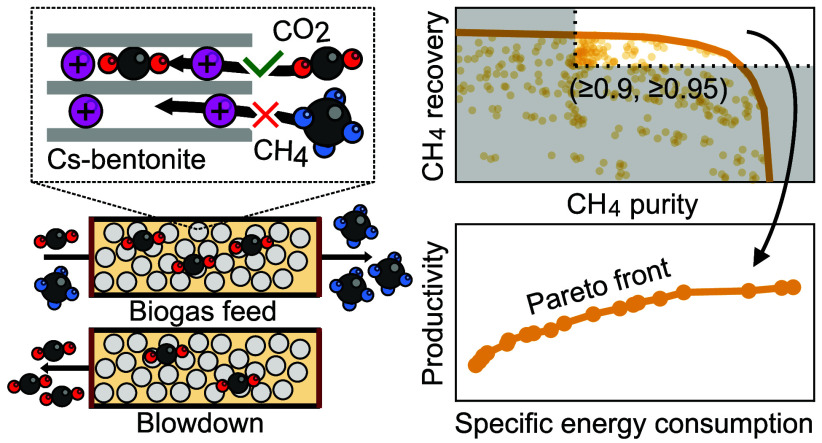
Abstract
Biogas upgrading by vacuum-pressure swing adsorption involves the selective adsorption of CO2 over CH4 on a sorbent material to separate both components. This work assesses numerically the performance of the previously characterized Cs-exchanged bentonite clay for this separation. This benchmarking study includes the effect of the process cycle configuration (seven different configurations using one stage and up to three columns), the ambient temperature (15 or 25 °C), the feed biogas composition (CO2 mole fraction of 0.35 or 0.45, balance CH4), and the process operating parameters. Specific constraints on CH4 purity and CH4 recovery provide Pareto fronts for maximum productivity and minimum specific energy consumption. A two-column unit operated at ambient feed pressure can upgrade 0.097 Nm3 feed biogas (CO2 mole fraction of 0.45, balance CH4) per kg sorbent per h to a bio-CH4 product with a purity of 0.906 and with a CH4 recovery of 0.967 at a comparatively low specific energy consumption of only 0.072 kWh per produced Nm3 of CH4. Using more columns and pressure equalization steps further enhances the CH4 recovery. The low bentonite cost, the comparatively low specific energy consumption due to the favorable linear CO2 adsorption isotherms, and the high recovery due to the high CO2/CH4 selectivity make Cs-bentonite an excellent alternative for conventional sorbent materials.
1. Introduction
Biogas is a gas mixture produced by the anaerobic digestion of organic matter (organic waste, wastewater sludge, manure, landfill waste, etc.). It comprises mainly CH4 (mole fraction of 0.50–0.70) and CO2 (mole fraction of 0.30–0.50), as well as other minor contaminants like H2S and H2O.1−3 Separation of the CH4 and CO2 into a bio-CH4 stream that can be used as a natural gas substitute (e.g., for gas grid injection) and a high-purity CO2 stream (e.g., used for plant or algae cultivation, chemical production, or sequestration) increases the value of and number of use cases for biogas.1−5 This separation is known as biogas upgrading.
Established methods for biogas upgrading include absorption (water, chemical, or physical scrubbing), membrane separation, and ((vacuum) pressure and/or temperature swing) adsorption.1−7 All of these methods have their (dis)advantages.2−6 However, the adsorption method has been considered of interest because of the compactness of the equipment, low energy consumption and capital costs, safety, and ability to achieve high purification levels.2−4,8,9 The adsorption method is a cyclic process. In short, for biogas upgrading, it requires first the selective adsorption of CO2 on an adsorbent material, thereby producing high-purity CH4. Subsequently, it requires the regeneration of the adsorbent material at reduced (CO2 partial) pressure and/or elevated temperature, thereby producing high-purity CO2. Therefore, the performance of this method depends strongly on the adsorbent material that is used.3,5,7,10−14 Conventional adsorbents used or studied for biogas upgrading include activated carbons (AC),12,15−21 various zeolites,9,10,13,16,22−36 carbon molecular sieves (CMS),10−12,14,25,30,32,37−43 and various other materials.8,10,16,28,44−50Table S1 presents performance indicators (if available) from previous works on these different sorbent materials.
Recently, we proposed (particles of) montmorillonite (MMT) or MMT-rich bentonite clay as a low-cost and readily available adsorbent for biogas upgrading.51 MMT is a natural layered material of which the sorption capacity for nonpolar molecules like CO2 and CH4 can be tuned by varying the height of its interlayer galleries. This is achieved by exchanging its natural interlayer cations for others of different sizes. Cs+ is particularly suitable to achieve high sorption capacities for CO2 while excluding the larger CH4 molecules from the interlayer galleries.51−53 Consequently, Cs-bentonite demonstrates an equilibrium CO2/CH4 selectivity under near-ambient conditions of up to ∼35 (here defined as the ratio of [single-component] equilibrium CO2 sorption to equilibrium CH4 sorption at equal pressure and temperature).51 Importantly, it shows favorable regeneration within several minutes only under ambient-temperature N2 purge or vacuum conditions51 due to the only relatively weak adsorption of CO2 (|ΔH|≈30 kJ mol–1). Together, the rather high CO2/CH4 selectivity, the fast CO2 adsorption and desorption kinetics,51 and the low cost and high availability of the bentonite material suggest that Cs-bentonite could be an appealing alternative adsorbent for biogas upgrading.
However, the actual performance of an adsorbent in a vacuum pressure swing adsorption (VPSA) unit also depends crucially on the VPSA cycle configuration and the operating conditions (e.g., ambient temperature, feed biogas composition) and operating parameters (e.g., pressure, flow rate, step duration). Ultimately, the key process performance indicators are minimal specific energy consumption (here: energy use per amount of CH4 recovered in the output bio-CH4 product) and maximal productivity (here: the amount of feed biogas treated per kg sorbent per hour) while the specific requirements on product purity and component recovery are satisfied. (The product purity is the mole fraction of the desired component in that output product. The component recovery is the mole fraction of the component feed recovered in the appropriate output product.) Given the large number of possible VPSA cycle configurations, a range of possible operating conditions and parameters, and requirements on product purity and component recovery that differ per purpose (e.g., grid injection, liquefaction, chemical production) and between countries,2,3,5 this is most easily assessed numerically.
This work aims to (i) perform the numerical assessment of Cs-bentonite for biogas upgrading in a VPSA process, (ii) identify the optimal VPSA cycle configuration and operating parameters under the specific requirements on product purity and component recovery, and (iii) compare the performance of Cs-bentonite against conventional sorbents. We specifically focus on the production of bio-CH4 with a purity ≥0.90 that is compatible with grid injection in, e.g., The Netherlands.2,3 Simultaneously, in the light of (possibly forthcoming) strict regulations on CH4 emissions and the possibility to utilize a high-purity CO2 product, we also focus on high CH4 recovery ≥0.95 (i.e., high compared to earlier works, Section 3.5 and Table S1). We limit this benchmarking study to a small and simple unit with a target productivity of several Nm3 h–1 (normal cubic meters per hour) input biogas. This unit features a single upgrading stage and up to three columns. Hereon, we test seven different basic cycle configurations. While more complex cycle configurations,7,38,54 multiple units or upgrading stages (possibly hybrid, featuring different adsorbents or even different methods),2,3,9,44,45,55 auxiliary equalization tanks,23,37 and/or layered beds30 may achieve higher purity, recovery, and/or productivity, this is beyond the scope of the current work. We also consider the effects of the operating conditions, ambient temperature and feed biogas composition (i.e., CO2 fraction), on the process performance. The assessment makes use of a newly developed model that is validated against the experimental breakthrough and regeneration curves published in ref (51). Our numerical results confirm the excellent performance of Cs-bentonite for biogas upgrading and show particularly low specific energy consumption compared to conventional adsorbents.
2. Theoretical Basis
2.1. Model Assumptions
Various mathematical models to analyze biogas upgrading using a range of adsorbents were used in previous works. All these models had different levels of complexity and underlying assumptions, see, e.g., refs (56 and 57). In this article, we assess the VPSA process by using a newly developed nonisothermal and nonisobaric model. Herein, the following is assumed.
The gas flow is represented by an axially dispersed plug flow model.
The gas phases are described by the ideal gas law, P = ∑iCg,iRT.
The mass transfer between the gas phases in the column void and the particle pore space and between the gas phase in the particle pore space and the adsorbed phase is described by linear driving force (LDF) models.
The pressure gradient along the column is described by the Ergun equation.58
Thermal equilibrium between the solid, gas, and adsorbed phases is established instantaneously. The local temperature of the solid, gas, and adsorbed phases is equal and described by a single energy balance.
Heat exchange with the column wall occurs. The temperature of the column wall is constant and equal to the ambient temperature T0.
There are no concentration, pressure, and temperature gradients in the radial direction of the column; i.e., the model is 1-dimensional (axial; z) in space along the length of the column.
The properties of the column are uniform. The adsorbent particles are homogeneous, spherically symmetric, and equally sized.
The input biogas is completely dry and devoid of minor impurities like H2S.
2.2. Model
The assumptions above result in mass balances for each of the different components in the gas phases and in the adsorbed phase, a momentum balance in the gas phase in the column void, and a combined energy balance for the solid, gas, and adsorbed phases. Figure 1 provides a schematic overview of the transport phenomena and coefficients and column and particle properties that are described and used in the model. The physical properties of the gas components (Table S2) and relations for the physical properties of the gas mixture (eqs S1–S6) and the transport coefficients (eqs S7–S14) are taken from the literature and discussed in the Supporting Information.
Figure 1.
Schematic illustration of transport phenomena, coefficients, and properties on/in the (left) column scale, (center) column cross section, and (right) particle scale. (Details in the main text.)
