Abstract
We evaluated, for the first time in Latin America, the performance of a commercial enzyme immunoassay (EIA) (Calypte Biomedical Corporation, Berkeley, Calif.) that detects human immunodeficiency virus type 1 (HIV-1)-specific antibodies in urine in comparison to standard serological assays (two commercial EIAs and a commercial Western blot [WB] assay). Paired serum and urine specimens were collected from two different groups of Brazilian patients: 225 drug users with unknown HIV status who attended drug treatment centers in Rio de Janeiro, Brazil, and 135 subjects with known HIV status. Patients showing positive results in the serum EIAs and/or in the urine EIA were serologically confirmed by WB assay. For 135 individuals with known HIV status, the urine EIA showed 100% sensitivity (74 positive samples) and 95.1% specificity (58 of 61 negative specimens). For 225 drug users, the test showed 100% sensitivity (2 positive samples) and 98.7% specificity (220 of 223 negative samples) compared to WB-confirmed serological EIA results. Thus, in a total of 360 samples, the urine EIA correctly identified all 76 HIV-positive samples and 278 of 284 negative samples (100% sensitivity and 97.9% specificity). Detailed analysis of the urine EIA results indicates that an increase of the recommended cutoff value might raise the specificity of the assay without affecting its sensitivity. Our results suggest that the HIV-1 urine EIA is a good screening test suitable for developing countries like Brazil. However, as for all other HIV screening tests on the market, it is not specific enough to be used as a one-step test and therefore requires confirmation.
Testing for human immunodeficiency virus (HIV)-specific antibodies continues to be the most important measure in diagnosis and epidemic surveillance of AIDS. Normally, antibodies are detected in serum or plasma samples. However, other body fluids, such as urine (3, 6, 7, 13, 14) and saliva (9, 10, 18), may serve as alternatives to serum for HIV antibody detection. The advantages of the other body fluids lie in the safety and noninvasiveness with which they can be obtained, even in precarious settings by personnel with little or no training, thus reducing the risk of accidental infection and the costs involved in sample collection and testing. In addition, venipuncture is not easily accepted by injecting drug users (1), who are reminded of their experiences, and in populations where religious and/or cultural habits discourage the donation of blood.
Urine and saliva both contain detectable amounts of specific immunoglobulins of different classes. However, saliva presents the disadvantage that it needs special collection devices and cannot be easily obtained from children (8). In this context, urine is particularly interesting, due to the ease of its collection without the need of special devices, as well as the absence of infectious virus particles (17). There is therefore no risk of exposure for health care workers and laboratory staff, and the material involved can be disposed of as regular waste. The majority of the antibodies detectable in urine would be of the immunoglobulin A (IgA) isotype locally produced in the mucosa, but small amounts of IgG can also be found in urine, due to its extravasation from the serum into the mucosa (16). In addition, it is well documented that urine is highly suitable for diagnosing a wide range of sexually transmitted diseases either by culture or by amplification techniques, such as PCR and the ligase chain reaction (1), thus making it a valuable specimen for multiple diagnoses. Nonetheless, to date the Brazilian Ministry of Health has not approved any antibody detection assay that uses saliva or urine as a specimen.
For any newly developed antibody detection assay, it is important to conduct a background evaluation study of the local population to assess specificity and to evaluate the cutoff values preset by the manufacturer. Normally, diagnostic tests are evaluated in developed countries by using samples locally obtained from well-defined populations in which major parasitic infections are absent. On the other hand, in developing countries, parasitic infections are frequent and lead, in conjunction with poor nutrition, to increased polyclonal antibody stimulation in the affected individual that can remain throughout life. The increase in nonspecific antibody titers can interfere with the performance of any antibody detection assay (22). In addition, the performance of such assays is influenced by the fact that the genetic makeup of the major histocompatibility complex is population dependent (11). As a consequence, the cutoff values established by the manufacturer should be reevaluated in different contexts and have to be adapted by receiver operating characteristics analysis. This was discussed by one of us (2) during an evaluation of the performance of commercial human T-cell lymphotrophic virus (HTLV) screening assays in Brazil. The authors showed that a simple recalculation of the cutoff value significantly improved the performance of one of the tests evaluated.
