Abstract
Extended-spectrum β-lactamase-producing Klebsiella pneumoniae (EPKP) strains are frequently implicated in outbreaks in neonatal intensive care units (NICUs). During the period from 1997 to 1998, 21 infections and 23 colonizations with EPKP were recorded in the NICU of a children's hospital in Athens, Greece. Seventeen of the infected and 12 of the colonized neonates had been referred from other hospitals. The remaining infections and colonizations occurred during the current hospitalization. Pulsed-field gel electrophoresis typing showed that the latter cases were due to an outbreak strain that persisted in the unit, while the repeated introduction of EPKP carriers was mostly due to clonal outbreaks in two maternity hospitals.
Klebsiella pneumoniae has been recognized as an important cause of infections in hospitalized neonates (8). Various environmental reservoirs have occasionally been identified in outbreaks caused by K. pneumoniae in such settings (6, 11). Irrespective of the primary source, it seems that the most significant reservoir of the microorganism is the digestive tract of colonized patients and that transmission occurs mostly via the hands of nursing staff (3, 8). During the past decade, strains of K. pneumoniae exhibiting resistance to newer cephalosporins due to the production of extended-spectrum β-lactamases (ESBLs) have been frequently implicated in outbreaks in pediatric hospitals and neonatal intensive care units (NICUs) (1, 15, 16, 20). These strains usually exhibit cross-resistance to other antibiotics, such as aminoglycosides. Therapeutic options are therefore limited.
Up to 1996, broad-spectrum cephalosporin-resistant K. pneumoniae strains (i.e., putative ESBL producers) were rarely isolated in one of the major children's hospitals in Athens, Greece. However, during 1998, a relatively high rate of isolation of ESBL-producing K. pneumoniae (EPKP) strains in the NICU was observed. With the aid of molecular typing, we showed in the present study that most EPKP acquired during hospitalization originated from a single strain. We also found that the majority of EPKP isolated on admission belonged to a limited number of strains that were endemic in referring maternity hospitals.
MATERIALS AND METHODS
Setting.
P. & A. Kyriakou Children's Hospital is one of the two major pediatric hospitals in Greece. It is a 358-bed tertiary-care institution with an average yearly admission of 22,000 patients. In the NICU, with about 700 admissions per year, there are 30 incubators, 11 of which provide intensive care facilities. As there is no attached maternity unit, all babies admitted to the NICU are referred for tertiary care from the greater Athens region, parts of southern Greece, and many of the islands.
Isolation of EPKP.
EPKP strains were isolated from clinical material submitted to the hospital microbiology laboratory or from body surface cultures (rectal and umbilical swab and nasopharyngeal secretion samples) used to screen colonized patients on admission to the hospital. The latter cultures were done with McConkey agar containing ceftazidime (1 μg/ml) to facilitate isolation of EPKP. All cases of colonization or infection were classified as referred to or acquired in the hospital. Referred cases were those from which an EPKP strain was isolated from surface cultures upon admission or within 48 h after admission. Attempts to isolate EPKP strains from the environment were also made. Environmental screening was performed by using swabs moistened with sterile saline and included work surfaces, sinks, incubators, solutions, and equipment used in intubation. Hand impressions were also taken in order to examine carriage of EPKP by the medical and nursing staff of the NICU. Samples were cultured on ceftazidime-containing medium as described above. Microorganisms were identified with the API 20E system (bioMerieux, Marcy l'Etoile, France).
Susceptibility testing and ESBL detection.
Susceptibility to antimicrobial drugs was tested by a disk diffusion method with Mueller-Hinton agar (14). Production of ESBLs was detected by the double-disk synergy test (DDST) (9) with disks of amoxicillin-clavulanic acid surrounded at a radius of 30 mm by cefotaxime, ceftazidime, aztreonam, and cefepime. This test was integrated in the routine susceptibility testing. When necessary, ESBL production was confirmed by using ESBL-detecting Etest strips containing ceftazidime and clavulanic acid (AB Biodisk, Solna, Sweden). MICs of various antibiotics, including ceftazidime and cefotaxime, were determined by using the Etest (AB Biodisk).
Typing.
