Abstract
A 5′ nuclease assay was developed to detect Lawsonia intracellularis in porcine fecal samples. The specific probe and primers were chosen by using the 16S ribosomal DNA gene as a target. The 5′ nuclease assay was used with a total of 204 clinical samples, and the results were compared to those of immunohistochemistry (IM) on ileal sections of the same animals. There was 91% agreement between the results of IM and the 5′ nuclease assay. In the 5′ nuclease assay, 111 (54%) of the pigs tested positive for L. intracellularis infection, with a mean cycle threshold (Ct) value of 27.2, whereas 98 (48%) of the pigs tested positive by IM. On average, the Ct and ΔRn values for the positive samples were 27.2 (standard deviation [SD], 3.7) and 1.6 (SD, 0.7), respectively. A Ct value of 27.2 corresponds to a fecal excretion of approximately 107 L. intracellularis cells per g of feces. Furthermore, a total of 40 fecal samples derived from a herd known to be free from infection with L. intracellularis all tested negative, with a Ct value of 40. By using a Ct value of 36 as the cutoff limit, the detection limit of the assay was 1 L. intracellularis cell per PCR tube. In conclusion, the 5′ nuclease assay that has been developed represents an applicable fast method for detection of L. intracellularis in fecal samples, with a sensitivity and specificity comparable to those of IM.
Porcine proliferative enteropathy (PPE) is a transmissible enteric disease of growing pigs characterized by adenomatous proliferation of immature intestinal epithelial cells in the distal small intestines, particularly in the ileum. The disease is caused by infection with the intracellular bacterium Lawsonia intracellularis. The infection is widely distributed among pig herds in many pig-producing countries, and affected pigs may exhibit impaired growth and diarrhea (19, 23). Postmortem diagnosis of PPE involves macroscopic pathological findings and detection of L. intracellularis intracellularly in the mucosal epithelium by Warthin-Starry silver staining (23), immunohistochemistry (IM) with monoclonal antibodies (11, 20), or in situ hybridization (3, 7). However, for epidemiological and herd surveillance studies and for detection of subclinical infections, antemortem diagnostic methods are necessary.
Until now, it has only been possible to grow L. intracellularis in vitro in cell cultures, and this method is not adaptable for routine procedures. For that reason, antemortem detection of the bacterium must be based on culture-independent techniques. Several methods have been described for detection of L. intracellularis in fecal samples. These methods include immunofluorescent staining of fecal smears (15, 20), dot blot hybridization (13), PCR (5, 14, 15, 21), and PCR-enzyme-linked oligosorbent assay (26). The primers for the PCR methods have been directed towards the 16S ribosomal DNA (rDNA) gene and a chromosomal gene sequence of L. intracellularis (5, 14). Although the PCR assay offers high degrees of specificity and sensitivity, the processing of a large number of samples is a very time-consuming procedure. Furthermore, the detection of the PCR product by gel electrophoresis or hybridization adds time to the analysis and may lead to laboratory contamination.
An assay applicable to real-time quantitative PCR which uses the 5′ nuclease activity of the Taq polymerase has been described previously (9, 10). In this assay, a specific oligonucleotide probe labeled with two fluorescent dyes (TaqMan probe) anneals between the two primer pairs (9). One fluorescent dye is referred to as the reporter, and its emission spectrum is quenched by the other fluorescent dye (the quencher) due to the proximity of the two dyes. During elongation of the PCR primers, the probe is cleaved and the reporter and quencher molecules are released. This results in an increased emission from the reporter relative to that of the quencher. The amount of reporter released is proportional to the amount of DNA being amplified by PCR and increases for each cycle. The results are expressed as a value termed ΔRn, representing the normalized reporter signal minus the baseline signal before amplification. The cycle threshold (Ct) value is defined as the cycle number, where ΔRn exceeds a threshold defined as 10 standard deviations (SD) above the mean baseline emission observed for nontemplate controls. The baseline value is set between cycles 3 and 15, which is the software's default value (9). The purpose of the present study was to develop and evaluate a 5′ nuclease assay for the detection of L. intracellularis in fecal samples. The assay was compared to the detection of L. intracellularis in intestinal tissue of the same animals by IM.