In order to address the performance and feasibility of a protocol using a urine assay in different settings, as well as to investigate the effects of population-dependent immune responses and HIV strain diversity, we carried out an evaluation of the Calypte (Berkeley, Calif.) HIV type 1 (HIV-1) urine enzyme immunoassay (EIA) using samples obtained in Brazil. The present study was designed to (i) evaluate the performance of the test with Brazilian patients with known HIV status and (ii) test the applicability of the kit for the screening of drug users with unknown HIV status recruited from drug treatment centers and taking part in a study of sexually transmitted and blood-borne infections (1). To our knowledge, the present study is the first evaluation of urine diagnosis of HIV in Latin America.
(The results of this study were presented in part as a poster at the XIIIth International Conference on AIDS in Durban, South Africa, in July 2000.)
MATERIALS AND METHODS
Study population and sample collection.
Two groups of patients were enrolled in the study after providing informed consent. Group A consisted of 135 subjects with known HIV status who attended either the outpatient service at the Evandro Chagas Hospital or a church-sponsored hospice. Sixty-three of them were HIV-positive patients who were receiving regular treatment at the hospital, 11 were AIDS patients hospitalized at the hospice for treatment who presented viral loads of more than 10,000 virus particles/ml, and 61 were either healthy students or volunteers from among the hospital staff or patients who were being treated for other tropical diseases (e.g., leishmaniasis, paracoccidoidomycosis, or tuberculosis) but were negative for HIV.
Group B included 225 drug users recruited from drug treatment centers in Rio de Janeiro and taking part in a study addressing the relationship between sexual and drug-taking behavior and infection rates for HIV and other blood-borne and sexually transmitted infections (1). At the time of sample collection, the participants also answered a detailed questionnaire. Twenty-four patients (among 225) reported a lifetime history of drug injection, of whom 8 had injected drugs during the 6 months preceding the interview.
Paired urine and serum samples were collected from all participants. Serum samples were stored at−20°C until use. Urine was kept at +4°C, without the addition of any preservative, until testing was performed within 24 h after collection.
Urinalysis.
The commercial HIV-1 urine EIA (Calypte Biomedical Corporation) contains a recombinant envelope protein (gp160) of HIV-1 adsorbed to the insides of the wells of a microtiter plate. In accordance with the instructions supplied with the test, 200 μl of chemically unpreserved and uncentrifuged urine was added to 25 μl of proprietary sample buffer in the wells of the plate. After incubation for 60 min at 37°C, the wells were washed and a goat anti-human immunoglobulin-alkaline phosphatase conjugate was added and incubated for another hour at 37°C. After an additional wash step, the immune complexes were detected by adding p-nitrophenylphosphate. The reaction was stopped after 30 min at 37°C by EDTA addition and the results were read at 405 nm on a 3550-UV microplate reader (Bio-rad, Richmond, Calif.), Cutoff values were determined by adding the constant 0.180 to the average absorbance value of a triplicate analysis of a single negative control supplied with the assay. Samples with absorbance values less than the cutoff were considered negative. Samples with values equal to or greater than the cutoff were considered initially reactive and were retested in duplicate.
In order to address interassay variability, samples from a proficiency panel provided by the manufacturer were included in each run.
Serology.
