Abstract
The relationship of initial concentration of Staphylococcus epidermidis in blood cultures and time to positivity (TTP) in an automated, continuously monitored blood culture system was assessed. Blood and 1 to 1,000 CFU of S. epidermidis per ml in stationary or exponential phase were inoculated in BACTEC Pediatric Plus F bottles and incubated. The TTP was inversely proportional to the initial inoculated concentration. Blood culture bottles with initial bacterial densities of <10 CFU/ml had a TTP of >20 h (upper limit of 95% prediction interval, 20.7 h) and bottles with initial bacterial densities of ≥50 CFU/ml had a TTP of ≤15 h (lower limit of 95% prediction interval, 13.4 h).
Coagulase-negative staphylococci (CoNS) are ubiquitous skin commensals, and their isolation from a blood culture frequently reflects contamination. CoNS can also cause sepsis, particularly in premature infants, immunocompromised hosts, and patients with intravascular devices such as vascular catheters. In fact, CoNS are the most common cause of nosocomial bacteremia (6) as well as the most common blood culture contaminant.
The significance of CoNS grown from blood cultures from febrile patients can be difficult to determine because their recovery may reflect bloodstream infection or blood culture contamination (or colonization of intravascular catheters or transient bacteremia). The differentiation is particularly difficult in pediatric patients, from whom only a single blood culture is frequently obtained. Quantitative cultures can aid interpretation. For example, in neonates a peripheral blood culture with ≥50 CFU of CoNS per ml of blood is generally considered indicative of infection (15), whereas a blood culture with <5 to 10 CFU/ml of blood is often considered indicative of contamination (8, 12, 15).
Quantitative blood cultures can also aid in determining if bacteremia, occurring in either pediatric or adult patients, is vascular catheter related. A blood culture drawn via a central catheter with >100 CFU/ml of blood is suggestive of catheter-related bloodstream infection (4, 7, 8, 12, 15). Catheter-related bloodstream infection is likely when quantitative cultures reveal that blood obtained via a catheter has ≥4 to 10 times greater bacterial density than found in peripheral blood (3, 4, 7, 9).
Quantitative blood cultures are not routinely available to clinicians. However, automated continuous-monitoring blood culture systems are in widespread use. Although these systems do not provide quantitative data, the incubation time to positivity (TTP), the time the blood culture bottle incubates in the instrument before microbial activity is detected, is available (5). TTP has been suggested as a surrogate for the initial bacterial density (2, 7, 11) and may potentially provide useful information to aid in interpreting the clinical significance of blood cultures growing CoNS and the diagnosis of vascular catheter-associated CoNS bacteremia.
In the present study, the TTP of simulated blood cultures was determined by inoculating culture bottles with human blood and known numbers of Staphylococcus epidermidis to determine the relationship between the initial bacterial density and TTP.
MATERIALS AND METHODS
Bacteria and growth conditions.
A clinical isolate of S. epidermidis from a febrile, neutropenic child with vascular catheter-related sepsis was used in these studies. To obtain bacteria in the exponential phase of growth, the organism was grown on 5% sheep blood agar (BBL, Becton Dickinson and Co., Cockeysville, Md.) for 18 h. The isolate was then suspended in Trypticase soy broth (BBL) and adjusted to an optical density (OD) of ≈0.2 at 490 nm using a Spectronic 20 spectrophotometer (Bausch and Lomb, Rochester, N.Y.). The bacterial suspension was then incubated at 37°C for an additional 3 to 5 h to achieve an OD of 0.38 (≈108/ml). Bacteria in the stationary phase of growth were obtained by harvesting colonies from sheep blood agar after ≈18 h of incubation at 35°C and suspending them in 0.01 M phosphate-buffered saline (PBS, pH 7.2).
Bacterial suspensions were diluted in PBS to attain the desired number of bacteria for blood culture bottle inoculation. The bacterial concentration was determined by a quantitative plate count method: 0.1 ml of serial 10-fold dilutions was spread on blood agar, and following overnight incubation at 35°C, colonies were counted on plates containing 40 to 200 colonies.
Simulated blood cultures.
A blood culture sample was simulated according to the following method. Human blood was obtained from outdated units of packed red blood cells from the hospital blood bank. Blood cells were warmed to 37°C prior to use. A bacterial suspension of known density was prepared in PBS and served as a seed inoculum. In each of four trials conducted, initial bacterial densities of 1 to 1,000 CFU/ml were used. Two milliliters of red blood cells and either 0.1 or 0.2 ml of the seed inoculum were injected aseptically into BACTEC Pediatric Plus F bottles (Becton Dickinson Microbiology Systems, Sparks, Md.). The inoculum is reported as CFU per milliliter of blood, and each inoculum tested in the study was assayed in triplicate. The blood culture bottles were placed in the BACTEC 9240 instrument within 2 h after inoculation.