MATERIALS AND METHODS
Clinical samples.
The material used for the study was obtained from routine submissions of whole carcasses or entire intestines received at the Danish Veterinary Laboratory from July to December 1999. The material originated from growing pigs (weight, 20 to 90 kg) with or without suspicion of intestinal disorders. For the 5′ nuclease assay, rectal fecal samples were taken, and when these were not available, colon contents were used instead. For IM, a section of ileum was fixed in neutral buffered formalin. In general, samples were processed on the day of arrival at the laboratory. When this was not possible, samples were stored at 5°C overnight and processed the following day. The sample used as the positive control was derived from mucosal scrapings of ileum from a pig affected by PPE, as diagnosed on the basis of gross lesions and IM findings. Negative fecal samples were collected from a herd known to be free from infection with L. intracellularis after eradication and repeated negative test results by PCR and serology (16, 21). To elucidate the variability of the test, 20 positive samples were tested in duplicate.
Optimization of sample preparation.
For extraction of DNA from fecal samples, three different methods were tested initially. The samples used were clinical samples that initially tested positive by IM. In method I, approximately 0.1 g of feces was thoroughly mixed with 200 μl of lysis buffer (50 mM Tris-HCl [pH 8.4], 1 mM EDTA, 0.5% Tween 20). The samples were boiled for 10 min, and 2 μl of the lysate was diluted in 200 μl of Milli-Q water. In method II, approximately 0.1 g of feces was suspended in an aqueous two-phase system containing polyethylene glycol and dextran, as described by Lantz et al. (17). After addition of the fecal sample, the contents of the aqueous two-phase system were mixed by inversion approximately 20 times and then left at room temperature for 30 min to separate. In method III, the BactXtractor solution (QRAB, Baålsta, Sweden) for separation and isolation of bacteria from food and feces was used. Samples were prepared according to the manufacturer's guidelines. Tenfold dilutions of the DNA preparations obtained from the three methods were subjected to the 5′ nuclease assay, and the differences in Ct values between method I and the two other methods were determined.
Primers and probe.
The primers and probe were based on the 16S rDNA gene. The sequence of the 16S rDNA gene of L. intracellularis has been published previously and was available through GenBank, accession no. L15739 (6).
The primers and probe were chosen by using Primer Express software (Perkin- Elmer, Foster City, Calif.). The following are the primer and probe sequences: forward primer, 5′-GCGCGCGTAGGTGGTTATAT-3′ (nucleotides 583 to 602 in GenBank sequence accession no. L15739); reverse primer, 5′-GCCACCCTCTCCGATACTCA-3′ (nucleotides 661 to 680 in GenBank no. L15739); probe, 5′-FAM-CACCGCTTAACGGTGGAACAGCCTT-TAMRA-3′ (nucleotides 621 to 645 in GenBank no. L15739). To prevent elongation, the probe had a 3′ phosphate block. The reporter dye used was 6-carboxyfluorescein (FAM); the quencher dye used was 6-carboxytetramethylrhodamine (TAMRA). The probe and primers were obtained from DNA Technology (Forskerparken, Aarhus, Denmark).
The size of the expected amplicon was 98 bp. The identity of the PCR product was confirmed by sequencing selected PCR products with the Taq Dye Deoxy Terminator Cycle Sequencing Kit and a 373A DNA Sequencer (Perkin-Elmer).
The 5′ nuclease assay.
The assay was carried out in an ABI PRISM sequence detection system. The assay was performed on 5 μl of lysate with 0.025 U of AmpliTaq Gold polymerase in a total volume of 50 μl containing 1× TaqMan buffer A, 5 mM MgCl2, 200 μM (each) dATP, dCTP, and dGTP, 400 μM dUTP, and 8% glycerol. Primer and probe concentrations were optimized according to the manufacturer's guidelines. Primer concentrations were 900 nM each, and the probe concentration was 200 nM. Amplification was performed in a 96-well plate with optical caps at the following settings: 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min of annealing and extension at 62°C.