Sera were tested by using the HIV-1/-2 Ab capture EIA (Ortho Diagnostic Systems, Raritan, N.J.) (Ortho EIA) and the Vironostika HIV Uni-Form II Plus O (Organon Teknika BV, Boxtel, The Netherlands) (Organon EIA). The Ortho EIA employs as antigens a mixture of two recombinant envelope proteins and one recombinant core protein from HIV-1 plus one recombinant HIV-2 envelope protein. The Organon EIA utilizes a mixture of HIV-1 antigens (p24, gp160, and the ANT70 peptide) and a peptide derived from the envelope protein of HIV-2 (amino acids 592 to 603). The results were confirmed by a laboratory different from the one that evaluated the urine EIA (MGM at the Department of Immunology, IOC, FIOCRUZ) using a Western blot (WB) assay (Cambridge Biotech Co., Worcester, Mass.). All assays were carried out strictly following the instructions supplied by the manufacturers. All serological tests employed in this study are dual-detection assays. However, to date, no HIV-2 has been reported in Brazil (4, 5, 12).
Diagnostic procedure.
At the time of sample testing, the investigators were unaware of the HIV status of the participants. Participants from group B had their sera simultaneously tested by the Ortho EIA and the Organon EIA. For economic reasons, patients with well-documented HIV status (group A) had their serum samples analyzed with the Ortho EIA only. Urine samples of all participants were screened by the urine EIA. Samples initially reactive with any of the EIAs were retested in duplicate. Samples repeatedly reactive in either of the serum EIAs and/or in the urine EIA were serologically confirmed by WB assay.
Data analysis and statistical procedures.
Data were entered into two spreadsheets. Group B patients were recruited by an epidemiological study, and their demographic, sociobehavioral, and laboratory data were analyzed as described by Bastos et al. (1). For group A patients, only descriptive statistics were provided.
Laboratory data were processed using an Excel spreadsheet and the SPSS statistical software package, generating graphics and test performance results. The analyses are descriptive, and no statistical test was performed for this study.
RESULTS
The results of the present study are summarized in Tables 1 and 2 and Fig. 1. Of 135 subjects with known HIV status (group A), the urine EIA correctly identified all 74 HIV-positive and 58 out of 61 HIV-negative subjects. Three subjects with EIA-positive urine samples were serologically negative, both by the Ortho EIA and the WB assay, although one serum reacted with the p24 band.
TABLE 1.
Performance of EIAs in different patient groups
| Kit employed | Resulta | No. of patients positive or negativeb
|
|||
|---|---|---|---|---|---|
| Group A (n = 135)
|
Group B (n = 225)
|
||||
| HIV positive | HIV negative | HIV positive | HIV negative | ||
| Organon EIA | Positive | ND | ND | 2 | 6 |
| Negative | ND | ND | 0 | 217 | |
| Ortho EIA | Positive | 74 | 0 | 2 | 0 |
| Negative | 0 | 61 | 0 | 223 | |
| Urine EIA | Positive | 74 | 3 | 2 | 3 |
| Negative | 0 | 58 | 0 | 220 | |
Positive, repeatedly positive by EIA; negative, negative by EIA.
ND, not done. The “gold standards” were the serum EIA result (EIA-negative samples) and the WB assay result (EIA-positive samples).
TABLE 2.
Performance characteristics of EIAs in different patient groups
| Characteristica | Valueb
|
|||||
|---|---|---|---|---|---|---|
| Ortho
|
Organon
|
Calypte
|
||||
| Group A | Group B | Group A | Group B | Group A | Group B | |
| Sensitivity (%) | 100 | 100 | NA | 100 | 100 | 100 |
| Specificity (%) | 100 | 100 | NA | 97.3 | 95.1 | 98.7 |
| NPV | 1.0 | 1.0 | NA | 1.0 | 1.0 | 1.0 |
| PPV | 1.0 | 1.0 | NA | 0.25 | 0.96 | 0.4 |
Sensitivity, true positives/(true positives + false negatives); specificity, true negatives/(true negatives + false positives); NPV, negative predictive value [true negatives/(true negatives + false negatives)]; PPV, positive predictive value [true positives/(true positives + false positives)].
NA, not applicable.
