparation of soft-boiled eggs can uncover additional enzyme cleavage sites, thereby optimizing protein digestibility. Nonetheless, prolonged exposure to PHT may trigger protein aggregation and interactions with sugars or fats, ultimately diminishing digestibility. Nevertheless, this process also fosters lipid oxidation, which contributes to the development of specific flavors (Zhang, Li, et al., 2023).
Poultry eggs, valued for their high-quality proteins, have functional properties like gelation, foaming, emulsification, and digestibility influenced by temperature, pressure, storage, and chemical agents. Protein oxidation begins during egg formation, storage, heating, drying, and marination, leading to protein carbonylation and reduced free thiol content. Moderate oxidation can enhance egg white protein digestibility by increasing sensitivity to gastric proteases, while also improving emulsification and foaming through altered protein interactions. However, research on protein oxidation in traditional Chinese egg products, such as salted eggs and century eggs, remains limited. The patterns of oxidation during processing and their effects on functional properties are not well understood, highlighting the need for further in-depth studies to fill this knowledge gap.
5. Protein oxidation and its effect on functional properties during the processing and storage of dairy products
Milk, rich in unsaturated fatty acids, flavorful compounds, and metal elements, is highly prone to oxidation (Berton-Carabin, Schröder, Rovalino-Cordova, Schroën, & Sagis, 2016). Whey protein isolate, in particular, is vulnerable to oxidative damage during processing, storage, and use due to its high content of amino and sulfhydryl groups. This oxidation not only changes the physicochemical properties of whey protein isolates but also significantly limits their functionality and application in the food industry (Jiang et al., 2023). Controlling oxidation is essential to preserving the quality and versatility of whey protein isolates.
5.1. Emulsifying properties
Whey proteins predominantly consist of globular proteins rich in cystine and disulfide bonds within their molecular framework. During thermal sterilization processes, milk proteins and other constituents undergo chemical alterations, resulting in the restructuring of protein spatial configurations. Consequently, this brings about changes in protein conformation and a subsequent loss of activity (Liu et al., 2022). In conditions of elevated temperature processing like cooking and baking, proteins face an increased likelihood of exposure to oxidative environments. This exposure triggers disulfide bond cleavage in α-lactoprotein, conformational shifts in β-lactoglobulin disulfide bonds, and modifications to endogenous tryptophan residues. Furthermore, the intensity of oxidation amplifies with higher temperatures (Kramer et al., 2017). Research conducted by Ma et al. (Ma et al., 2019) unveiled that heating treatments reduce the sulfhydryl content of whey protein, while controlled protein oxidation can enhance solubility, bolster emulsification capabilities, and fortify stability. Within condensed milk, lipid peroxidation instigates the generation of methionine sulfoxide, subsequently giving rise to ROS. Methionine residues are susceptible to oxidation by ROS to form methionine sulfoxide via hydrogen peroxide or electron transfer mechanisms, which can further progress to methionine sulfone (Dyer et al., 2016). Such oxidation processes hamper the proteins' capacity to construct tightly packed, interlinked viscoelastic films at the oil-water interface, leading to reduced interfacial elasticity and aggregate formation. These alterations markedly impact their stability within food emulsification systems (Berton-Carabin, Schröder, Rovalino-Cordova, Schroën, & Sagis, 2016).
Furthermore, specific amino acids within proteins, such as tryptophan and tyrosine, possess the capability to directly absorb ultraviolet light and transition into excited states, thus undergoing photoionization and oxidation processes (Obando et al., 2015). Simultaneously, photosensitive compounds like vitamin B2 can absorb both visible and ultraviolet light, transitioning into excited states to catalyze protein oxidation. Within retail dairy showcases, dairy items frequently encounter light exposure emanating from fluorescent lamps or light-emitting diodes (leds), supplying light energy across ultraviolet and visible wavelength spectrums. Prolonged exposure to light precipitates the photooxidation of vitamin B2 and other photosensitive compounds in dairy goods, yielding singlet oxygen and thereby diminishing the nutritional quality of dairy products. With extended periods of storage, the risk associated with light exposure escalates significantly (Schiano et al., 2019). In the case of β-casein and β-lactoglobulin, the existence of H2O2 and Cu2+ hastens the depletion of tryptophan induced by ultraviolet rays and fosters protein oxidation (Scheidegger et al., 2016).
The process of oxidation not only alters the internal structure and composition of proteins but also impacts their functional attributes. When subjected to extrusion at 50 °C, whey protein isolate, indirectly affected by lipid oxidation byproducts, demonstrates optimized hydrophobicity, net charge, and emulsification characteristics (Jiang et al., 2023). Exposure to ultra-high magnetic fields (UMF) at intensities of 5, 10, 15, and 20 T induces the transformation of C—C bonds in whey protein into C-O-C(H), further progressing to COOH; concurrently, the oxidation of the N-C=O group leads to COOH formation. Furthermore, the sulfhydryl group undergoes oxidation to form disulfide bonds. Following the 10 T treatment, whey protein evolves into a highly condensed polymer with a particle size 36 times larger than that of the untreated specimen, significantly enhancing water/oil absorption capacity and consequently improving milk emulsification capability (Liu, Hou, et al., 2023). By delving into the protein oxidation paradigm, the impact of oxidation on whey protein emulsification was scrutinized. Results revealed a notable enhancement in the emulsification of whey protein with H2O2 concentration of 5 mmol/L and an oxidation duration of 5 h (Kong et al., 2011).
