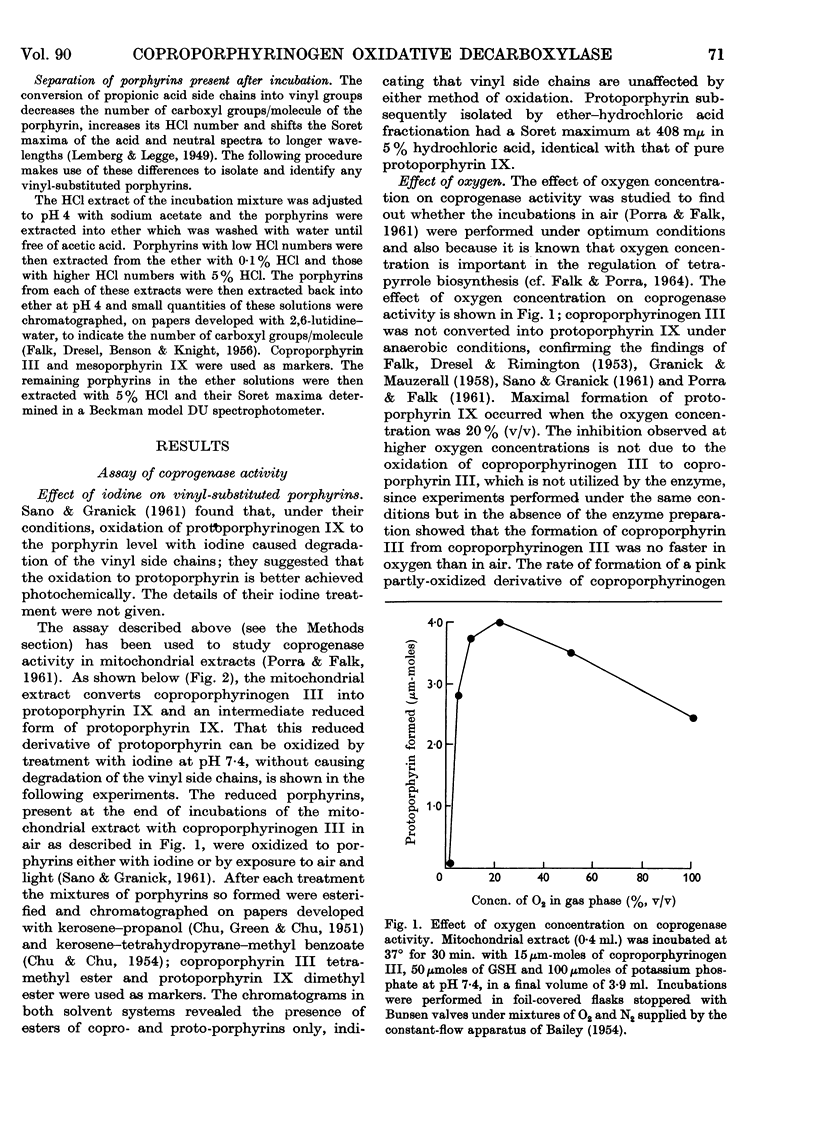
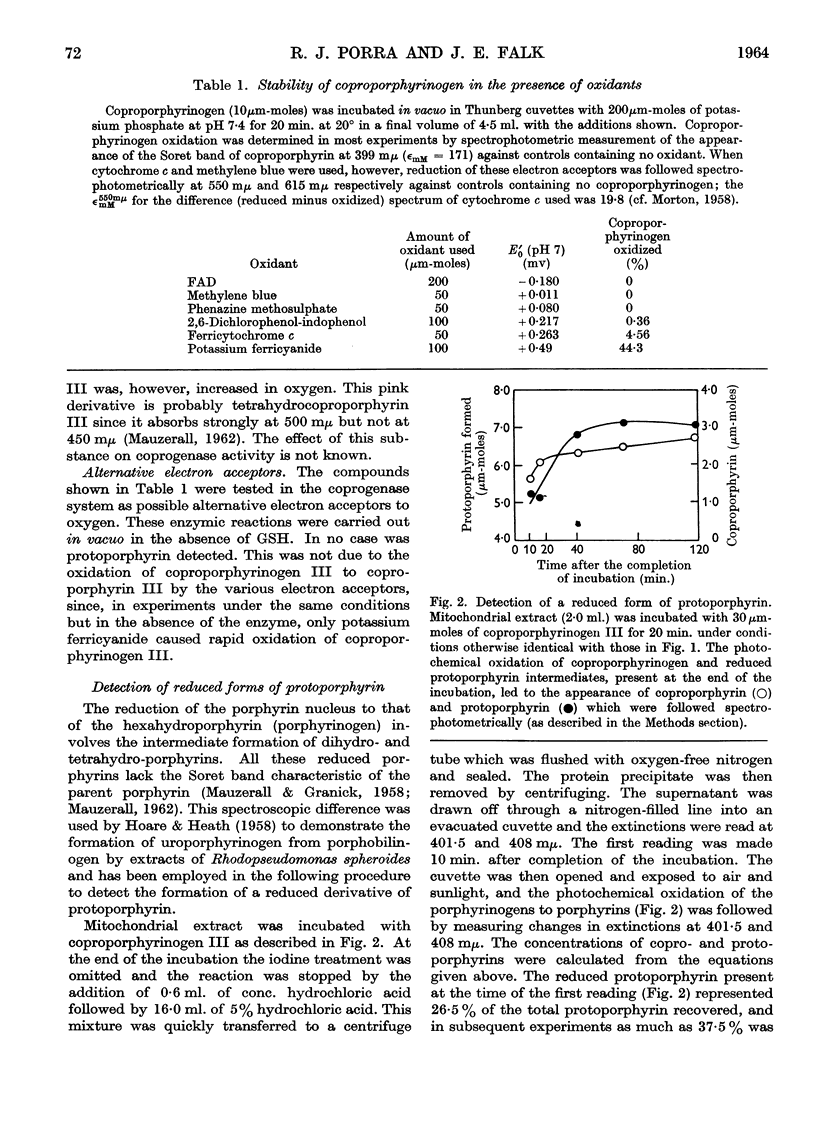
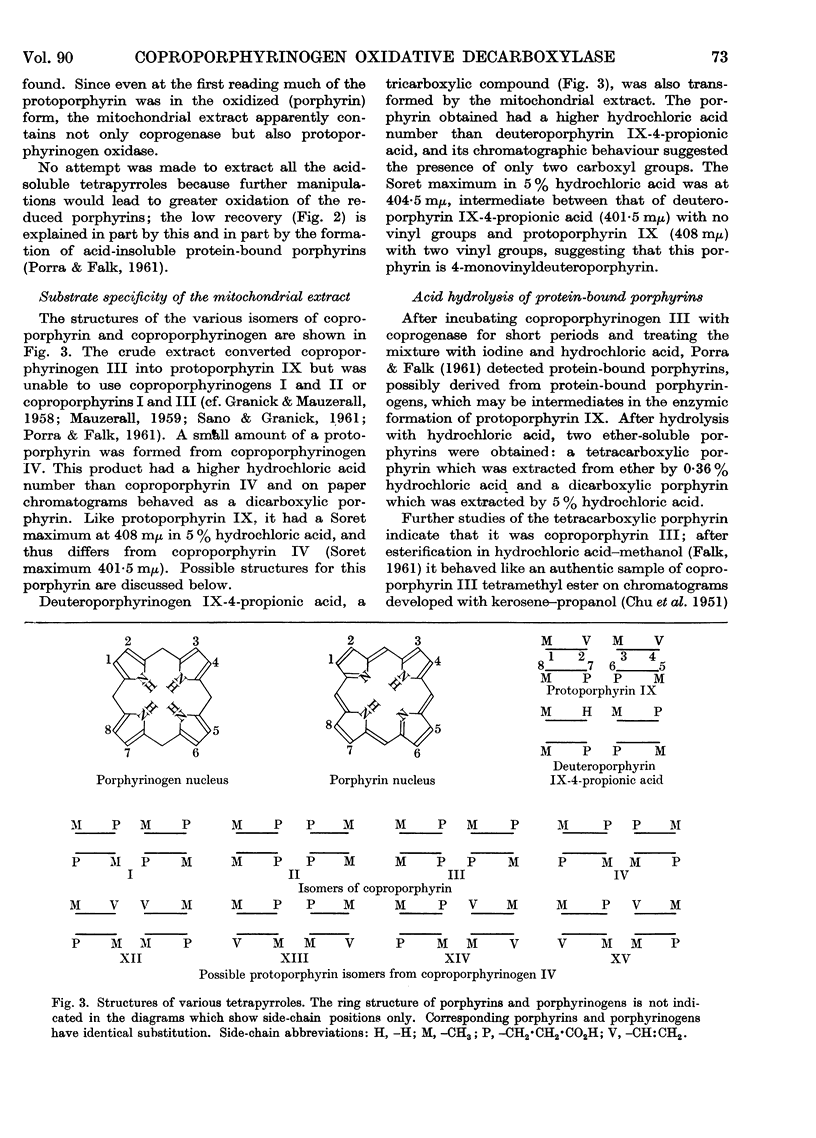
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOGORAD L., GRANICK S. Protoporphyrin precursors produced by a Chlorella mutant. J Biol Chem. 1953 Jun;202(2):793–800. [PubMed] [Google Scholar]
- Bogorad L., Granick S. The Enzymatic Synthesis of Porphyrins from Porphobilinogen. Proc Natl Acad Sci U S A. 1953 Dec;39(12):1176–1188. doi: 10.1073/pnas.39.12.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU T. C., CHU E. J. H. Paper chromatography of methyl esters of dicarboxylic porphyrins. J Biol Chem. 1954 Jun;208(2):537–541. [PubMed] [Google Scholar]
- CHU T. C., SISTER A A GREEN, CHU E. J. Paper chromatography of methyl esters of porphyrins. J Biol Chem. 1951 Jun;190(2):643–646. [PubMed] [Google Scholar]
- FALK J. E., BENSON A. Separation of uroporphyrin esters I and III by paper chromatography. Biochem J. 1953 Aug;55(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- FALK J. E., DRESEL E. I., BENSON A., KNIGHT B. C. Studies on the biosynthesis of blood pigments. 