In separate experiments to determine the effect of delayed incubation on the TTP, blood culture bottles inoculated with 10 or 100 CFU of exponential-phase CoNS per ml were allowed to remain at controlled room temperature for 6 and 12 h prior to incubation in the BACTEC 9240 instrument. TTP was compared with that of inoculated blood culture bottles placed in the incubator within 1 h after inoculation. In these experiments, TTP was determined in triplicate in two different trials at initial bacterial densities of ≈10 and ≈100 CFU/ml. Incubation time to detection of bacterial growth (TTP), which is calculated by the instrument, was recorded.
Statistical analyses were performed using the Mann-Whitney rank sum test, and SigmaPlot 2000 (SPSS, Inc., Chicago, Ill.) was used for linear regression, 95% prediction intervals, 95% confidence intervals, and preparation of the figure.
RESULTS
The growth phase of CoNS circulating in the blood of patients, colonizing intravascular devices, or colonizing the surface of the skin is unknown. Therefore, in the present study, simulated cultures grown to both exponential and stationary phase were used. Four trials were conducted with exponential-phase and stationary-phase bacteria using initial cell densities of 1 to ≈1,000 CFU/ml of blood.
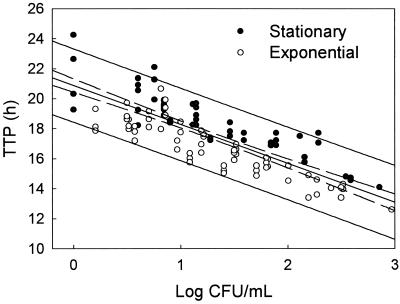
All cultures with initial bacterial densities of ≥5 CFU/ml yielded a positive result, as did 22 of 25 cultures with initial densities of <5 CFU/ml of blood. All positive cultures were detected within 25 h of incubation. Figure 1 demonstrates a significant inverse correlation between the logarithm of the initial bacterial density and TTP (P < 0.0001) for CoNS in either phase of growth. Over the range of initial bacterial densities tested, exponential-phase bacteria exhibited a TTP 1.5 to 2 h shorter than stationary-phase bacteria. TTP for cultures with an initial density of <10 CFU/ml of blood was always >16.5 h for exponential-phase bacteria and >18 h for stationary-phase bacteria. TTP for cultures with an initial density of ≥100 CFU/ml blood was <15.5 h for exponential-phase bacteria and <17.8 h for stationary-phase bacteria.
FIG. 1.
TTP for blood culture bottles inoculated with 2 ml of blood containing various initial concentrations of exponential-phase (open circles) or stationary-phase (solid circles) S. epidermidis. Each circle represents the result of a single culture. The dashed lines indicate the 95% confidence intervals for the regression line of the data points. The solid lines represent the 95% prediction intervals. The combined r = 0.84.
Using the 95% prediction intervals (solid lines in the figure), there is a 95% likelihood that an initial bacterial density of <10 CFU/ml of blood will result in a TTP of >20.7 h, an initial bacterial density of ≥50 CFU/ml will result in a TTP of ≤14.0 h, and an initial bacterial density of ≥100 CFU/ml will result in a TTP of ≤13.4 h.
In clinical practice, there may be considerable variation in the time interval between obtaining the blood culture and placement of the blood culture bottle in the BACTEC instrument. This variation may be due in part to delays in transport to the microbiology laboratory or limitations imposed by laboratory hours of operation. Therefore, the effect of such delays in incubation was also studied. Blood culture bottles placed in the BACTEC 9240 instrument at 6 or 12 h postinoculation were compared to those incubated within 1 h of inoculation. Simulated blood cultures containing approximately 10 CFU/ml demonstrated a TTP that was shortened by a mean of 1.8 h (range, 0.87 to 3.4 h) after an incubation delay of 6 h. Similarly, a mean decrease in TTP of 2.1 h (range, 1.1 to 3.3 h) was observed after a 12-h delay. Introduction of an inoculum containing ≈100 CFU/ml into the blood culture bottle resulted in a TTP that was shortened by a mean of 1.3 h (range, 0.13 to 1.5 h) and 1.5 h (range, −0.21 to 2.6 h) after a delay of 6 and 12 h, respectively.
DISCUSSION
In this study, blood cultures from children were simulated by inoculating 2 ml of human blood, a typical volume obtained from a infant or young child, along with the bacterial inoculum. The inoculum density used was determined by measuring viable count rather than by optical density measurement to ensure that TTP correlated with viable, metabolically active bacterial cells. This method was chosen because BACTEC 9240 and other continuous-monitoring blood culture systems are based on detection of bacterial metabolic activity (10). Because it is not known if bacteria circulating in the bloodstream are in an exponential or stationary phase of growth, S. epidermidis was studied at both phases of growth. It is likely that CoNS colonizing the skin are the reservoir of blood culture contaminants, and it is plausible that skin contaminants are more likely to be in a stationary phase of growth than are clinically significant CoNS recovered from the blood.
