Abstract
The role of wild mammals, such as roe deer (Capreolus capreolus) and chamois (Rupicapra rupicapra), in the epidemiology of granulocytic ehrlichiae in Switzerland was investigated. We tested blood samples for Ehrlichia phagocytophila genogroup 16S rRNA gene sequences by PCR and for immunoglobulin G antibodies against granulocytic ehrlichiae by indirect fluorescent-antibody assay (IFA). Overall means of 60.9% of 133 roe deer serum samples and 28.2% of 39 chamois serum samples were seroreactive by IFA. PCR results were positive for 18.4% of 103 roe deer serum samples as well. None of the 24 chamois blood samples tested were positive by PCR. Partial 16S rRNA gene and groESL heat shock operon sequences of three roe deer samples tested showed strong degrees of homology (≥99.7 and ≥98.6%, respectively) with the sequences of granulocytic ehrlichiae isolated from humans. These results confirm that chamois, and particularly roe deer, are commonly infected with granulocytic ehrlichiae and provide evidence that these wild mammals are potential reservoirs for granulocytic ehrlichiae in Switzerland.
Granulocytic ehrlichiae (GE) are tick-transmitted, obligate intracellular bacteria which have been associated with illnesses of veterinary importance for decades. In the early 1990s, a human infection with a granulocytic Ehrlichia species was recognized to be an emerging human disease, and to date, more than 600 cases of human granulocytic ehrlichiosis (HGE) have been identified, mostly in the upper Midwest and in the Northeast of the United States (3). Insufficient differences exist between the HGE agent, Ehrlichia phagocytophila, responsible for tick-borne fever in ruminants, and Ehrlichia equi, the agent of the equine granulocytic ehrlichiosis, to support separate species designations; and the 16S rRNA sequences of this group are at least 98.2% similar to those of any Anaplasma species (14). These 16S rRNA gene analyses are strongly supported by similar groESL clades, as well as biological and antigenic characteristics. Very recently, Dumler et al. (14) proposed that the genus Anaplasma be emended to include Anaplasma (Ehrlichia) phagocytophila comb. nov., which also encompasses the species formerly known as E. equi and the HGE agent.
Since 1995, serological evidence of HGE has been demonstrated in several European countries (3, 7, 11, 15, 16, 20, 23, 40, 49, 50), including Switzerland (9, 29, 45), in areas of known endemicity for Lyme borreliosis. Clinical and laboratory-confirmed HGE cases have been reported to occur in patients from Slovenia (42), The Netherlands (51), and Sweden (6).
In the United States, infections with GE in humans and animals have often been associated with Ixodes scapularis and Ixodes pacificus ticks, which serve as the primary vectors. Transovarial transmission of Ehrlichia species appears to be inefficient in ticks (33), and mammalian hosts are therefore presumed to play an important role in the maintenance and propagation of Ehrlichia species in nature. The white-footed mouse (Peromyscus leucopus), besides being a major host for immature I. scapularis, is apparently a main reservoir host for species of GE. Large mammals, including the white-tailed deer (Odocoileus virginianus), are implicated as natural reservoirs as well (5, 34, 53). In Europe, very little is known about the animal reservoirs or the ecology of GE. Studies that have used PCR methods have demonstrated the role of I. ricinus as a potential vector (12, 19, 28, 39, 41, 43, 44, 46, 52). There is some evidence of the role of small mammals, particularly rodents, in the transmission of GE in Switzerland (28), and in the United Kingdom (39). In the latter country, field evidence that roe deer (Capreolus capreolus) are a potential natural host for GE was shown (2). To our knowledge, except for the latter British studies, no other studies on wild large mammals and infections with GE in Europe have been published.
The purpose of the present study was to investigate the role of wild ruminants, such as roe deer and chamois, common hosts of I. ricinus ticks, as potential reservoirs for GE by quantifying the prevalence of infection of these animals with GE by PCR and serologic testing in order to better understand the epidemiology of granulocytic ehrlichiosis caused by species of the E. phagocytophila genogroup.
MATERIALS AND METHODS
Study areas and sample collection.
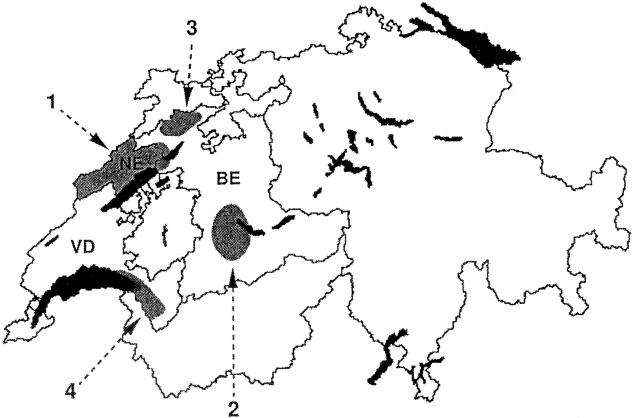
This study was conducted in four areas located in three Swiss cantons (territorial subdivisions): area 1 (canton of Neuchâtel), area 2 (southern canton of Bern), area 3 (northern canton of Bern), and area 4 (canton of Vaud) (Fig. 1). On the basis of previous studies with small mammals, ticks, and cattle, these areas are known to be endemic for granulocytic ehrlichiosis (27, 28).
FIG. 1.