4. The nature of the porphyrins formed on incubation of chicken erythrocyte preparations with glycine, delta-aminolaevulic acid or porphobilinogen. Biochem J. 1956 May;63(1):87–94. doi: 10.1042/bj0630087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK J. E., DRESEL E. I., RIMINGTON C. Porphobilinogen as a porphyrin precursor, and interconversion of porphyrins, in a tissue system. Nature. 1953 Aug 15;172(4372):292–294. doi: 10.1038/172292a0. [DOI] [PubMed] [Google Scholar]
- Falk J. E., Porra R. J. The effects of oxygen concentration on porphyrin biosynthesis in chicken-erythrocyte preparations. Biochem J. 1964 Jan;90(1):66–69. doi: 10.1042/bj0900066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARE D. S., HEATH H. Intermediates in the biosynthesis of porphyrins from porphobilinogen by Rhodopseudomonas spheroides. Nature. 1958 Jun 7;181(4623):1592–1593. doi: 10.1038/1811592a0. [DOI] [PubMed] [Google Scholar]
- LEMBERG R., FALK J. E. Comparison of haem a, the dichroic haem of heart muscle, and of porphyrin a with compounds of known structure. Biochem J. 1951 Oct;49(5):674–683. doi: 10.1042/bj0490674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEILANDS J. B., TUPPY H. Crystalline synthetic porphyrin c. Biochim Biophys Acta. 1960 Feb 26;38:351–353. doi: 10.1016/0006-3002(60)91256-7. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., FALK J. E. Protein-bound porphyrins associated with protoporphyrin biosynthesis. Biochem Biophys Res Commun. 1961 Jun 28;5:179–184. doi: 10.1016/0006-291x(61)90106-1. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 2. An in vestigation of the role offerrochelatase in the biosynthesis of various haem prosthetic groups. Biochem J. 1963 Apr;87:186–192. doi: 10.1042/bj0870186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIMINGTON C., TOOTH B. E. Role of mitochondria in the in vitro formation of protoporphyrin and haem. J Biochem. 1961 Jun;49:456–467. doi: 10.1093/oxfordjournals.jbchem.a127328. [DOI] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- SUGITA Y. The role of particulate bound protoporphyrin in biosynthesis of heme. J Biochem. 1962 Jun;51:436–440. doi: 10.1093/oxfordjournals.jbchem.a127559. [DOI] [PubMed] [Google Scholar]