Specificity of the 5′ nuclease assay.
On two separate days, 20 fecal samples were obtained from the L. intracellularis-free herd and tested with the 5′ nuclease assay. In addition, the 5′ nuclease assay was used on clinical isolates of the following bacterial species (with the number of isolates shown in parentheses): hemolytic Escherichia coli (n = 3), nonhemolytic E. coli (n = 2), Salmonella enterica serovar Typhimurium (n = 3), Campylobacter coli (n = 5), Campylobacter jejuni (n = 3), Clostridium perfringens types A, B, and C (n = 5), Brachyspira hyodysenteriae (n = 3), and weakly β-hemolytic spirochetes of groups II, III, and IV (n = 5).
IM.
The results of the assay were compared to detection of L. intracellularis by immunohistochemical staining of ileal sections from the same animal. Thus, at necropsy, an approximately 2-cm-long sample of the ileum was fixed in 10% neutral buffered formalin for immunohistochemical examination (12). IM was performed by standard operating procedure on all samples by using an indirect fluorescein isothiocyanate immunofluorescence procedure (11) with mouse monoclonal antibodies to L. intracellularis (20). For epifluorescence microscopy, a DMRB microscope (Leica Microsystems AG, Wetzlar, Germany) equipped with a 100-W halogen lamp was used.
Detection limit.
For determination of the detection limit of the assay, dilutions of L. intracellularis were added to feces derived from the L. intracellularis-free herd. The L. intracellularis isolate, originating from a Danish pig, was derived from cell culture in a rat enterocyte cell line (IEC 18), as described by Lawson et al. (18). Tenfold dilutions of the bacterium were prepared in saline. One hundred microliters of each dilution was added to 0.1 g of feces and thoroughly mixed. The samples were then prepared as previously described. Ten microliters of each dilution was bound to six-well, poly-l-lysine (Sigma Chemical, St. Louis, Mo.)- and Teflon-coated slides (NovaKemi AB, Enskede, Sweden), and the L. intracellularis cells were counted after visualization by immunofluorescence as described for tissue sections. The dilution experiments were performed in triplicate, and each sample was tested in at least three replicate runs.
Statistical analysis.
Descriptive statistical analyses were performed with Excel 97 (Microsoft Corp.). Regression analysis was performed with Prism 3.0 (GraphPad Software Inc., San Diego, Calif.). Differences in Ct values between sample preparation methods were determined by Student's t test.
RESULTS
Optimization of sample preparation.
A comparison of the Ct values of the three DNA extraction methods showed no significant difference (P > 0.05). The average Ct value of samples prepared by method I was 2.18 (SD, 2.16) lower than that of samples prepared by method II and 1.23 (SD, 1.87) lower than that of samples prepared by method III (n = 25). For that reason, method I was selected for further studies due to its simplicity and lowest cost.
Specificity of the 5′ nuclease assay.
None of the results for the 40 fecal samples derived from the L. intracellularis-free herd passed the threshold value during the 40-cycle run. All other bacterial species tested were negative by the 5′ nuclease assay, with a Ct value of 40.
Detection limit.
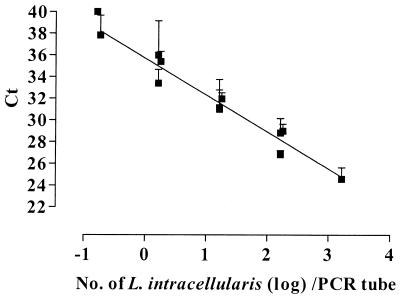
In the dilution experiments with suspensions of L. intracellularis, a log-linear relationship between the Ct value and the number of L. intracellularis cells in the PCR tube was observed (Fig. 1). For each 10-fold increase in the number of L. intracellularis cells, the average decrease in Ct value was 3.40 (SD, 0.19). This implies a highly efficient PCR amplification; with an optimal PCR efficiency, the expected increase in Ct value would be 3.32 (ln 10/ln 2). On average, the Ct value was 35.7 (SD, 0.30) with 1 L. intracellularis cell present in the PCR tube. When the samples were diluted further, the average Ct value gradually increased to 40. For that reason, the cutoff Ct value for a positive sample was set at 36, corresponding to a detection limit of 1 L. intracellularis cell per tube.