Molecular typing was carried out by pulsed-field gel electrophoresis (PFGE). Genomic DNA was prepared as described previously (17). After XbaI digestion, the DNA was electrophoresed through 1% agarose-0.5× Tris-borate-EDTA in a CHEF-DRIII apparatus (Bio-Rad Laboratories, Hercules, Calif.) under the following conditions: 14°C and 120° switch angle for 20 h with a linear switch time ramp of 5 to 55 s. Digitized gel images were processed by using the GelCompar software package (Applied Maths, Kortrijk, Belgium). Banding pattern similarities were estimated by using the Dice coefficcient. A 2% tolerance in band position differences was allowed. Matrices of similarity coefficients were clustered and a dendrogram was constructed by the unweighted pair-group method with arithmetic averages.
RESULTS
The first case of infection due to an EPKP strain was recognized in May 1997 (isolate 1221; Table 1). This neonate had been admitted to the NICU from the maternity unit of a general hospital (GH-I) due to sepsis caused by an EPKP. The next two patients infected by EPKP (isolates 1253 and 1517; Table 1) were referred from a maternity hospital (MH-II) in September 1997. Additionally, three sporadic cases of infection by EPKP (one in May 1997 and two in July 1997) occurred in other wards of P. & A. Kyriakou Children's Hospital. After these incidents, an attempt was made to strengthen the general hygienic measures (better adherence to hand-washing practices and more frequent changes of gloves) and to isolate or group EPKP-colonized patients. Also, the DDST was routinely applied for all enterobacterial isolates. Especially for the NICU, it was decided to examine the admitted neonates by body surface cultures for EPKP carriage and to perform periodically environmental and 1-day surveys. Examination for EPKP colonization started in November 1997 and ceased at the end of 1998.
TABLE 1.
Infections due to EPKP in the NICU
| Isolate | Patient age (days) at admission | Reason for admissiona | Admission dateb | Isolation dateb | Sample positive for EPKP | Possible place of acquisition | PFGE typec |
|---|---|---|---|---|---|---|---|
| 1221 | 25 | P/I | 14-05-97 | 16-05-97 | Blood, feces | GH-I | U |
| 1253 | 1 | P/R/I | 11-09-97 | 12-09-97 | Blood, urine, tracheal secretions | MH-II | C |
| 1517 | 1 | P/I | 12-09-97 | 14-09-97 | Blood, urine, tracheal secretions, feces | MH-II | C |
| 1329b | 1 | P/I | 23-03-98 | 24-03-98 | Tracheal secretions | MH-II | C |
| 1329a | 1 | P/D/I | 23-03-98 | 24-03-98 | Blood, tracheal secretions | MH-II | C |
| 1353 | 16 | I | 26-05-98 | 26-05-98 | Urine | MH-I | D |
| 1352 | 19 | P | 14-04-98 | 28-05-98 | Urine, blood | NICU | B |
| 1508 | 10 | I | 02-06-98 | 03-06-98 | Urine | MH-I | D |
| 1509 | 14 | I | 10-07-98 | 10-07-98 | Blood, feces | MH-I | — |
| 1411 | 14 | I | 23-07-98 | 23-07-98 | Urine, blood | MH-I | D |
| 1412 | 20 | I | 23-07-98 | 23-07-98 | Urine, blood | MH-I | D |
| 1446 | 1 | P/R | 03-04-98 | 20-08-98 | Blood, feces | NICU | A |
| 1439 | 30 | I | 21-08-98 | 22-08-98 | Urine | MH-I | D |
| 1466 | 12 | I | 27-08-98 | 27-08-98 | Urine, feces | MH-I | D |
| 1533b | 2 | D | 03-08-98 | 18-09-98 | Urine | NICU | A |
| 1560 | 30 | P/I | 08-10-98 | 12-10-98 | Pus, blood, feces | GH-II | U |
| 1561 | 13 | I | 12-10-98 | 12-10-98 | Blood, feces | GH-III | U |
| 1568 | 1 | P/R | 09-10-98 | 16-10-98 | Blood | NICU | — |
| 1593 | 23 | I | 24-10-98 | 25-10-98 | Urine | MH-I | — |
| 1594 | 15 | I | 27-10-98 | 28-10-98 | Urine | MH-I | — |
| 1612 | 30 | I | 17-11-98 | 18-11-98 | Urine | MH-I | — |
P, prematurity; D, dysmaturity; R, respiratory insufficiency; I, suspected infection.
