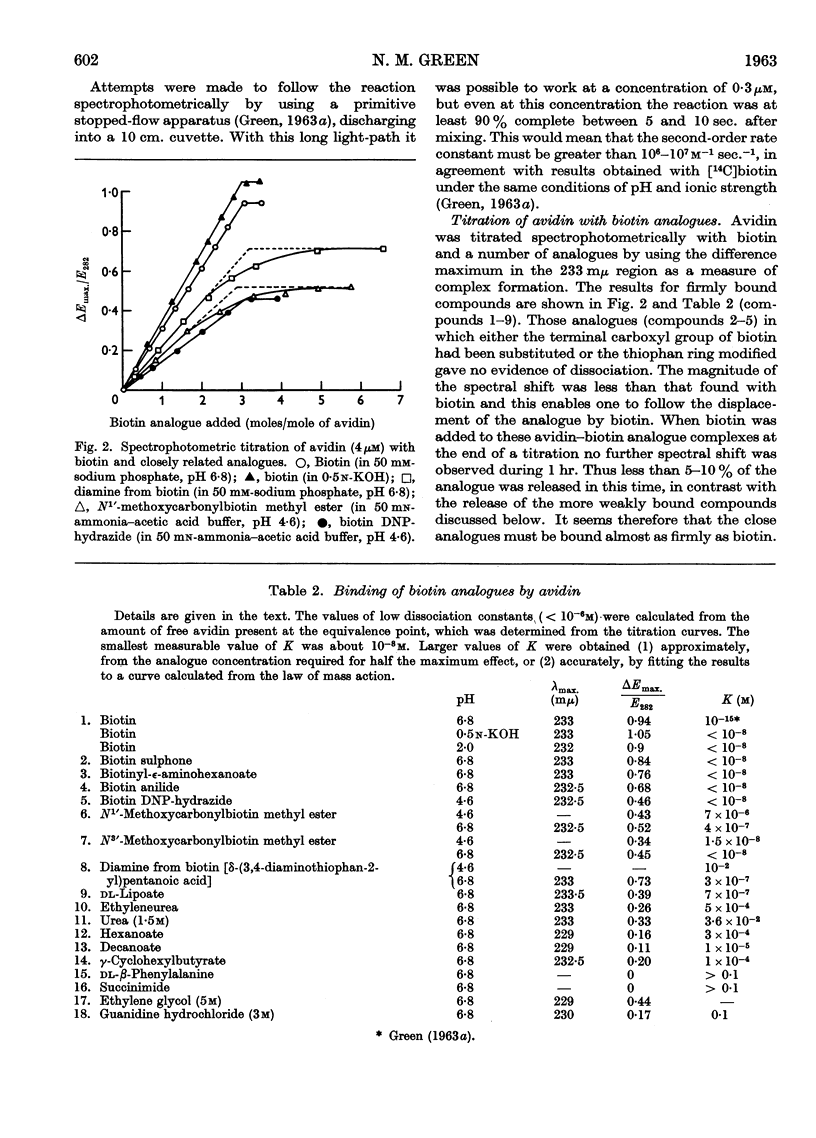
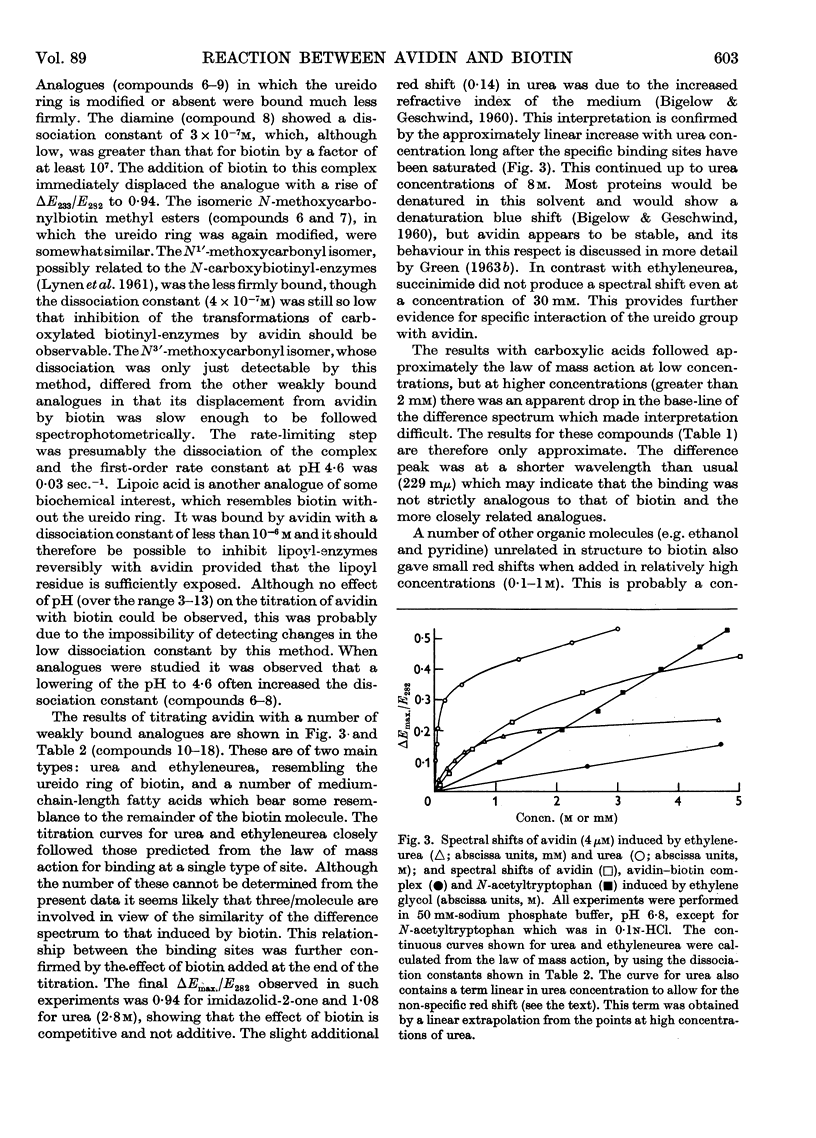
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGELOW C. C., GESCHWIND I. I. Difference specta of amino acids and proteins in aqueous media of high refractive index. C R Trav Lab Carlsberg. 1960;31:283–304. [PubMed] [Google Scholar]
- BIGELOW C. C., SONENBERG M. The effect of dodecyl sulfate on the ultraviolet spectra of proteins. Biochemistry. 1962 Mar;1:197–204. doi: 10.1021/bi00908a002. [DOI] [PubMed] [Google Scholar]
- GLAZER A. N., SMITH E. L. Studies on the ultraviolet difference spectra of proteins and polypeptides. J Biol Chem. 1961 Nov;236:2942–2947. [PubMed] [Google Scholar]
- GREEN N. M. AVIDIN. 1. THE USE OF (14-C)BIOTIN FOR KINETIC STUDIES AND FOR ASSAY. Biochem J. 1963 Dec;89:585–591. doi: 10.1042/bj0890585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN N. M. AVIDIN. 4. STABILITY AT EXTREMES OF PH AND DISSOCIATION INTO SUB-UNITS BY GUANIDINE HYDROCHLORIDE. Biochem J. 1963 Dec;89:609–620. doi: 10.1042/bj0890609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN N. M. Spectroscopic evidence for the participation of tryptophan residues in the binding of biotin by avidin. Biochim Biophys Acta. 1962 May 7;59:244–246. doi: 10.1016/0006-3002(62)90726-6. [DOI] [PubMed] [Google Scholar]
- HERSKOVITS T. T., LASKOWSKI M., Jr Location of chromophoric residues in proteins by solvent perturbation. I. Tyrosyls in serum albumins. J Biol Chem. 1962 Aug;237:2481–2492. [PubMed] [Google Scholar]