2.2.1. Mass Balance (Column Void)
The mass balances for each component (with i ∈ {CO2, CH4, N2}) in the gas phase in the column void include convection, axial dispersion, and mass transfer between the gas phases in the column void and in the particle pore space, eq 1.
| 1 |
Here, Cg,i is the concentration in the column void, Cp,i is the average concentration in the particle pore space, and yi is the mole fraction in the gas phase in the column void, all of component i. ϵb is the column void fraction, P is the local pressure in the column void, T is the local temperature, vs is the superficial gas velocity, and DMax is the axial mass dispersion coefficient. (The superficial gas velocity is the volumetric flow rate divided by the cross-sectional area of the column, without particles.) kf is the film LDF coefficient, rp is the particle radius, and Bii is the mass Biot number of component i (see below). The product 3kf/[rp(Bii/5 + 1)] describes film resistance and pore diffusivity in series (see below). Substitution of the ideal gas law, P = ∑iCg,iRT, into eq 1 yields eq 2.
| 2 |
2.2.2. Mass Balance (Particle)
The mass balances for each component in the particle pore space include adsorption and mass transfer to and from the gas phase in the column void, eq 3.
| 3 |
Here, ϵp and ρp are the particle porosity and density, respectively, qi is the particle-averaged adsorption, and kp,i is the pore LDF coefficient from Glueckauf’s approximation,59eq 4, both of component i.
| 4 |
Here, Deffm,i is the effective pore diffusion coefficient of component i (which includes molecular and Knudsen diffusion; see Supporting Information, eq S10). Equation 4 is valid for Deffm,it/rp2 > 0.1.56 Note that for Bii = kfrp/Deffm,i, eq 5 can be written:
| 5 |
Thus, the model describes film resistance and pore diffusion in series and mass is conserved between eqs 2 and 3. The rate of adsorption (i.e., mass transfer between the particle pore space and the adsorbed phase) is also described by an LDF model, eq 6.
| 6 |
Here, q*i is the equilibrium adsorption of component i at temperature T and average concentration of component i in the particle pore space Cp,i, as provided by the adsorption isotherms described in Section 2.3. kc,i is the adsorption LDF coefficient of component i.
2.2.3. Momentum Balance
The addition of the component mass balances in eq 2 under the condition ∑iyi = 1 and the substitution of eq 3 therein yield the total mass balance, eq 7.
| 7 |
The axial pressure gradient is related to the local superficial velocity by the semiempirical Ergun equation, eq 8.58
| 8 |
Here, μg and ρg are the local viscosity and density of the gas, respectively, and dp is the particle diameter. Equation 8 can be solved to find the local superficial velocity for a given pressure gradient. Alternatively, eq 8 provides a pressure gradient for a given (input) superficial velocity.
2.2.4. Energy Balance
The energy balance accounts for heat effects due to adsorption and desorption, dispersive and convective energy transport via the gas phase in the column void, heat exchange between the gas phase in the column void and the column wall, and mass transfer between the gas phases and the adsorbed phase, eq 9.
 |
9 |
Here, cP,p and cP,i are the heat capacities of the solid phase and of component i (assumed to be equal for the gas phases [in the column void and particle pore space] and the adsorbed phase), ΔHi is the enthalpy of adsorption of component i (assumed to be independent of loading), DTax is the axial heat dispersion coefficient, hw is the wall heat transfer coefficient, dr is the column diameter, and Tw = T0 is the wall temperature set equal to the ambient temperature.
2.2.5. Initial and Boundary Conditions
The system of partial differential equations (PDEs) discussed above requires suitable initial and boundary conditions. These depend on the specific steps in a VPSA cycle (configuration) that is defined by the order and direction in which these steps are executed. Figure 2 provides an overview of the specific steps that are used in this work and includes the abbreviations used to refer to these steps. The initial condition of the first step in the first simulated cycle is described by the absence of CO2 and CH4 in the column; instead, the column is saturated with N2 (assumed to be nonadsorbing) at the target regeneration pressure and at the ambient temperature. For each subsequent step, the final condition of the preceding step is used as an initial condition.
Figure 2.
Steps used in the VPSA cycles. All schematics show the steps executed in the “forward” direction. The colors inside the column illustrate the typical component concentration in the column voids at the end of the respective step. Red: CH4; blue: CO2; yellow: N2; deeper colors indicate higher pressures. The N2 regeneration feed is only used for model validation, Section 2.4.
Table 1 displays the boundary conditions for the specific steps when executed in the “forward” direction. Analogous boundary conditions with both ends interchanged apply to steps that are executed in the “backward” direction. In summary, when an inlet (z = 0) or outlet (z = Lr) is open, Danckwerts boundary conditions56,60 apply to the component mole fraction and the temperature. Specification of either the pressure or the gas velocity (effectively, the pressure gradient via eq 8) is required on both ends of the column. For a closed in- or outlet, vs = 0 via the vanishing pressure gradient. The PR, FE, BD, and PPE steps include check valves that permit only a positive or zero inflow or outflow gas velocity (i.e., a negative or zero pressure gradient) using the min and max functions. yin,i and Tin are the mole fraction of component i and the temperature, respectively, of the inlet gas stream. Ps,0 and ts,0 are the local pressure and the time at the beginning of the step, respectively. PPR, PBD, τPR, and τBD are the characteristic pressures and time scales within the respective steps.
Table 1. Boundary Conditions for Each Step Executed in the “Forward” Direction.
Some of the steps are coupled. First, the PPE and RPE require the conservation of mass and energy. The molar outflow rate during the PPE step is set proportional to the time-dependent pressure difference ΔP(t) between the outlet of that column and the inlet of the column in the RPE step. To calculate this pressure difference, the time evolution of the inlet pressure of the column in the RPE step in the preceding cycle is used. The molar outflow rate then is vsArP/(RT) = max{0, aΔP(t)}. Here, the coefficient a is analogous to a flow coefficient of a restricting device (we set a = 10–5 mol Pa–1 s–1). The output (i.e., the time-dependent molar flow rate, composition, and temperature) of the PPE step is then used as an input for the RPE step thereafter. This allows for multiple columns to be simulated iteratively by using a single column, instead of simultaneously. This (“store-and-retrieve”) method is known to introduce oscillations around the equalization pressure.61 However, in our simulations, these oscillations decay well before the final cycle is simulated. Second, (part of) the output product of a preceding step (i.e., feed or blowdown) can serve as an input for another step (i.e., pressurization or purge/rinse feed; the latter is not used in this work). In this case, the average composition of the output product is taken and recycled at the ambient temperature and pressure.
When a step needs to be simulated before the step from which it requires input has been simulated for the first time, an initial estimate is used. For the simulation of the PPE step(s), a constant pressure between the target feed and blowdown pressures is taken as the inlet pressure of the RPE step(s). In the case of recycled products, the composition of the biogas is taken instead. For some representative cases, we verified that the cyclic steady state is independent of these initial estimates.
2.2.6. Model Implementation
To solve the system of PDEs, the column was divided into N = 30 cells, each with a length Δz. The PDEs were spatially discretized using the weighted essentially non oscillatory (WENO)-finite volume method,62 as is detailed in the Supporting Information (eqs S15–S19). The discretized system of coupled ordinary differential equations was solved by using the ode15s solver in MATLAB (R2023b) with an absolute tolerance of 10–3. The solution was refined 10 times for higher accuracy of the Riemann-type integration for the in- and output flows. To ensure that the cyclic steady state was reached, 100 cycles were simulated in each simulation. The typical computation time of one simulation was around 1 h on a dedicated desktop PC with one Intel i7–7700 CPU (3.60 GHz) and 8.00 GB of RAM.
2.3. Separation System
The experimental adsorption isotherms of CO2 and CH4 on Cs-bentonite are presented and discussed in ref (51). Therein, each isotherm was fitted individually with a (single- or dual-site) Langmuir model (SSL and DSL). As the simulations require the isotherms at intermediate temperatures, the data is refitted with a temperature-dependent model, eq 10.
 |
10 |
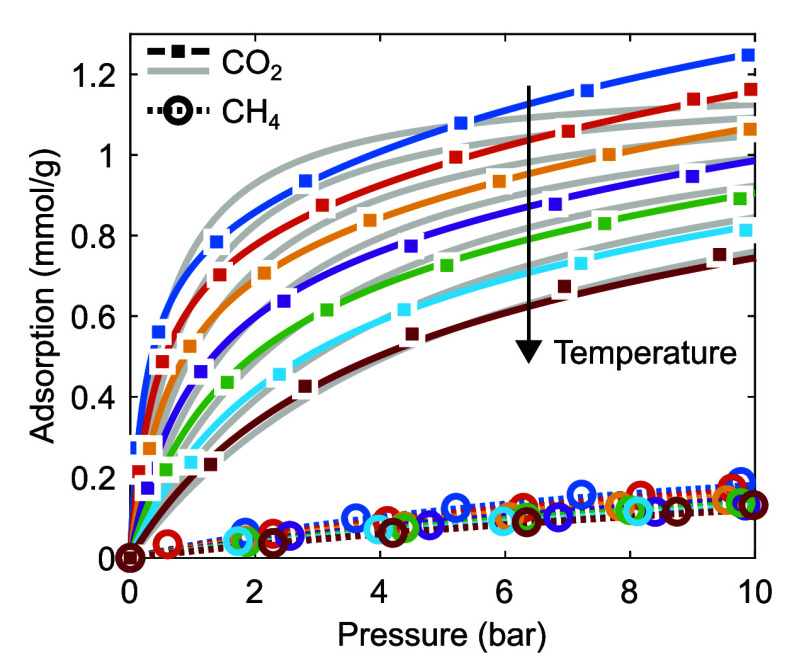
Here, the fit parameters are ni,k the number of adsorption sites per unit sorbent mass of type k for gas component i and b0,i,k and ΔHi,k the ratio between the adsorption and desorption attempt frequency and the enthalpy of adsorption for sites of type k for gas component i, respectively, and yi = 1. We fit both sets of adsorption isotherms with the SSL model (i.e., k = 1) and also fit a DSL model (i.e., k ∈ {1,2}) to the CO2 adsorption isotherms only, in line with ref (51). The fitted adsorption isotherms along with the experimental data in the temperature range of 10–70 °C and the corresponding fit parameters are presented in Figure 3 and Tables S3 and S4, respectively.
Figure 3.
Adsorption isotherms of CO2 and CH4 on binderless Cs-bentonite. For CO2, the arrow indicates increasing temperature from 10 to 70 °C at 10 °C increments. For CO2 and CH4, identical colors indicate equal temperatures. The experimental data is presented with solid squares (CO2) and open circles (CH4). The temperature-dependent fits are presented with colored solid lines (CO2; DSL), gray solid lines (CO2; SSL), and colored dashed lines (CH4; SSL).
The CO2 adsorption is described much better by the DSL model than by the SSL model—in particular at lower temperatures—due to the larger number of fitting parameters. We use the fitted DSL model to describe the equilibrium CO2 adsorption q*i in eq 6. In contrast, we use the ΔHi,1 fitted using the SSL model as an effective enthalpy of adsorption for the energy balance, eq 9. For CH4, the fitted SSL model is used for both the equilibrium adsorption and the energy balance. We further multiply the equilibrium adsorption by 0.91 to account for the (assumed passive) binder material in a mass fraction of 0.09 required to produce particles from the binderless Cs-bentonite in Figure 3.51
The synergistic and competitive adsorption of multiple components on MMTs is a complex process that cannot easily be described by competitive adsorption models. This is due to the violation of various assumptions made in such models. Most notably, the adsorption sites are not homogeneous and the number of available adsorption sites differs per component due to steric limitations—and may in fact increase (due to swelling) or decrease by the coadsorption of another component.51 In this previous work, we noted that these effects are minor for the coadsorption of CO2 and CH4 on Cs-bentonite. Hence, we choose not to include these minor effects in the model (i.e., the adsorption of the different gas components occurs independently).