Day-month-year.
U, unique; —, not tested.
From May 1997 to December 1998, 799 neonates were admitted to the NICU. Infection due to EPKP was documented in 19 patients; in 2 more patients, EPKP (isolates 1509 and 1561) was considered a potential cause of infection (Table 1). Fourteen of these 21 neonates had been referred from two maternity hospitals in Athens, MH-I (n = 10) and MH-II (n = 4). Three patients were referred from maternity units of general hospitals (GH-I, GH-II, and GH-III) also located in the Athens area. The remaining four strains were most likely acquired during hospitalization in the NICU, a conclusion corroborated by the fact that body surface cultures upon admission were negative for EPKP. An EPKP was likely to be responsible for urinary tract infection in 12 neonates, for bacteremia or sepsis in 6 neonates, and for respiratory tract infection in 3 neonates. Empirical treatment was initiated with ampicillin plus gentamicin. Upon confirmation that the strain was an ESBL producer, the therapy was switched to imipenem and/or an aminoglycoside, depending on the susceptibility profile and the type of infection.
From November 1997 to December 1998, it was feasible to examine by body surface cultures on admission 390 of 567 neonates. The remaining patients were not examined due to limited staff during nighttime and weekends. Twelve neonates were found colonized with EPKP (Table 2). Eight of them had been referred from MH-I, and three had been referred from MH-II. One patient was referred from a general hospital (GH-IV) outside Athens. Additionally, 11 colonized patients who most likely acquired EPKP during their stay in the NICU were detected in the four 1-day surveys conducted in 1998 (9 January, three isolates; 20 February, three isolates; 25 July, two isolates; and 10 September, three isolates). In nine patients, the initial surface cultures were negative for EPKP. The remaining two patients were not examined upon admission. Nineteen of the 23 isolates obtained from colonizations were found either in feces only or in feces and nasopharyngeal secretions.
TABLE 2.
Colonizations with EPKP in the NICU
| Isolate | Patient age (days) at admission | Reason for admissiona | Admission dateb | Isolation dateb | Sample positive for EPKP | Place of acquisition | PFGE typec |
|---|---|---|---|---|---|---|---|
| 1113 | 9 | I | 08-12-97 | 08-12-97 | Feces | MH-II | C |
| 436 | 1 | P | 14-11-97 | 09-01-98 | Feces | NICU | — |
| 1300 | 2 | I | 02-01-98 | 09-01-98 | Feces | NICU | — |
| 5 | 1 | P/D | 02-01-98 | 09-01-98 | Feces, nasopharyngeal secretions | NICU | — |
| 1039 | 7 | P | 11-02-98 | 11-02-98 | Feces, nasopharyngeal secretions | MH-I | D |