FIG. 1.
Relationship between the Ct value in the 5′ nuclease assay and the number of L. intracellularis cells in the PCR tube. Tenfold dilutions of L. intracellularis in feces were prepared in triplicate. Results are mean Ct values for at least three replicate runs, with error bars representing 1 SD. The linear regression line is indicated.
Test of clinical samples.
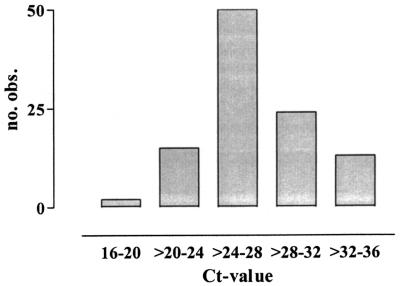
A total of 204 pigs were examined. The samples used for the 5′ nuclease assay consisted of 180 rectal fecal samples and 24 samples of colon content. The samples included intestines with PPE-associated gross lesions of various severity, normally appearing intestines, and intestines affected by catarrhal enteritis or swine dysentery. Gel electrophoresis of selected positive samples confirmed the presence of an amplicon of approximately 98 bp. By subsequent sequencing of these amplicons, they were identified as the target sequence from L. intracellularis. A total of 20 positive samples were tested in duplicate, and the average differences in Ct and ΔRn values were 0.62 (SD, 0.56) and 0.10 (SD, 0.07), respectively. The number of positive samples in the 5′ nuclease assay was 111. On average, the Ct and ΔRn values for the positive samples were 27.2 (SD, 3.7) and 1.6 (SD, 0.7), respectively. In Fig. 2, the distribution of these samples with regard to Ct value is illustrated. Forty-five percent of the samples were positive, with Ct values of 24 to 28. A comparison of the results from IM and the 5′ nuclease assay is shown in Table 1. With the assumption that an ileal sample and a fecal sample always share the same true infection status in mind, the sensitivity, specificity, and positive and negative predictive values of the 5′ nuclease assay for detection of L. intracellularis-infected pigs compared with those of IM were 97, 85, 86, and 97%, respectively. There was 91% agreement between the results of IM and the 5′ nuclease assay: 95 pigs, including 89 with PPE-associated gross lesions, were positive by both tests, whereas 90 pigs, all without PPE-associated gross lesions, were negative. Ileum samples from three pigs, all without gross lesions, were positive by IM, but fecal samples from the same pigs were negative by the 5′ nuclease assay. A total of 16 pigs were positive by the 5′ nuclease assay only. These 16 pigs included 2 with PPE-associated gross lesions in sections of the intestines other than the ileum, 12 without PPE-associated gross lesions, and 2 with gross lesions that possibly reflected healed PPE changes. The mean Ct and ΔRn values for the 16 samples from these pigs were 32.4 (SD, 3.1) and 1.0 (SD, 0.6), respectively.
FIG. 2.
Distribution of positive samples with regard to Ct value. A total of 111 samples were included. These samples all tested positive for L. intracellularis infection in the 5′ nuclease assay. obs., observed.
TABLE 1.
Comparison of results of IM and 5′ nuclease assay for detection of L. intracellularis in clinical samples from pigsa
| Results of 5′ nuclease assay | No. (%) of pigs
|
Total | |
|---|---|---|---|
| IM+ | IM− | ||
| + | 95 | 16 | 111 (54) |
| − | 3 | 90 | 93 (46) |
| Total | 98 (48) | 106 (52) | 204 |
IM was performed on sections of ileum, and the 5′ assay was performed on rectal fecal samples (n = 180) or colon content (n = 24).