5.2. Other characteristics
During the initial phase of ROS-induced protein oxidation (1−2h), proteins come together to form supple aggregates characterized by commendable solubility and robust stability. Yet, as oxidation progresses (3–4 h), these supple aggregates gradually evolve into compact insoluble formations through covalent interactions (such as protein-protein cross-linking, carbonyl cross-linking, and aromatic side chain cross-linking), leading to diminished solubility and stability (Feng et al., 2022). In an exploration by Semagoto et al. (Semagoto et al., 2014) discernible protein oxidation was identified in milk powder following 6 days of ultraviolet exposure, where the carbonyl group content surged from 2 nmol/mg to 15 nmol/mg. As the duration of ultraviolet exposure extends, the carbonyl content persistently rises, causing protein structural unfolding and exposure of hydrophobic groups, consequently impacting the thermal stability of β-lactoglobulin (Fitzner et al., 2023). Moreover, the extent of photooxidation in milk is influenced by the oxygen concentration, while the intensity of light governs the pace of the photooxidation process. Findings indicate a positive correlation between the level of oxidation and both light intensity and oxygen content. Protein oxidation not only impacts the structural integrity and thermal resilience of proteins but also brings about substantial alterations in the foam-forming capacity of whey protein. In a study by Segat et al. (Segat et al., 2014), it was observed that ozone treatment instigated the oxidation of whey protein isolates, characterized by heightened surface hydrophobicity and a decline in free sulfhydryl group levels, thus enhancing their foam-forming attributes.
Oxidative stress significantly affects milk proteins, altering their solubility, thermal stability, emulsification, and foaming properties. Key oxidation sites include cysteine, methionine, tryptophan, and tyrosine, with oxidation intensifying at higher temperatures and longer heating times. Milk's high fat content facilitates lipid peroxidation, converting methionine into methionine sulfoxide and sulfone, which reduces proteins' ability to form stable films at oil-water interfaces, decreasing interfacial elasticity and emulsification efficiency. Photosensitive substances like vitamin B2 absorb light, triggering oxidative reactions with proteins. Moderate oxidation can improve milk's thermal stability, foaming, and emulsification. However, excessive oxidation causes protein denaturation, degradation, and oxidation, which continue during storage, shortening shelf life and compromising quality and nutritional value. Controlling oxidation is crucial to maintaining milk's functional and nutritional properties.
6. Conclusions and prospects
Protein oxidation in livestock products, influenced by processing and storage conditions, significantly affects their functional properties. Moderate oxidation can enhance functionality by unfolding protein structures and exposing hydrophobic groups, improving edibility. However, excessive oxidation, caused by prolonged processing, high intensities, or temperatures, risks protein carbonylation, degrading product quality and nutrition. To mitigate this, strategies like reducing oxidative stress, removing pro-oxidants, and adding antioxidants are employed to control oxidation levels and preserve product integrity.
Despite extensive research on protein oxidation, critical challenges remain. First, there is limited understanding of the relationship between protein oxidation and functional properties in dairy and egg products, particularly in traditional Chinese egg processing. Further studies are needed to explore how oxidation affects gelation and digestibility. Second, the mechanisms of oxidation-induced protein oxidation in livestock products are not fully understood, requiring deeper investigation into the varying effects of different oxidants. The complexity of protein oxidation products, due to multiple oxidation sites, underscores the need for reliable markers to distinguish protein oxidation from other reactions.
A key priority for future research is developing standardized methods to accurately measure moderate to excessive protein oxidation levels during livestock product processing and storage. Such advancements would enhance quality control and drive innovation in processing technologies, ensuring better preservation of product quality and nutritional value.
CRediT authorship contribution statement
Jiamei Wang: Writing – review & editing, Writing – original draft. Na Wu: Writing – review & editing. Yao Yao: Writing – review & editing. Shuping Chen: Writing – review & editing. Lilan Xu: Writing – review & editing. Yan Zhao: Writing – review & editing, Supervision, Project administration, Funding acquisition. Yonggang Tu: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge the support provided by the National Natural Science Foundation of China (Grant Nos. 32260608 and 32472395), the Training Project of High-level and High-skilled Leading Talents of Jiangxi Province (29202300002) and the Jiangxi Provincial Key Research and Development Project (20232BBF60025).
Contributor Information
Yan Zhao, Email: zhaoyan@jxau.edu.cn.
Yonggang Tu, Email: tygzy1212@jxau.edu.cn.
Data availability
No data was used for the research described in the article.