Map of Switzerland showing the four areas located in three cantons where roe deer and chamois blood samples were collected; area 1 (canton of Neuchâtel [NE]), area 2 (southern canton of Bern [BE]), area 3 (northern canton of Bern), and area 4 (canton of Vaud [VD]). Original map copyright GEOSTAT, Office Fédéral de la Statistique. Adapted with permission of GEOSTAT.
In the autumn of 1992, during the regular hunting season, roe deer and chamois were collected by gunshot. Hunters were recruited to collect roe deer and chamois blood samples. Several recruitment strategies were used, including presentations to local hunting groups and direct mailing to members of hunting organizations. Blood collection kits were distributed at group meetings. Each kit consisted of two blood tubes (one for serum and one containing K-EDTA), a 20-ml plastic syringe with a needle inside a biohazard bag with instructions, and a form for the collection of the date of collection and demographic data (age and sex of the animal, altitude and place where the animal was shot). Hunters were asked to obtain blood samples from the animals by cardiac puncture when dressing freshly killed animals in the field. They were instructed to place the tubes in the biohazard bag (one bag for each shot animal to avoid cross-contamination) and to keep the tubes cool after sample collection by refrigerating them as soon as possible. Multiple sites where the blood samples could be dropped off were available. Samples were sent by mail to our laboratory as well. Each animal was examined visually for the presence of ticks by the hunters. For this reason, the identification of tick species was unfeasible. Thus, it is assumed that the great majority, if not all, were I. ricinus ticks, a common species infesting wild mammals. Ticks were also sent to our laboratory for species determination.
IFA.
Serum was harvested from clotted blood after centrifugation at 1,000 × g for 15 min and stored at −80°C prior to testing. Samples were tested for antibodies to the E. phagocytophila genogroup by an indirect fluorescent-antibody test (IFA) (27). Samples were screened at dilutions of 1/40 and 1/80 in phosphate-buffered saline (PBS; pH 7.3) on spot slides of neutrophils infected with a Swiss bovine isolate of E. phagocytophila (27). Fluorescein isothiocyanate rabbit anti-bovine immunoglobulin G (IgG; heavy and light chains) conjugate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was diluted to 1/400 in PBS. Samples were considered positive when their titers were greater than or equal to 1/80. In order to determine end-point titers, positive samples were diluted twofold and the results were recorded as reciprocals of the highest dilution at which specific fluorescence of cytoplasmic ehrlichial morulae or individual organisms could be detected. Samples used as negative controls were from cattle with no history of ehrlichiosis, and samples used as positive controls were from calves experimentally infected with E. phagocytophila by using Swiss bovine blood stabilates.
DNA extraction.
Total DNA was extracted from blood samples by using a QIAamp Blood kit (Qiagen, Basel, Switzerland), according to the manufacturer's instructions. Briefly, DNA was extracted from 100- to 200-μl aliquots of blood. Detergent lysis was carried out in the presence of proteinase K for 15 min at 70°C. The lysed material was applied to a spin column containing a silica gel-based membrane and washed twice. Purified DNA was eluted from the columns in 200 μl of 10 mM Tris-HCl-0.5 mM EDTA (pH 9.0) and stored at −20°C until it was used as a template for PCR amplification.
PCR amplification.
Granulocytic ehrlichial infections in roe deer and chamois blood were detected with two sets of primers in a nested PCR format which specifically targets the 16S rRNA gene of the E. phagocytophila genogroup (10, 35). PCR amplifications were performed in a thermal cycler (Eppendorf Mastercycler Personnal; Eppendorf, Hamburg, Germany). Primary reactions used 5 μl of DNA extraction sample as the template in a total volume of 50 μl. The reaction mixtures contained 10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl (pH 8.3), each deoxynucleoside triphosphate (dNTP) at a concentration of 200 μM, 1.25 U of Taq polymerase, and each primer at a concentration of 0.5 μM. The outer primers were ge3a (5′-CACATGCAAGTCGAACGGATTATTC) and ge10r (5′-TTCCGTTAAGAAGGATCTAATCTCC), which produce a 932-bp product. Cycling conditions included an initial 2 min of denaturation at 95°C, followed by 40 cycles, each consisting of 30 s of denaturation at 94°C, 30 s of annealing at 55°C, and 1 min of extension at 72°C. These 40 cycles were followed by an extension period of 5 min at 72°C. For samples that had no amplified products after the initial PCR, reamplification was done by a nested PCR. The nested amplifications used 1 μl of the primary PCR product as the template in a total volume of 50μl. Each nested amplification contained 10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl (pH 8.3), each dNTP at a concentration of 200 μM, 1.25 U of Taq polymerase, and each internal primer (ge9f [5′-AACGGATTATTCTTTATAGCTTGCT] and ge2 [5′-GGCAGTATTAAAAGCAGCTCCAGG]) at a concentrations of 0.2 μM. These primers produce a 546-bp product. The cycling conditions for the nested amplification were as described above for the primary amplification, except that 30 cycles were used.