| 53 | 1 | D | 17-02-98 | 19-02-98 | Tracheal secretions | MH-II | — |
| 20 | 1 | P | 26-01-98 | 20-02-98 | Feces, nasopharyngeal secretions | NICU | — |
| 1315 | 11 | I | 12-01-98 | 20-02-98 | Feces | NICU | B |
| 1538 | 1 | P/D | 15-12-97 | 20-02-98 | Feces, nasopharyngeal secretions | NICU | A |
| 1223 | 14 | D | 21-03-97 | 21-03-98 | Feces | MH-I | D |
| 164 | 13 | I | 09-05-98 | 10-05-98 | Nasopharyngeal secretions | MH-I | — |
| 197 | 9 | I | 04-06-98 | 04-06-98 | Feces, nasopharyngeal secretions | MH-I | — |
| 1114 | 10 | I | 16-06-98 | 16-06-98 | Feces | MH-I | D |
| 1417 | 10 | I | 16-07-98 | 17-07-98 | Nasopharyngeal secretions | MH-I | D |
| 1406 | 12 | I | 12-07-98 | 25-07-98 | Feces | NICU | A |
| 217 | 5 | Other | 18-06-98 | 25-07-98 | Feces | NICU | — |
| 288 | 8 | Other | 02-08-98 | 02-08-98 | Nasopharyngeal secretions | GH-IV | — |
| 1440 | 11 | I | 12-08-98 | 13-08-98 | Feces | MH-II | C |
| 1476 | 5 | I | 25-06-98 | 10-09-98 | Feces, nasopharyngeal secretions | NICU | A |
| 1482 | 1 | P | 30-05-98 | 10-09-98 | Feces | NICU | A |
| 1501 | 6 | P | 26-08-98 | 10-09-98 | Feces | NICU | A |
| 1600 | 5 | P | 28-10-98 | 29-10-98 | Feces | MH-I | — |
| 1601 | 5 | P | 28-10-98 | 29-10-98 | Feces | MH-I | — |
The two environmental surveys conducted in 1998 (20 February and 25 July) as well as examination of the hands of NICU personnel were carried out in parallel with the respective 1-day surveys mentioned above. The first environmental survey yielded no EPKP, while an EPKP isolate was obtained from a weighing scale in the NICU during the second environmental survey (isolate 1459; Fig. 1). No EPKP isolates were recovered from the hands of NICU personnel.
FIG. 1.
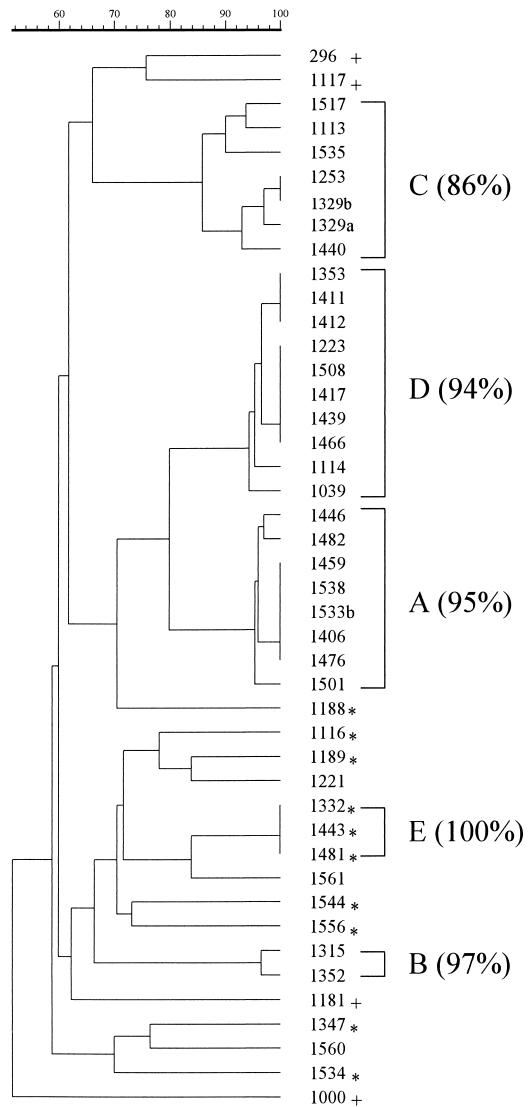
Dendrogram of isolate similarity based on PFGE patterns. Clusters of isolates displaying at least 85% similarity are indicated by brackets, with the similarity index in parentheses. The similarity scale is shown above the dendrogram. Asterisks indicate EPKP isolates from hospital wards other than the NICU; plus signs indicate non-EPKP control isolates.
Forty-four isolates were available for typing by PFGE. Sixteen and 12 isolates were from infections (Table 1) and colonizations (Table 2), respectively. The EPKP environmental isolate as well as 11 EPKP clinical isolates found in other hospital wards during the study period were also typed by PFGE. Four additional epidemiologically unrelated EPKP strains were included for control purposes. PFGE revealed five clusters (A to E) of isolates with identical or highly similar (>80%) PFGE fingerprints (Fig. 1 and 2).
FIG. 2.