DISCUSSION
The 5′ nuclease assay developed in the present study represents a fast and accurate method for antemortem detection of L. intracellularis infection in pigs. In a comparison of the assay to IM, there was a good correlation between the results of the two tests. In the 5′ nuclease assay, 111 (54%) of the pigs tested positive, whereas 98 pigs (48%) were positive by IM. Thus, it appears that the sensitivity of the 5′ nuclease assay is at least comparable to the sensitivity of the IM test. In accordance, previous studies comparing IM and PCR have reported a similar agreement between results (11, 12, 15). However, in these earlier studies, PCR was performed on mucosal scrapings of ileum, which would be expected to contain a higher number of L. intracellularis cells than fecal samples.
A discrepancy between the results of the two tests was observed in 9% of the samples; 16 samples tested positive only by the 5′ nuclease assay, whereas 3 samples were positive only by IM. The majority of these samples showed no evidence of PPE-associated gross lesions. Thus, it seems that the discrepancy between the two tests is confined to pigs without PPE-associated gross lesions. Besides reflecting a difference in sensitivity between the two tests, this discrepancy may also be due to the different sampling sites of the two tests. IM is performed on a 3-μm-thick cross section of the ileum; hence, sampling of the correct tissue is crucial for the results of this test. However, by IM, it is possible to detect as few as one L. intracellularis-infected crypt in a whole cross section (11). Although focal lesions are detectable by IM, the lesions may not result in a fecal excretion of a sufficient number of L. intracellularis cells to be detected by the 5′ nuclease assay. The higher number of samples that tested positive by the 5′ nuclease assay might also be the result of a lower specificity or may indicate an intestinal transfer of the bacterium due to a contaminated environment. However, intestinal transfer of L. intracellularis has not previously been reported, not even after the inoculation of pigs with huge doses of the bacterium in experimental studies (16, 24).
Upon testing of pure cultures of other bacterial species and fecal samples from an L. intracellularis-free herd, the results of the 5′ nuclease test were negative, indicating a high specificity. Moreover, according to the ARB database (Department of Microbiology, Technische Universität München [http://www.mikro.biologie.tu-muenchen.de]), which currently holds 10,073 bacterial 16S rDNA species, including 47 species belonging to Desulfovibrio and 2 species of Bilophila wadsworthia, the forward primer as well as the probe has two or more sequence mismatches to all other bacterial species and three or more mismatches to Desulfovibrio spp. and Bilophila spp. The reverse primer has one or more sequence mismatches to all other species in the database. Furthermore, the higher number of samples found positive is in accordance with previous comparisons of IM and PCR-based assays (11, 15). Thus, it is reasonable that the high number of pigs that tested positive by the 5′ nuclease assay without PPE-associated gross lesions is due to the excretion of L. intracellularis organisms from multiple small foci of infected intestinal mucosae.
The detection limit of the 5′ nuclease assay was determined to be 1 L. intracellularis cell per PCR tube, which is equal to a fecal excretion of approximately 4 × 104 L. intracellularis cells per g of feces. For comparison, challenge experiments have shown that pigs may excrete L. intracellularis from 2 to 10 weeks after infection, in the range of 5 × 104 to 7 × 108 organisms per g of feces (24).
In dilution experiments, a log-linear relationship between the Ct value and the number of L. intracellularis cells in the PCR tube was observed (Fig. 1). The linearity was seen over a range of 1 to 103 L. intracellularis cells per tube, with 103 being the maximum number tested. The applicability of the Ct value for quantification of target DNA and/or RNA has been demonstrated in several studies (1, 8, 9). Other studies have used the ΔRn value for quantification (2, 4, 22, 25), but Heid et al. (9) reported that using the Ct value for quantification permits a larger assay range than that permitted by using the ΔRn value directly. In our dilution experiments, some variation was observed in the ΔRn values between different runs, depending on the age and batch of the probe and, to a lesser extent, the primers. This variation was not observed for the Ct value. When the clinical samples diagnosed as positive in the 5′ nuclease assay were stratified with regard to Ct value, the samples were distributed around an average Ct value of 27.2. According to the dilution experiments, a Ct value of 27.2 corresponds to approximately 300 L. intracellularis cells per PCR tube (log 2.5), which is equal to a fecal excretion of approximately 107 L. intracellularis cells per g of feces.