For further characterization, randomly selected DNA samples positive for the E. phagocytophila genogroup 16S rRNA gene were tested by PCR assays with primers designed to amplify the groESL heat shock operon of Ehrlichia spp. The assay was conducted in a nested format with primers HS1a (5′-AITGGGCTGGTAITGAAAT) and HS6a (5′-CCICCIGGIACIAIACCTTC) (the primers were modified from those described previously [48]) (37) in the first round and primers HS43 [5′-AT(A/T)GC(A/T)AA(G/A)GAAGCATAGTC] and HSVR (5′-CTCAACAGCAGCTCTAGTAGC) (32) in the nested amplification reactions. The nested primers amplify a 1,297-bp region of the heat shock operon that includes the end of the groES gene, the spacer region between the groES and groEL genes, and approximately three-fourths of the groEL gene. All reaction mixtures for PCR assays were prepared with commercial amplification kits (Ready-To-Go PCR Beads; Amersham Pharmacia Biotech, Piscataway, N.J.). Two microliters of DNA template was added to 23 μl of a reaction mixture containing 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, each dNTP at a concentration of 200 μM, each primer at a concentration of 1 μM, and 1.5 U of Taq polymerase. PCRs were performed in a thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.). Cycling conditions involved three preliminary cycles, each consisting of 1 min of denaturation at 94°C, 2 min of annealing at 48°C, and 1.5 min of extension at 68°C, followed by 37 amplification cycles, each consisting of 1 min of denaturation at 88°C, 2 min of annealing at 48°C, and 1.5 min of extension at 68°C. These cycles are followed by an additional extension period of 5 min at 68°C. During the second (nested) amplification round, 1 μl of the finished primary reaction mixture was added to 24 μl of the reaction mixture. The annealing temperature of the reaction was increased to 55°C, and the temperature used for extension was increased to 72°C.
All amplified products were subsequently maintained at 4°C until they were analyzed by agarose gel electrophoresis or purified for DNA sequencing.
For quality control both positive and negative control specimens that were amplified by PCR in parallel with all specimens were used. In order to minimize the potential for contamination, DNA extractions, PCR setup, and agarose gel electrophoresis were performed in three separate rooms. Precautions were taken to prevent contamination of samples, including the use of aerosol-barrier pipette tips.
DNA sequencing and data analysis.
Sequencing of the PCR products was conducted by the purification methods and automated sequencing techniques described previously (35, 48). We partially sequenced the 16S rRNA gene and groESL heat shock operon from three roe deer samples (samples 56, 58, and 99). The 16S rRNA gene and groESL heat shock operon sequences used for comparison were obtained from the GenBank database. Nucleotide sequence homology searches were made through the National Center for Biotechnology Information BLAST network service.
Statistical analysis.
Fisher's exact test was used to compare the proportions. P values of <0.05 were considered statistically significant.
Nucleotide sequence accession numbers.
The sequences of the GE obtained from three roe deer samples (samples 56, 58, and 99) are available in GenBank under accession nos. AF384212, AF384213, and AF284214, respectively, for the 16S rRNA gene (rDNA) sequences and accession nos. AF383225, AF383226, and AF383227, respectively, for the groESL heat shock operon sequences.
RESULTS
Chamois and roe deer samples.
A total of 133 roe deer and 39 chamois samples were collected. The sex and age compositions of the roe deer and chamois population are presented by area in Table 1.
TABLE 1.
Numbers of roe deer and chamois collected from four Swiss areas, by sex and age
| Area | No. of animals collecteda
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex
|
Age (yr)
|
|||||||||
| Male
|
Female
|
0-1
|
≥1-2
|
≥2
|
||||||
| rd | ch | rd | ch | rd | ch | rd | ch | rd | ch | |
| 1 | 33 | 16 | 14 | 4 | 4 | 1 | 8 | 4 | 35 | 15 |
| 2 | 23 | 6 | 15 | 11 | 14 | 0 | 3 | 9 | 21 | 8 |
| 3 | 24 | 1 | 15 | 1 | 12 | 0 | 7 | 2 | 20 | 0 |
| 4 | 6 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | 6 | 0 |
| Total | 86 | 23 | 47 | 16 | 30 | 1 | 21 | 15 | 82 | 23 |
rd, roe deer (n = 133); ch, chamois (n = 39).
Ticks collected from animals.
Of the 133 roe deer and 39 chamois, 61 (45.9%) and 2 (5.1%), respectively, were infested with ticks (Table 2). All the ticks (n = 85) sent by hunters to our laboratory were identified as engorged female I. ricinus ticks. If we exclude the data for area 4 (where no chamois samples were collected), roe deer were significantly more infested with ticks than chamois (P < 0.000001) in all collections areas. Furthermore, roe deer shot in areas below 1,000 m in altitude were significantly more infested with ticks than those shot above that altitude (P < 0.015) (Table 2).
TABLE 2.
Prevalence of roe deer and chamois infested with ticks, by altitude, in four Swiss areas
| Area | No. of roe deer infested with ticks/no. of roe deer examined (%)
|
No. of chamois infested with ticks/no. of chamois examined (%)
|
||
|---|---|---|---|---|
| ≤1,000 m | >1,000 m | ≤1,000 m | >1,000 m | |
| 1 | 11/29 | 6/18 | 1/4 | 0/17 |
| 2 | 15/20 | 5/18 | 0/0 | 1/16 |
| 3 | 22/37 | 1/2 | 0/1 | 0/1 |
| 4 | 0/5 | 1/4 | 0/0 | 0/0 |
| Total | 48/91 (52.7) | 13/42 (31.0) | 1/5 (20) | 1/34 (2.9) |
Direct microscopic detection of GE.
Direct microscopic examinations of Giemsa-stained buffy coat blood smears from roe deer and chamois never demonstrated any intragranulocytic ehrlichial inclusions (morulae).
Antibody detection.