2.4. Model Validation
To validate the model and to estimate kc and dm (i.e., the typical pore diameter; an input parameter for Deffm,i), we compare the output flow compositions of experimental consecutive (cyclic) breakthrough and regeneration (in N2) measurements51 with simulated output flow compositions under the same conditions, Figure 4. Table 2 lists the estimated material properties.
Figure 4.
Comparison between experimental51 and simulated cyclic breakthrough and regeneration measurements on 50 g of Cs-bentonite particles, T0 ≈ 23 °C. Column dimensions: dr = 1.3 cm and Lr = 60 cm. Each cycle consisted of (i) a 8 min forward feed step with an inlet gas mixture containing only CO2 (mole fraction of 0.50 ± 0.02) and CH4 (mole fraction of 0.50 ± 0.02) at a total flow rate of 0.40 L min–1, (ii) a 1 min forward idle step (gray background), (iii) a 10 min forward feed step with N2 (for regeneration) at a flow rate of 0.60 L min–1, and (iv) a 1 min forward idle step (gray background). The volumetric flow rates are actual values. The experimental cycles are shifted on top of each other. The estimated material properties are tabulated in Table 2.
Table 2. Material Properties of Cs-Bentonite Particlesa.
| dm (nm) | 15 |
| kc (s–1)b | 0.10 |
| dp (mm) | 3.0 |
| ρp (kg m–3) | 1400 |
| ρc (kg m–3)c | 270063,64 |
| ϵp (−) | 0.48 |
| τ(−)d | 2.0 |
| cP,p(kJ kg–1 K–1) | 0.8065 |
dm and kc are estimated from the experimental breakthrough curves, while all other properties are estimated from the literature or macroscopic properties of the materials.
Set equal for CO2 and CH4 and independent of temperature.
Crystal density.
Particle tortuosity.
The simulated outputs can describe the experimental
outputs well,
both during the feed step with the mixture of CO2 and CH4 and during the regeneration step using N2 feed.
To cross-validate the estimated values for dm (that ultimately affects the diffusional transport in particles)
and kc (that sets the “intrinsic”
adsorption kinetics, i.e., into the interlayer galleries), we compare
the corresponding LDF coefficients with independent kinetic experiments
of CO2 adsorption on powders and small and large particles
of Cs-bentonite, Figure 4 in ref (51). As for the diffusional transport limitations,
for CO2 and at 20 °C, typically BiCO2≫1 and the molecular and Knudsen diffusivities of CO2 are on the order of 1.6 × 10–5 m2 s–1 and 1.9 × 10–6 m2 s–1, respectively (see Supporting
Information, eqs S7 and S11). This indicates
that mass transfer between the gas phase in the column void and the
particle pore space is mostly limited by Knudsen diffusion in the
particle pore space. For ϵp = 0.48 and τ =
2.0, the product  , i.e., approximately the inverse of the
pore LDF coefficient, yields typical time scales of 0.7 and 18 s for
small particles with rp = 2 mm and large
particles with rp = 1 cm, respectively.
These are in line with the previous observations that for small particles,
the CO2 adsorption nearly follows the adsorption on a powder,
and for large particles, the CO2 adsorption follows the
adsorption on a powder within ∼10–20 s. Moreover, the
estimated effective pore diameter dm =
15 nm is close to the typical mesopore throat size and between the
typical micro- and mesopore body sizes of MMT66 (see also Figure 4.10 in ref (67)). As for the “intrinsic” adsorption, kc–1 = 10 s agrees well with the fast CO2 adsorption and desorption
on powders (and small particles) that follows the CO2 concentration
of the environment within ∼10 s. Ultimately, these independent
kinetic experiments thus confirm the (order of magnitude) of our estimated
values for dm and kc.
, i.e., approximately the inverse of the
pore LDF coefficient, yields typical time scales of 0.7 and 18 s for
small particles with rp = 2 mm and large
particles with rp = 1 cm, respectively.
These are in line with the previous observations that for small particles,
the CO2 adsorption nearly follows the adsorption on a powder,
and for large particles, the CO2 adsorption follows the
adsorption on a powder within ∼10–20 s. Moreover, the
estimated effective pore diameter dm =
15 nm is close to the typical mesopore throat size and between the
typical micro- and mesopore body sizes of MMT66 (see also Figure 4.10 in ref (67)). As for the “intrinsic” adsorption, kc–1 = 10 s agrees well with the fast CO2 adsorption and desorption
on powders (and small particles) that follows the CO2 concentration
of the environment within ∼10 s. Ultimately, these independent
kinetic experiments thus confirm the (order of magnitude) of our estimated
values for dm and kc.
2.5. Process Definition
2.5.1. VPSA Cycle Configurations
We consider seven different cycle configurations that are also illustrated schematically in Figure S1.
-
1.
(i) Forward pressurization with biogas (PR); (ii) forward feed with biogas (FE; CH4 product collection); and (iii) backward blowdown (BD; CO2 product collection).
-
2.
As configuration 1 but (i) backward pressurization with the CH4 product (PR).
-
3.
(i) Forward pressurization with biogas (PR); (ii) forward feed with biogas (FE; CH4 product collection); (iii) forward provide pressure equalization (PPE); (iv) backward blowdown (BD; CO2 product collection); and (v) forward receive pressure equalization (RPE).
-
4.
As configuration 3 but (v) backward receive pressure equalization (RPE).
-
5.
As configuration 3 but (i) backward pressurization with the CH4 product (PR).
-
6.
As configuration 3 but (i) backward pressurization with the CH4 product (PR) and (v) backward receive pressure equalization (RPE).
-
7.
(i) Forward pressurization with biogas (PR); (ii) forward feed with biogas (FE; CH4 product collection); (iii) forward provide pressure equalization (PPE); (iv) backward provide pressure equalization (PPE); (v) backward blowdown (BD; CO2 product collection); (vi) forward receive pressure equalization (RPE); (vii) idle (ID; required for column synchronization); and (viii) backward receive pressure equalization (RPE).
Configuration 1 is the minimal configuration for a VPSA process. For configurations 2 and 5–6, the pressurization with the CH4 product (instead of biogas) possibly increases the CH4 purity.41,43 This is, to a certain extent, similar to the often applied CH4 purge step that improves the removal of CO2 from the column in particular at the end where the CH4 product is collected.8,10,22,23,25,26,36,37,39−41,45,46 We do not consider product purge/rinse steps in the current work. For configurations 3–7, the pressure equalization steps possibly increase the CH4 recovery and decrease the specific energy consumption.6,7,10,22,24,25,34,37−40,44−46,61,68,69 These pressure equalization steps require two columns to be connected at specific times and, thereby, the synchronization of multiple columns. The synchronization scheme for two (one PE step; configurations 3–6) or three (two PE steps; configuration 7) columns is given in Table 3.
Table 3. Column Synchronization Scheme.
| column | configurations 1–2 | ||
|---|---|---|---|
| 1 | PR | FE | BD |
| column | configurations 3–6 | |||||
|---|---|---|---|---|---|---|
| 1 | PR | FE | PPE | BD | BD | RPE |
| 2 | BD | BD | RPE | PR | FE | PPE |
| column | configuration 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | PR | FE | PPE | PPE | BD | BD | RPE | ID | RPE |
| 2 | RPE | ID | RPE | PR | FE | PPE | PPE | BD | BD |
| 3 | PPE | BD | BD | RPE | ID | RPE | PR | FE | PPE |
2.5.2. Operating Parameters
The process
operating parameters include the pressure (evolution) during, duration
of, and inflow rate during each step. Four of these operating parameters,
namely, the (target) pressurization pressure PPR = 1–4 bar, the (target) blowdown pressure PBD = 0.05–0.15 bar, Qin = 0.3–1.0 (see below), and the feed duration tFE = 1–10 min, are varied in our simulations
within the indicated ranges. Qin is a
fraction that relates the CO2 inflow during the feed step
to the estimated equilibrium CO2 working capacity of the
sorbent,  . Then, the inlet superficial velocity
. Then, the inlet superficial velocity 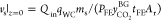 . Here, ms is
the sorbent mass in the column,
. Here, ms is
the sorbent mass in the column,  is the CO2 mole fraction in
the biogas, and Ar is the cross-sectional
area of the column. By increasing Qin,
the column utilization thus increases and the (expected) position
of the CO2 breakthrough front at the end of the FE step
shifts to (or beyond) the outlet end of the column. Moreover, this
position is (nearly) independent of PPR, PBD, and tFE for a fixed Qin. However, Qin does not take into account the CO2 that
is provided during any other step between BD and FE.
is the CO2 mole fraction in
the biogas, and Ar is the cross-sectional
area of the column. By increasing Qin,
the column utilization thus increases and the (expected) position
of the CO2 breakthrough front at the end of the FE step
shifts to (or beyond) the outlet end of the column. Moreover, this
position is (nearly) independent of PPR, PBD, and tFE for a fixed Qin. However, Qin does not take into account the CO2 that
is provided during any other step between BD and FE.
The other operating parameters are either set constant or depend only on the previously discussed operating parameters. These are (i) the duration of the PR, PPE, and RPE steps that are fixed at 1 min, (ii) the duration of the BD step that is set equal to the combined duration of the PR and FE steps for column synchronization (Table 3), and (iii) the FE pressure that is set equal to the (target) PR pressure. Furthermore, the characteristic pressurization and blowdown times for the exponential pressure in- or decrease, τPR and τBD, are set to 1/5th of the duration of the respective steps. At the end of these steps, the target pressure is then achieved within around 99.5%.