References
- Ai M., Jiang A. Phosphorylation modification affects the gelation behavior of alkali-induced duck egg white gels. Food Chemistry. 2021;340 doi: 10.1016/j.foodchem.2020.128185. [DOI] [PubMed] [Google Scholar]
- Akagawa M. Protein carbonylation: Molecular mechanisms, biological implications, and analytical approaches. Free Radical Research. 2021;55(4):307–320. doi: 10.1080/10715762.2020.1851027. [DOI] [PubMed] [Google Scholar]
- Alavi F., Emam-Djomeh Z., Momen S., Mohammadian M., Salami M., Moosavi-Movahedi A.A. Effect of free radical-induced aggregation on physicochemical and interface-related functionality of egg white protein. Food Hydrocolloids. 2019;87:734–746. doi: 10.1016/j.foodhyd.2018.08.048. [DOI] [Google Scholar]
- Al-Dalali S., Li C., Xu B. Effect of frozen storage on the lipid oxidation, protein oxidation, and flavor profile of marinated raw beef meat. Food Chemistry. 2022;376 doi: 10.1016/j.foodchem.2021.131881. [DOI] [PubMed] [Google Scholar]
- Bao Y., Ertbjerg P. Effects of protein oxidation on the texture and water-holding of meat: A review. Critical Reviews in Food Science and Nutrition. 2019;59(22):3564–3578. doi: 10.1080/10408398.2018.1498444. [DOI] [PubMed] [Google Scholar]
- Bao Z.-J., Wu J.-P., Cheng Y., Chi Y.-J. Effects of lipid peroxide on the structure and gel properties of ovalbumin. Process Biochemistry. 2017;57:124–130. doi: 10.1016/j.procbio.2017.03.009. [DOI] [Google Scholar]
- Baraibar M.A., Ladouce R., Friguet B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. Journal of Proteomics. 2013;92:63–70. doi: 10.1016/j.jprot.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Bax M.-L., Aubry L., Ferreira C., Daudin J.-D., Gatellier P., Rémond D., Santé-Lhoutellier V. Cooking temperature is a key determinant of in vitro meat protein digestion rate: Investigation of underlying mechanisms. Journal of Agricultural and Food Chemistry. 2012;60(10):2569–2576. doi: 10.1021/jf205280y. [DOI] [PubMed] [Google Scholar]
- Bekhit A.E.D.A., Hopkins D.L., Fahri F.T., Ponnampalam E.N. Oxidative processes in muscle systems and fresh meat: Sources, markers, and remedies. Comprehensive Reviews in Food Science and Food Safety. 2013;12(5):565–597. doi: 10.1111/1541-4337.12027. [DOI] [PubMed] [Google Scholar]
- Berton-Carabin C.C., Schröder A., Rovalino-Cordova A., Schroën K., Sagis L. Protein and lipid oxidation affect the viscoelasticity of whey protein layers at the oil–water interface. European Journal of Lipid Science and Technology. 2016;118(11):1630–1643. doi: 10.1002/ejlt.201600066. [DOI] [Google Scholar]
- Bhat Z.F., Morton J.D., Bekhit A.E.D.A., Kumar S., Bhat H.F. Effect of processing technologies on the digestibility of egg proteins. Comprehensive Reviews in Food Science and Food Safety. 2021;20(5):4703–4738. doi: 10.1111/1541-4337.12805. [DOI] [PubMed] [Google Scholar]
- Chen G., Xu C., Wang Z., Han Z., Xia Q., Wei S.…Liu S. Effect of MDA-mediated oxidation on the protein structure and digestive properties of golden pomfret. Food Chemistry. 2024;443 doi: 10.1016/j.foodchem.2024.138563. [DOI] [PubMed] [Google Scholar]
- Chen X., He Z., Wang Z., Li H. The effect of the purslane polyphenols on the structure of rabbit meat myofibrillar protein under malondialdehyde-induced oxidative stress. Journal of Food Science. 2023;88(5):1924–1938. doi: 10.1111/1750-3841.16510. [DOI] [PubMed] [Google Scholar]
- Cheng J., Sun J., Yao K., Xu M., Dai C. Multi-task convolutional neural network for simultaneous monitoring of lipid and protein oxidative damage in frozen-thawed pork using hyperspectral imaging. Meat Science. 2023;201 doi: 10.1016/j.meatsci.2023.109196. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chi Y., Geng X., Chi Y. Effect of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) induced oxidation on the physicochemical properties, in vitro digestibility, and nutritional value of egg white protein. LWT. 2021;143 doi: 10.1016/j.lwt.2021.111103. [DOI] [Google Scholar]
- Dalsgaard T.K., Larsen L.B. Effect of photo-oxidation of major milk proteins on protein structure and hydrolysis by chymosin. International Dairy Journal. 2009;19(6–7):362–371. doi: 10.1016/j.idairyj.2008.12.005. [DOI] [Google Scholar]
- Davies M.J. Protein oxidation and peroxidation. Biochemical Journal. 2016;473(7):805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Shao Y., Xu M., Yao Y., Wu N., Hu H.…Tu Y. Effects of metal ions on the physico-chemical, microstructural and digestion characteristics of alkali-induced egg white gel. Food Hydrocolloids. 2020;107 doi: 10.1016/j.foodhyd.2020.105956. [DOI] [Google Scholar]
- Domínguez R., Gómez M., Fonseca S., Lorenzo J.M. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Science. 2014;97(2):223–230. doi: 10.1016/j.meatsci.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Domínguez R., Pateiro M., Munekata P.E.S., Zhang W., Garcia-Oliveira P., Carpena M.…Lorenzo J.M. Protein oxidation in muscle foods: A comprehensive review. Antioxidants. 2021;11(1) doi: 10.3390/antiox11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongdong L., Yaling P., Haihong Z., Yingmei T., Dunhua L. Relationship between quality changes of post-rigor tan mutton and myofibrillar protein following high-pressure treatment. CyTA - Journal of Food. 2021;19(1):378–388. doi: 10.1080/19476337.2020.1863476. [DOI] [Google Scholar]