A total of 133 serum samples from roe deer and 39 serum samples from chamois were tested by IFA (Table 3). Of these, 81 (60.9%) roe deer and 11 (28.2%) chamois were found to contain IgG antibodies reactive to GE by IFA. The titers of the 81 positive roe deer samples ranged from 80 to 2,560, with 45.9% (61 of 133) of the titers being greater than or equal to 160 (geometric mean titer = 137). The titers for the 11 positive chamois samples ranged from 80 to 1,280, with 25.6% (10 of 39) of the titers being greater than or equal to 160 (geometric mean titer = 76). Depending on the areas, variations in the prevalence of infection were observed: 53.2 to 77.8% for roe deer and 11.8 to 50.0% for chamois. No significant differences in the prevalence of infection were found by age or sex for either mammalian species (data not shown). The overall prevalence of antibodies reactive to GE was significantly higher in roe deer than in chamois (P < 0.0005). Furthermore, the prevalence in roe deer and chamois coming from areas below 1,000 m, where they are likely to be in contact with ticks, was significantly higher than that in animals coming from areas above 1,000 m (P < 0.02) (Table 4).
TABLE 3.
Prevalence of IgG antibodies reactive to GE among roe deer and chamois in four Swiss areasa
| Area | No. positive/no. tested (%)
|
Geometric mean titerb
|
Maximum reciprocal titer
|
|||
|---|---|---|---|---|---|---|
| rd | ch | rd | ch | rd | ch | |
| 1 | 25/47 (53.2) | 8/20 (40.0) | 112 | 80 | 640 | 640 |
| 2 | 27/38 (71.1) | 2/17 (11.8) | 187 | 57 | 2,560 | 640 |
| 3 | 22/39 (56.4) | 1/2 (50.0) | 116 | 190 | 2,560 | 1,280 |
| 4 | 7/9 (77.8) | 0/0 | 187 | 0 | 2,560 | 0 |
| Total | 81/133 (60.9) | 11/39 (28.2) | 137 | 76 | 2,560 | 1,280 |
rd, roe deer; ch, chamois.
A value of 40, the reciprocal titer for negative sera, was used for each negative sample in the analyses.
TABLE 4.
Prevalence of IgG antibodies reactive to GE among roe deer and chamois, by altitude, in four Swiss areas
| Area | No. of roe deer positive/no. of roe deer tested (%)
|
No. of chamois positive/no. of chamois tested (%)
|
||
|---|---|---|---|---|
| ≤1,000 m | >1,000 m | ≤1,000 m | >1,000 m | |
| 1 | 19/29 | 6/18 | 3/4 | 5/16 |
| 2 | 18/20 | 9/18 | 0/0 | 2/17 |
| 3 | 21/37 | 1/2 | 1/1 | 0/1 |
| 4 | 4/5 | 3/4 | 0/0 | 0/0 |
| Total | 62/91 (68.1) | 19/42 (45.2) | 4/5 (80.0) | 7/34 (20.6) |
PCR detection.
None of the 24 chamois blood samples tested positive for GE by PCR. However, granulocytic ehrlichial DNA was detected in 18.4% (19 of 103) of blood samples from roe deer, with the range being from 10.0 to 44.4%, depending on the collection area (Table 5). Comparison of the results for roe deer by Fisher's exact test indicated agreement between the results of PCR and those of IFA (P < 0.02). As for individual animals, seropositivity did not always correlate with PCR positivity of the blood samples, although 84.2% of PCR-positive roe deer were also positive by IFA and 26.2% of the seropositive roe deer were positive by PCR.
TABLE 5.
PCR examination and serology for granulocytic ehrlichial DNA in roe deer and chamois blood samples in four Swiss areas
| Area | No. (%) of animalsa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Roe deer (%)
|
Chamois (%)
|
|||||||
| PCR + | Sero + | PCR and Sero + | Tested | PCR + | Sero + | PCR and Sero + | Tested | |
| 1 | 3 | 13 | 3 | 30 | 0 | 4 | 0 | 10 |
| 2 | 7 | 25 | 6 | 33 | 0 | 0 | 0 | 10 |
| 3 | 5 | 17 | 4 | 31 | 0 | 1 | 0 | 4 |
| 4 | 4 | 7 | 3 | 9 | 0 | 0 | 0 | 0 |
| Total | 19 (18.4) | 62 (60.2) | 16 (15.5) | 103 | 0 | 5 (20.8) | 0 | 24 |
+, positive; Sero, serology.
Sequence analysis.
The 16S rDNA sequences and groESL heat shock operon sequences from three roe deer (samples 56, 58, and 99) were compared to each other and to sequences in the nucleotide sequence databases. The partial 16 rDNA sequences (881 bp between the GE3A and GE9 primer sites) from samples 56 and 58 were identical, and sequence homology searches found one identical sequence (GenBank accession no. AJ242784) that was designated Swedish tick Ehrlichia type IIb (52). The sequence from sample 99 differed from those from samples 56 and 58 by 2 nucleotides (99.8% identity), and no exact match was found between the sequence from sample 99 and those in the databases. Sequence ESP242784 was the most similar sequence from the databases with the greatest similarity to sequences from samples collected in this study. Overall, the 16S rRNA gene sequences of the three samples revealed 99.7 to 100% homologies with known granulocytic ehrlichial DNA sequences.