2.5.3. Performance Indicators
The purity and recovery of product and component i are calculated from the cumulative molar output ni,out and input ni,in of component i over all steps in one cycle in the cyclic steady state. Superscripts producti and BG indicate whether the out/input is directed to/from product i or from biogas (see Section 2.5.1). The recovery of component i is expressed as the ratio between the recovered component in output product i (after accounting for any recycled stream) and the input of that component, eq 11.
| 11 |
Here, k sums over all steps in one cycle in the cyclic steady state. The purity of product i is expressed as the ratio between the desirably collected component i and the total collected product, eq 12.
| 12 |
Here, k sums over all steps in one cycle in the cyclic steady state and j sums over all gas components. The compression and evacuation processes are approximated to be adiabatic processes, eq 13. The required power p is calculated under the assumption that the feed gas pressure and the vacuum pump discharge pressure are equal to the ambient pressure P0.
 |
13 |
Here, γ is the adiabatic constant (we assume γ = 1.3) and η the mechanical efficiency (we assume η = 0.8). Pin and vs,in and Pout and vs,out are the inlet and outlet pressure and superficial velocity, respectively. Compression of the product(s) beyond ambient pressure is not considered. The PPE and RPE step pair, any idle step, pressurization and feed to/at (sub)atmospheric pressure, and blowdown to(ward) atmospheric pressure do not require power. The specific energy consumption (SEC) is defined as the energy consumption per Nm3 (normal cubic meter; i.e., at 15 °C and 1 atm) collected CH4 in the CH4 product, eq 14.
| 14 |
Here, ts is the duration of the step. The productivity is defined as the amount of input biogas (in Nm3) per kg adsorbent per hour, eq 15.
| 15 |
2.5.4. Column Sizing
To match (within the order of magnitude) the capacity of a small “farm-scale” unit, we simulate a column with Lr = 2.0 m and dr = 20 cm, loaded with 55 kg of Cs-bentonite particles (ϵb = 0.375). For reference, the CO2 adsorption capacity of the column at the typical temperature of 15 °C and a CO2 partial pressure of 0.45 bar is around 27.5 mol or 0.65 Nm3 CO2.
3. Results and Discussion
3.1. VPSA Cycle
To illustrate the principle of the VPSA process, Figure 5 shows the results of one representative simulation using configuration 3 at an ambient temperature of 15 °C and with biogas feed mole fractions of 0.45 CO2 and 0.55 CH4. Specifically, Figure 5a–d show the input gas flow, pressure, output gas flow, and power consumption, respectively, during the final five simulated cycles (i.e., cycles 96–100). Figure 5e–h show the adsorbed CO2, adsorbed CH4, CO2 mole fraction in the column void, and temperature, respectively, along the axial dimension and at the end of each step (corresponding to the downward triangles in Figure 5a–d) during the final five simulated cycles. As all these results overlap between each of the final five simulated cycles, we conclude that the cyclic steady state was achieved.
Figure 5.
Simulation results (CSS) of configuration 3,  , T0 = 15 °C,
and operating parameters PPR = 2 bar, PBD = 0.05 bar, tFE = 2 min, and Qin = 0.5. (a) Input gas
flows, (b) pressure, (c) output gas flows, and (d) power consumption
over time. (Steps are indicated by the alternating background colors.)
(e–h) Profiles along the column axial dimension at the end
of each step, of (e) CO2 adsorbed, (f) CH4 adsorbed,
(g) CO2 mole fraction in the column void, and (h) temperature.
Line colors in (e–h) correspond to the color of the downward
triangles in (a–d).
, T0 = 15 °C,
and operating parameters PPR = 2 bar, PBD = 0.05 bar, tFE = 2 min, and Qin = 0.5. (a) Input gas
flows, (b) pressure, (c) output gas flows, and (d) power consumption
over time. (Steps are indicated by the alternating background colors.)
(e–h) Profiles along the column axial dimension at the end
of each step, of (e) CO2 adsorbed, (f) CH4 adsorbed,
(g) CO2 mole fraction in the column void, and (h) temperature.
Line colors in (e–h) correspond to the color of the downward
triangles in (a–d).
First, during the PR step, biogas is provided at z = 0 while the other end of the column is closed. This increases the column pressure until the desired PPR is (nearly) reached. The CO2 in the biogas is mostly adsorbed near the column inlet. As a consequence, also the temperature and CO2 gas fraction increase only near the column inlet. In turn, the adsorbed CH4 and CH4 gas fraction increase in the center and toward the opposite end of the column.
Second, during the FE step, biogas is provided at z = 0 and the CH4 product is collected at z = Lr. The column pressure remains nearly constant, besides the (slightly varying) pressure drop over the column that is of the order ∼10 mbar. The CO2 adsorption front and the temperature front progress synchronously along the axial dimension. The FE step is stopped before the CO2 adsorption front reaches the column outlet. Meanwhile, the FE step also reduces the amount of adsorbed and gaseous CH4 in the column. However, the amount of CO2 that is collected in the CH4 product, while much smaller than in the biogas, is still significant due to the incomplete regeneration of the sorbent during the BD step (see below).
Third, during the PPE step, the column is connected at z = Lr to another column that was previously evacuated in a BD step and is now in the RPE step (Table 3). The pressure difference between both columns facilitates the mass transfer from this column to the column in the RPE step until a pressure equilibrium is reached. The gas flow is relatively rich in CH4. As this CH4 is now not collected in the CO2 product (in the BD step hereafter), this significantly increases the CH4 recovery. In contrast, most of the (adsorbed) CO2 is retained in the column.
Fourth, during the BD step, the CO2 product is collected by backward evacuation at z = 0 while the other end of the column is closed. This decreases the column pressure until the desired PBD is (nearly) reached. The lower pressure reduces the adsorbed CO2 to ∼0.2 mol kg–1 and thereby results in a decrease of the temperature. Essentially no CH4 is retained in the column at the end of the BD step, i.e., the CO2 gas fraction is near unity. Nevertheless, the amount of CH4 that is collected in the CO2 product is small as most CH4 was removed from the column in the preceding PPE step.
Fifth, during the RPE step, the input gas is provided at z = 0 by another column that is in the PPE step until a pressure equilibrium is reached between both columns. The input gas that is relatively rich in CH4 (see above) increases the amount of adsorbed CH4 and decreases the CO2 gas fraction. The amount of adsorbed CO2 and the temperature remain nearly constant due to the only small amount of CO2 in the input gas. At this point, one cycle is completed and another is started with the PR step.
As for energy consumption, the PR and FE steps require input gas at above-ambient pressure and thus compression power. Similarly, the BD step is performed at subambient pressure and requires evacuation power. In contrast, the PPE and RPE steps require no power, as they are driven by an initial pressure difference between both columns.
The performance indicators are obtained by the integration of the
input and output gas flows and the power consumption. For this specific
configuration and this specific combination of operating parameters,
the CH4 purity is 0.9568, the CH4 recovery is
0.9387, the SEC is  , and the productivity is 0.1133 Nm3BG kg–1 h–1. The performance indicators can possibly,
however, be enhanced when a different combination of operating parameters
is used. The effect of changing only one of the operating parameters
while all others are fixed is discussed in the Supporting Information, Figure S2 and Table S5. These results suggest
(i) a trade-off between performance indicators (e.g., CH4 purity versus CH4 recovery and SEC versus productivity)
and (ii) interdependence of the (optimal) operating parameters. Thus,
one-dimensional sensitivity analyses do likely not provide the optimal
combination of operating parameters.
, and the productivity is 0.1133 Nm3BG kg–1 h–1. The performance indicators can possibly,
however, be enhanced when a different combination of operating parameters
is used. The effect of changing only one of the operating parameters
while all others are fixed is discussed in the Supporting Information, Figure S2 and Table S5. These results suggest
(i) a trade-off between performance indicators (e.g., CH4 purity versus CH4 recovery and SEC versus productivity)
and (ii) interdependence of the (optimal) operating parameters. Thus,
one-dimensional sensitivity analyses do likely not provide the optimal
combination of operating parameters.
3.2. Effect of Cycle Configuration
The process performance depends crucially on the VPSA cycle configuration and the operating parameters and conditions. Maximizing the performance, therefore, requires (i) selecting for each configuration the combination of operating parameters that result in the maximum productivity and minimum SEC under the specific constraints that are set on output gas purity and recovery and based thereon (ii) identifying the optimal configuration, all under the given operating conditions. To this end, each configuration was first simulated with all element combinations of the operating parameter sets PPR = {1,1.5,2,4} bar, PBD = {0.05,0.10,0.15} bar, tFE = {1,2,5,10} min, and Qin = {0.4,0.5,0.6,0.65,0.7,0.8} and optionally Qin = {0.3,0.9,1.0} (i.e., ≥288 combinations per configuration), again at an ambient temperature of 15 °C and with biogas feed mole fractions of 0.45 CO2 and 0.55 CH4. We refer to these combinations of operating parameters as the “seed”. Additional, more favorable combinations of operating parameters were selected from an interpolation of the output performance indicators of the initial seed simulations (and any other preceding generation of simulations) on a refined grid of operating parameters using the griddatan function in MATLAB. The selected combinations of operation parameters were then used as inputs for additional simulations. The interpolation method allows for a relatively rapid screening of combinations of operating parameters. This method is reasonably accurate, as demonstrated in the parity plots between the interpolated (based on the initial seed only) and additionally simulated (all simulations other than the initial seed) outputs in Figure S3.
Figure 6a,b display, for all simulated combinations of operating parameters and for all configurations, the CH4 recovery as a function of the CH4 purity and the CO2 recovery as a function of the CO2 purity, respectively (opaque dots). Most of the simulated combinations result in CH4 recoveries and/or CH4 purities that do not satisfy our constraints (indicated by the top-right box in Figure 6a). The maximum component recovery as a function of the component purity is for each configuration indicated by the solid lines. For both CH4 and CO2, the maximum recovery decreases with increasing purity. This is in accordance with previous studies on various sorbent materials8,10,14,20,22,24,26,32,37,39,40,42,45 and with the trade-off between both performance indicators suggested by Figure S2 and Table S5. By comparing the different configurations, we conclude the following. First, the pressure equalization steps in configurations 3–7 significantly increase the maximum CH4 recovery and CO2 purity, as was also found in previous studies.22,37,38 This increase is due to the removal of CH4 from the column during the PPE step before the CO2 product is collected during the BD step. Second, the pressurization with the CH4 product in configurations 2 and 5–6 can improve the maximum CH4 purity and CO2 recovery. A (relatively pure) CH4 product then displaces the CO2 adsorption front from the column end where the CH4 product is collected. Third, the RPE step in the forward direction (configurations 3 and 5) can improve the combination CH4 recovery and CH4 purity and the combination CO2 recovery and CO2 purity as compared to the RPE step in backward direction (configurations 4 and 6) when the CH4 recovery is relatively high. In contrast, a RPE step in the backward direction can improve the maximum CH4 purity and CO2 recovery when the CH4 recovery is relatively low, similar to the pressurization with the CH4 product. The effect of the RPE direction is discussed further in the Supporting Information, Figure S4. For a minimal CH4 purity of 0.90, the maximum CH4 recovery is ∼0.913 for configurations 1–2, ∼0.975 for configurations 3–6, and ∼0.992 for configuration 7. In other words, CH4 recovery above ∼0.91 requires at least two connected columns (i.e., configurations 3–7). Similarly, CH4 recovery above ∼0.97 requires at least three connected columns (configuration 7).
Figure 6.