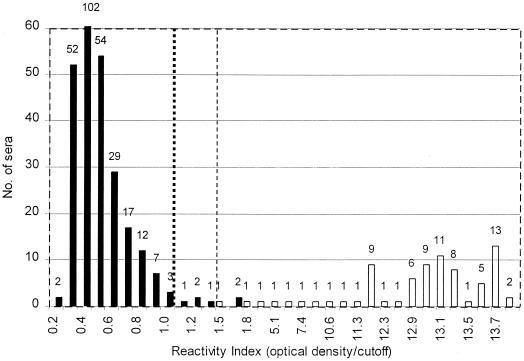
FIG. 1.
Histogram indicating distribution of positive (open bars) and negative (solid bars) sera relative to the RI. The total number of sera with a given RI is indicated above each column. The dotted line indicates the cutoff preset by the manufacturer, and the dashed line indicates the cutoff suggested by the authors. For better visualization of the bars representing only one serum, the ordinate was truncated at a value of 60.
Of 225 drug users (group B), 2 patients were found to be positive in all serological assays (Ortho EIA, Organon EIA, and WB assay) and the urine EIA (prevalence, 0.9%), and 214 patients were negative in all assays employed. Of the remaining nine subjects, three were positive by the urine EIA only and six were positive by the Organon EIA only. All of them were negative by the Ortho EIA and by WB assay, although five of the six Organon EIA-positive samples reacted with the p24 band. Thus, in a total of 360 samples, the urine EIA correctly identified 76 of 76 HIV-positive samples and 278 of 284 negative samples, giving an overall sensitivity of 100.0% and a specificity of 97.9%. Figure 1 shows the distribution of positive and negative urine samples and their respective absorbance values. To permit the comparison of results obtained on different days with different plates, the results were expressed as reactivity indices (RI) by dividing the absorbance values of the individual test results by the cutoff value of the respective plate. All samples obtained from HIV-infected subjects, but from only one of the uninfected subjects, showed RI greater than 1.5.
When the RI was calculated by dividing the absorbance value of an individual test result by the cutoff value of the assay (Fig. 1), six samples from negative group A patients showed RI values ranging from 1.1 to 1.9 in the urine EIA. On the other hand, with the exception of two samples (see below), urine from HIV-positive patients showed RI values higher than 11.0 (i.e., absorbance values greater than 3.500 (limit of the microplate reader) and cutoff values varying around 0.275).
In order to monitor assay performances over the study period, at least three out of five urine samples from a proficiency panel were included in each run. The panel consisted of three positive (high, medium, and low) and two negative samples. Analysis of the RI obtained for the proficiency panel and the positive and negative controls provided with the kit demonstrated a consistently high performance (data not shown).
DISCUSSION
In the present study, we evaluated the applicability of a HIV-1 EIA for urine samples as a screening assay in a group of 225 drug users recruited from drug treatment centers in Rio de Janeiro, Brazil. Due to the low prevalence of HIV infection in the study group (0.9%; 2 of 225), we also tested the diagnostic performance of the assay on samples obtained from 135 Brazilians with known HIV status (prevalence, 54.8%; 74 of 135). The urine test identified all 76 positive samples (sensitivity, 100%) and 278 of 284 negative samples (specificity, 97.9%).
Although to date HIV-2 has not been isolated in Brazil (4, 5, 12), a large number of HIV-1 subtypes circulate in different parts of the country (15). A diagnostic test suitable for Brazil should be able to detect antibodies against all circulating subtypes. The urine EIA employs the recombinant gp160 HIV-1 envelope protein as an antigen (21). Our results show that the assay correctly identified all 76 HIV-positive samples tested, thus presenting a sensitivity of 100%. For 12 of these patients, HIV subtyping revealed the existence of the HIV-1 subtypes B (6 patients), B" (5 patients), and F (1 patient) in the study population (15). Therefore, the urine EIA is capable of detecting antibodies to the major HIV-1 subtypes that circulate in the Brazilian population.