The groESL sequences amplified from the three roe deer samples were more divergent. The sequences were of equal lengths, 1,256 bp between nested PCR primers HS43 and HSVR. These primers amplify a portion of the groESL operon that includes 50 bp of the 52-bp intergenic spacer sequence between the groES and groEL genes and 1,206 bp of the groEL-coding sequence. Sequences from samples 56 and 58 were most similar, differing by 5 nucleotides (99.6% identity), and the deduced amino acid sequences for the portion of groEL amplified (402 amino acid residues) were identical. As with the 16S rRNA gene sequences, the sequence from sample 99 was less similar, differing from the sequence from sample 56 by 25 nucleotides (98.0% identity) and from the sequence from sample 58 by 22 nucleotides (98.2% identity). The sequences from the spacer region were identical. Nucleotide differences occurred in the groEL-coding sequence and were silent, with one exception. The deduced amino acid sequence for sample 99 differed from the others by 1 residue. Alanine was replaced by serine at position 242. The deduced amino acid sequence for sample 99 was identical to those of most sequences of GE in the databases.
In summary, the sequences obtained from the PCR products are compatible with sequences previously described for GE, and this confirms that for the PCR tests the correct targets were amplified. It is difficult to judge the significance of the sequence variations observed among the PCR products.
DISCUSSION
The life cycle of GE involves a complex interaction between natural hosts that act as potential reservoirs and tick vectors that progress through multiple life stages and that may transmit the infection to humans. In many parts of Europe, roe deer appear to be an important maintenance host for I. ricinus tick reproduction (essentially nymphs and adults, but also larvae) and are strongly associated with other tick-borne pathogens such as Borrelia burgdorferi, the causative agent of Lyme disease, although the competence of roe deer as a reservoir is still discussed (18, 22). In both Europe and North America, the magnitude of Ixodes vector infestations appears to be related to the local abundance of deer (38, 54). This study provides evidence supporting the role of chamois, and particularly the role of roe deer, in the maintenance cycle of GE in Switzerland. To our knowledge, the present publication mentions for the first time the presence of tick infestation in chamois and evidence of infection of chamois with GE.
Cervids, such as white-tailed deer, have been initially implicated as suspected reservoir hosts for Ehrlichia chaffeensis, an agent of human monocytic ehrlichiosis, in the United States. Depending on the areas, rates of seropositivity for E. chaffeensis infection ranging from 4.4 to 92% were described (21, 24, 26, 30, 31, 36, 47); E. chaffeensis 16S rDNA was detected by PCR in the blood, spleens, or lymph nodes of white-tailed deer (24, 30) or in archived tissue (25), and E. chaffeensis from deer was isolated in cell culture (26, 30) as well. Furthermore, a novel Ehrlichia-like organism closely related to the E. phagocytophila genogroup by 16S rRNA gene sequence comparisons was found to be infecting white-tailed deer distributed throughout the southeastern United States (8, 13, 26, 31) and other wild cervid species from California (17, 24).
In the United States, white-tailed deer participate in the maintenance of the Ixodes species life cycle, but their role as a reservoir host for GE is still undetermined. By the use of specific 16S rDNA primers, granulocytic ehrlichial DNA was detected in deer blood samples from Connecticut, and among them, samples were positive for the DNA of the 44-kDa gene, which is unique to the E. phagocytophila group. The same study showed that deer serum samples contained antibodies to either or both of the BDS or NCH-1 strains of GE (34). Studies carried out in Wisconsin (5, 35, 54) showed a seroprevalence among white-tailed deer between 8 and 60%, and a seroprevalence of 25% was found among white-tailed deer in Maryland (53). Ehrlichial GE DNA was detected by PCR in 9.4% of deer blood samples in Maryland (35) and in 15% (5) and 64% (4) of deer blood samples in Wisconsin. The latter study also described three patients with HGE who had extensive exposure to deer blood presumed to be infected with the causative agent. Seropositive white-tailed deer were found in Georgia as well (26).
In Europe, studies linking large wild mammals infected with GE are nearly nonexistent. In the United Kingdom, the prevalence of infection in blood and spleen samples from culled roe deer collected from nine sites was determined by serology and PCR. Means of 58% of 102 plasma or serum samples were seroreactive by IFA, and means of 38% of 84 blood samples and 29% of 82 spleen samples were positive by PCR (2). A Dutch study that used a sensitive and specific PCR hybridization assay showed that roe deer may infect tick vectors: 45% of I. ricinus ticks collected on roe deer were positive. More than half of these positive ticks carried species of GE with 16S rDNA sequences closely related to those of E. phagocytophila and the HGE agent. The majority of the other positive ticks were infected with a newly identified Ehrlichia-like species (46). Our serologic results for roe deer are identical to those presented in the British study, but the PCR prevalence in Switzerland seems lower.
Roe deer are highly adaptable species found in a wide variety of habitats, ranging from open moors to thickly covered areas in coniferous or deciduous woodlands. Chamois live in mountain pastures and rocky areas above the tree line to 3,500 m, not far from the snow line. In winter, they descend to lower altitudes, below 1,500 m, into forested regions, although they remain near steep cliffs or open slopes cleaned of snow by the wind. During the summer months, herds of chamois wander alpine meadows above 1,800 m. The I. ricinus tick is the most common tick species in Switzerland, living in wooded areas with abundant low-stratum vegetation and generally deciduous woodland, but it also lives in coniferous or mixed forests. The altitude acts as a restrictive factor for this tick species (1). In Switzerland, the tick population decreases rapidly beyond 1,000 m and disappears above 1,500 m. In areas 1, 3, and 4 examined in the present study, the altitude does not exceed 1,200 m. Roe deer and chamois populations often live in the same geographic sites, and during the entire year, both species may be in contact with ticks infected with GE. In area 2, however, the altitude can reach more than 3,000 m. Most of the year, chamois live at altitudes where ticks are not present. The decrease in the tick population with altitude is confirmed by the tick infestation rates of roe deer and chamois. Roe deer were significantly more infested with ticks than chamois (45.9 and 5.1%, respectively), this was the case regardless of the area studied (Table 2). The animals coming from areas below 1,000 m had more ticks than those coming from studied areas above 1,000 m (P < 0.02) (Table 2). This difference of habitats is reflected in the prevalence of infection with GE. The overall prevalence of antibodies reactive to GE was higher in roe deer than in chamois (P < 0.0005) (Table 3), and none of the chamois blood samples analyzed by PCR tested positive for GE. We detected current infection in 18.4% of roe deer by PCR (Table 5). Among these animals, 84.2% (16 of 19) were IFA positive, 79.0% came from areas situated below 1,000 m, and 47.3% were infested with ticks.