Simulated (opaque
dots) and maximum (solid lines) component recovery
as a function of component purity for (a) CH4 and (b) CO2. T0 = 15 °C;  . The different colors indicate the different
configurations (Section 2.5.1).
. The different colors indicate the different
configurations (Section 2.5.1).
For each configuration, Figure 7a displays the Pareto front for the maximum
productivity
and minimum SEC (or maximum SEC–1), all under the
constraints CH4 recovery ≥0.95 and CH4 purity ≥0.90 (top-right box in Figure 6a). As configurations 1–2 never satisfy
these constraints, these do not show in this figure. The maximum productivity
decreases with decreasing SEC. This is similar to the trade-off between
product purity and recovery and was also suggested by Figure S2 and Table S5. Remarkably, configurations
3–6 show a nearly identical decrease of the maximum productivity
with decreasing SEC. To understand this similarity, we compared the
CO2 adsorption profiles (as in Figure 5e) specifically for simulations around the
center of the Pareto front (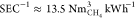 ). This revealed quite similar profiles
between the different configurations at the end of the FE, PPE, and
BD steps. Apparently, for the current, relatively mild constraint
on CH4 purity ≥0.90, the pressurization gas and
the direction of the RPE step do not significantly affect the process
performance (see Supporting Information; Figure S4). Configuration 7 shows a lower productivity for a given
SEC than configurations 3–6, despite its ability to achieve
higher CH4 recovery. This reduced productivity can be attributed
to the longer cycle duration due to the required “idle”
step for column synchronization and to the presence of two PPE and
RPE steps in this configuration, see also refs (37 and 69). Ultimately, these results imply
that the VPSA cycle configuration should be tailored toward the specific
requirements on product purity and component recovery and that the
configuration that yields the highest product purity and/or component
recovery does not necessarily provide the optimal process performance.
). This revealed quite similar profiles
between the different configurations at the end of the FE, PPE, and
BD steps. Apparently, for the current, relatively mild constraint
on CH4 purity ≥0.90, the pressurization gas and
the direction of the RPE step do not significantly affect the process
performance (see Supporting Information; Figure S4). Configuration 7 shows a lower productivity for a given
SEC than configurations 3–6, despite its ability to achieve
higher CH4 recovery. This reduced productivity can be attributed
to the longer cycle duration due to the required “idle”
step for column synchronization and to the presence of two PPE and
RPE steps in this configuration, see also refs (37 and 69). Ultimately, these results imply
that the VPSA cycle configuration should be tailored toward the specific
requirements on product purity and component recovery and that the
configuration that yields the highest product purity and/or component
recovery does not necessarily provide the optimal process performance.
Figure 7.
(a) Pareto
fronts for the maximum productivity and minimal SEC
under the constraints CH4 purity ≥0.90 and CH4 recovery ≥0.95. (b) Operating parameters along the
Pareto fronts. T0 = 15 °C;  . The different line colors indicate the
different configurations (Section 2.5.1).
. The different line colors indicate the
different configurations (Section 2.5.1).
Figure 7b shows
the operating parameters along the Pareto fronts. PPR, PBD, and tFE do not depend strongly on the specific configuration
but vary along the Pareto front. When high productivity is desired
over low SEC (left side in Figure 7a–b), PPR is high
and PBD and tFE are at or close to their lower bounds of 0.05 bar and 1 min, respectively.
With decreasing SEC and productivity, first PPR decreases toward its lower bound of 1 bar, while tFE increases only slightly and PBD remains at its lower bound (up to the black dotted
vertical line at  ). Upon further decreasing the SEC at the
expense of productivity (right side in Figure 7a–b), both PBD and tFE increase while PPR remains at its lower bound of 1 bar. This
indeed confirms that the operating parameters are interdependent and
that each individual operating parameter should not be “optimized”
using a one-dimensional sensitivity analysis only. In contrast to
the other operating parameters, Qin is
relatively constant along the Pareto front but differs between configurations.
Specifically, Qin ≈ 0.56 for configurations
3–4, Qin ≈ 0.65 for configurations
5–6, and Qin ≈ 0.46 for
configuration 7. Recall that Qin reflects
the fraction of the expected CO2 working capacity that
is provided during the FE step only. The differences between the configurations
can then be attributed to the amount of CO2 that is already
provided during the PR and RPE steps. Compared to configurations 3–4
in which the PR step uses biogas, less CO2 is provided
during the PR step in configurations 5–6 that use the CH4 product instead. This permits a larger Qin in configurations 5–6 before CO2 contaminates
the CH4 product. Compared to configurations 3–4
that use one RPE step, more CO2 is provided during the
two RPE steps in configuration 7. This permits a smaller Qin in configuration 7 before CO2 contaminates
the CH4 product.
). Upon further decreasing the SEC at the
expense of productivity (right side in Figure 7a–b), both PBD and tFE increase while PPR remains at its lower bound of 1 bar. This
indeed confirms that the operating parameters are interdependent and
that each individual operating parameter should not be “optimized”
using a one-dimensional sensitivity analysis only. In contrast to
the other operating parameters, Qin is
relatively constant along the Pareto front but differs between configurations.
Specifically, Qin ≈ 0.56 for configurations
3–4, Qin ≈ 0.65 for configurations
5–6, and Qin ≈ 0.46 for
configuration 7. Recall that Qin reflects
the fraction of the expected CO2 working capacity that
is provided during the FE step only. The differences between the configurations
can then be attributed to the amount of CO2 that is already
provided during the PR and RPE steps. Compared to configurations 3–4
in which the PR step uses biogas, less CO2 is provided
during the PR step in configurations 5–6 that use the CH4 product instead. This permits a larger Qin in configurations 5–6 before CO2 contaminates
the CH4 product. Compared to configurations 3–4
that use one RPE step, more CO2 is provided during the
two RPE steps in configuration 7. This permits a smaller Qin in configuration 7 before CO2 contaminates
the CH4 product.
The effects of the individual operating parameters on the performance indicators are for all configurations illustrated in Figures S5–S9. We can now generalize these along all dimensions and for all configurations. In summary, (i) increasing PPR increases the productivity and SEC and generally decreases the CH4 recovery while the effect on CH4 purity is nontrivial and depends on the other operating parameters; (ii) increasing PBD decreases the CH4 recovery and productivity and almost always decreases the CH4 purity and the SEC (configurations 2 and [less so] 5–6 that use CH4 product pressurization deviate at high PPR only); (iii) increasing tFE increases the CH4 purity and decreases the productivity; and (iv) increasing Qin increases the CH4 recovery at the expense of CH4 purity, increases the productivity, and decreases the SEC. These trends are also in accordance with those presented in Figure 7b for fixed constraints on CH4 recovery and purity and largely in line with the observations for configuration 3 along one dimension only (i.e., Figure S2 and Table S5).
3.3. Effects of Ambient Temperature and Biogas Composition
To illustrate the effects of the operating conditions
ambient temperature and biogas composition on the process, we performed
additional simulations with (i) T0 = 25
°C and  and (ii)
and (ii)  and T0 = 15
°C, in addition to (iii) the previously discussed conditions
(T0 = 15 °C,
and T0 = 15
°C, in addition to (iii) the previously discussed conditions
(T0 = 15 °C, 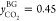 ; all balance CH4). We restrict
to configuration 3 for three main reasons. First, two connected columns
(configurations 3–6) result in the highest productivity for
a given SEC under the constraints CH4 purity ≥0.90
and CH4 recovery ≥0.95. Second, the RPE step in
the forward direction (configurations 3 and 5) can improve the combination
CH4 recovery and CH4 purity in the relevant
domain as compared to the RPE step in the backward direction (configurations
4 and 6). Third, configuration 3 (and 4) excludes CH4 product
refluxes and hence simplifies the unit (as compared to configurations
5–6).
; all balance CH4). We restrict
to configuration 3 for three main reasons. First, two connected columns
(configurations 3–6) result in the highest productivity for
a given SEC under the constraints CH4 purity ≥0.90
and CH4 recovery ≥0.95. Second, the RPE step in
the forward direction (configurations 3 and 5) can improve the combination
CH4 recovery and CH4 purity in the relevant
domain as compared to the RPE step in the backward direction (configurations
4 and 6). Third, configuration 3 (and 4) excludes CH4 product
refluxes and hence simplifies the unit (as compared to configurations
5–6).
Figure 8a,b display for each simulated combination of operating parameters and for the three conditions the component recovery as a function of the component purity (similar to Figure 6). The different temperature and biogas composition only have limited effects on the maximum CH4 recovery and CH4 purity (Figure 8a). Consequently, under all three conditions, the constraints CH4 purity ≥0.90 and CH4 recovery ≥0.95 can be satisfied. In contrast, but as expected, a smaller CO2 fraction in the biogas reduces the maximum CO2 recovery and CO2 product purity (Figure 8b).
Figure 8.
Simulated (opaque dots) and maximum (solid lines) component recovery as a function of component purity for (a) CH4 and (b) CO2. (c) Pareto fronts for the maximum productivity and minimal SEC under the constraints CH4 purity ≥0.90 and CH4 recovery ≥0.95. (d) Operating parameters along the Pareto fronts. Configuration 3; ambient and feed biogas conditions as detailed in legend.
Figure 8c displays the Pareto front for the maximum productivity and minimal SEC under the constraints CH4 recovery ≥0.95 and CH4 purity ≥0.90 (similar to Figure 7a). First, a higher ambient temperature slightly decreases the productivity and/or increases the SEC. This reduced performance can be attributed to the reduced CO2 adsorption at higher temperatures, Figure 3.51 This contrasts some other sorbents for which higher temperatures that facilitate their regeneration and/or enhance the diffusivity of CO2 therein are preferred.22,24,36,42 Second, a smaller CO2 fraction in the biogas strongly increases the productivity and/or decreases the SEC, in accordance with earlier studies.6,8 This improved performance can be attributed to the larger amount of CH4 that can be processed per unit adsorbed CO2. Figure 8d shows the operating parameters along the Pareto front. The trends herein largely resemble those for the different configurations (Figure 7b) but are shifted toward lower SEC for a smaller biogas CO2 fraction.
3.4. Alternative Requirements on Product Purity and Component Recovery
The requirements on product purity
and component recovery are generally set by the specific product purpose
and country-specific regulations. Alternative requirements will affect
the optimal configuration and combination of operating parameters
and ultimately the process performance. To illustrate these effects, Figures 9 and S10 and S11 show the Pareto fronts for the maximum
productivity and minimum SEC under alternative constraints on CH4 purity and CH4 recovery for configuration 3 and
for the other configurations, respectively (now again, T0 = 15 °C and 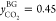 ). These Pareto fronts are based on interpolated
results (to limit the required number of simulations); therefore,
we only discuss these qualitatively.
). These Pareto fronts are based on interpolated
results (to limit the required number of simulations); therefore,
we only discuss these qualitatively.