- Du X.J., Sun Y.Y., Pan D.D., Wang Y., Ou C.R., Cao J.X. The effect of structural change on the digestibility of sarcoplasmic proteins in Nanjing dry-cured duck during processing. Poultry Science. 2018;97(12):4450–4457. doi: 10.3382/ps/pey316. [DOI] [PubMed] [Google Scholar]
- Duan X., Li M., Shao J., Chen H., Xu X., Jin Z., Liu X. Effect of oxidative modification on structural and foaming properties of egg white protein. Food Hydrocolloids. 2018;75:223–228. doi: 10.1016/j.foodhyd.2017.08.008. [DOI] [Google Scholar]
- Dyer J.M., Clerens S., Grosvenor A., Thomas A., Callaghan C., Deb-Choudhury S., Haines S. Proteomic tracking of hydrothermal Maillard and redox modification in lactoferrin and beta-lactoglobulin: Location of lactosylation, carboxymethylation, and oxidation sites. Journal of Dairy Science. 2016;99(5):3295–3304. doi: 10.3168/jds.2015-10273. [DOI] [PubMed] [Google Scholar]
- Estévez M., Díaz-Velasco S., Martínez R. Protein carbonylation in food and nutrition: A concise update. Amino Acids. 2021;54(4):559–573. doi: 10.1007/s00726-021-03085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjami T., Babaei J., Nau F., Dupont D., Madadlou A. Effects of thermal, non-thermal and emulsification processes on the gastrointestinal digestibility of egg white proteins. Trends in Food Science & Technology. 2021;107:45–56. doi: 10.1016/j.tifs.2020.11.029. [DOI] [Google Scholar]
- Feng X., Chen L., Lei N., Wang S., Xu X., Zhou G., Li Z. Emulsifying properties of oxidatively stressed myofibrillar protein emulsion gels prepared with (−)-epigallocatechin-3-gallate and NaCl. Journal of Agricultural and Food Chemistry. 2017;65(13):2816–2826. doi: 10.1021/acs.jafc.6b05517. [DOI] [PubMed] [Google Scholar]
- Feng X., Li C., Ullah N., Cao J., Lan Y., Ge W.…Chen L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. Journal of Dairy Science. 2015;98(11):7602–7613. doi: 10.3168/jds.2015-9814. [DOI] [PubMed] [Google Scholar]
- Feng Y., Wang J., Hu H., Yang C. Effect of oxidative modification by reactive oxygen species (ROS) on the aggregation of whey protein concentrate (WPC) Food Hydrocolloids. 2022;123 doi: 10.1016/j.foodhyd.2021.107189. [DOI] [Google Scholar]
- Fitzner L., Kühl T., Hasler M., Imhof D., Schwarz K., Keppler J.K. Modification and oxidative degradation of β-lactoglobulin by UVB irradiation. Food Chemistry. 2023;136698 doi: 10.1016/j.foodchem.2023.136698. [DOI] [PubMed] [Google Scholar]
- Fu Z.X., Cai Y.L., Zhang Y., Chen W.J., Meng F.B., Liu D.Y. Nontargeted metabolomics reveals dynamic changes in the quality of fresh yak meat during ice-temperature preservation. LWT. 2024 doi: 10.1016/j.lwt.2024.116579. [DOI] [Google Scholar]
- Ganjeh A.M., Pinto C.A., Casal S., Saraiva J.A. The effects of pressure-based processing technologies on protein oxidation. Food Bioscience. 2024;59 doi: 10.1016/j.fbio.2024.103963. [DOI] [PubMed] [Google Scholar]
- Guo W., Zhao Y., Yao Y., Wu N., Xu M., Du H., Tu Y. Relationship between protein structure changes and in vitro digestion of preserved egg white during pickling. International Journal of Biological Macromolecules. 2019;138:116–124. doi: 10.1016/j.ijbiomac.2019.07.057. [DOI] [PubMed] [Google Scholar]
- Hellwig M. The chemistry of protein oxidation in food. Angewandte Chemie International Edition. 2019;58(47):16742–16763. doi: 10.1002/ange.201814144. [DOI] [PubMed] [Google Scholar]
- Hellwig M. Analysis of protein oxidation in food and feed products. Journal of Agricultural and Food Chemistry. 2020;68(46):12870–12885. doi: 10.1021/acs.jafc.0c00711. [DOI] [PubMed] [Google Scholar]
- Hong Z., Kong Y., Guo R., Huang Q. Stabilizing effect of silver carp myofibrillar protein modified by high intensity ultrasound on high internal phase emulsions: Protein denaturation, interfacial adsorption and reconfiguration. International Journal of Biological Macromolecules. 2024;265 doi: 10.1016/j.ijbiomac.2024.130896. [DOI] [PubMed] [Google Scholar]
- Huang Y., Sarkhel S., Roy A., Mohan A. Interrelationship of lipid aldehydes (MDA, 4-HNE, and 4-ONE) mediated protein oxidation in muscle foods. Critical Reviews in Food Science and Nutrition. 2023;1-17 doi: 10.1080/10408398. [DOI] [PubMed] [Google Scholar]
- Huang Z., Guan W., Lyu X., Chen R., Wu Y., Mao L. Impacts of acute ammonia-N exposure on the muscle quality of whiteleg shrimp (Penaeus vannamei): Novel insights into lipid and protein oxidation. Food Chemistry. 2023;137781 doi: 10.1016/j.foodchem.2023.137781. [DOI] [PubMed] [Google Scholar]
- Jiang J., Xia M., Gong H., Ma J., Sun W. Effect of magnetic field modification on oxidative stability of myoglobin in sarcoplasm systems. Food Chemistry. 2024;436 doi: 10.1016/j.foodchem.2023.137691. [DOI] [PubMed] [Google Scholar]
- Jiang J., Yang K., Gong H., Ma J., Hu X., Zhou Y.…Sun W. The conformational modification of myofibrillar protein by magnetic field improves its emulsification properties. International Journal of Biological Macromolecules. 2024;277 doi: 10.1016/j.ijbiomac.2024.134114. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Meng Y., Hou C., Gantumur M.-A., Gao Y., Huang Y.…Mu Z. Extrusion for reducing malondialdehyde-induced whey protein isolate oxidation in relation with its physicochemical, functional and intro digestive properties. Food Hydrocolloids. 2023;142 doi: 10.1016/j.foodhyd.2023.108730. [DOI] [Google Scholar]