In Switzerland, roe deer are relatively abundant in diverse biotopes and serve as hosts for large numbers of all stages of I. ricinus ticks, which may become infected with GE. For these reasons, roe deer are likely to be competent reservoirs of granulocytic ehrlichial infection. This study suggest that chamois, and particularly roe deer, are naturally infected with GE in diverse geographical regions of Switzerland and that these animals, and potentially humans, are exposed to infected ticks at a high frequency in nature.
Acknowledgments
We appreciate the technical assistance of Aurélie Jeanneret-Gris with the PCR assays and are grateful to all the hunters who participated in this study by providing roe deer and chamois blood samples for analysis. We gratefully acknowledge Lise Gern, University of Neuchâtel, for support and review of the manuscript.
This study was supported by the Swiss National Science Foundation (grant 32-52744.97).
REFERENCES
- 1.Aeschlimann, A. 1972. Ixodes ricinus, Limmeus, 1758 (Ixodoidea: Ixodidae). Preliminary study of the biology of the species in Switzerland. Acta Trop. 29:321-340. (In French.) [PubMed]
- 2.Alberdi, M. P., A. R. Walker, and K. A. Urquhart. 2000. Field evidence that roe deer (Capreolus capreolus) are a natural host for Ehrlichia phagocytophila. Epidemiol. Infect. 124:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakken, J. S., J. Krueth, R. L. Tilden, J. S. Dumler, and B. E. Kristiansen. 1996. Serological evidence of human granulocytic ehrlichiosis in Norway. Eur. J. Clin. Microbiol. Infect. Dis. 15:829-832. [DOI] [PubMed] [Google Scholar]
- 4.Bakken, J. S., J. K. Krueth, T. Lund, D. Malkovitch, K. Asanovich, and J. S. Dumler. 1996. Exposure to deer blood may be a cause of human granulocytic ehrlichiosis. Clin. Infect. Dis. 23:198.. [DOI] [PubMed] [Google Scholar]
- 5.Belongia, E. A., K. D. Reed, P. D. Mitchell, C. P. Kolbert, D. H. Persing, J. S. Gill, and J. J. Kazmierczak. 1997. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J. Clin. Microbiol. 35:1465-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjoersdorff, A., J. Berglund, B. E. Kristiansen, C. Soderstrom, and I. Eliasson. 1999. Varying clinical picture and course of human granulocytic ehrlichiosis. Twelve Scandinavian cases of the new tick-borne zoonosis are presented. Lakartidningen 96:4200-4204. (In Swedish.) [PubMed]
- 7.Bjoersdorff, A., P. Brouqui, I. Eliasson, R. F. Massung, B. Wittesjo, and J. Berglund. 1999. Serological evidence of Ehrlichia infection in Swedish Lyme borreliosis patients. Scand. J. Infect. Dis. 31:51-55. [DOI] [PubMed] [Google Scholar]
- 8.Brandsma, A. R., S. E. Little, J. M. Lockhart, W. R. Davidson, D. E. Stallknecht, and J. E. Dawson. 1999. Novel Ehrlichia organism (Rickettsiales: Ehrlichieae) in white-tailed deer associated with lone star tick (Acari: Ixodidae) parasitism. J. Med. Entomol. 36:190-194. [DOI] [PubMed] [Google Scholar]
- 9.Brouqui, P., J. S. Dumler, R. Lienhard, M. Brossard, and D. Raoult. 1995. Human granulocytic ehrlichiosis in Europe. Lancet 346:782-783. [DOI] [PubMed] [Google Scholar]
- 10.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christova, I. S., and J. S. Dumler. 1999. Human granulocytic ehrlichiosis in Bulgaria. Am. J. Trop. Med. Hyg. 60:58-61. [DOI] [PubMed] [Google Scholar]
- 12.Cinco, M., D. Padovan, R. Murgia, M. Maroli, L. Frusteri, M. Heldtander, K. E. Johansson, and E. Olsson Engwall. 1997. Coexistence of Ehrlichia phagocytophila and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Italy as determined by 16S rRNA gene sequencing. J. Clin. Microbiol. 35:3365-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson, J. E., C. K. Warner, V. Baker, S. A. Ewing, D. E. Stallknecht, W. R. Davidson, A. A. Kocan, J. M. Lockhart, and J. G. Olson. 1996. Ehrlichia-like 16S rDNA sequence from wild white-tailed deer (Odocoileus virginianus). J. Parasitol. 82:52-58. [PubMed] [Google Scholar]