Several other studies have evaluated the Calypte HIV-1 urine EIA (3, 7, 14, 19-21; C. M. Lowndes, L. Mukenge, F. Bernier, E. Lafia, S. Anagonou, C. Gnintoungbé, J. R. Joly, and M. Alary, abstract from the 8th Annual Conference of the Canadian Association for HIV Research, Can. J. Infect. Dis., Suppl. B, abstr. C157P, p. 57B, 1999). While most studies were carried out using samples from patients in the United States (3, 7, 19-21), two studies were carried out in Africa (14; Lowndes et al., Can. J. Infect. Dis. Suppl. B, 1999).
When the abilities of certain commercial EIAs to detect HIV-specific antibodies in urine samples were evaluated, the urine EIA showed a sensitivity of 98.9% and a specificity of 94.2% (7). In a larger validation study, Berrios and coauthors (3) reported a sensitivity and specificity of 100% for the urine EIA when the results were confirmed by a urine WB assay and a specificity of 95.2% for the unconfirmed urine EIA.
Urnovitz and others (19) focused on a detailed follow-up analysis of seven subjects who were repeatedly reactive in the experimental version of the urine EIA but were negative or indeterminate in the serum EIA and WB assay. The authors suggested that a compartmentalized response can in some cases result in the production of HIV-1 envelope antibodies detectable in urine but absent from serum. In two large-scale studies (20, 21), the same authors reported urine-positive, serum-negative results for 1.3% of 11,896 individuals at different risks for HIV exposure, for 1.0% of 25,132 individuals at low risk, and for 10.8% of 859 individuals at high risk for HIV infection.
In the present study, the first to be carried out in Latin America, the urine EIA showed an overall specificity of 97.9% (278 of 284 samples). Although our study group was small, no false-negative results were obtained. The six patients who were considered false positive in the urine EIA were confirmed to be negative by WB serology. However, we cannot rule out the possibility that these six patients presented with a compartmentalized immune response, since IgA and IgG, the major isotypes that can be found in urine, would be detected by the anti-human immunoglobulin conjugate supplied with the urine EIA. However, at the time of the study we were unable to carry out isotyping or urine WB analysis of the fresh urine samples. Therefore, we assume that the six patients were false positive in the urine EIA.
Two urine samples obtained from HIV-infected group A patients gave results close to the cutoff value. One patient presented with the final stage of AIDS and suffered from multidrug-resistant tuberculosis. He died 1 month after sample collection. His urine gave an RI value of 1.5, which was consistent with his anergic status and low antibody titers due to immune suppression. The other patient presented an RI of 1.9. She was being treated on a combined regimen of zidovudine and lamivudine and presented at the time of sample collection with a completely controlled HIV infection, with an undetectable viral load and a CD4 count of 2,185. The high CD4 count associated with an undetectable viral load has been consistently maintained over the last 3 years, suggesting very low antigenic stimulation. We cannot exclude the possibility that the patient suffered from undetected urine infection at the time of sample collection, which could interfere with specific antibody detection.
On the other hand, analysis of the absorbance values obtained in this study indicates that the specificity of the urine EIA could be improved by simply increasing the cutoff value (Fig. 1). In our study, a shift of the cutoff RI from 1.0, as proposed by the manufacturer, to 1.5 would increase the specificity of the test from 97.9 to 99.3% (282 of 284 samples) without affecting its sensitivity. This could be achieved, for instance, by using a higher conjugate dilution. However, a number of samples larger than that of the present study would be necessary to confirm these interesting results.
Taking the data together, the urine EIA proved capable of correctly classifying all positive samples in three groups of patients with different prevalences of HIV infection. As for all other HIV screening tests on the market, the urine EIA is not specific enough to be used as a one-step test and therefore requires confirmation. However, the ease of sample collection and the high sensitivity of the test make it very useful for large-scale epidemiological investigations.
Acknowledgments
We thank Maria Inez Linhares de Carvalho, the staff of the Ambulatório da Providência, and the staffs of the Evandro Chagas Hospital and the drug treatment centers for their unrestricted cooperation. Diagnostic kits for HIV-1 detection in urine were provided by Calypte Biomedical Corporation.