Compared to traditional PCR-based methods, the 5′ nuclease assay of fecal samples represents a fast and labor-saving procedure for detection of L. intracellularis in pigs, with a reduced risk of laboratory contamination. The method is suitable for processing a large number of samples. The sensitivity of the assay is comparable to that of IM, and the application of an internal specific probe offers the possibility of making the assay more specific than traditional PCR is. Furthermore, the assay offers the possibility for quantification of fecally excreted L. intracellularis cells.
REFERENCES
- 1.Angen, Ø., J. Jensen, and D. T. Lavritsen. 2001. Evaluation of 5′ nuclease assay for detection of Actinobacillus pleuropneumoniae. J. Clin. Microbiol. 39:260-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassler, H. A., S. J. A. Flood, K. J. Livak, J. Marmaro, R. Knorr, and C. A. Batt. 1995. Use of a fluorogenic probe in a PCR-based assay for the detection of Listeria monocytogenes. Appl. Environ. Microbiol. 61:3724-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boye, M., T. K. Jensen, K. Møller, T. D. Leser, and S. E. Jorsal. 1998. Specific detection of Lawsonia intracellularis in porcine proliferative enteropathy inferred from fluorescent rRNA in situ hybridization. Vet. Pathol. 35:153-156. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S., A. Yee, M. Griffiths, C. Larkin, C. T. Yamashiro, R. Behari, C. Paszko-Kolva, K. Rahn, and S. A. De Grandis. 1997. The evaluation of a fluorogenic polymerase chain reaction assay for the detection of Salmonella species in food commodities. Int. J. Food Microbiol. 35:239-250. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, D. M., D. L. Swanson, and C. J. Gebhart. 1997. Diagnosis of proliferative enteritis in frozen and formalin-fixed, paraffin-embedded tissues from a hamster, horse, deer and ostrich using Lawsonia intracellularis-specific multiplex PCR assay. Vet. Microbiol. 54:47-62. [DOI] [PubMed] [Google Scholar]
- 6.Gebhart, C. J., S. M. Barns, S. McOrist, G.-F. Lin, and G. H. K. Lawson. 1993. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int. J. Syst. Bacteriol. 43:533-538. [DOI] [PubMed] [Google Scholar]
- 7.Gebhart, C. J., S. McOrist, G. H. K. Lawson, J. E. Collins, and G. E. Ward. 1994. Specific in situ hybridization of the intracellular organism of porcine proliferative enteropathy. Vet. Pathol. 31:462-467. [DOI] [PubMed] [Google Scholar]
- 8.Gibson, U. E. M., C. A. Heid, and P. M. Williams. 1996. A novel method for real time quantitative RT-PCR. Genome Res. 6:995-1001. [DOI] [PubMed] [Google Scholar]
- 9.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 10.Holland, P. M., R. D. Abramson, R. Watson, and D. H. Gelfand. 1991. Detection of specific polymerase chain reaction product by utilizing the 5′-3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 88:7276-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen, T. K., K. Møller, T. D. Leser, and S. E. Jorsal. 1997. Comparison of histology, immunohistochemistry and polymerase chain reaction for detection of Lawsonia intracellularis in natural porcine proliferative enteropathy. Eur. J. Vet. Pathol. 3:115-123. [Google Scholar]
- 12.Jensen, T. K., K. Møller, R. H. Lindecrona, and S. E. Jorsal. 2000. Detection of Lawsonia intracellularis in the tonsils of pigs with proliferative enteropathy. Res. Vet. Sci. 68:23-26. [DOI] [PubMed] [Google Scholar]