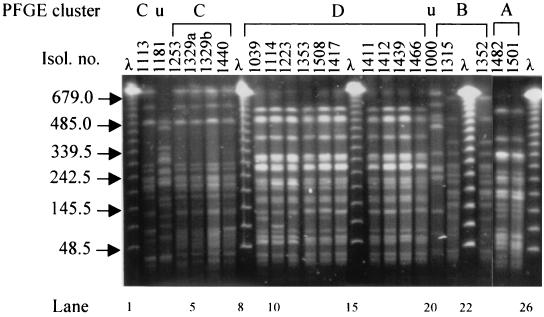
PFGE analysis of representative isolates. The sizes, in kilobases, of lambda concatemers are shown to the left of the gel. PFGE clusters as well as two distinct unique patterns (u) are indicated above the gel. Isol. no., isolate number. All lanes are from the same gel.
Eleven of 13 tested isolates derived from infections or colonizations that had occurred during hospitalization in the NICU belonged to PFGE cluster A (95% similarity of band patterns). The EPKP isolate obtained from the surface of a weighing scale during the second environmental screening also belonged to this cluster. Isolation dates spanned a period of 8 months (from February to September 1998). Notably, isolates of cluster A appeared susceptible to broad-spectrum cephalosporins upon standard susceptibility testing (MICs for ceftazidime and cefotaxime ranged from 4 to 8 and from 2 to 8 μg/ml, respectively). Nevertheless, they were positive in the DDST. The results of confirmatory testing by using ESBL-detecting Etest strips were also positive. These isolates were resistant to all tested aminoglycosides (gentamicin, netilmicin, tobramycin, and amikacin).
EPKP isolates 1352 and 1315 (Tables 1 and 2, respectively), which were also acquired during hospitalization in the NICU, were indistinguishable (97% similarity), composing PFGE cluster B (Fig. 1 and 2). Both were resistant to ceftazidime and cefotaxime (MICs, >32 μg/ml) as well as to all four aminoglycosides tested.
Six isolates imported from MH-II, spanning isolation dates from December 1997 to August 1998, were grouped together in PFGE cluster C (86% similarity) (Fig. 1 and 2). This cluster also included one nosocomial isolate obtained from a medical ward (isolate 1535; Fig. 1) in September 1998. It can therefore be considered to represent an EPKP clone endemic in MH-II but which nevertheless spread to another ward of P. & A. Kyriakou Children's Hospital. All six cluster C isolates were resistant to ceftazidime and cefotaxime (MICs, >32 μg/ml). They were also resistant to netilmicin, tobramycin, and amikacin but susceptible to gentamicin.
Similarly, PFGE cluster D consisted of highly related isolates (94%), all of which were imported from MH-I and could therefore be considered representative of a clone endemic in that hospital. Cluster D included 10 isolates that were obtained throughout 1998 and that exhibited a multidrug resistance phenotype similar to that of cluster C isolates.
Cluster E was composed of three nosocomially acquired isolates with indistinguishable PFGE patterns (100% similarity). They were obtained from children hospitalized in the oncology ward and the internal medicine ward between April and September 1998. Their resistance pattern was similar to that of cluster B isolates. The remaining seven EPKP isolates derived from wards other than the NICU during the study period exhibited unique PFGE types (Fig. 1).
Isolates of all clusters were susceptible to cefepime, imipenem, and the combination of piperacillin and tazobactam. However, susceptibilities to amoxicillin-clavulanate varied, even within clusters. All isolates were also susceptible to ciprofloxacin.
DISCUSSION
Infections due to multiantibiotic-resistant K. pneumoniae are frequent in Greek hospitals (7, 12, 13, 18, 19). Control of epidemics due to this pathogen may benefit from detailed typing. As also demonstrated here, PFGE is effective in delineating the epidemiology of K. pneumoniae nosocomial infections (6, 15). The results provided by PFGE showed that a distinct “in-house” strain (cluster A) was responsible for most colonizations and infections that occurred during hospitalization. It is not inconceivable that this K. pneumoniae strain had been established in the NICU earlier than 1997, since it would be easily overlooked due to its apparent susceptibility to the broad-spectrum cephalosporins. This “discrepancy” is encountered more frequently in SHV-type ESBL producers (2). Thus, the application of DDST was helpful in identifying and tracing this endemic clone. The usefulness of synergy tests in detecting ESBL producers with low-level resistance to newer cephalosporins was also emphasized in a previous study (4). The origin of this resident strain and its mode of spread in the NICU were not elucidated. Isolation from the surface of a weighing scale indicated that occasional environmental contamination might also have contributed to its persistence in the NICU. Notwithstanding its presence in the NICU for at least 1 year, this strain had not spread to other wards. Conversely, a strain of PFGE type E that did persist for at least 5 months in other wards of the hospital was never isolated in the NICU.