Financial support for this study was provided by the Brazilian Ministry of Health and the Brazilian National Research Council (CNPq), and the FIOCRUZ AIDS program (PIAF). M.A. was the recipient of a research scholarship from Fonds de Recherche en Santé de Québec (no. 970097). W.M.R.O., M.G.M, and F.I.B. are recipients of research scholarships from CNPq.
REFERENCES
- 1.Bastos, F. I., C. M. Lowndes, M. Derrico, L. R. R. Castello-Branco, M.-I. Linhares-de-Carvalho, W. Oelemann, F. Bernier, M. G. Morgado, C. F. Yoshida, T. Rozental, and M. Alary. 2000. Sexual behaviour and infection rates for HIV, blood-borne and sexually transmitted infections among patients attending drug treatment centers in Rio de Janeiro, Brazil. Int. J. STD AIDS 11:383-392. [DOI] [PubMed] [Google Scholar]
- 2.Beck, A., M. C. C. Pereira Lima, M. C. Castro, D. X. Drummond, M. L. A. Oliveira, W. Oelemann, G. Pauli, and Y. S. M. Van Tilburg Bernardes. 1996. Performance of HTLV-1 screening assays in Brazil. Zentbl. Bakteriol. 283:340-346. [DOI] [PubMed] [Google Scholar]
- 3.Berrios, D. C., A. L. Avins, K. Haynes-Sanstad, R. Eversley, and W. J. Woods. 1995. Screening for human immunodeficiency virus antibody in urine. Arch. Pathol. Lab. Med. 119:139-141. [PubMed] [Google Scholar]
- 4.Brazilian Network for the HIV-1 Isolation and Characterization. 2000. HIV-1 diversity in Brazil: genetic, biologic, and immunologic characterization of HIV-1 strains in three potential HIV vaccine evaluation sites. J. Acquir. Immune Defic. Syndr. 23:184-193. [DOI] [PubMed] [Google Scholar]
- 5.Broutet, N., A. de Queiroz Souza, F. P. Basilio, H. L. Sa, F. Simon, and F. Dabis. 1996. Prevalence of HIV-1, HIV-2 and HTLV antibody in Fortaleza, Ceara, Brazil, 1993-1994. Int. J. STD AIDS 7:365-369. [DOI] [PubMed] [Google Scholar]
- 6.Connell, J. A., J. V. Parry, P. P. Mortimer, R. J. S. Duncan, K. A. McLean, A. A. Johnson, M. H. Hambling, J. Barbara, and C. P. Farrington. 1990. Preliminary report: accurate assays for anti-HIV in urine. Lancet 335:1366-1369. [DOI] [PubMed] [Google Scholar]
- 7.Desai, S., H. Bates, and F. J. Michalski. 1991. Detection of antibody to HIV-1 in urine. Lancet 337:183-184. [DOI] [PubMed] [Google Scholar]
- 8.Flaitz, C. M., M. J. Hicks, A. B. Carter, S. N. Rossmann, G. J. Demmler, C. L. Simon, S. G. Cron, W. T. Shearer, and M. W. Kline. 1998. Saliva collection technique for cytologic, microbiologic and viral evaluation in pediatric HIV infection. ASDC J. Dent. Child. 65:318-324, 355. [PubMed] [Google Scholar]
- 9.Frerichs, R. R., N. Eskes, and M. T. Htoon. 1994. Validity of three assays for HIV-1 antibodies in saliva. J. Acquir. Immune Defic. Syndr. 7:522-525. [PubMed] [Google Scholar]
- 10.Frerichs, R. R., N. Silarug, N. Eskes, P. Pagcharoenpol, A. Rodklai, S. Thangsupachai, and C. Wongba. 1994. Saliva-based HIV-antibody testing in Thailand. AIDS 8:885-894. [DOI] [PubMed] [Google Scholar]
- 11.Gottfried, T. D., and H. B. Urnovitz. 1990. HIV-1 testing: product development strategies. Trends Biotechnol. 8:35-40. [DOI] [PubMed] [Google Scholar]