A significant inverse correlation was found between TTP and initial bacterial density. All cultures with a TTP of >20 h (and TTP of >20.7 h based on the upper limit of the 95% prediction interval) had initial densities of <10 CFU/ml, a concentration generally indicative of CoNS contamination (8, 12, 15). All blood cultures with a TTP of <15 h (and TTP of <14 h based on the lower limit of the 95% prediction interval) had initial bacterial densities of ≥50 CFU/ml. A bacterial density of this magnitude is generally indicative of bloodstream infection (4, 8, 12, 15).
Our findings are similar to those of Rogers and Oppenheim (11), who inoculated S. epidermidis into bottles from a different continuous-monitoring blood culture system (BacT/Alert). They found that inoculated concentrations of ≤10 CFU/ml were detected in ≥16.3 h and concentrations of ≥100 CFU/ml were detected in ≤14.8 h. Schelonka et al. (13) inoculated Pedi-BacT/Alert culture bottles with 2 ml of blood and small numbers (≤5 CFU/2 ml of blood) of S. epidermidis organisms (or other microorganisms), which were then placed in the BacT/Alert instrument. After inoculating S. epidermidis, they found a median TTP of 22.5 h, with the 25th to 75th percentile range of 20.0 to 28.7 h, findings comparable to the TTP observed after inoculation of similarly low colony counts in the present study.
In a previous clinical study of the TTP of pediatric blood cultures yielding CoNS from patients with vascular catheter-related bacteremia or patients with a CoNS contaminant, all blood cultures having a TTP of ≤13 h and 85% of blood cultures with a TTP of ≤17 h were found to be from patients with catheter-related bacteremia (Y. Haimi-Cohen and L. G. Rubin, Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am., abstr. 254, 1999). In a clinical study of adults with blood cultures positive for CoNS after incubation in the BacT/Alert continuous-monitoring system, no difference in median TTP was found between isolates judged to represent true CoNS bacteremia and isolates considered to be blood culture contaminants (14). However, the data were not analyzed to determine what proportion of CoNS pathogens and CoNS contaminants had a TTP of >20.7 h or <14 h.
Thus, the present findings suggest that a pediatric BACTEC blood culture yielding CoNS with a TTP of >20.7 h (in the absence of confounding factors such as current treatment with an antibiotic active against the CoNS isolate) is likely to indicate a low initial colony count. A culture with a TTP of <14 h is likely to indicate a high initial colony count. The initial bacterial density of TTPs in the intermediate range of 14 to 20.7 h is “indeterminant” and may not be helpful to the clinician faced with deciding whether a CoNS blood culture isolate is a pathogen or contaminant.
A 4- to 10-fold-higher bacterial density obtained from a blood culture drawn through a vascular catheter compared to venipuncture is considered evidence of catheter-related bloodstream infection (3, 4, 7, 9). In the present study, a 10-fold difference in the inoculated bacterial density resulted in a differential TTP of >2.3 h and 2.6 h for exponential-phase and stationary-phase bacteria, respectively. A fivefold difference in the inoculated density resulted in a differential TTP of 1.6 h and 1.8 h for exponential-phase and stationary-phase bacteria, respectively.
Blot et al. (2) studied patients with suspected catheter-related bacteremia by inoculating blood cultures with blood drawn through the catheter and from peripheral veins and incubating them in an automated continuous-monitoring blood culture system (Vital; bioMérieux, France). Patients were infected with a variety of pathogens, including several with S. epidermidis. For patients with vascular catheter-related bloodstream infection, a differential TTP of >2 h for blood drawn through the catheter compared with blood from a peripheral blood vessel had a 100% sensitivity and a 96% specificity for diagnosis of vascular catheter-related sepsis (1, 2).
A 6- or 12-h delay in incubation following inoculation of the blood culture bottle with ≈10 CFU/ml shortened the TTP by 1.8 and 2.1 h, respectively. This should have no effect on interpretation of the TTP of paired blood cultures obtained from a catheter and a peripheral vein because it is likely that both cultures would be subjected to the same delay and should not affect the differential TTP, the relevant time for assessing if the blood culture isolate is catheter related. However, a decrease in the true TTP of ≈2 h would affect interpretation of the initial bacterial density for reported TTPs in the range of 18.7 to 20.6 h (resulting in an interpretation of indeterminant rather than <10 CFU/ml) and reported TTPs of 12 to 14 h (resulting in an interpretation of >50 CFU/ml rather than indeterminant).