A notable finding was the repeated isolation of EPKP from neonates upon admission from two large maternity hospitals in Athens (MH-I and MH-II). Molecular typing provided evidence that this finding was due to ongoing clonal outbreaks that presumably had been going unnoticed. Extensive dissemination of the EPKP clones was also indicated by the fact that approximately 2% of the neonates admitted from MH-I and MH-II were colonized by EPKP. Both implicated maternity hospitals were notified of the problem. Action on their part may well have contributed to the drastic reduction in the numbers of EPKP-infected or -colonized neonates admitted to the NICU from December 1998 to February 1999. Screening of patients upon admission also ceased at the same time; therefore, the reduction of the extent of colonization cannot be estimated. While we were aware of the low value of body surface cultures in predicting microorganisms causing severe infections in neonates (5, 10), this approach enabled us to identify EPKP carriers in a timely fashion. The control measures taken, including either isolation or grouping of these patients, may have been responsible, at least in part, for the fact that none of the imported strains was established in the NICU. Unexpectedly, the strain endemic in MH-II did appear in a single patient in the general pediatric ward. The origin of this phenomenon could not be ascertained.
The high prevalence of EPKP in Athens tertiary-care hospitals was documented in previous studies (12, 13). Measures implemented since 1991 have been aimed at the reduction of cephalosporin usage and the strengthening of infection control, primarily in intensive care units, considered the main foci of ESBL-producing enterobacteria (21). So far, a systematic evaluation of the effectiveness of these measures has not been undertaken. However, the spread and persistence of EPKP in major pediatric and maternity hospitals as well as in maternity units of general hospitals around Athens indicate that the policy followed has largely failed and must be revised.
REFERENCES
- 1.Bingen, E. H., P. Desjardins, G. Arlet, F. Bourgeois, P. Mariani-Kurkdjian, N. Y. Lambert-Zechovsky, E. Denamur, A. Philippon, and J. Elion. 1993. Molecular epidemiology of plasmid spread among extended broad-spectrum β-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J. Clin. Microbiol. 31:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush, K. 1996. Is it important to identify extended-spectrum beta-lactamase-producing isolates? Eur. J. Clin. Microbiol. Infect. Dis. 15:361-364. [DOI] [PubMed] [Google Scholar]
- 3.Coovadia, Y. M., A. P. Johnson, R. H. Bhama, G. R. Hutchinson, R. C. George, and I. E. Hafferjee. 1992. Multiresistant Klebsiella pneumoniae in a neonatal nursery: the importance of maintenance of infection control policies and procedures in the prevention of outbreaks. J. Hosp. Infect. 22:197-205. [DOI] [PubMed] [Google Scholar]
- 4.Coudron, P. E., E. S. Moland, and C. C. Sanders. 1997. Occurrence and detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae at a veterans medical center: seek and you may find. J. Clin. Microbiol. 35:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulgitini, V. A., and C. G. Ray. 1988. Body surface cultures in the newborn infant. Am. J. Dis. Child. 142:19-20. [DOI] [PubMed] [Google Scholar]