Figure 9.
Interpolated Pareto fronts for the maximum
productivity and minimal
SEC under the constraints (a) CH4 recovery ≥0.90
and variable minimal CH4 purity and (d) CH4 purity
≥0.90 and variable minimal CH4 recovery. (b,c) Operating
parameters along the Pareto fronts in (a). (e,f) Operating parameters
along the Pareto fronts in (d). Configuration 3; T0 = 15 °C;  . The arrows are detailed in the main text.
. The arrows are detailed in the main text.
First, we consider increasingly strict constraints on CH4 purity (here, CH4 recovery ≥0.90), Figure 9a–c. On the one end, these reduce the maximum productivity (arrow I; left side of Figure 9a–b). This reduction is initially due to the smaller Qin to move the CO2 adsorption front away from the column outlet and ultimately due to the longer tFE to sharpen the CO2 adsorption front. Both reduce the amount of CO2 collected in the CH4 product but adversely affect the amount of CH4 product that is collected per cycle or per unit time (see also Figure S2). For maximum productivity, PPR and PBD are always near their upper and lower bounds, respectively. On the other end, stricter constraints on CH4 purity increase the minimum SEC (arrow II; bottom side of Figure 9a,c). This increase is due to (i) similarly the smaller Qin and (ii) first the lower PBD and then the higher PPR that both increase the energy consumption. For minimum SEC, tFE is mostly constant and approaches its upper bound. For intermediate productivity and SEC, all operating parameters vary interdependently.
In contrast, increasingly strict constraints on CH4 recovery (here, CH4 purity ≥0.90) have less of an effect on the trade-off between SEC and productivity, Figure 9d–f. As long as PPR is not too high, high CH4 recovery is effectively provided “for free” by the pressure equalization steps. The maximum productivity is only reduced by the inability of higher PPR to satisfy these increasingly strict constraints (arrow III). This inability is due to the lower selectivity of the sorbent at higher pressure.51 The reduction of the maximum productivity, however, merely shrinks the compatible domain but does not decrease the maximum productivity for a given SEC as such. Only the strictest constraint on CH4 recovery (here, 0.97) requires an increase of Qin and tFE and decrease of PBD to provide a sharp CO2 adsorption front that displaces more CH4 from the column during the FE step.
3.5. Comparison with Alternative Adsorbents
The preceding analysis puts us in the position to compare the performance indicators for Cs-bentonite to those of alternative adsorbents. Before we do so, recall that all of the performance indicators are interdependent, i.e., within specific bounds, some performance indicators can be improved at the expense of others. This, together with varying biogas compositions, temperatures, and target product specifications between different studies, complicates a quantitative comparison to other published works.
Table 4 tabulates the performance indicators for Cs-bentonite for several different conditions and product purity and component recovery levels along the Pareto fronts in Figures 7a and 8c at the point where PPR tends to 1 bar (except for the high productivity case). This point is often around the midpoint of the Pareto front. It is of further interest because a process at ambient pressure may as well reduce capital and maintenance costs due to a possible simplification of the unit, e.g., enabling the use of a blower or simply the overpressure from the digester instead of a compressor.
Table 4. Simulated Performance Indicators for Cs-Bentonite and for Several Conditions and Product Purity and Component Recovery Levels.
| CH4/CO2 | T0 (°C) | Ca | CH4 pur | CH4 rec | CO2 pur | CO2 rec | SECb | prodc | notes |
|---|---|---|---|---|---|---|---|---|---|
| 55/45 | 15 | 3 | 0.906 | 0.967 | 0.957 | 0.878 | 0.072 | 0.097 | |
| 55/45 | 25 | 3 | 0.904 | 0.964 | 0.952 | 0.874 | 0.071 | 0.082 | higher temperature |
| 65/35 | 15 | 3 | 0.904 | 0.964 | 0.926 | 0.809 | 0.046 | 0.115 | larger CH4 feed fraction |
| 55/45 | 15 | 7 | 0.908 | 0.981 | 0.975 | 0.878 | 0.079 | 0.055 | high CH4 recovery |
| 55/45 | 15 | 6 | 0.962 | 0.906 | 0.892 | 0.955 | 0.083 | 0.056 | high CH4 purity |
| 55/45 | 15 | 3 | 0.908 | 0.952 | 0.937 | 0.881 | 0.121 | 0.161 | high productivity |
Configuration.
Units: 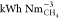 .
.
Units: Nm3BG kg–1 h–1.
In addition to Cs-bentonite, ref (51) also considered tetramethylammonium (TMA)-bentonite for biogas upgrading. However, in contrast to Cs-bentonite, the interlayer galleries of TMA-bentonite are accessible to CH4 and, therefore, this material shows a lower CO2/CH4 selectivity of ∼7 (Figure S12). In the Supporting Information, we provide a similar assessment for this material as for Cs-bentonite. We indeed find that its high CH4 adsorption capacity makes TMA-bentonite inappropriate for biogas upgrading (Figure S14).
By comparing our results in Table 4 with the results of the previous works in Table S1, we conclude the following. First, most of the previous works focused on high CH4 purity ≥0.97. While not the main focus of this work, by using CH4 product pressurization (i.e., configurations 5–6), also a CH4 purity of 0.962 was achieved (for a CH4 recovery of 0.906; higher CH4 purity is possible at the expense of CH4 recovery). On the other hand, the CH4 recovery obtained in this work is high compared to most of the previous works, with the notable exceptions of the dual-PSA units (refs (9 and 45)). We attribute this on the one hand to the relatively high CO2/CH4 selectivity up to ∼35 of Cs-bentonite and on the other hand to the use of the pressure equalization steps that were not used in some of the previous works. While not used in this work, product purge/rinse steps and/or dual-VPSA units to possibly further increase the CH4 purity and/or recovery should be the subject of a future study.
Second, the productivity of Cs-bentonite is mostly lower than or at most comparable to the conventional sorbents. This can mainly be attributed to its comparatively low CO2 adsorption capacity. However, two additional aspects should be noted here. (i) The duration of the PR, PPE, and RPE steps is in our simulations set to 1 min. The productivity can likely be increased by a reduction thereof, e.g., to ∼15–30 s for the PPE and RPE steps as in some previous works.9,10,14,22,23,26,32,37,39,40,44,45 (ii) To compare the required equipment size, the productivity can instead be expressed per unit sorbent volume. Sorbents with a high volumetric adsorption capacity (i.e., high adsorption capacity and particle density) are then favored. On the one hand, the particle density of Cs-bentonite (∼1400 kg m–3) is comparable to some zeolites,10,22,31,34 silica gel,47 and MOF-508b.10 On the other hand, it is (significantly) higher than, e.g., that of some other zeolites,9,13,24,25,28,33 MOFs,46,49 porous polymeric beads,44 silicalites,8 and CMS.10−12,14,25,36−43
Third, the SEC is always significantly lower for Cs-bentonite than for the conventional sorbents. We attribute this to the CO2 adsorption isotherms that are relatively linear in the relevant pressure domain. They thereby facilitate the sorbent regeneration already under weak vacuum conditions. Indeed, the benefits of sorbents with relatively linear CO2 adsorption isotherms for their easy regeneration (or the reverse) were suggested in various previous studies.7,10,16,22,25,36,44,45,47,49,50
Thus, Cs-bentonite generally shows excellent performance as compared to the conventional sorbents. These alternative materials often suffer from a trade-off between high CO2/CH4 selectivity and easy regeneration.12,16,49,70 (For example, AC shows easy regeneration but low CO2/CH4 selectivity ≲5,12,16−19 whereas zeolite 13X shows higher CO2/CH4 selectivity, but its regeneration is impeded by its rather steep CO2 adsorption isotherms.10,16,25,26,28,29,36) Therefore, we attribute the excellent performance of Cs-bentonite to the rather unique combination of high CO2/CH4 selectivity and easy regeneration.
4. Conclusions
The simulations presented in this work demonstrate the ability of Cs-bentonite to separate the CH4 and CO2 in biogas (i.e., biogas upgrading). A sufficiently high CH4 purity for grid injection in, e.g., The Netherlands, and a high CH4 recovery can be reached at significantly lower specific energy consumption than for conventional adsorbent materials. This is even possible at ambient feed pressure, without product purge and rinse steps, and by using a single upgrading stage. The high CH4 recovery and low specific energy consumption are due to (i) the high CO2/CH4 selectivity and (ii) the linear CO2 adsorption isotherms that facilitate the regeneration under weak vacuum conditions at ambient temperature. In the case of high CH4 recovery, a CO2 product with a typical purity of ∼0.93–0.97 is coproduced. Such a CO2 product can, for example, be used for CO2 sequestration to actually produce carbon-negative bio-CH4.44
The process performance depends crucially on the VPSA cycle configuration and the operating parameters that should, therefore, be tailored toward the specific requirements on product purity and component recovery. Pressure equalization steps between multiple columns are essential for high CH4 recovery but should be avoided when high CH4 recovery is not required. For CH4 recovery ≥0.95, a two-column system with one PE step is desired. Bio-CH4 product refluxes do not significantly improve the productivity and specific energy consumption for the production of bio-CH4 with a purity ≳0.90. However, bio-CH4 product refluxes can improve these performance indicators when higher CH4 purity is required and/or increase the maximum attainable CH4 purity. Also, the operating conditions affect the process performance; smaller CO2 fractions in the feed biogas (i.e., within the typical range of biogas composition) and lower temperatures (i.e., within the typical range of ambient temperatures) increase the productivity and/or decrease the specific energy consumption.
Interestingly, for most operating conditions and constraints on product purity and component recovery, there exists a domain along the (productivity-specific energy consumption) Pareto front in which the process does not require feed pressures above atmospheric. This enables the use of a rather simple unit to reduce capital and maintenance cost (e.g., not requiring a compressor). However, the current work did not take into account the pressurization of the CH4 product that is required for certain downstream applications. Should this be accounted for, then elevated feed pressures may be desirable to increase productivity as the CH4 product is then available at that pressure.
The current work provides a strong case for using Cs-bentonite in biogas upgrading and discusses several aspects that should be taken into account when designing a VPSA unit to do so. The design and commissioning of a pilot plant that is based on the current simulation in- and outputs should test this material on a larger scale with actual biogas and provide input parameters for further process development. We hope that, ultimately, the use of low-cost and low specific energy consumption sorbent materials like bentonite results in the wider use of biogas upgrading for the energy and chemical transitions and beyond.
Acknowledgments
We thank the University of Twente and the Faculty of Science and Technology for their financial support. We thank the Physics of Complex Fluids and Sustainable Process Technology groups at the University of Twente for their continuous cooperation. We acknowledge funding from the Dutch Research Council (NWO) for financially supporting the commercialization efforts of the technology (NWO Demonstrator, grant number: 20745).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.iecr.4c04491.