- Kehm R., Baldensperger T., Raupbach J., Höhn A. Protein oxidation - formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biology. 2021;42 doi: 10.1016/j.redox.2021.101901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B., Xiong Y.L., Cui X., Zhao X. Hydroxyl radical-stressed whey protein isolate: Functional and rheological properties. Food and Bioprocess Technology. 2011;6(1):169–176. doi: 10.1007/s11947-011-0674-8. [DOI] [Google Scholar]
- Kramer A.C., Torreggiani A., Davies M.J. Effect of oxidation and protein unfolding on cross-linking of β-lactoglobulin and α-lactalbumin. Journal of Agricultural and Food Chemistry. 2017;65(47):10258–10269. doi: 10.1021/acs.jafc.7b03839. [DOI] [PubMed] [Google Scholar]
- Li C., Xiong Y.L., Chen J. Oxidation-induced unfolding facilitates myosin cross-linking in Myofibrillar protein by microbial transglutaminase. Journal of Agricultural and Food Chemistry. 2012;60(32):8020–8027. doi: 10.1021/jf302150h. [DOI] [PubMed] [Google Scholar]
- Li G., Mi S., Zeng Q., Wang L., Liu X., Zhang M.…Guo Y. Quantitative proteomics provides insights into the mechanism of the differences in heat-induced gel properties for egg white proteins with different interior quality during ageing in laying hens. Food Chemistry. 2023;419 doi: 10.1016/j.foodchem.2023.136031. [DOI] [PubMed] [Google Scholar]
- Li J., Wang Z., Xiao N., Guo S., Ai M. Endogenous reactive oxygen species (ROS)-driven protein oxidation regulates emulsifying and foaming properties of liquid egg white. International Journal of Biological Macromolecules. 2024;268 doi: 10.1016/j.ijbiomac.2024.131843. [DOI] [PubMed] [Google Scholar]
- Li Q., Sun X., Mubango E., Zheng Y., Liu Y., Zhang Y.…Hong H. Effects of protein and lipid oxidation on the water holding capacity of different parts of bighead carp: Eye, dorsal, belly and tail muscles. Food Chemistry. 2023;423 doi: 10.1016/j.foodchem.2023.136238. [DOI] [PubMed] [Google Scholar]
- Li S., Guo X., Shen Y., Pan J., Dong X. Effects of oxygen concentrations in modified atmosphere packaging on pork quality and protein oxidation. Meat Science. 2022;189 doi: 10.1016/j.meatsci.2022.108826. [DOI] [PubMed] [Google Scholar]
- Li S., Huang Y., An F., Huang Q., Geng F., Ma M. Hydroxyl radical-induced early stage oxidation improves the foaming and emulsifying properties of ovalbumin. Poultry Science. 2019;98(2):1047–1054. doi: 10.3382/ps/pey370. [DOI] [PubMed] [Google Scholar]
- Lin H., Zhang J., Huang Y., Wang F., Liu Y., Niu L. Effects of acetic acid and citric acid on quality properties of ground pork during storage and subsequent commercial sterilization. Journal of Food Measurement and Characterization. 2022;17(1):155–166. doi: 10.1007/s11694-022-01607-8. [DOI] [Google Scholar]
- Liu H., Xu Y., Zu S., Wu X., Shi A., Zhang J.…He N. Effects of high hydrostatic pressure on the conformational structure and gel properties of myofibrillar protein and meat quality: A review. Foods. 2021;10(8):1872. doi: 10.3390/foods10081872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang J., Wang H., Chen Q., Kong B. High-intensity ultrasound improves the physical stability of myofibrillar protein emulsion at low ionic strength by destroying and suppressing myosin molecular assembly. Ultrasonics Sonochemistry. 2021;74 doi: 10.1016/j.ultsonch.2021.105554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Han L., Cheng D., Li S., Chen X., Yu Y.…Zhang T. Incorporating ε-polylysine hydrochloride, tea polyphenols, nisin, and ascorbic acid into edible coating solutions: Effect on oxidation and structure of marinated egg proteins. Food Control. 2024;161 doi: 10.1016/j.foodcont.2024.110395. [DOI] [Google Scholar]
- Liu P., Hou M., Yue Y., Tong Y., Zhang T., Lu Z., Yang L. Effects of ultrahigh magnetic field on the structure and properties of whey protein. LWT. 2023;177 doi: 10.1016/j.lwt.2023.114590. [DOI] [Google Scholar]
- Liu Y., Hettinga K., Liu D., Zhang L., Zhou P. Current progress of emerging technologies in human and animals’ milk processing: Retention of immune-active components and microbial safety. Comprehensive Reviews in Food Science and Food Safety. 2022;21(5):4327–4353. doi: 10.1111/1541-4337.13019. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang K., Ma J., Wang Z., Zhu Q., Jin Y. Effect of yolk spheres as a key histological structure on the morphology, character, and oral sensation of boiled egg yolk gel. Food Chemistry. 2023;424 doi: 10.1016/j.foodchem.2023.136380. [DOI] [PubMed] [Google Scholar]
- Liu Z., Xiong Y.L., Chen J. Morphological examinations of oxidatively stressed pork muscle and myofibrils upon salt marination and cooking to elucidate the water-binding potential. Journal of Agricultural and Food Chemistry. 2011;59(24):13026–13034. doi: 10.1021/jf2041017. [DOI] [PubMed] [Google Scholar]