There are several limitations to our study. Only a single CoNS strain was used. It is possible that other CoNS may exhibit different growth kinetics in this blood culture system. In addition, we only studied a single blood culture medium and a single continuous-monitoring blood culture system. However, the studies cited above using different CoNS strains, different media, and different systems had remarkably similar results. This in vitro study using simulated blood cultures may not accurately reflect the TTP that would be observed with clinical specimens yielding CoNS.
The clinical utility of our findings needs to be validated with clinical studies. However, our results support the concept that the TTP of CoNS-positive blood cultures using BACTEC Pediatric Plus F blood cultures bottles in the BACTEC 9240 continuous-monitoring blood culture system serves as a surrogate for a quantitative culture. A TTP of <14 h or >20.7 h is likely to represent high-density and low-density CoNS bacteremia, respectively. This information may be helpful when combined with other clinical and laboratory data for distinguishing CoNS contaminants from pathogens. A differential TTP of >2.6 h between simultaneously obtained blood cultures is likely to indicate at least a 10-fold difference in initial bacterial density and may be useful in determining if CoNS bacteremia is vascular catheter related.
REFERENCES
- 1.Blot, F., G. Nitenberg, E. Chachaty, B. Raynard, N. Germann, S. Antoun, A. Laplanche, C. Brun-Buisson, and C. Tancrede. 1999. Diagnosis of catheter-related bacteremia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet 354:1071-1077. [DOI] [PubMed] [Google Scholar]
- 2.Blot, F., E. Schmidt, G. Nitenberg, C. Tancrede, B. Leclercq, A. Laplanche, and A. Andremont. 1998. Earlier positivity of central venous versus peripheral blood cultures is highly predictive of catheter-related sepsis. J. Clin. Microbiol. 36:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capdevila, J. A. 1998. Catheter-related infection: an update on diagnosis, treatment, and prevention. Int. J. Infect. Dis. 2:230-236. [DOI] [PubMed] [Google Scholar]
- 4.Capdevila, J. A., A. M. Planes, M. Palomar, I. Gasser, B. Almirante, A. Pahissa, E. Crespo, and J. M. Martinez-Vazquez. 1992. Value of differential quantitative blood cultures in the diagnosis of catheter-related sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 11:403-407. [DOI] [PubMed] [Google Scholar]
- 5.Doern, G. V., A. Barton, and S. Rao. 1998. Controlled comparative evaluation of BacT/Alert FAN and ESP 80A aerobic media as means for detecting bacteremia and fungemia. J. Clin. Microbiol. 36:2686-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 7.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 8.Phillips, S. E., and J. S. Bradley. 1990. Bacteremia detected by lysis direct plating in a neonatal intensive care unit. J. Clin. Microbiol. 28:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quilici, N., G. Audibert, M.-C. Conroy, P.-E. Bollaert, F. Guillemin, P. Welfringer, J. Garric, M. Weber, and M.-C. Laxenaire. 1997. Differential quantitative blood cultures in the diagnosis of catheter-related sepsis in intensive care units. Clin. Infect. Dis. 25:1066-1070. [DOI] [PubMed] [Google Scholar]
- 10.Reisner, B. S., G. L. Woods, R. B. Thomson, Jr., D. H. Larone, L. S. Garcia, and R. Y. Shimuzu. 1999. Specimen processing, p. 67. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 11.Rogers, M. S., and B. A. Oppenheim. 1998. The use of continuous monitoring blood culture systems in the diagnosis of catheter related sepsis. J. Clin. Pathol. 51:635-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruderman, J. W., M. A. Morgan, and A. H. Klein. 1988. Quantitative blood cultures in the diagnosis of sepsis in infants with umbilical and Broviac catheters. J. Pediatr. 112:748-751. [DOI] [PubMed] [Google Scholar]
- 13.Schelonka, R. L., M. K. Chai, B. A. Yoder, D. Hensley, R. M. Brockett, and D. P. Ascher. 1996. Volume of blood required to detect common neonatal pathogens. J. Pediatr. 129:275-278. [DOI] [PubMed] [Google Scholar]
- 14.Souvenir, D., D. E. Anderson, S. Palpant, H. Mroch, S. Askin, J. Anderson, J. Claridge, J. Eiland, C. Malone, M. W. Garrison, P. Watson, and D. M. Campbell. 1998. Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J. Clin. Microbiol. 36:1923-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St. Geme, J. W., III, L. M. Bell, S. Baumgart, C. T. D'Angio, and M. C. Harris. 1990. Distinguishing sepsis from blood culture contamination in young infants with blood cultures growing coagulase-negative staphylococci. Pediatrics 86:157-162. [PubMed] [Google Scholar]