- 14.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, description of six new species combinations and designation of Ehrlichia equi and “HGE agent” as synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 15.Dumler, J. S., L. Dotevall, R. Gustafson, and M. Granstrom. 1997. A population-based seroepidemiologic study of human granulocytic ehrlichiosis and Lyme borreliosis on the west coast of Sweden. J. Infect. Dis. 175:720-722. [DOI] [PubMed] [Google Scholar]
- 16.Fingerle, V., J. L. Goodman, R. C. Johnson, T. J. Kurtti, U. G. Munderloh, and B. Wilske. 1997. Human granulocytic ehrlichiosis in southern Germany: increased seroprevalence in high-risk groups. J. Clin. Microbiol. 35:3244-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley, J. E., J. E. Barlough, R. B. Kimsey, J. E. Madigan, E. DeRock, and A. Poland. 1998. Ehrlichia spp. in cervids from California. J. Wildl. Dis. 34:731-737. [DOI] [PubMed] [Google Scholar]
- 18.Gern, L., A. Estrada-Pena, F. Frandsen, J. S. Gray, T. G. Jaenson, F. Jongejan, O. Kahl, E. Korenberg, R. Mehl, and P. A. Nuttall. 1998. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentbl. Bakteriol. Parasitenko. Infektkrankh. Hyg. Abt. 1 Orig. 287:196-204. [DOI] [PubMed] [Google Scholar]
- 19.Guy, E., S. Tasker, and D. H. Joynson. 1998. Detection of the agent of human granulocytic ehrlichiosis (HGE) in UK ticks using polymerase chain reaction. Epidemiol. Infect. 121:681-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunfeld, K. P., R. Allwinn, S. Peters, P. Kraiczy, and V. Brade. 1998. Serologic evidence for tick-borne pathogens other than Borrelia burgdorferi (TOBB) in Lyme borreliosis patients from midwestern Germany. Wien Klin. Wochenschr. 110:901-908. [PubMed] [Google Scholar]
- 21.Irving, R. P., R. R. Pinger, C. N. Vann, J. B. Olesen, and F. E. Steiner. 2000. Distribution of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaeceae) in Amblyomma americanum in southern Indiana and prevalence of E. chaffeensis-reactive antibodies in white-tailed deer in Indiana and Ohio in 1998. J. Med. Entomol. 37:595-600. [DOI] [PubMed] [Google Scholar]
- 22.Jaenson, T. G., and L. Talleklint. 1992. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J. Med. Entomol. 29:813-817. [DOI] [PubMed] [Google Scholar]
- 23.Lebech, A. M., K. Hansen, P. Pancholi, L. M. Sloan, J. M. Magera, and D. H. Persing. 1998. Immunoserologic evidence of human granulocytic ehrlichiosis in Danish patients with Lyme neuroborreliosis. Scand. J. Infect. Dis. 30:173-176. [DOI] [PubMed] [Google Scholar]
- 24.Little, S. E., J. E. Dawson, J. M. Lockhart, D. E. Stallknecht, C. K. Warner, and W. R. Davidson. 1997. Development and use of specific polymerase reaction for the detection of an organism resembling Ehrlichia sp. in white-tailed deer. J. Wildl. Dis. 33:246-253. [DOI] [PubMed] [Google Scholar]
- 25.Little, S. E., and E. W. Howerth. 1999. Ehrlichia chaffeensis in archived tissues of a white-tailed deer. J. Wildl. Dis. 35:596-599. [DOI] [PubMed] [Google Scholar]
- 26.Little, S. E., D. E. Stallknecht, J. M. Lockhart, J. E. Dawson, and W. R. Davidson. 1998. Natural coinfection of a white-tailed deer (Odocoileus virginianus) population with three Ehrlichia spp. J. Parasitol. 84:897-901. [PubMed] [Google Scholar]
- 27.Liz, J. S. 1994. Ehrlichia phagocytophila: epidemiological, haematological, and serological aspects of the infection in cattle in Switzerland. Ph.D. thesis. University of Neuchâtel, Neuchâtel, Switzerland.