- KENDREW J. C., WATSON H. C., STRANDBERG B. E., DICKERSON R. E., PHILLIPS D. C., SHORE V. C. The amino-acid sequence x-ray methods, and its correlation with chemical data. Nature. 1961 May 20;190:666–670. doi: 10.1038/190666a0. [DOI] [PubMed] [Google Scholar]
- LYNEN F., KNAPPE J., LORCH E., JUETTING G., RINGELMANN E., LACHANCE J. P. [On the biochemical function of biotin. II. Purification and mode of action of beta-methyl-crotonyl-carboxylase]. Biochem Z. 1961;335:123–167. [PubMed] [Google Scholar]
- MELAMED M. D., GREEN N. M. AVIDIN. 2. PURIFICATION AND COMPOSITION. Biochem J. 1963 Dec;89:591–599. doi: 10.1042/bj0890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENHECK K., DOTY P. The far ultraviolet absorption spectra of polypeptide and protein solutions and their dependence on conformation. Proc Natl Acad Sci U S A. 1961 Nov 15;47:1775–1785. doi: 10.1073/pnas.47.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON H. C., KENDREW J. C. The amino-acid sequence of sperm whale myoglobin. Comparison between the amino-acid sequences of sperm whale myoglobin and of human hemoglobin. Nature. 1961 May 20;190:670–672. doi: 10.1038/190670a0. [DOI] [PubMed] [Google Scholar]
- WEBER G. Fluorescence-polarization spectrum and electronic-energy transfer in proteins. Biochem J. 1960 May;75:345–352. doi: 10.1042/bj0750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITKOP B. Nonenzymatic methods for the preferential and selective cleavage and modification of proteins. Adv Protein Chem. 1961;16:221–321. doi: 10.1016/s0065-3233(08)60031-5. [DOI] [PubMed] [Google Scholar]