- 6.Gaillot, O., C. Maruejouls, E. Abachin, F. Lecuru, G. Arlet, M. Simonet, and P. Berche. 1998. Nosocomial outbreak of Klebsiella pneumoniae producing SHV-5 extended-spectrum β-lactamase, originating from a contaminated ultrasonography coupling gel. J. Clin. Microbiol. 36:1357-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazouli, M., M. Kaufmann, E. Tzelepi, H. Dimopoulou, O. Paniara, and L. S. Tzouvelekis. 1997. Study of an outbreak of cefoxitin-resistant Klebsiella pneumoniae in a general hospital. J. Clin. Microbiol. 35:508-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart, C. A. 1993. Klebsiellae and the neonate. J. Hosp. Infect. 23:83-86. [DOI] [PubMed] [Google Scholar]
- 9.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 10.Jolley, A. E. 1993. The value of surveillance cultures on neonatal intensive care units. J. Hosp. Infect. 25:153-159. [DOI] [PubMed] [Google Scholar]
- 11.Lalitha, M. K., J. Kenneth, A. K. Jana, M. V. Jesudason, K. A. Kuruvilla, K. Jacobson, I. Kuhn, and G. Kronvall. 1999. Identification of an IV-dextrose solution as the source of an outbreak of Klebsiella pneumoniae sepsis in a newborn nursery. J. Hosp. Infect. 43:70-73. [DOI] [PubMed] [Google Scholar]
- 12.Legakis, N. J., A. Maniatis, and L. S. Tzouvelekis. 1996. Prevalent mechanisms of resistance among enterobacteria isolated in Greek hospitals. Microb. Drug Resist. 1:331-333. [DOI] [PubMed] [Google Scholar]
- 13.Legakis, N. J., L. S. Tzouvelekis, G. Hatzoudis, E. Tzelepi, A. Gourkou, T. L. Pitt, and A. C. Vatopoulos. 1995. Dissemination of plasmids encoding an SHV-5-type β-lactamase among Klebsiella pneumoniae strains isolated in Greek hospitals. J. Hosp. Infect. 31:177-187. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1997. Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard M2-A6 (M100-S7). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Royle, J., S. Halasz, G. Eagles, G. Gilbert, D. Dalton, P. Jelfs, and D. Isaacs. 1999. Outbreak of extended spectrum β lactamase producing Klebsiella pneumoniae in a neonatal unit. Arch. Dis. Child. Fetal Neonatal Ed. 80:F64-F68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabo, D., Z. Filetoth, J. Szentandrassy, M. Nenedi, E. Toth, C. Jeney, G. Kispal, and F. Rozgonyi. 1999. Molecular epidemiology of a cluster of cases due to Klebsiella pneumoniae producing SHV-5 extended-spectrum β-lactamase in the premature intensive care unit of a Hungarian hospital. J. Clin. Microbiol. 37:4167-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tassios, P. T., A. Markogiannakis, A. C. Vatopoulos, E. Katsanikou, E. N. Velonakis, K. Kourea-Kremastinou, and N. J. Legakis. 1997. Molecular epidemiology of antibiotic resistance of Salmonella enteritidis during a 7-year period in Greece. J. Clin. Microbiol. 35:1316-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzouvelekis, L. S., E. Tzelepi, E. Prinarakis, M. Gazouli, A. Katrahoura, P. Giakkoupi, O. Paniara, and N. J. Legakis. 1998. Sporadic emergence of cefepime- and cefpirome-resistant Klebsiella pneumoniae strains in Greek hospitals. J. Clin. Microbiol. 36:266-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vatopoulos, A. C., A. Philippon, L. S. Tzouvelekis, Z. Komninou, and N. J. Legakis. 1990. Prevalence of a transferable SHV-5 type β-lactamase in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Greece. J. Antimicrob. Chemother. 26:635-648. [DOI] [PubMed] [Google Scholar]
- 20.Venezia, R. A., F. J. Scarano, K. E. Preston, L. M. Steele, T. P. Root, R. Limberger, W. Archinal, and M. A. Kacica. 1995. Molecular epidemiology of an SHV-5 extended-spectrum beta-lactamase in Enterobacteriaceae isolated from infants in neonatal intensive care unit. Clin. Infect. Dis. 21:915-923. [DOI] [PubMed] [Google Scholar]
- 21.Yuan, M., H. Aucken, L. M. C. Hall, T. L. Pitt, and D. M. Livermore. 1998. Epidemiological typing of klebsiellae with extended-spectrum β-lactamases from European intensive care units. J. Antimicrob. Chemother. 41:427-439. [DOI] [PubMed] [Google Scholar]