- 28.Liz, J. S., L. Anderes, J. W. Sumner, R. F. Massung, L. Gern, B. Rutti, and M. Brossard. 2000. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J. Clin. Microbiol. 38:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liz, J. S., B. Rutti, and M. Brossard. 1997. Human granulocytic ehrlichiosis in Switzerland: serological evidences. Clin. Microbiol. Infect. 3:343. [Google Scholar]
- 30.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, J. E. Dawson, and E. W. Howerth. 1997. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J. Clin. Microbiol. 35:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lockhart, J. M., W. R. Davidson, D. E. Stallknecht, J. E. Dawson, and S. E. Little. 1997. Natural history of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in the piedmont physiographic province of Georgia. J. Parasitol. 83:887-894. [PubMed] [Google Scholar]
- 32.Lotric-Furlan, S., M. Petrovec, T. A. Zupanc, W. L. Nicholson, J. W. Sumner, J. E. Childs, and F. Strle. 1998. Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings for four patients from Slovenia. Clin. Infect. Dis. 27:424-428. [DOI] [PubMed] [Google Scholar]
- 33.MacLeod, J., and W. S. Gordon. 1933. Studies in tick-borne fever of sheep. I. Transmission by the tick, Ixodes ricinus, with a description of the disease produced. Parasitology 25:273-283. [Google Scholar]
- 34.Magnarelli, L. A., J. W. Ijdo, K. C. Stafford, and E. Fikrig. 1999. Infections of granulocytic ehrlichiae and Borrelia burgdorferi in white-tailed deer in Connecticut. J. Wildl. Dis. 35:266-274. [DOI] [PubMed] [Google Scholar]
- 35.Massung, R. F., K. Slater, J. H. Owens, W. L. Nicholson, T. N. Mather, V. B. Solberg, and J. G. Olson. 1998. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller-Anneling, L., M. J. Gilchrist, and P. S. Thorne. 2000. Ehrlichia chaffeensis antibodies in white-tailed deer, Iowa, 1994 and 1996. Emerg. Infect. Dis. 6:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson, W. L., M. B. Castro, V. L. Kramer, J. W. Sumner, and J. E. Childs. 1999. Dusky-footed wood rats (Neotoma fuscipes) as reservoirs of granulocytic ehrlichiae (Rickettsiales: Ehrlichieae) in northern California. J. Clin. Microbiol. 37:3323-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Connell, S., M. Granstrom, J. S. Gray, and G. Stanek. 1998. Epidemiology of European Lyme borreliosis. Zentbl. Bakteriol. Parasiten kd. Infektkrankh. Hyg. Abt. 1 Orig. 287:229-240. [DOI] [PubMed] [Google Scholar]
- 39.Ogden, N. H., K. Bown, B. K. Horrocks, Z. Woldehiwet, and M. Bennett. 1998. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the U.K. Med. Vet. Entomol. 12:423-429. [DOI] [PubMed] [Google Scholar]
- 40.Oteo, J. A., J. R. Blanco, V. Martinez de Artola, and V. Ibarra. 2000. First report of human granulocytic ehrlichiosis from southern Europe (Spain). Emerg. Infect. Dis. 6:430-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parola, P., L. Beati, M. Cambon, P. Brouqui, and D. Raoult. 1998. Ehrlichial DNA amplified from Ixodes ricinus (Acari: Ixodidae) in France. J. Med. Entomol. 35:180-183. [DOI] [PubMed] [Google Scholar]
- 42.Petrovec, M., S. Lotric Furlan, T. A. Zupanc, F. Strle, P. Brouqui, V. Roux, and J. S. Dumler. 1997. Human disease in Europe caused by a granulocytic Ehrlichia species. J. Clin. Microbiol. 35:1556-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovec, M., J. W. Sumner, W. L. Nicholson, J. E. Childs, F. Strle, J. Barlic, S. Lotric-Furlan, and T. Avsic-Zupanc. 1999. Identity of ehrlichial DNA sequences derived from Ixodes ricinus ticks with those obtained from patients with human granulocytic ehrlichiosis in Slovenia. J. Clin. Microbiol. 37:209-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pusterla, N., C. M. Leutenegger, J. B. Huder, R. Weber, U. Braun, and H. Lutz. 1999. Evidence of the human granulocytic ehrlichiosis agent in Ixodes ricinus ticks in Switzerland. J. Clin. Microbiol. 37:1332-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pusterla, N., R. Weber, C. Wolfensberger, G. Schar, R. Zbinden, W. Fierz, J. E. Madigan, J. S. Dumler, and H. Lutz. 1998. Serological evidence of human granulocytic ehrlichiosis in Switzerland. Eur. J. Clin. Microbiol. Infect. Dis. 17:207-209. [DOI] [PubMed] [Google Scholar]
- 46.Schouls, L. M., I. Van De Pol, S. G. Rijpkema, and C. S. Schot. 1999. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 37:2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steiner, F. E., R. R. Pinger, and C. N. Vann. 1999. Infection rates of Amblyomma americanum (Acari: Ixodidae) by Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) and prevalence of E. chaffeensis-reactive antibodies in white-tailed deer in southern Indiana, 1997. J. Med. Entomol. 36:715-719. [DOI] [PubMed] [Google Scholar]
- 48.Sumner, J. W., W. L. Nicholson, and R. F. Massung. 1997. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 35:2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sumption, K. J., D. J. M. Wright, S. J. Cutler, and B. A. S. Dale. 1995. Human ehrlichiosis in the UK. Lancet 346:1487-1488. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, D. R., M. Sillis, T. J. Coleman, S. M. Kench, N. H. Ogden, R. L. Salmon, P. Morgan-Capner, P. Softley, and D. Meadows. 1998. Low rates of ehrlichiosis and Lyme borreliosis in English farmworkers. Epidemiol. Infect. 121:609-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Dobbenburgh, A., A. P. van Dam, and E. Fikrig. 1999. Human granulocytic ehrlichiosis in western Europe. N. Engl. J. Med. 340:1214-1216. [DOI] [PubMed] [Google Scholar]
- 52.Von Stedingk, L. V., M. G. Gürtleschmid, H. S. Hanson, R. Gustafson, L. Dotevall, E. Olsson Engwall, and M. Granstrom. 1997. The human granulocytic ehrlichiosis (HGE) agent in Swedish ticks. Clin. Microbiol. Infect. 3:573-574. [DOI] [PubMed] [Google Scholar]
- 53.Walls, J. J., K. M. Asanovich, J. S. Bakken, and J. S. Dumler. 1998. Serologic evidence of a natural infection of white-tailed deer with the agent of human granulocytic ehrlichiosis in Wisconsin and Maryland. Clin. Diagn. Lab. Immunol. 5:762-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson, M. L., A. M. Ducey, T. S. Litwin, T. A. Gavin, and A. Spielman. 1990. Microgeographic distribution of immature Ixodes dammini ticks correlated with that of deer. Med. Vet. Entomol. 4:151-159. [DOI] [PubMed] [Google Scholar]