- 12.Hendry, R. M., D. E. Parks, D. L. Mello, G. V. Quinnan, and B. G. Castro. 1991. Lack of evidence for HIV-2 infection among at-risk individuals in Brazil. J. Acquir. Immune Defic. Syndr. 4:623-627. [PubMed] [Google Scholar]
- 13.Martinez, P. M., A. R. Torres, R. O. de Lejarazu, A. Montoya, J. F. Martin, and J. M. Eiros. 1999. Human immunodeficiency virus antibody testing by enzyme-linked fluorescent and Western blot assays using serum, gingival-crevicular transudate, and urine samples. J. Clin. Microbiol. 37:1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meehan, M. P., N. K. Sewankambo, M. J. Wawer, D. McNairn, T. C. Quinn, T. Lutalo, S. Kalibbala, C. Li, D. Serwadda, F. Wabwire-Mangen, The Rakai Project Team, and R. H. Gray. 1999. Sensitivity and specificity of HIV-1 testing of urine compared with serum specimens: Rakai, Uganda. Sex. Transm. Dis. 26:590-592. [DOI] [PubMed] [Google Scholar]
- 15.Morgado, M. G., M. L. Guimarães, C. B. C. Gripp, C. I. Costa, I. Neves, Jr., V. G. Veloso, M.-I. Linhares-Carvalho, L. R. R. Castello-Branco, F. I. Bastos, C. Kuiken, E. A. Castilho, B. Galvao-Castro, V. Bongertz, and Evandro Chagas Hospital AIDS Clinical Research Group. 1998. Molecular epidemiology of HIV-1 in Brazil: high prevalence of HIV-1 subtype B and identification of an HIV-1 subtype D infection in the city of Rio de Janeiro, Brazil. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:488-494. [DOI] [PubMed] [Google Scholar]
- 16.Pinho, R. T., R. C. Pedrosa, P. Costa-Martins, and L. R. R. Castello-Branco. 1999. Saliva ELISA: a method for the diagnosis of chronic Chagas' disease in endemic areas. Acta Tropica 72:31-38. [DOI] [PubMed] [Google Scholar]
- 17.Skolnik, P. R., B. R. Kosloff, L. J. Bechtel, K. R. Huskins, T. Flynn, N. Karthas, K. McIntosh, and M. S. Hirsch. 1989. Absence of infectious HIV-1 in the urine of seropositive viremic subjects. J. Infect. Dis. 160:1056-1060. [DOI] [PubMed] [Google Scholar]
- 18.Tribble, D. R., G. R. Rodier, M. D. Saad, G. Binson, F. Marrot, S. Salah, C. Omar, and R. R. Arthur. 1997. Comparative field evaluation of HIV rapid diagnostic assays using serum, urine, and oral mucosal transudate specimens. Clin. Diagn. Virol. 7:127-132. [DOI] [PubMed] [Google Scholar]
- 19.Urnovitz, H. B., M. Clerici, G. M. Shearer, T. D. Gottfried, D. J. Robinson, L. I. Lutwick, L. Montagnier, and D. V. Landers. 1993. HIV-1 antibody serum negativity with urine positivity. Lancet 342:1458-1459. [DOI] [PubMed] [Google Scholar]
- 20.Urnovitz, H. B., J. C. Sturge, and T. D. Gottfried. 1997. Increased sensitivity of HIV-1 antibody detection. Nat. Med. 3:1258.. [DOI] [PubMed] [Google Scholar]
- 21.Urnovitz, H. B., J. C. Sturge, T. D. Gottfried, and W. H. Murphy. 1999. Urine antibody tests: new insights into the dynamics of HIV-1 infection. Clin. Chem. 45:1602-1613. [PubMed] [Google Scholar]
- 22.Watt, G., P. Chanbacherd, and A. E. Brown. 2000. Human immunodeficiency virus type 1 test results in patients with malaria and dengue infections. Clin. Infect. Dis. 30:819.. [DOI] [PubMed] [Google Scholar]