- Luna C., Arjona A., Dueñas C., Estevez M. Allysine and α-Aminoadipic acid as markers of the Glyco-oxidative damage to human serum albumin under pathological glucose concentrations. Antioxidants. 2021;10(3) doi: 10.3390/antiox10030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund M., Baron C. Chemical deterioration and physical instability of food and beverages. Elsevier; 2010. Protein oxidation in foods and food quality; pp. 33–69. [Google Scholar]
- Lund M.N., Lametsch R., Hviid M.S., Jensen O.N., Skibsted L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Science. 2007;77(3):295–303. doi: 10.1016/j.meatsci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Jiang Y., Chi Y. Effect of dry heating on the aggregation behaviour and aggregate morphologies of ovalbumin. Food Chemistry. 2019;285:296–304. doi: 10.1016/j.idairyj.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Manheem K., Adiamo O., Roobab U., Mohteshamuddin K., Hassan H.M., Nirmal N.P., Maqsood S. A comparative study on changes in protein, lipid and meat-quality attributes of camel meat, beef and sheep meat (mutton) during refrigerated storage. Animals. 2023;13(5):904. doi: 10.3390/ani13050904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor A., Haque A., Ahmad S., Hopkins D.L. Incorporation of betel leaf extract provides oxidative stability and improves phytochemical, textural, sensory and antimicrobial activities of buffalo meat sausages. Meat Science. 2023;200 doi: 10.1016/j.meatsci.2023.109157. [DOI] [PubMed] [Google Scholar]
- Nawaz A., Irshad S., Ali Khan I., Khalifa I., Walayat N., Muhammad Aadil R.…Lorenzo J.M. Protein oxidation in muscle-based products: Effects on physicochemical properties, quality concerns, and challenges to food industry. Food Research International. 2022;157 doi: 10.1016/j.foodres.2022.111322. [DOI] [PubMed] [Google Scholar]
- Nie X., Liu H., Yu N., Tang Q., Wu C., Meng X. Effect of high pressure homogenization on aggregation, conformation, and interfacial properties of bighead carp myofibrillar protein. Journal of Food Science. 2021;86(12):5318–5328. doi: 10.1111/1750-3841.15965. [DOI] [PubMed] [Google Scholar]
- Nolasco E., Yang J., Ciftci O., Vu D.C., Alvarez S., Purdum S., Majumder K. Evaluating the effect of cooking and gastrointestinal digestion in modulating the bio-accessibility of different bioactive compounds of eggs. Food Chemistry. 2021;344 doi: 10.1016/j.foodchem.2020.128623. [DOI] [PubMed] [Google Scholar]
- Obando M., Papastergiadis A., Li S., De Meulenaer B. Impact of lipid and protein co-oxidation on digestibility of dairy proteins in oil-in-water (O/W) emulsions. Journal of Agricultural and Food Chemistry. 2015;63(44):9820–9830. doi: 10.1021/acs.jafc.5b03563. [DOI] [PubMed] [Google Scholar]
- Park D., Xiong Y.L. Oxidative modification of amino acids in porcine myofibrillar protein isolates exposed to three oxidizing systems. Food Chemistry. 2007;103(2):607–616. doi: 10.1016/j.foodchem.2006.09.004. [DOI] [Google Scholar]
- Pathania S., Parmar P., Tiwari B.K. Stability of proteins during processing and storage. Proteins: Sustainable source, processing and applications. Elsevier; 2019. pp. 295–330. [DOI] [Google Scholar]
- Pérez-Palacios T., Ruiz J., Martín D., Barat J., Antequera T. Pre-cure freezing effect on physicochemical, texture and sensory characteristics of Iberian ham. Food Science and Technology International. 2011;17(2):127–133. doi: 10.1177/1082013210381435. [DOI] [PubMed] [Google Scholar]
- Poojary M.M., Lund M.N. Chemical stability of proteins in foods: Oxidation and the Maillard reaction. Annual Review of Food Science and Technology. 2022;13(1):35–58. doi: 10.1146/annurev-food-052720-104513. [DOI] [PubMed] [Google Scholar]
- Rannou C., Queveau D., Beaumal V., David-Briand E., Le Borgne C., Meynier A.…Loisel C. Effect of spray-drying and storage conditions on the physical and functional properties of standard and n− 3 enriched egg yolk powders. Journal of Food Engineering. 2015;154:58–68. doi: 10.1016/j.jfoodeng.2014.11.002. [DOI] [Google Scholar]
- Rowe L., Maddock K., Lonergan S.M., Huff-Lonergan E. Influence of early postmortem protein oxidation on beef quality. Journal of Animal Science. 2004;82(3):785–793. doi: 10.1093/ansci/82.3.785. [DOI] [PubMed] [Google Scholar]
- Rubén Domínguez M.P., Carpena M., Prieto M.A., Munekata P.E.S., Zhang W., Garcia-Oliveira P., Lorenzo J.M. Protein oxidation in muscle foods: A comprehensive review. Antioxidants, 2021. 2022;11(1):60. doi: 10.3390/antiox11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger D., Larsen G., Kivatinitz S.C. Oxidative consequences of UV irradiation on isolated milk proteins: Effects of hydrogen peroxide and bivalent metal ions. International Dairy Journal. 2016;55:64–71. doi: 10.1016/j.idairyj.2015.12.005. [DOI] [Google Scholar]
- Schiano A.N., Jo Y., Barbano D.M., Drake M.A. Does vitamin fortification affect light oxidation in fluid skim milk? Journal of Dairy Science. 2019;102(6):4877–4890. doi: 10.3168/jds.2018-15594. [DOI] [PubMed] [Google Scholar]
- Segat A., Misra N., Fabbro A., Buchini F., Lippe G., Cullen P.J., Innocente N. Effects of ozone processing on chemical, structural and functional properties of whey protein isolate. Food Research International. 2014;66:365–372. doi: 10.1016/j.foodres.2014.10.002. [DOI] [Google Scholar]