Previously reported performance indicators for various sorbent materials; physical properties of gas components and mixtures and heat and mass transport coefficients; details of the model implementation; Langmuir adsorption isotherms fit parameters; schematic of process cycle configurations; effect of individual operating parameters; parity plots simulation and interpolation; effect of RPE direction; multidimensional sensitivity analyses; Pareto fronts under alternative recovery and purity constraints; assessment of tetramethylammonium-bentonite (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Angelidaki I.; Treu L.; Tsapekos P.; Luo G.; Campanaro S.; Wenzel H.; Kougias P. G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. 10.1016/j.biotechadv.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Awe O. W.; Zhao Y.; Nzihou A.; Minh D. P.; Lyczko N. A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valorization 2017, 8, 267–283. 10.1007/s12649-016-9826-4. [DOI] [Google Scholar]
- Kapoor R.; Ghosh P.; Kumar M.; Vijay V. K. Evaluation of biogas upgrading technologies and future perspectives: a review. Environ. Sci. Pollut. Res. 2019, 26, 11631–11661. 10.1007/s11356-019-04767-1. [DOI] [PubMed] [Google Scholar]
- Mulu E.; M’Arimi M. M.; Ramkat R. C. A review of recent developments in application of low cost natural materials in purification and upgrade of biogas. Renew. Sustain. Energy Rev 2021, 145, 111081. 10.1016/j.rser.2021.111081. [DOI] [Google Scholar]
- Khan I. U.; Othman M. H. D.; Hashim H.; Matsuura T.; Ismail A. F.; Rezaei-DashtArzhandi M.; Azelee I. W. Biogas as a renewable energy fuel–A review of biogas upgrading, utilisation and storage. Energy Convers. Manage. 2017, 150, 277–294. 10.1016/j.enconman.2017.08.035. [DOI] [Google Scholar]
- Bauer F.; Persson T.; Hulteberg C.; Tamm D. Biogas upgrading–technology overview, comparison and perspectives for the future. Biofuels, Bioprod. Biorefin. 2013, 7, 499–511. 10.1002/bbb.1423. [DOI] [Google Scholar]
- Grande C. A. Biogas upgrading by pressure swing adsorption. Biofuel’s Eng. Process Technol. 2011, 65–84. [Google Scholar]
- Delgado J. A.; Uguina M. A.; Sotelo J. L.; Ruiz B.; Gomez J. M. Fixed-bed adsorption of carbon dioxide/methane mixtures on silicalite pellets. Adsorption 2006, 12, 5–18. 10.1007/s10450-006-0134-3. [DOI] [Google Scholar]
- Augelletti R.; Conti M.; Annesini M. C. Pressure swing adsorption for biogas upgrading. A new process configuration for the separation of biomethane and carbon dioxide. J. Cleaner Prod. 2017, 140, 1390–1398. 10.1016/j.jclepro.2016.10.013. [DOI] [Google Scholar]
- Wu B.; Zhang X.; Xu Y.; Bao D.; Zhang S. Assessment of the energy consumption of the biogas upgrading process with pressure swing adsorption using novel adsorbents. J. Cleaner Prod. 2015, 101, 251–261. 10.1016/j.jclepro.2015.03.082. [DOI] [Google Scholar]
- Rocha L. A. M.; Andreassen K. A.; Grande C. A. Separation of CO2/CH4 using carbon molecular sieve (CMS) at low and high pressure. Chem. Eng. Sci. 2017, 164, 148–157. 10.1016/j.ces.2017.01.071. [DOI] [Google Scholar]
- Rainone F.; D’Agostino O.; Erto A.; Balsamo M.; Lancia A. Biogas upgrading by adsorption onto activated carbon and carbon molecular sieves: Experimental and modelling study in binary CO2/CH4 mixture. J. Environ. Chem. Eng. 2021, 9, 106256. 10.1016/j.jece.2021.106256. [DOI] [Google Scholar]
- Cavenati S.; Grande C. A.; Rodrigues A. E. Removal of Carbon Dioxide from Natural Gas by Vacuum Pressure Swing Adsorption. Energy Fuels 2006, 20, 2648–2659. 10.1021/ef060119e. [DOI] [Google Scholar]
- Canevesi R. L. S.; Andreassen K. A.; da Silva E. A.; Borba C. E.; Grande C. A. Pressure swing adsorption for biogas upgrading with carbon molecular sieve. Ind. Eng. Chem. Res. 2018, 57, 8057–8067. 10.1021/acs.iecr.8b00996. [DOI] [Google Scholar]
- Grande C. A.; Blom R.; Möller A.; Möllmer J. High-pressure separation of CH4/CO2 using activated carbon. Chem. Eng. Sci. 2013, 89, 10–20. 10.1016/j.ces.2012.11.024. [DOI] [Google Scholar]
- Bacsik Z.; Cheung O.; Vasiliev P.; Hedin N. Selective separation of CO2 and CH4 for biogas upgrading on zeolite NaKA and SAPO-56. Appl. Energy 2016, 162, 613–621. 10.1016/j.apenergy.2015.10.109. [DOI] [Google Scholar]
- Peredo-Mancilla D.; Ghimbeu C. M.; Ho B.-N.; Jeguirim M.; Hort C.; Bessieres D. Comparative study of the CH4/CO2 adsorption selectivity of activated carbons for biogas upgrading. J. Environ. Chem. Eng. 2019, 7, 103368. 10.1016/j.jece.2019.103368. [DOI] [Google Scholar]
- Vivo-Vilches J. F.; Pérez-Cadenas A. F.; Maldonado-Hódar F. J.; Carrasco-Marín F.; Faria R. P.; Ribeiro A. M.; Ferreira A. F.; Rodrigues A. E. Biogas upgrading by selective adsorption onto CO2 activated carbon from wood pellets. J. Environ. Chem. Eng. 2017, 5, 1386–1393. 10.1016/j.jece.2017.02.015. [DOI] [Google Scholar]
- Gil M. V.; Álvarez-Gutiérrez N.; Martínez M.; Rubiera F.; Pevida C.; Morán A. Carbon adsorbents for CO2 capture from bio-hydrogen and biogas streams: Breakthrough adsorption study. Chem. Eng. J. 2015, 269, 148–158. 10.1016/j.cej.2015.01.100. [DOI] [Google Scholar]
- Durán I.; Rubiera F.; Pevida C. Modeling a biogas upgrading PSA unit with a sustainable activated carbon derived from pine sawdust. Sensitivity analysis on the adsorption of CO2 and CH4 mixtures. Chem. Eng. J. 2022, 428, 132564. 10.1016/j.cej.2021.132564. [DOI] [Google Scholar]
- Alvarez-Gutierrez N.; Garcia S.; Gil M. V.; Rubiera F.; Pevida C. Dynamic performance of biomass-based carbons for CO2/CH4 separation. Approximation to a pressure swing adsorption process for biogas upgrading. Energy Fuels 2016, 30, 5005–5015. 10.1021/acs.energyfuels.6b00664. [DOI] [Google Scholar]
- Santos M. S.; Grande C. A.; Rodrigues A. E. Pressure swing adsorption for biogas upgrading. Effect of recycling streams in pressure swing adsorption design. Ind. Eng. Chem. Res. 2011, 50, 974–985. 10.1021/ie100757u. [DOI] [Google Scholar]
- Khunpolgrang J.; Yosantea S.; Kongnoo A.; Phalakornkule C. Alternative PSA process cycle with combined vacuum regeneration and nitrogen purging for CH4/CO2 separation. Fuel 2015, 140, 171–177. 10.1016/j.fuel.2014.09.100. [DOI] [Google Scholar]
- Jiang Y.; Ling J.; Xiao P.; He Y.; Zhao Q.; Chu Z.; Liu Y.; Li Z.; Webley P. A. Simultaneous biogas purification and CO2 capture by vacuum swing adsorption using zeolite NaUSY. Chem. Eng. J. 2018, 334, 2593–2602. 10.1016/j.cej.2017.11.090. [DOI] [Google Scholar]
- Grande C. A.; Rodrigues A. E. Biogas to fuel by vacuum pressure swing adsorption I. Behavior of equilibrium and kinetic-based adsorbents. Ind. Eng. Chem. Res. 2007, 46, 4595–4605. 10.1021/ie061341+. [DOI] [Google Scholar]
- Canevesi R.; Grande C. A. Biogas upgrading by pressure swing adsorption using zeolite 4A. Effect of purge on process performance. Sep. Purif. Technol. 2023, 309, 123015. 10.1016/j.seppur.2022.123015. [DOI] [Google Scholar]
- Montanari T.; Finocchio E.; Salvatore E.; Garuti G.; Giordano A.; Pistarino C.; Busca G. CO2 separation and landfill biogas upgrading: A comparison of 4A and 13X zeolite adsorbents. Energy 2011, 36, 314–319. 10.1016/j.energy.2010.10.038. [DOI] [Google Scholar]
- Sonnleitner E.; Schöny G.; Hofbauer H. Assessment of zeolite 13X and Lewatit® VP OC 1065 for application in a continuous temperature swing adsorption process for biogas upgrading. Biomass Convers. Biorefin. 2018, 8, 379–395. 10.1007/s13399-017-0293-3. [DOI] [Google Scholar]
- Li Y.; Yi H.; Tang X.; Li F.; Yuan Q. Adsorption separation of CO2/CH4 gas mixture on the commercial zeolites at atmospheric pressure. Chem. Eng. J. 2013, 229, 50–56. 10.1016/j.cej.2013.05.101. [DOI] [Google Scholar]
- Grande C. A.; Rodrigues A. E. Layered vacuum pressure-swing adsorption for biogas upgrading. Ind. Eng. Chem. Res. 2007, 46, 7844–7848. 10.1021/ie070942d. [DOI] [Google Scholar]
- Ferella F.; Puca A.; Taglieri G.; Rossi L.; Gallucci K. Separation of carbon dioxide for biogas upgrading to biomethane. J. Cleaner Prod. 2017, 164, 1205–1218. 10.1016/j.jclepro.2017.07.037. [DOI] [Google Scholar]
- Arya A.; Divekar S.; Rawat R.; Gupta P.; Garg M. O.; Dasgupta S.; Nanoti A.; Singh R.; Xiao P.; Webley P. A. Upgrading biogas at low pressure by vacuum swing adsorption. Ind. Eng. Chem. Res. 2015, 54, 404–413. 10.1021/ie503243f. [DOI] [Google Scholar]