- 13.Jones, G. F., G. E. Ward, C. J. Gebhart, M. P. Murtaugh, and J. E. Collins. 1993. Use of a DNA probe to detect the intracellular organism of proliferative enteritis in swine feces. Am. J. Vet. Res. 54:1585-1590. [PubMed] [Google Scholar]
- 14.Jones, G. F., G. E. Ward, M. P. Murtaugh, G. Lin, and C. J. Gebhart. 1993. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis in feces by polymerase chain reaction. J. Clin. Microbiol. 31:2611-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan, D. M., J. P. Knittel, M. B. Roof, K. Schwartz, D. Larson, and L. J. Hoffman. 1999. Detection of Lawsonia intracellularis in swine using polymerase chain reaction methodology. J. Vet. Diagn. Investig. 11:45-49. [DOI] [PubMed] [Google Scholar]
- 16.Knittel, J. P., D. M. Jordan, K. J. Schwartz, B. H. Janke, M. B. Roof, S. McOrist, and D. L. Harris. 1998. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am. J. Vet. Res. 59:722-726. [PubMed] [Google Scholar]
- 17.Lantz, P. G., F. Tjerneld, E. Borch, B. Hahn-Hägerdahl, and P. Rådström. 1994. Enhanced sensitivity in PCR detection of Listeria monocytogenes in soft cheese through use of an aqueous two-phase system as a sample preparation method. Appl. Environ. Microbiol. 60:3416-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson, G. H. K., S. McOrist, S. Jasni, and R. A. Mackie. 1993. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J. Clin. Microbiol. 31:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson, G. H. K., and C. J. Gebhart. 2000. Proliferative enteropathy. J. Comp. Pathol. 122:77-100. [DOI] [PubMed] [Google Scholar]
- 20.McOrist, S., R. Boid, G. H. K. Lawson, and I. McConnell. 1987. Monoclonal antibodies to intracellular campylobacter-like organisms of the porcine proliferative enteropathies. Vet. Rec. 121:421-422. [DOI] [PubMed] [Google Scholar]
- 21.Møller, K., T. K. Jensen, S. E. Jorsal, T. D. Leser, and B. Carstensen. 1998. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly β-haemolytic intestinal spirochaetes, Salmonella enterica, and haemolytic Escherichia coli from swine herds with and without diarrhoea among growing pigs. Vet. Microbiol. 62:59-72. [DOI] [PubMed] [Google Scholar]
- 22.Oberst, R. D., M. P. Hays, L. K. Bohra, R. K. Phebus, C. T. Yamashiro, C. Paszko-Kolva, S. J. A. Flood, J. M. Sargeant, and J. R. Gillespie. 1998. PCR-based DNA amplification and presumptive detection of Escherichia coli O157:H7 with an internal fluorogenic probe and the 5′ nuclease (TaqMan) assay. Appl. Environ. Microbiol. 64:3389-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowland, A. C., and G. H. K. Lawson. 1992. Porcine proliferative enteropathies, p. 560-569. In A. D. Leman et al. (ed.), Diseases of swine, 7th ed. Iowa State University Press, Ames.
- 24.Smith, S. H., and S. McOrist. 1997. Development of persistent intestinal infection and excretion of Lawsonia intracellularis by piglets. Res. Vet. Sci. 62:6-10. [DOI] [PubMed] [Google Scholar]
- 25.Witham, P. K., C. T. Yamashiro, K. J. Livak, and C. A. Batt. 1996. A PCR-based assay for the detection of Escherichia coli Shiga-like toxin genes in ground beef. Appl. Environ. Microbiol. 62:1347-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, P., C. J. Gebhart, D. Burden, and G. E. Duhamel. 2000. Improved diagnosis of porcine proliferative enteropathy caused by Lawsonia intracellularis using polymerase chain reaction-enzyme-linked oligosorbent assay (PCR-ELOSA). Mol. Cell. Probes 14:101-108. [DOI] [PubMed] [Google Scholar]