- Semagoto H.M., Liu D., Koboyatau K., Hu J., Lu N., Liu X.…Zhou P. Effects of UV induced photo-oxidation on the physicochemical properties of milk protein concentrate. Food Research International. 2014;62:580–588. doi: 10.1016/j.foodres.2014.04.012. [DOI] [Google Scholar]
- Sheng M., Lin S., Ma T., Qin L., Chang Y., Chen D. The improvement effects of Lentinus edodes powder marination on sous vide cooked chicken patties: Physicochemical attributes, oxidative properties and flavor characteristics. Food Chemistry. 2024;444 doi: 10.1016/j.foodchem.2024.138689. [DOI] [PubMed] [Google Scholar]
- Soladoye O.P., Juárez M.L., Aalhus J.L., Shand P., Estévez M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Comprehensive Reviews in Food Science and Food Safety. 2015;14(2):106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Soyer A., Özalp B., Dalmış Ü., Bilgin V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chemistry. 2010;120(4):1025–1030. doi: 10.1016/j.foodchem.2009.11.042. [DOI] [Google Scholar]
- Tian Y., Lin S., Bao Z. Characterization and mechanism of gel deterioration of egg yolk powder during storage. Foods. 2023;12(13):2477. doi: 10.3390/foods12132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utrera M., Armenteros M., Ventanas S., Solano F., Estévez M. Pre-freezing raw hams affects quality traits in cooked hams: Potential influence of protein oxidation. Meat Science. 2012;92(4):596–603. doi: 10.1016/j.meatsci.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Utrera M., Estévez M. Oxidative damage to poultry, pork, and beef during frozen storage through the analysis of novel protein oxidation markers. Journal of Agricultural and Food Chemistry. 2013;61(33):7987–7993. doi: 10.1021/jf402220q. [DOI] [PubMed] [Google Scholar]
- Van der Plancken I., Van Loey A., Hendrickx M.E. Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. Journal of Agricultural and Food Chemistry. 2005;53(14):5726–5733. doi: 10.1021/jf050289+. [DOI] [PubMed] [Google Scholar]
- Wang D., Cheng F., Wang Y., Han J., Gao F., Tian J.…Jin Y. The changes occurring in proteins during processing and storage of fermented meat products and their regulation by lactic acid Bacteria. Foods. 2022;11(16) doi: 10.3390/foods11162427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhao Y., Niu S., Wang X., Chen F. Effect of oxidation induced by hydroxyl radical-mediated model on molecular structural and physical character of egg white powder. International Journal of Food Science & Technology. 2018;53(10):2282–2289. doi: 10.1111/ijfs.13818. [DOI] [Google Scholar]
- Wang X., Yu Q., He L., Zhang Q., Ma J. Effects of nitrite concentrations on the quality and protein oxidation of salted meat. Journal of Food Science. 2022;87(9):3978–3994. doi: 10.1111/1750-3841.16177. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wu Z., Tu J., Xu B. Muscle food and human health: A systematic review from the perspective of external and internal oxidation. Trends in Food Science & Technology. 2023;138:85–99. doi: 10.1016/j.tifs.2023.06.006. [DOI] [Google Scholar]
- Wongnen C., Panpipat W., Saelee N., Rawdkuen S., Grossmann L., Chaijan M. A novel approach for the production of mildly salted duck egg using ozonized brine salting. Foods. 2023;12(11):2261. doi: 10.3390/foods12112261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Kong B., Liu J., Diao X., Liu Q. Influence of different thawing methods on physicochemical changes and protein oxidation of porcine longissimus muscle. LWT - Food Science and Technology. 2012;46(1):280–286. doi: 10.1016/j.lwt.2011.09.018. [DOI] [Google Scholar]
- Xie Y., Wang J., Wang Y., Wu D., Liang D., Ye H.…Geng F. Effects of high-intensity ultrasonic (HIU) treatment on the functional properties and assemblage structure of egg yolk. Ultrasonics Sonochemistry. 2020;60 doi: 10.1016/j.ultsonch.2019.104767. [DOI] [PubMed] [Google Scholar]
- Xiong Y.L., Guo A. Animal and plant protein oxidation: Chemical and functional property significance. Foods. 2020;10(1) doi: 10.3390/foods10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H., Han T., Zhang G., Hu X., Li R., Liu H.…Zhao Y. Combined effects of NaOH, NaCl, and heat on the characteristics of ovalbumin gel and the exploration of the mechanism of transparent gel formation. Food Hydrocolloids. 2023;140 doi: 10.1016/j.foodhyd.2023.108589. [DOI] [Google Scholar]
- Xue H., Liu H., Zhang G., Tu Y., Zhao Y. Formation mechanism of salted egg yolk mudding during storage: Protein oxidation, gel structure, and conformation. Food Chemistry. 2023;413 doi: 10.1016/j.foodchem.2023.135632. [DOI] [PubMed] [Google Scholar]
- Xue S., Xu X., Shan H., Wang H., Yang J., Zhou G. Effects of high-intensity ultrasound, high-pressure processing, and high-pressure homogenization on the physicochemical and functional properties of myofibrillar proteins. Innovative Food Science & Emerging Technologies. 2018;45:354–360. doi: 10.1016/j.ifset.2017.12.007. [DOI] [Google Scholar]
- Yang S., Takeuchi M., Friedrich H., van Duynhoven J.P., Hohlbein J. Unravelling mechanisms of protein and lipid oxidation in mayonnaise at multiple length scales. Food Chemistry. 2023;402 doi: 10.1016/j.foodchem.2022.134417. [DOI] [PubMed] [Google Scholar]