- Remy T.; Gobechiya E.; Danaci D.; Peter S. A.; Xiao P.; Van Tendeloo L.; Couck S.; Shang J.; Kirschhock C. E. A.; Singh R. K.; et al. Biogas upgrading through kinetic separation of carbon dioxide and methane over Rb-and Cs-ZK-5 zeolites. RSC Adv. 2014, 4, 62511–62524. 10.1039/c4ra12460j. [DOI] [Google Scholar]
- Campo M. C.; Ribeiro A. M.; Ferreira A. F.; Santos J. C.; Lutz C.; Loureiro J. M.; Rodrigues A. E. Carbon dioxide removal for methane upgrade by a VSA process using an improved 13X zeolite. Fuel Process. Technol. 2016, 143, 185–194. 10.1016/j.fuproc.2015.11.024. [DOI] [Google Scholar]
- Alonso-Vicario A.; Ochoa-Gómez J. R.; Gil-Río S.; Gómez-Jiménez-Aberasturi O.; Ramírez-López C. A.; Torrecilla-Soria J.; Domínguez A. Purification and upgrading of biogas by pressure swing adsorption on synthetic and natural zeolites. Microporous Mesoporous Mater. 2010, 134, 100–107. 10.1016/j.micromeso.2010.05.014. [DOI] [Google Scholar]
- Cavenati S.; Grande C. A.; Rodrigues A. E. Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 2006, 61, 3893–3906. 10.1016/j.ces.2006.01.023. [DOI] [Google Scholar]
- Canevesi R. L. S.; Andreassen K. A.; Silva E. A.; Borba C. E.; Grande C. A. Evaluation of simplified pressure swing adsorption cycles for bio-methane production. Adsorption 2019, 25, 783–793. 10.1007/s10450-019-00049-x. [DOI] [Google Scholar]
- Chouikhi N.; Brandani F.; Pullumbi P.; Perre P.; Puel F. Biomethane production by adsorption technology: New cycle development, adsorbent selection and process optimization. Adsorption 2020, 26, 1275–1289. 10.1007/s10450-020-00250-3. [DOI] [Google Scholar]
- Kim Y. J.; Nam Y. S.; Kang Y. T. Study on a numerical model and PSA (pressure swing adsorption) process experiment for CH4/CO2 separation from biogas. Energy 2015, 91, 732–741. 10.1016/j.energy.2015.08.086. [DOI] [Google Scholar]
- Kim M.-B.; Bae Y.-S.; Choi D.-K.; Lee C.-H. Kinetic separation of landfill gas by a two-bed pressure swing adsorption process packed with carbon molecular sieve: nonisothermal operation. Ind. Eng. Chem. Res. 2006, 45, 5050–5058. 10.1021/ie0511074. [DOI] [Google Scholar]
- Cavenati S.; Grande C. A.; Rodrigues A. E. Upgrade of methane from landfill gas by pressure swing adsorption. Energy Fuels 2005, 19, 2545–2555. 10.1021/ef050072h. [DOI] [Google Scholar]
- Jayaraman A.; Chiao A. S.; Padin J.; Yang R. T.; Munson C. L. Kinetic separation of methane/carbon dioxide by molecular sieve carbons. Sep. Sci. Technol. 2002, 37, 2505–2528. 10.1081/SS-120004450. [DOI] [Google Scholar]
- Kapoor A.; Yang R. T. Kinetic separation of methane—carbon dioxide mixture by adsorption on molecular sieve carbon. Chem. Eng. Sci. 1989, 44, 1723–1733. 10.1016/0009-2509(89)80014-4. [DOI] [Google Scholar]
- Golmakani A.; Nabavi S. A.; Manović V. Production of negative-emission biomethane by twin double-bed pressure swing adsorption with tail gas sequestration. Chem. Eng. J. 2021, 408, 127312. 10.1016/j.cej.2020.127312. [DOI] [Google Scholar]
- Shen Y.; Shi W.; Zhang D.; Na P.; Fu B. The removal and capture of CO2 from biogas by vacuum pressure swing process using silica gel. J. CO2 Util. 2018, 27, 259–271. 10.1016/j.jcou.2018.08.001. [DOI] [Google Scholar]
- Ferreira A. F. P.; Ribeiro A. M.; Kulaç S.; Rodrigues A. E. Methane purification by adsorptive processes on MIL-53 (Al). Chem. Eng. Sci. 2015, 124, 79–95. 10.1016/j.ces.2014.06.014. [DOI] [Google Scholar]
- Grande C. A.; Morence D. G. B.; Bouzga A. M.; Andreassen K. A. Silica gel as a selective adsorbent for biogas drying and upgrading. Ind. Eng. Chem. Res. 2020, 59, 10142–10149. 10.1021/acs.iecr.0c00949. [DOI] [Google Scholar]
- Rieß M.; Siegel R.; Senker J.; Breu J. Diammonium-pillared MOPS with dynamic CO2 selectivity. Cell Rep. Phys. Sci. 2020, 1, 100210. 10.1016/j.xcrp.2020.100210. [DOI] [Google Scholar]
- Cavenati S.; Grande C. A.; Rodrigues A. E.; Kiener C.; Müller U. Metal organic framework adsorbent for biogas upgrading. Ind. Eng. Chem. Res. 2008, 47, 6333–6335. 10.1021/ie8005269. [DOI] [Google Scholar]
- Pires J.; Saini V. K.; Pinto M. L. Studies on selective adsorption of biogas components on pillared clays: approach for biogas improvement. Environ. Sci. Technol. 2008, 42, 8727–8732. 10.1021/es8014666. [DOI] [PubMed] [Google Scholar]
- Mendel N.; Sîretanu I.; Mugele F.; Brilman D. W. F. Biogas Upgrading Using Cation-Exchanged Bentonite Clay. Ind. Eng. Chem. Res. 2023, 62, 17883–17892. 10.1021/acs.iecr.3c01635. [DOI] [Google Scholar]
- Mendel N.; Sîretanu D.; Sîretanu I.; Brilman D. W. F.; Mugele F. Interlayer cation-controlled adsorption of carbon dioxide in anhydrous montmorillonite clay. J. Phys. Chem. C 2021, 125, 27159–27169. 10.1021/acs.jpcc.1c06746. [DOI] [Google Scholar]
- Ziemiański P. P.; Derkowski A.; Szczurowski J.; Kozieł M. The structural versus textural control on the methane sorption capacity of clay minerals. Int. J. Coal Geol. 2020, 224, 103483. 10.1016/j.coal.2020.103483. [DOI] [Google Scholar]
- Santos M. S.; Grande C. A.; Rodrigues A. E. New cycle configuration to enhance performance of kinetic PSA processes. Chem. Eng. Sci. 2011, 66, 1590–1599. 10.1016/j.ces.2010.12.032. [DOI] [Google Scholar]
- Grande C. A.; Blom R. Dual pressure swing adsorption units for gas separation and purification. Ind. Eng. Chem. Res. 2012, 51, 8695–8699. 10.1021/ie300341v. [DOI] [Google Scholar]
- Shafeeyan M. S.; Daud W. M. A. W.; Shamiri A. A review of mathematical modeling of fixed-bed columns for carbon dioxide adsorption. Chem. Eng. Res. Des. 2014, 92, 961–988. 10.1016/j.cherd.2013.08.018. [DOI] [Google Scholar]
- Hosseini S. S.; Denayer J. F. M. Biogas upgrading by adsorption processes: Mathematical modeling, simulation and optimization approach–A review. J. Environ. Chem. Eng. 2022, 10, 107483. 10.1016/j.jece.2022.107483. [DOI] [Google Scholar]
- Ergun S. Fluid flow through packed columns. Chem. Eng. Prog. 1952, 48, 89. 10.1021/ie50474a011. [DOI] [Google Scholar]
- Glueckauf E. Theory of chromatography. Part 10.—Formulæ for diffusion into spheres and their application to chromatography. Trans. Faraday Soc. 1955, 51, 1540–1551. 10.1039/TF9555101540. [DOI] [Google Scholar]
- Wehner J. F.; Wilhelm R. H. Boundary conditions of flow reactor. Chem. Eng. Sci. 1995, 50, 3885–3888. 10.1016/0009-2509(96)81815-X. [DOI] [Google Scholar]
- Gibson A. S.; Todd R. S. Improved method to converge pressure equalization steps when simulating a cyclic adsorption process. Comput. Chem. Eng. 2019, 125, 185–203. 10.1016/j.compchemeng.2019.03.021. [DOI] [Google Scholar]
- Haghpanah R.; Majumder A.; Nilam R.; Rajendran A.; Farooq S.; Karimi I. A.; Amanullah M. Multiobjective optimization of a four-step adsorption process for postcombustion CO2 capture via finite volume simulation. Ind. Eng. Chem. Res. 2013, 52, 4249–4265. 10.1021/ie302658y. [DOI] [Google Scholar]
- Jeon P. R.; Choi J.; Yun T. S.; Lee C.-H. Sorption equilibrium and kinetics of CO2 on clay minerals from subcritical to supercritical conditions: CO2 sequestration at nanoscale interfaces. Chem. Eng. J. 2014, 255, 705–715. 10.1016/j.cej.2014.06.090. [DOI] [Google Scholar]
- Hwang J.; Joss L.; Pini R. X2and CH4 on montmorillonite source clay. Microporous Mesoporous Mater. 2019, 273, 107–121. 10.1016/j.micromeso.2018.06.050. [DOI] [Google Scholar]
- Skauge A.; Fuller N.; Hepler L. G. Specific heats of clay minerals: sodium and calcium kaolinites, sodium and calcium montmorillonites, Illite, and attapulgite. Thermochim. Acta 1983, 61, 139–145. 10.1016/0040-6031(83)80310-4. [DOI] [Google Scholar]
- Kuila U.; Prasad M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. 10.1111/1365-2478.12028. [DOI] [Google Scholar]
- Mendel N.Smectite-rich Clays for CO2 Adsorption and Separation; University of Twente, 2024. [Google Scholar]
- Yavary M.; Ebrahim H. A.; Falamaki C. The effect of number of pressure equalization steps on the performance of pressure swing adsorption process. Chem. Eng. Process. 2015, 87, 35–44. 10.1016/j.cep.2014.11.003. [DOI] [Google Scholar]
- Ntiamoah A.; Ling J.; Xiao P.; Webley P. A.; Zhai Y. CO2 capture by vacuum swing adsorption: role of multiple pressure equalization steps. Adsorption 2015, 21, 509–522. 10.1007/s10450-015-9690-8. [DOI] [Google Scholar]
- Tagliabue M.; Farrusseng D.; Valencia S.; Aguado S.; Ravon U.; Rizzo C.; Corma A.; Mirodatos C. Natural gas treating by selective adsorption: Material science and chemical engineering interplay. Chem. Eng. J. 2009, 155, 553–566. 10.1016/j.cej.2009.09.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.