- Yeasmin Akter A.K., Omar H., Sazili A.Q. Effect of storage time and temperature on the quality characteristics of chicken eggs. Journal of Food, Agriculture & Environment. 2015;12(3&4):87–92. [Google Scholar]
- Yin Y., Xing L., Zhang W. Moderate protein oxidation improves bovine myofibril digestibility by releasing peptides in the S2 region of myosin: A peptidomics perspective. Journal of Agricultural and Food Chemistry. 2023;71(5):2514–2522. doi: 10.1021/acs.jafc.2c07708. [DOI] [PubMed] [Google Scholar]
- Yu Q., Hong H., Liu Y., Monto A.R., Gao R., Bao Y. Oxidation affects pH buffering capacity of myofibrillar proteins via modification of histidine residue and structure of myofibrillar proteins. International Journal of Biological Macromolecules. 2024;260 doi: 10.1016/j.ijbiomac.2024.129532. [DOI] [PubMed] [Google Scholar]
- Zamuz S., López-Pedrouso M., Barba F.J., Lorenzo J.M., Domínguez H., Franco D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Research International. 2018;112:263–273. doi: 10.1016/j.foodres.2018.06.053. [DOI] [PubMed] [Google Scholar]
- Zhang D., Wu Z.-C., Xu J.-B., Huang N.-X., Tang Y., Su C.…Li H.-J. Effect of different addition amounts of capsaicin on the structure, oxidation sites, and gel properties of myofibrillar proteins under oxidative conditions. Journal of Agricultural and Food Chemistry. 2024 doi: 10.1021/acs.jafc.4c06603. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li Z., Ahn D.U., Huang X. The effect of pressurized heat treatment on the textural, flavor, nutritional, and digestive characteristics of soft-boiled and marinated eggs. Food Science of Animal Products. 2023;1(3) doi: 10.26599/FSAP.2023.9240026. [DOI] [Google Scholar]
- Zhang L., Li Q., Bao Y., Tan Y., Lametsch R., Hong H., Luo Y. Recent advances on characterization of protein oxidation in aquatic products: A comprehensive review. Critical Reviews in Food Science and Nutrition. 2022;1-20 doi: 10.1080/10408398.2022.2117788. [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhao D., Zhu S., Nian Y., Xu X., Zhou G., Li C. Overheating induced structural changes of type I collagen and impaired the protein digestibility. Food Research International. 2020;134 doi: 10.1016/j.foodres.2020.109225. [DOI] [PubMed] [Google Scholar]
- Zhang R., Xing L., Kang D., Zhou L., Wang L., Zhang W. Effects of ultrasound-assisted vacuum tumbling on the oxidation and physicochemical properties of pork myofibrillar proteins. Ultrasonics Sonochemistry. 2021;74 doi: 10.1016/j.ultsonch.2021.105582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Gong P., Wang Y., Jiang H., Zhang M., Yang M.…Liu X. Oxidation-mediated structure and molecular interaction transformation of egg white protein: The underlying mechanism of functional properties and in vitro gastric digestibility improvement. Food Chemistry. 2023;405 doi: 10.1016/j.foodchem.2022.134874. [DOI] [PubMed] [Google Scholar]
- Zhang W., Xiao S., Ahn D.U. Protein oxidation: Basic principles and implications for meat quality. Critical Reviews in Food Science and Nutrition. 2013;53(11):1191–1201. doi: 10.1080/10408398.2011.577540. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yang Y., Zhou P., Zhang X., Wang J. Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chemistry. 2017;217:678–686. doi: 10.1016/j.foodchem.2016.09.040. [DOI] [PubMed] [Google Scholar]
- Zhao D., He J., Zou X., Xie Y., Xu X., Zhou G., Li C. Influence of hydrothermal treatment on the structural and digestive changes of actomyosin. Journal of the Science of Food and Agriculture. 2019;99(14):6209–6218. doi: 10.1002/jsfa.9893. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Tu Y., Xu M., Li J., Du H. Physicochemical and nutritional characteristics of preserved duck egg white. Poultry Science. 2014;93(12):3130–3137. doi: 10.3382/ps.2013-03823. [DOI] [PubMed] [Google Scholar]
- Zheng X., Zou B., Zhang J., Cai W., Na X., Du M.…Wu C. Recent advances of ultrasound-assisted technology on aquatic protein processing: Extraction, modification, and freezing/thawing-induced oxidation. Trends in Food Science & Technology. 2024;144 doi: 10.1016/j.tifs.2023.104309. [DOI] [Google Scholar]
- Zhu H., Zhang Q., Ding Y., Liu S., Zhu S., Zhou X. Myofibrillar protein oxidation associated with surimi processing and emerging control techniques: A review. Trends in Food Science & Technology. 2024;149 doi: 10.1016/j.tifs.2024.104560. [DOI] [Google Scholar]
- Zhu M., Xing Y., Zhang J., Li H., Kang Z., Ma H.…Jiao L. Low-frequency alternating magnetic field thawing of frozen pork meat: Effects of intensity on quality properties and microstructure of meat and structure of myofibrillar proteins. Meat Science. 2023;204 doi: 10.1016/j.meatsci.2023.109241. [DOI] [PubMed] [Google Scholar]
- Zhu X., Gallogly M.M., Mieyal J.J., Anderson V.E., Sayre L.M. Covalent cross-linking of glutathione and carnosine to proteins by 4-oxo-2-nonenal. Chemical Research in Toxicology. 2009;22(6):1050–1059. doi: 10.1021/tx9000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Zhang J., Zhang X., Dai Q., Fu Q. Effects of 2, 2′-Azobis (2-methylpropionamidine) Dihydrochloride stress on the gel properties of duck Myofibrillar protein isolate. Molecules. 2023;28(18):6721. doi: 10.3390/molecules28186721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.